Abstract
Background
The antiretroviral treatment (ART) combination of stavudine, lamivudine and nevirapine (d4T/3TC/NVP) is the most frequently used initial regimen in many Asian countries. There are few data on the outcome of this treatment in clinic cohorts in this region.
Methods
We selected patients from the TREAT Asia HIV Observational Database (TAHOD) who started their first ART regimen with d4T/3TC/NVP. Treatment change was defined as cessation of therapy or the addition or change of one or more drugs. Clinical failure was defined as diagnosis with an AIDS defining illness, or death while on d4T/3TC/NVP treatment.
Results
The rate of treatment change among TAHOD patients starting d4T/3TC/NVP as their first antiretroviral treatment was 22.3 per 100 person-years, with lower baseline haemoglobin (i.e. anaemia) associated with slower rate of treatment change. The rate of clinical failure while on d4T/3TC/NVP treatment was 7.3 per 100 person-years, with baseline CD4 cell count significantly associated with clinical failure. After d4T/3TC/NVP was stopped, nearly 40% of patients did not restart any treatment and, of those who changed to other treatment, the majority changed to zidovudine (ZDV)/3TC/NVP and less than 3% of patients changed to a protease inhibitor (PI)-containing regimen. The rates of disease progression on the second-line regimen were similar to those on the first-line regimen.
Conclusions
These real-life data provide an insight into clinical practice in Asia and the Pacific region. d4T/3TC/NVP is maintained longer than other first-line regimens and change is mainly as a result of adverse effects rather than clinical failure. There is a need to develop affordable second-line antiretroviral treatment options for patients with HIV infection in developing countries.
Keywords: adverse effects, antiretroviral treatment, Asia-Pacific region, stavudine / lamivudine / nevirapine, treatment change
Introduction
The combination of stavudine, lamivudine and nevirapine (d4T/3TC/NVP) is the most popular combination currently in use in many Asian countries because the individual drugs are available as generics and also in a convenient fixed-dose combination [1,2]. Although increasingly widely used, the regimen has some theoretical disadvantages. d4T and NVP do not have the optimal toxicity profile of drugs within their class [3]. Furthermore, 3TC and NVP have a low genetic barrier to resistance and failure may involve and compromise further treatment with two of the major classes of drugs [nucleoside/ nucleotide reverse transcriptase inhibitors (NRTIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs)]. The third class of drugs, protease inhibitors (PIs), are expensive, and hence the management of patients who fail this regimen may be especially challenging in resourcepoor settings.
In spite of the widespread adoption of this regimen in antiretroviral therapy (ART) scale-up programmes, there is a paucity of published data on the use and outcome of this regimen in real-life clinical settings outside that of randomized controlled trials. Information about how well the regimen is tolerated and sustained in clinical practice is essential for evaluating the likely success of large-scale treatment programmes, and for understanding the magnitude of the need for second-line therapy or alternative drugs. The TREAT Asia HIV Observational Database (TAHOD) is a collaborative study by the TREAT Asia network, a co-operative network of clinicians throughout Asia and the Pacific, funded by the American Foundation of AIDS Research (amfAR) [4]. In this study, we describe the rate of treatment change among patients taking d4T/3TC/NVP in TAHOD and determine factors associated with d4T/3TC/NVP treatment change. We also describe the spectrum of first regimen changes selected after d4T/3TC/NVP change and examine the outcome with the second-line regimen.
Methods
TAHOD is a collaborative observational cohort study involving 12 sites in the Asia and Pacific region (see the Appendix). Detailed methods are published elsewhere [4], but briefly, each site recruited 200 patients, both treated and untreated with antiretroviral (ARV) drugs. Recruitment was based on a consecutive series of patients regularly attending a given site from a particular start-up time.
The following data were collected: (1) patient demographics: date of the clinical visit, age, gender, ethnicity, exposure category, date of first positive HIV test, HIV-1 subtype, and date and result of hepatitis B virus, hepatitis C virus and syphilis serology; (2) stage of disease: CD4 and CD8 cell count, HIV viral load estimation, prior AIDSdefining illness, and date and cause of death; (3) treatment history: prior and current prescribed antiretroviral treatments, reason for treatment changes (e.g. treatment failure, clinical failure and adverse events) and prophylactic treatments for opportunistic infection.
A modified version of the 1993 Centers for Disease Control and Prevention (CDC) AIDS case definition [5] was adopted, in which a presumptive diagnosis was available for most illnesses. All data were entirely observational, with test or intervention performed only according to the clinical guideline at each site. It was also noted that the laboratory methods varied across the sites. For example, HIV viral load estimations were obtained using a Roche Amplicor monitor (Roche Molecular Systems Inc., Branchburg, NJ, USA) or Quantiplex bDNA assay (Chiron Diagnostics, East Walpole, MA, USA).
Patients who started their first ARV combination with d4T/3TC/NVP were included in the study. Because of the limited number of patients who initiated treatment with d4T/3TC/NVP after recruitment to TAHOD, analysis was carried out on both retrospective and prospective data. Endpoints were defined as follows:
Treatment change: either stopping the d4T/3TC/NVP combination, or any addition or change of at least one drug. Follow-up was censored at the time of treatment change or death, or at the date of most recent data transfer (October 2004). Clinical failure: diagnosis with an AIDS-defining illness, or death while on d4T/3TC/NVP treatment. Follow-up was censored at the time of clinical failure while on treatment, or at the time of treatment change. Virological failure: while on d4T/3TC/NVP treatment, an HIV viral load measurement of 4 400 HIV-1 RNA copies/mL, obtained at least 6 months after treatment was started. Follow-up was censored at virological failure, or at the time of treatment change. Immunological failure: while on d4T/3TC/NVP treatment, three successive decreasing CD4 cell counts (the first obtained at least 6 months after treatment was started). Follow-up was censored at immunological failure or at the time of treatment change. Clinical failure after first treatment change: diagnosis with an AIDS-defining illness or death after d4T/3TC/ NVP was stopped. Follow-up was censored at the time of AIDS or death, or at the date of most recent data transfer (October 2004).
