Key Points
Question
Does early metformin initiation improve glycemic control and reduce insulin use in pregnant individuals with gestational diabetes?
Findings
In this randomized clinical trial, the composite outcome of insulin initiation and a fasting glucose level of 5.1 mmol/L (92 mg/dL) or greater at gestation weeks 32 or 38 was not significantly different between groups. Secondary outcomes of maternal glycaemic control, weight gain, and infant size were lower in the metformin group. There was no difference in maternal or neonatal morbidities.
Meaning
Early metformin initiation did not reduce the occurrence of the combination of a fasting glucose level of 5.1 mmol/L or greater at gestation weeks 32 or 38 or insulin initiation. Ongoing review of rates of small for gestational age should continue when using metformin.
Abstract
Importance
Gestational diabetes is a common complication of pregnancy and the optimal management is uncertain.
Objective
To test whether early initiation of metformin reduces insulin initiation or improves fasting hyperglycemia at gestation weeks 32 or 38.
Design, Setting, and Participants
Double-blind, placebo-controlled trial conducted in 2 centers in Ireland (one tertiary hospital and one smaller regional hospital). Participants were enrolled from June 2017 through September 2022 and followed up until 12 weeks’ postpartum. Participants comprised 510 individuals (535 pregnancies) diagnosed with gestational diabetes based on World Health Organization 2013 criteria.
Interventions
Randomized 1:1 to either placebo or metformin (maximum dose, 2500 mg) in addition to usual care.
Main Outcomes And Measures
The primary outcome was a composite of insulin initiation or a fasting glucose level of 5.1 mmol/L or greater at gestation weeks 32 or 38.
Results
Among 510 participants (mean age, 34.3 years), 535 pregnancies were randomized. The primary composite outcome was not significantly different between groups and occurred in 150 pregnancies (56.8%) in the metformin group and 167 pregnancies (63.7%) in the placebo group (between-group difference, −6.9% [95% CI, −15.1% to 1.4%]; relative risk, 0.89 [95% CI, 0.78-1.02]; P = .13). Of 6 prespecified secondary maternal outcomes, 3 favored the metformin group, including time to insulin initiation, self-reported capillary glycemic control, and gestational weight gain. Secondary neonatal outcomes differed by group, with smaller neonates (lower mean birth weights, a lower proportion weighing >4 kg, a lower proportion in the >90% percentile, and smaller crown-heel length) in the metformin group without differences in neonatal intensive care needs, respiratory distress requiring respiratory support, jaundice requiring phototherapy, major congenital anomalies, neonatal hypoglycemia, or proportion with 5-minute Apgar scores less than 7.
Conclusion and relevance
Early treatment with metformin was not superior to placebo for the composite primary outcome. Prespecified secondary outcome data support further investigation of metformin in larger clinical trials.
Trial Registration
ClinicalTrials.gov Identifier: NCT02980276; EudraCT: 2016-001644-19
This randomized clinical trial compares use of metformin vs placebo in treating the maternal and neonatal outcomes associated with gestational diabetes.
Introduction
Gestational diabetes mellitus, defined as carbohydrate intolerance resulting in hyperglycemia with onset during pregnancy,1 is a global health problem estimated to annually affect 2.93 million pregnancies worldwide. The major burden of cases occur in low- and middle-income countries.2 Gestational diabetes is associated with an increased risk of several adverse maternal pregnancy outcomes including weight gain, cesarean birth, and preeclampsia, and an increased risk of adverse fetal outcomes requiring neonatal intensive care unit admission (eg, birth injuries and respiratory distress).3,4 Moreover, pregnant individuals and their offspring are at increased long-term risk of type 2 diabetes.5,6,7
While there is convincing evidence that improved glycemic control is associated with improved maternal and fetal outcomes,8,9 there is uncertainty about the optimal management approach following a gestational diabetes diagnosis. Currently, gestational diabetes is initially managed with medical nutritional therapy and exercise,10 and pharmacotherapy is reserved for individuals who do not achieve glycemic control after lifestyle management. When pharmacotherapy is required, insulin is recommended.10 Insulin is effective in improving the rate of several perinatal outcomes11 but is associated with increased rates of maternal and infant hypoglycemia, excess gestational weight gain, higher rates of cesarean birth, and treatment in a neonatal intensive care unit.12 An alternate therapeutic approach is oral therapies, usually with metformin, which has been evaluated in phase 3 randomized clinical open-label trials.13,14,15 Compared with insulin, metformin is reported to be associated with improved maternal and fetal metabolic outcomes,14 although concerns about a higher rate of spontaneous preterm birth13,14 and small for gestational age have been reported.16 Metformin crosses the placenta and activates AMP-activated protein kinase, which leads to alterations in the mammalian target of rapamycin pathways, which regulate placental amino acid transport.17
A limitation to the current approach to management, ie, only prescribing pharmacotherapy following unsuccessful lifestyle modification, is that it results in a number of weeks of hyperglycemia in a large proportion of individuals (approximately 50%) during their pregnancy. Routine earlier initiation of metformin (at the time of diagnosis) may improve glycemic control, reduce the need for insulin therapy, and may have clinical advantages beyond glycemic control, such as reducing gestational weight gain. Accordingly, we hypothesized that metformin at the time of diagnosis would be associated with superior clinical outcomes in pregnant individuals with gestational diabetes.