Rate of treatment change, and clinical, virological and immunological failures were measured using the persontime method. Factors associated with treatment change and clinical failure were assessed by univariate and multivariate analyses using Cox proportional hazard models. Covariates included in the analysis were demographics, time since HIV diagnosis, clinical disease at time of ART start, CD4 cell count and viral load prior to initiation of ART, nutritional status (body mass index), hepatitis B and C virus infection status, and haemoglobin level. Multivariate models were built using forward stepwise techniques. Statistical significance was taken as Po0.05.
Results
From September 2003 to 15 October 2004, 2088 patients were recruited to TAHOD. A total of 1702 patients were treated with ARV drugs (Table 1). The proportion of treated patients receiving monotherapy or double therapy decreased from 72% before 2000 to around 10% in recent years. From 2000 onwards, the majority of treated patients started with a NNRTI-based treatment combination, with three or more drugs containing at least one NNRTI and at least one NRTI, but without a PI. A total of 404 patients (23.7% of all patients starting their first treatment) started ARV treatment with d4T/3TC/NVP, which has been the major NNRTI-based treatment since 2002, with nearly half of all patients receiving the treatment. Treatment change and reasons: Among the 404 patients who started d4T/3TC/NVP as their first antiretroviral treatment, there were 131 patients who stopped the combination over a total of 586.9 person-years, a rate of 22.3 [95% confidence interval (CI) 18.8–26.5] per 100 person-years. The median duration of d4T/3TC/NVP treatment among the patients who stopped was 262 days (range 2–1519 days). The rate of treatment change of d4T/3TC/NVP was compared with that of other initial ARV regimens (Table 2, Fig. 1). Monotherapy and double therapy had the highest rate of treatment change, with almost all patients stopping within a year. The rate of change was lower in treatment with NNRTI-based triple or more combination therapy than in treatment with PI-based treatment, or in treatment containing three or more NRTIs. Treatment with d4T/3TC/NVP showed a lower rate of change than treatment with most other NNRTI-based regimens (Table 2). Baseline haemoglobin was the only significant risk factor associated with treatment change, with patients who were anaemic when d4T/3TC/NVP was started having a longer duration on this treatment (Table 3).
Table 1.
Type of first antiretroviral treatment, by year in which treatment started
| Year started | Mono/double antiretroviral | 3+ (NRTI ± PI – NNRTI)* | 3+ (NRTI+NNRTI – PI)† | 3+(NNRTI+PI ± NRTI)‡ | ||
|---|---|---|---|---|---|---|
| d4T/3TC/NVP | 3TC/ZDV/NVP | Others | ||||
|
| ||||||
| ≤1999 | 281 (72%) | 101 (26%) | 6 (2%) | 0 (0%) | ||
|
|
||||||
| 1 (17%) | 1 (17%) | 4 (66%) | ||||
|
|
||||||
| 2000 | 48 (29%) | 27 (16%) | 89 (54%) | 0 (0%) | ||
|
|
||||||
| 22 (25%) | 8 (9%) | 59 (66%) | ||||
|
|
||||||
| 2001 | 38 (17%) | 39 (18%) | 140 (64%) | 3 (1%) | ||
|
|
||||||
| 33 (24%) | 19 (14%) | 88 (63%) | ||||
|
|
||||||
| 2002 | 31 (8%) | 36 (10%) | 297 (81%) | 3 (1%) | ||
|
|
||||||
| 140 (47%) | 30 (10%) | 127 (43%) | ||||
|
|
||||||
| 2003 | 30 (7%) | 21 (4%) | 403 (88%) | 4 (1%) | ||
|
|
||||||
| 174 (43%) | 50 (12%) | 179 (45%) | ||||
|
|
||||||
| 2004 | 15 (14%) | 6 (6%) | 81 (77%) | 3 (3%) | ||
|
|
||||||
| 34 (42%) | 12 (15%) | 35 (43%) | ||||
3+ (NRTI ± PI – NNRTI), three or more drugs including at least one nucleoside reverse transcriptase inhibitor (NRTI) and/or a protease inhibitor (PI), but without a nonnucleoside reverse transcriptase inhibitor (NNRTI).
3+ (NRTI + NNRTI – PI), three or more drugs including at least one NNRTI, but without a PI.
3+ (NNRTI + PI ± NRTI), three or more drugs including at least one NNRTI and one PI, with or without an NRTI.
d4T, stavudine; 3TC, lamivudine; NVP, nevirapine; ZDV, zidovudine.
Table 2.
Rate of change of first antiretroviral treatment
| First antiretroviral combination | Number of patients | Number stopped | Follow up (years) | Rate (per 100 person-years) | 95% CI (per 100 person-years) |
|---|---|---|---|---|---|
|
| |||||
| NNRTI-based | 1016 | 395 | 1504.6 | 26.3 | 23.8–29.0 |
| d4T/3TC/NVP | 404 | 131 | 586.9 | 22.3 | 18.8–26.5 |
| Other NNRTI-based* | 612 | 264 | 917.7 | 28.8 | 25.5–32.5 |
| 3TC/ZDV/EFV | 201 | 67 | 309.9 | 21.6 | 17.0–27.5 |
| 3TC/ZDV/NVP | 120 | 51 | 160.6 | 31.8 | 24.1–41.8 |
| 3TC/d4T/EFV | 112 | 38 | 180.7 | 21.0 | 15.3–28.9 |
| ddI/d4T/EFV | 76 | 48 | 151.8 | 31.6 | 23.8–42.0 |
| ddI/d4T/NVP | 56 | 35 | 47.9 | 73.1 | 52.5–101.8 |
| Other | 47 | 25 | 66.8 | 37.4 | 25.3–55.4 |
| PI-based | 13 | 5 | 13.5 | 36.9 | 15.4–88.7 |
| Three or more, NRTI only | 230 | 181 | 334.9 | 54.1 | 46.7–62.5 |
| Mono/double therapy | 443 | 422 | 424.4 | 99.4 | 90.4–109.4 |
| Total | 1702 | 1003 | 2277.4 | 44.0 | 41.4–46.9 |
Excluding d4T/3TC/NVP.
CI, confidence interval; d4T, stavudine; 3TC, lamivudine; NVP, nevirapine; ZDV, zidovudine; EFV, efavirenz; ddI, didanosine; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Fig. 1.
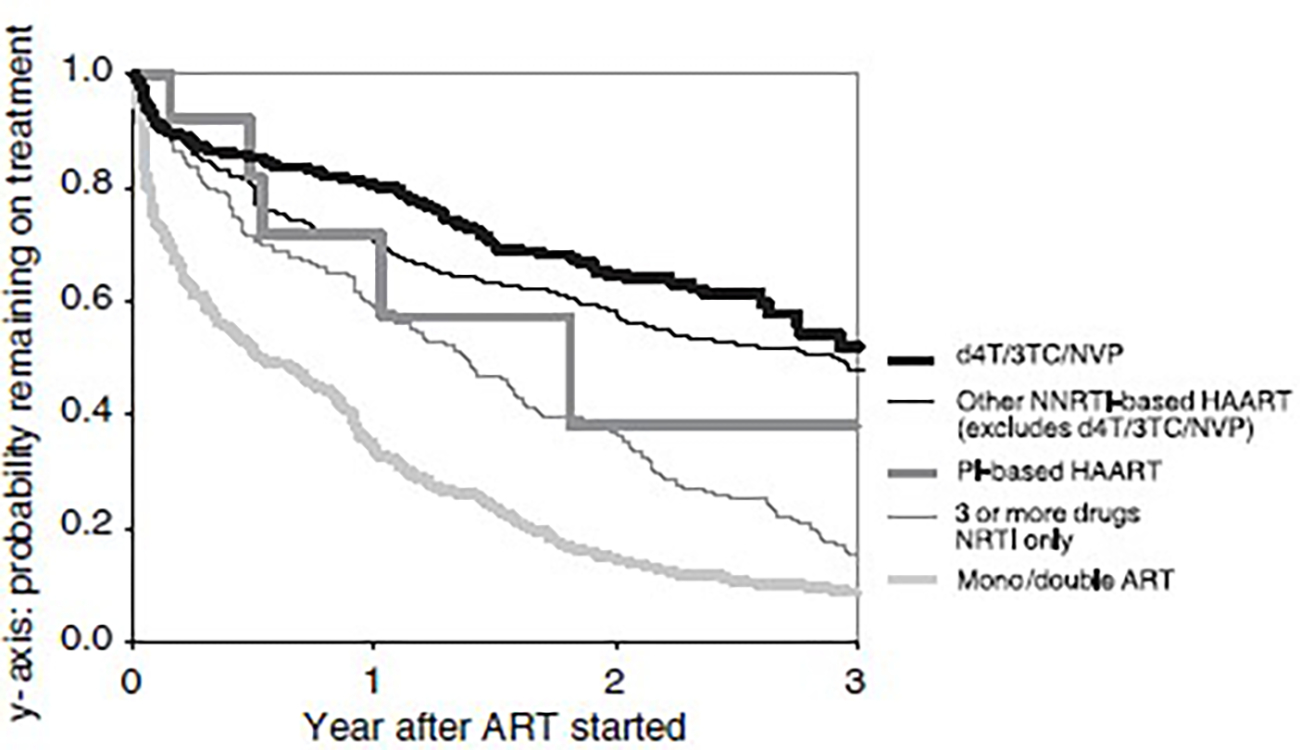
Duration of first antiretroviral treatment. d4T, stavudine; 3TC, lamivudine; NVP, nevirapine; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; HAART, highly active antiretroviral therapy; PI, protease inhibitor; ART, antiretroviral therapy.
Table 3.
Predictors of treatment change among patients starting stavudine/lamivudine/nevirapine (d4T/3TC/NVP) as their first antiretroviral treatment
| Number of patients | Follow up (years) | Number of events | Rate* | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|---|
| HR | P-value | HR (95% CI) | P-value | |||||
|
| ||||||||
| Total | 404 | 586.9 | 131 | 22.3 | ||||
| Gender | ||||||||
| Male | 299 | 434.6 | 98 | 22.5 | 1.00 | 1.00 | ||
| Female | 105 | 152.3 | 33 | 21.7 | 0.96 | 0.822 | 0.92 (0.62–1.37) | 0.677 |
| Age when treatment started (years) | ||||||||
| ≤30 | 129 | 206.8 | 40 | 19.3 | 1.00 | 1.00 | ||
| 31–40 | 202 | 275.8 | 67 | 24.3 | 1.22 | 0.322 | 1.26 (0.84–1.87) | 0.260 |
| ≥41 | 73 | 104.4 | 24 | 23.0 | 1.16 | 0.571 | 1.18 (0.71–1.96) | 0.517 |
| Ethnicity | ||||||||
| Thai | 222 | 345.2 | 79 | 22.9 | 1.00 | 1.00 | ||
| Indian | 128 | 188.4 | 34 | 18.0 | 0.78 | 0.223 | 0.69 (0.45–1.06) | 0.089 |
| Other | 54 | 53.3 | 18 | 33.8 | 1.33 | 0.272 | 1.12 (0.65–1.95) | 0.682 |
| Exposure category | ||||||||
| Heterosexual contact | 363 | 534.7 | 118 | 22.1 | 1.00 | 1.00 | ||
| Other | 41 | 52.2 | 13 | 24.9 | 1.07 | 0.823 | 1.01 (0.56–1.82) | 0.983 |
| Time between HIV treatment and treatment initiation (years) | ||||||||
| <1 | 239 | 360.7 | 77 | 21.3 | 1.00 | 1.00 | ||
| 1–3 | 119 | 154.1 | 39 | 25.3 | 1.14 | 0.497 | 1.10 (0.74–1.62) | 0.638 |
| ≥4 | 43 | 69.4 | 14 | 20.2 | 0.94 | 0.843 | 0.95 (0.54–1.68) | 0.854 |
| Test missing | 3 | 2.7 | 1 | 36.4 | 1.60 | 0.640 | 1.76 (0.24–12.8) | 0.577 |
| Prior AIDS when treatment started | ||||||||
| No | 215 | 334.1 | 72 | 21.6 | 1.00 | 1.00 | ||
| Yes | 189 | 252.8 | 59 | 23.3 | 1.08 | 0.667 | 1.21 (0.84–1.73) | 0.308 |
| CD4 count prior to treatment (cells/μL)† | ||||||||
| <50 | 81 | 104.1 | 23 | 22.1 | 1.00 | 1.00 | ||
| 50–99 | 43 | 63.3 | 11 | 17.4 | ||||
| 100–199 | 69 | 93.2 | 22 | 23.6 | ||||
| ≥200 | 42 | 56.6 | 19 | 33.6 | ||||
| ≥50 | 154 | 213.1 | 52 | 24.4 | 1.12 | 0.650 | 1.00 (0.61–1.66) | 0.973 |
| Not tested | 169 | 269.8 | 56 | 20.8 | 0.98 | 0.936 | 0.90 (0.51–1.59) | 0.706 |
| HIV viral load prior to treatment (copies/mL)† | ||||||||
| ≤150 000 | 25 | 36.0 | 8 | 22.2 | 1.00 | 1.00 | ||
| ≥150 001 | 27 | 28.1 | 12 | 42.7 | 1.81 | 0.193 | 2.20 (0.88–5.45) | 0.090 |
| Not tested | 352 | 522.8 | 111 | 21.2 | 0.93 | 0.842 | 1.05 (0.50–2.20) | 0.901 |
| BMI‡ prior to treatment | ||||||||
| Underweight | 57 | 82.2 | 15 | 18.2 | 1.00 | 1.00 | ||
| Normal | 61 | 84.0 | 17 | 20.2 | 1.09 | 0.806 | 0.93 (0.46–1.91) | 0.853 |
| Overweight | 13 | 9.8 | 3 | 30.8 | 1.40 | 0.597 | 0.97 (0.27–3.51) | 0.961 |
| Not tested | 273 | 410.9 | 96 | 23.3 | 1.27 | 0.397 | 1.24 (0.67–2.30) | 0.501 |
| Hepatitis B virus infection§ | ||||||||
| Negative | 140 | 167.4 | 50 | 29.9 | 1.00 | 1.00 | ||
| Positive | 11 | 21.8 | 4 | 18.3 | 0.62 | 0.363 | 0.64 (0.23–1.79) | 0.395 |
| Not tested | 253 | 297.7 | 77 | 19.4 | 0.67 | 0.035 | 0.73 (0.50–1.07) | 0.107 |
| Hepatitis C virus infection§ | ||||||||
| Negative | 98 | 115.8 | 31 | 26.8 | 1.00 | 1.00 | ||
| Positive | 12 | 15.3 | 5 | 32.7 | 1.18 | 0.731 | 1.13 (0.43–2.95) | 0.801 |
| Not tested | 294 | 455.8 | 95 | 20.8 | 0.81 | 0.320 | 0.85 (0.57–1.28) | 0.442 |
| Haemoglobin¶ prior to treatment | ||||||||
| Normal | 61 | 68.7 | 21 | 37.8 | 1.00 | 1.00 | ||
| Anaemia | 155 | 215.9 | 41 | 19.0 | 0.53 | 0.013 | 0.53 (0.32–0.87) | 0.013 |
| Not tested | 188 | 302.3 | 64 | 21.2 | 0.61 | 0.034 | 0.61 (0.38–0.96) | 0.034 |
Rate per 100 person-years.
CD4 count measured within 180 days before treatment started; HIV viral load measured within 365 days before treatment started.
Body mass index (BMI)=weight (kg)/[height (m)]2: Underweight, BMI < 18.5; normal, BMI 18.5–24.9; overweight, BMI>25.0.
Hepatitis B virus infection: a positive hepatitis B surface antigen (HBsAg) test; hepatitis C virus infection: a positive anti-hepatitis C virus antibody test.
Haemoglobin tested within 180 days before treatment started. Definition of anaemia: normal, haemoglobin>13 g/dL for male and 12 g/dL for female subjects; anaemia, 8–13 g/dL for male and 8–12 g/dL for female subjects; severe anaemia, <8 g/dL for both male and female subjects.
CI, confidence interval; HR, hazard ratio.
Table 4 summarizes the reasons for d4T/3TC/NVP treatment change and duration of treatment. Adverse effects (62.6% of patients stopped d4T/3TC; 20.3% of the 404 patients included in the study) were the major reason for treatment change, among which lipodystrophy was the most common, followed by hepatitis, rash and peripheral neuropathy. Drug interaction was not reported as a reason for treatment change. There were large variations of duration on treatment before the treatment change. Clinical failure: Among the 404 patients who started d4T/3TC/NVP as their first antiretroviral treatment, two patients died and 38 patients developed AIDS during treatment over 547.7 person-years of follow-up, giving a rate of clinical failure of 7.3 per 100 person-years (95% CI 5.4–10.0). The majority of newly diagnosed AIDS cases after treatment were a result of tuberculosis (39.5%), followed by cytomegalovirus retinitis (15.8%), extrapulmonary cryptococcosis (10.5%) and toxoplasmosis (10.5%). Baseline CD4 cell count was significantly associated with disease progression (data not shown).
Table 4.
Reasons for stavudine/lamivudine/nevirapine (d4T/3TC/NVP) treatment change and duration of treatment
| Reason for treatment change | No. (%) | Days on treatment [median (range)] | No. (%) who stopped treatment permanently* |
|---|---|---|---|
|
| |||
| Total | 131 (100) | 262 (2–1519) | 15 (11.5) |
| Adverse effects | 82 (62.6) | 295 (2–1519) | 2 (2.4) |
| Lipodystrophy | 26 (31.7) | 546 (30–1519) | 0 (0.0) |
| Hepatitis | 10 (12.2) | 27.5 (14–1297) | 0 (0.0) |
| Rash | 12 (14.6) | 14 (10–108) | 0 (0.0) |
| Peripheral neuropathy | 9 (11.0) | 407 (117–532) | 0 (0.0) |
| Lactic acidosis | 6 (7.3) | 416 (204–951) | 0 (0.0) |
| Pancreatitis | 5 (6.1) | 55 (5–343) | 1 (20.0) |
| Nausea and vomiting | 4 (4.9) | 17.5 (2–49) | 0 (0.0) |
| Fever | 2 (2.4) | 19 (11–27) | 0 (0.0) |
| Allergy | 1 (1.2) | 61 | 0 (0.0) |
| Insomnia | 1 (1.2) | 42 | 0 (0.0) |
| Missing | 6 (7.3) | 67.5 (21–491) | 1 (16.7) |
| Patient’s decision/request | 18 (13.7) | 195.5 (6–1005) | 7 (38.9) |
| Treatment failure | 5 (3.8) | 457 (138–672) | 0 (0.0) |
| Clinical progression/hospitalization | 3 (2.3) | 85 (34–322) | 1 (33.3) |
| Compliance difficulties | 2 (1.5) | 135.5 (9–262) | 0 (0.0) |
| Other | 18 (13.7) | 250 (13–1071) | 4 (22.2) |
| Financial problem | 3 (16.7) | 467 (264–549) | 1 (33.3) |
| Doctor’s decision | 2 (11.1) | 809 (547–1071) | 0 (0.0) |
| Pregnancy | 2 (11.1) | 646.5 (286–1007) | 0 (0.0) |
| Lost to follow up | 1 (5.6) | 29 | 0 (0.0) |
| Patient died | 1 (5.6) | 236 | 1 (100) |
| Not specified | 9 (50.0) | 155 (13–415) | 2 (22.2) |
| Not reported | 3 (2.3) | 211 (88–545) | 1 (33.3) |
As a percentage of the total number of patients who changed treatment for a specific reason (in the second column).
Among the 38 patients who had an AIDS diagnosis, 13 stopped d4T/3TC/NVP at various stages, but none of these 13 patients stopped d4T/3TC/NVP because of the AIDS diagnosis, which was defined as an endpoint in the analysis. Reported reasons for stopping varied from the patient’s decision to adverse effects or clinical failure at a later stage (nearly 1 year after the AIDS diagnosis).
Virological and immunological failure (among patients with a viral load or CD4 cell measurement after starting d4T/3TC/NVP)
There were 57 patients who had at least one HIV viral load assessment 6 months after starting d4T/3TC/NVP. Five patients had an HIV viral load of more than 400 copies/mL while on treatment (over 132.3 person-years), giving a rate of 3.7 per 100 person-years (95% CI 1.6–9.1). There were 56 patients who had at least three CD4 cell counts 6 months after starting d4T/3TC/NVP. During the period of d4T/3TC/NVP treatment (over 148.5 person-years), 10 patients had three consecutive decreasing CD4 cell counts, giving a rate of 6.7 per 100 person-years (95% CI 3.6–12.5).
Most of these patients either did not stop d4T/3TC/NVP treatment because of the virological or immunological failure, or stopped long after the failure, with the reasons for treatment change mainly not related to virological failure (data not shown). Adverse effects (mainly lipodystrophy) were again the major reason for treatment change.
Treatment change and outcome
Table 5 summarizes the ARV treatment used after d4T/3TC/NVP was stopped and the duration on d4T/3TC/NVP before stopping. Nearly 40% of the 131 patients stopped taking any treatment, at least for some period of time. Among those who changed to other treatment, the majority changed to zidovudine (ZDV)/3TC/NVP. Only three patients (< 3%) changed to a combination containing a PI.
Table 5.
Antiretroviral treatment after stavudine/lamivudine/nevirapine (d4T/3TC/NVP) stopped and duration on d4T/3TC/MVP before treatment changed
| Antiretroviral treatment after d4T/3TC/NVP stopped | No. of patients | % of patients | Duration on d4T/3TC/NVF before Stopping [median (range)] |
|---|---|---|---|
|
| |||
| Not treated | 49 | 37.4 | 83 (2–549) |
| Stopped treatment permanently | 15 | 11.4 | 343 (23–545) |
| Changed to other treatment | 34 | 26.0 | 27.5 (2–549) |
| after some time | |||
| d4T/3TC/NVP | 11 | 8.4 | |
| d4T/3TC/EFV | 11 | 8.4 | |
| 3TC/ZDV/EFV | 4 | 3.1 | |
| d4T/3TC | 3 | 2.3 | |
| ddI/3TC/NVP | 1 | 0.8 | |
| ddI/d4T/SQV/RTV | 1 | 0.8 | |
| d4T/3TC/IDV/RTV | 1 | 0.8 | |
| 3TC/ZDV/NVP | 1 | 0.8 | |
| 3TC/NVP | 1 | 0.8 | |
| 3TC/ZDV/NVP | 51 | 38.9 | 469 (5–1519) |
| Stopped d4T/3TC/NVP | 44 | 33.4 | 470.5 (5–1519) |
| because of adverse effects | |||
| Lipodvstrophy | 23 | 17.6 | 618 (406-1S19) |
| Peripheral neuropathy | 9 | 6.9 | 407 (117–532) |
| Lactic acidosis | 6 | 4.6 | 416 (204–951) |
| d4T/3TC | 12 | 9.2 | 32.5 (11–95) |
| d4T/3TC/EFV | 6 | 4.6 | 60.5 (14–1297) |
| 3TC/ZDV/EFV | 5 | 3.8 | 418 (138–672) |
| 3TC/NVP | 3 | 2.3 | 547 (14–1007) |
| ABC/3TC/NVP | 1 | 0.8 | 552 |
| d4T/3TC/NVP/IDV | 1 | 0.8 | 202 |
| d4T/3TC/NVP/RTV | 1 | 0.8 | 88 |
| d4T/3TC/NVP/5QV/RTV | 1 | 0.8 | 211 |
| d4T/NVP | 1 | 0.8 | 42 |
ABC, abacavir; ddI, didanosine; EFV, efavirenz; IDV, indinavir; RTV, ritonavir; SQV, saquinavir; ZDV, zidovudine.
The major reason for treatment change among those patients who changed to ZDV/3TC/NVP was adverse effects (86%, P<0.001), over half of which were lipodystrophy (52%), followed by peripheral neuropathy (20%) and lactic acidosis (14%). Patients who changed to ZDV/3TCNVP had remained on d4T/3TC/NVP for a significantly longer time (median 469 days; range 5–1398 days) than had the patients who changed to treatment other than ZDV/3TC/NVP after stopping d4T/3TC/NVP (median 79 days; range 2–1297 days; P<0.001).
A total of 20 patients developed AIDS and three died (one patient died with an AIDS diagnosis on the same day) after stopping d4T/3TC/NVP, over a total of 152.0 person-years of follow up, giving a rate of clinical failure of 14.5 per 100 person-years (95% CI 9.5–22.0). Patients receiving ZDV/3TC/NVP after stopping d4T/3TC/NVP had a lower rate of clinical failure than patients receiving no treatment (3.8 vs 27.3 per 100 person-years; P=0.004).
Discussion
Initial treatment with two NRTIs and one NNRTI is recommended by the World Health Organization (WHO) [6], as part of the ‘3 by 5’ strategy against HIV and AIDS [7]. Nearly one in four of the TAHOD patients started their ARV treatment with d4T/3TC/NVP. Data on whether the drug was generic or proprietary was not collected. However, in Asian countries such as India [1,2] and Thailand [8], the generic d4T/3TC/NVP combination, or GPO-VIR in Thailand, is the most popular combination currently in use.
In this study, the rate of treatment change among TAHOD patients starting d4T/3TC/NVP as the first ARV treatment was found to be 22.3 per 100 person-years. The rate of d4T/3TC/NVP treatment change was comparable to that seen in other studies. Using data from the Australia HIV Observational Database (AHOD), Petoumenos et al. [9] reported a rate of treatment change of 31% among patients who started their first NNRTI-based HAART combination. In the OzCombo 2 Study [10], 27% of the patients on d4T/3TC/NVP did not complete treatment over a 52-week clinical trial. In a study in India [1], among patients receiving a generic NVP-based HAART regimen (55% of them receiving NVP in combination with d4T/3TC), 16% stopped treatment. The substantial rate of change is of concern given the very limited options available in some settings in TAHOD, as many people who stop actually do not have a viable alternative treatment regimen. This also shows that a one-combination-fits-all policy is potentially going to deprive a substantial number of patients of effective long-term therapy. In AHOD, patients with a baseline CD4 count of<200 cells/μL had an earlier treatment change, while in this study a lower baseline haemoglobin level (i.e. anaemia) was associated with a later treatment change. This association with anaemia may be explained by the fact that the common alternative drug, ZDV, is contraindicated in patients with anaemia. Hence there might be greater reluctance to change d4T/3TC/NVP therapy in anaemic patients because there are few effective and affordable options.
The rate of treatment change was lower among patients starting d4T/3TC/NVP than among patients initiating most other NNRTI-based triple or more combination therapies, a PI-based regimen or a triple combination containing only NRTI. Similar to AHOD, patients receiving NNRTI-based HAART treatment had a slower change rate than patients receiving PI-based treatment. Because of the small number of patients who were receiving PI-based treatment, further comparison was not performed. The reason for a higher change rate for PI-based regimens might be toxicity [9] or the unsustainable expense for some patients.
In this study, the main reported reason for d4T/3TC/NVP treatment change was adverse effects, this reason being recorded for 20% of the 404 patients initiating d4T/3TC/NVP and 62.6% of the 131 patients who stopped d4T/3TC/NVP. In a study with a relatively short follow-up time of 24 weeks in treatment-naïve patients receiving a fixed-dosed combination of d4T/3TC/NVP in Thailand [8], 20% experienced adverse events and 15% withdrew from the study because of the adverse events. Side effects were also the major reason for discontinuing HAART treatment in Indian patients (64% of patients who discontinued a generic HAART regimen) [1]. Although lactic acidosis and lipodystrophy were not observed during this 24-week study, this is consistent with our observation that these are late events: the median duration on treatment for patients who stopped because of lactic acidosis was 416 (range 204–951) days and that for lipodystrophy was 546 (30–1519) days. Given the relatively short period of follow up in TAHOD, the true prevalence of adverse effects leading to long-term treatment change, such as lipodystrophy, lactic acidosis and peripheral neuropathy, may have been underestimated in this study, whereas early toxicities, such as hepatitis, rash and pancreatitis, may mainly have been captured. The prevalence of treatment-related hepatitis (2.5% of all patients receiving d4T/3TC/NVP) was similar to that found in a clinical trial setting in Thailand (5.8%) [11] and in observational studies in India (3.2%) [2] and Thailand (7%) [8]. However, without liver enzyme monitoring for all patients receiving treatment, this prevalence may be underestimated. The prevalence of lipodystrophy was low compared with prevalences for Western cohorts (38–50% [3,12,13]) but similar to those for other Asian patient populations [14,15]. This may reflect the fact that lipodystrophy is less common among nonwhite patients, and also the small proportion of patients receiving PI-based treatment in our cohort [15].
The rate of clinical failure while on d4T/3TC/NVP treatment and the relationship between baseline CD4 cell count and disease progression are similar to findings obtained in other studies [1,8,16,17]. It is possible that some of the events may have represented immune reconstitution syndrome. In a cohort of patients receiving fixed-dose combination therapy in India, 67% of clinical events were defined as immune reconstitution syndrome [2].
The rate of virological and immunological failure after 6 months of treatment was consistent with the findings of previous studies [8,16], although relatively few patients had measurements taken. It is interesting that neither disease progression nor virological or immunological failure was a major reason for treatment change, as patients generally continued their treatment after failure. When they finally stopped the regimen, it was mainly because of adverse effects. Another point worth noting is the relative paucity of data for HIV viral load and CD4 cell count change among patients receiving treatment, although TAHOD participating sites are by and large the better academic centres in the region. It is clear that many patients are managed without regular viral load and CD4 cell count monitoring, and yet seem to have similar rates of survival and response to treatment to patients who do receive such monitoring [4,17]. This shows that limitations in laboratory monitoring should not necessarily impede implementation of ART. However, the fact that early failure is not being detected in these patients by laboratory monitoring and the fact that treatment is maintained even in the patients in whom such failure is being detected suggest that many of these patients are likely to develop extensive resistance by the time a treatment change finally occurs.
After d4T/3TC/NVP was stopped, nearly 40% of patients ceased ART entirely. Among those who changed to other treatment, the majority changed to ZDV/3TC/NVP. This change is unlikely to be a result of early toxicity and is more likely to reflect a concern to avoid future lipodystrophy. When a cheap fixed-dose combination containing ZDV/3TC/NVP becomes more widely available, it is likely that this will be frequently used as the initial regimen because of this concern to avoid lipodystrophy. Only three patients changed to a combination containing PI. This may also reflect the limited choice of treatment among patients in the Asia and Pacific region. The rates of progression on the second-line regimen were similar to those on the first-line regimen, further confirming that patients who changed were probably not failing the first-line regimen, but changed mainly because of toxicity concerns.
Several limitations should be taken into consideration when interpreting the results. Firstly, the analysis was performed on both prospective and retrospective data. With data transfer each year, increasing amounts of prospective data will be available for better understanding of the efficacy and side effects of the d4T/3TC/NVP regimen and other treatment combinations. Secondly, the TAHOD patients, who are recruited if they have ‘good’ follow-up based on clinicians’ judgement, cannot be seen as entirely representative of HIV-infected patients in the Asia–Pacific region. However, studies on the natural history of HIV disease and responses to ARV treatment can still be derived from a cohort of TAHOD patients with good follow up, albeit with some limitations on the generalizability of the findings. Thirdly, although the overall follow-up rate is satisfactory (up to 90%), loss to follow-up must be considered when interpreting the results. AIDS or adverse effects might be underreported if a patient does not regularly visit the clinics or if a patient dies. Efforts have been made to maximize follow-up, with each site developing its own mechanism to contact patients whenever possible.
In summary, our study presents real-life data providing an insight into clinical practice in Asia and the Pacific region. It confirms the findings of earlier studies in African and Asian countries that have shown treatment with the simple fixed-dose combination of d4T/3TC/NVP to be safe and effective, with good adherence and tolerability [1,2,8,16]. It is of concern that there is a lack of systematic laboratory monitoring, together with very limited options for treatment changes, other than those implemented for side effects. It is also notable that adverse events are the most common reported reason for treatment change. As toxicity is likely to remain a problem, there is a need to develop affordable second-line ART options for patients with HIV infection in developing countries. Although d4T/3TC/NVP appears to be relatively well tolerated by the majority of patients, the current practice of treatment may well create extensive problems with resistance in the future.
Acknowledgements
TREAT Asia and TAHOD are funded by a grant from the American Foundation for AIDS Research. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales.
Appendix
Appendix: the TREAT Asia HIV Observational Database
C. V. Mean* and V. Saphonn*, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia; F. Zhang*, H. Zhao and N. Han, Beijing Ditan Hospital, Beijing, China; P. Li* and M. P. Lee, Queen Elizabeth Hospital, Hong Kong, China; N. Kumarasamy* and J. A. Cecelia, YRG Centre for AIDS Research and Education, Chennai, India; S. Pujari* and K. Joshi, HIV Project, Ruby Hall Clinic, Pune, India; T. P. Merati* and F. Yuliana, Faculty of Medicine, Udayana University & Sanglah Hospital, Bali, Indonesia; S. Oka* and M. Honda, International Medical Centre of Japan, Tokyo, Japan; C. K. C. Lee* and J. Pang, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; A. Kamarulzaman* and C. Sim, University of Malaya, Kuala Lumpur, Malaysia; R. Ditangco*† and R. Capistrano, Research Institute for Tropical Medicine, Manila, Philippine; Y. M. A. Chen*, W. W. Wong and Y. R. Chang, Taipei Veterans General Hospital and AIDS Prevention and Research Centre, National Yang-Ming University, Taipei, Taiwan; P. L. Lim*, C. C. Lee and S. M. Thitsar, Tan Tock Seng Hospital, Singapore; P. Phanuphak* and M. Khongphattanayothing, HIV-NAT/The Thai Red Cross AIDS Research Centre, Bangkok, Thailand; A. Vibhagool*, S. Kiertiburanakul and W. Kiatatchasai, Ramathibodi Hospital, Bangkok, Thailand; T. Sirianthana*, Research Institute for Health Sciences, Chiangmai, Thailand; J. Chuah*, Gold Coast Sexual Health Clinic, Miami, Queensland, Australia; K. Frost* and S. Wong, American Foundation for AIDS Research, New York, NY, USA; D. A. Cooper*, M. G. Law*, K. Petoumenos and J. Zhou*, National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, Australia.
*Steering Committee member; †current Steering Committee chair.
References
- 1.Kumarasamy N, Solomon S, Chaguturu SK et al. The safety, tolerability and effectiveness of generic antiretroviral drug regimens for HIV-infected patients in south India. AIDS 2003; 17: 2267–2269. [DOI] [PubMed] [Google Scholar]
- 2.Pujari SN, Patel AK, Naik E et al. Effectiveness of generic fixeddose combinations of highly active antiretroviral therapy for treatment of HIV infection in India. J Acquir Immune Defic Syndr 2004; 37: 1566–1569. [DOI] [PubMed] [Google Scholar]
- 3.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet 2000; 356: 1423–1430. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Kumarasamy N, Ditangco R et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr 2005; 38: 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1993. revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992; 41: 1–19. [PubMed] [Google Scholar]
- 6.World Health Organization. Scaling Up Antiretroviral Therapy in Resource-limited Settings. Treatment Guidelines for a Public Health Approach – 2003 Revision. Geneva, Switzerland: World Health Organization, 2004. [Google Scholar]
- 7.WHO/UNAIDS. Treating 3 Million by 2005: Making It Happen – The WHO Strategy. Geneva, 2003. [Google Scholar]
- 8.Anekthananon T, Ratanasuwan W, Techasathit W, Sonjai A, Suwanagool S. Safety and efficacy of a simplified fixeddose combination of stavudine, lamivudine and nevirapine (GPO-VIR) for the treatment of advanced HIV-infected patients: a 24-week study. J Med Assoc Thai 2004; 87: 760–767. [PubMed] [Google Scholar]
- 9.The Australian HIV Observational Database. Rates of combination antiretroviral treatment change in Australia, 1997–2000. HIV Med 2002; 3: 28–36. [DOI] [PubMed] [Google Scholar]
- 10.French M, Amin J, Roth N et al. Randomized, open-label, comparative trial to evaluate the efficacy and safety of three antiretroviral drug combinations including two nucleoside analogues and nevirapine for previously untreated HIV-1 Infection: the OzCombo 2 study. HIV Clin Trials 2002; 3: 177–185. [DOI] [PubMed] [Google Scholar]
- 11.Law WP, Dore GJ, Duncombe CJ et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996–2001. AIDS 2003; 17: 2191–2199. [DOI] [PubMed] [Google Scholar]
- 12.Carr A, Samaras K, Burton S et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 1998; 12: F51–F58. [DOI] [PubMed] [Google Scholar]
- 13.Carr A HIV protease inhibitor-related lipodystrophy syndrome. Clin Infect Dis 2000; 30 (Suppl. 2): S135–S142. [DOI] [PubMed] [Google Scholar]
- 14.Chang KH, Kim JM, Song YG, Hong SK, Lee HC, Lim SK. Does race protect an oriental population from developing lipodystrophy in HIV-infected individuals on HAART? J Infect 2002; 44: 33–38. [DOI] [PubMed] [Google Scholar]
- 15.Paton NI, Earnest A, Ng YM, Karim F, Aboulhab J. Lipodystrophy in a cohort of human immunodeficiency virusinfected Asian patients: prevalence, associated factors, and psychological impact. Clin Infect Dis 2002; 35: 1244–1249. [DOI] [PubMed] [Google Scholar]
- 16.Laurent C, Kouanfack C, Koulla-Shiro S et al. Effectiveness and safety of a generic fixed-dose combination of nevirapine, stavudine, and lamivudine in HIV-1-infected adults in Cameroon: open-label multicentre trial. Lancet 2004; 364: 29–34. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Kumarasamy N. Predicting short-term disease progression among HIV-infected patients in Asia and the Pacific region: preliminary results from the TREAT Asia HIV Observational Database. HIV Med 2005; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