Methods
Trial Design and Oversight
This trial, researching the effectiveness of early metformin in addition to usual care in the reduction of gestational diabetes effects (EMERGE), was a phase 3, parallel, superiority, randomized, double-blind, placebo-controlled trial of metformin (in addition to usual care), introduced at the time of gestational diabetes diagnosis and continued until delivery. The trial was conducted at 2 sites in Ireland—a tertiary university hospital referral center (average annual birth rate, 2800) and a smaller regional hospital (average annual birth rate, 1600). The geographical area and study population included participants from urban and rural locations and participants receiving either public or private health care.18 An independent steering committee and a data and safety monitoring committee oversaw the trial. Ethical approval was granted by the Clinical Research Ethics Committee of Galway University Hospital (2016-001644-19). The full protocol has been previously published (Supplement 1).18
Trial Population
Participants were aged 18 to 50 years diagnosed with gestational diabetes according to World Health Organization 2013 criteria1 (based on any one of the following glucose values: fasting ≥5.1 mmol/L, 1 hour ≥10 mmol/L, or 2 hour ≥8.5 mmol/L), were pregnant with a singleton fetus (confirmed by scan) with a gestation up to 28 weeks (+ 6 days). Throughout, to convert glucose from mmol/L to mg/dL, divide values by 0.0555.
Exclusion criteria included an established diagnosis of diabetes (type 1, type 2, monogenic or secondary), a fasting glucose level of 7 mmol/L or greater or a 2-hour value of 11.1 mmol/L on the oral glucose tolerance test (8-oz solution with 75 grams of sugar), or a known intolerance to metformin. Other key exclusion criteria are included in the eBox in Supplement 2. Written consent was obtained from each participant by either the principal investigator or a co-investigator and witnessed by a research assistant and in addition, a trial nurse or midwife. A translator service was provided for participants when needed.
Trial Procedures
Participants were assigned randomly on a 1:1 ratio to receive either placebo or metformin, in addition to usual care. A minimization strategy was used to balance proportion of participants with a body mass index (BMI) of less than or equal to 30 vs greater than 30 and a history of gestational diabetes between groups. A centralized system for secure, interactive web-based randomization was used to ensure random sequence and allocation concealment. Participating sites obtained allocated treatment numbers and participant ID numbers after confirming eligibility. Treatment allocation was masked from site investigators, site personnel, participants, and outcome assessors.
Usual care consisted of standardized advice on medical nutritional therapy and exercise, described in eAppendix 2 in Supplement 2. Participants performed daily 7-point glucose testing at meal time (before and 1 hour after) and before bed and were seen by a clinician at an antenatal diabetes clinic at 2- to 4-week intervals.
Metformin (or matched placebo, identical in taste, smell, appearance, and packing to metformin) was started at 500 mg daily, and titrated upwards every 2 days over 10 days, to a maximum of 2500 mg daily (5 tablets) in 2 doses (1500 mg in the morning and 1000 mg in the evening), taken until delivery. Participants with significant adverse effects were advised to reduce their dose to the maximum tolerated dose. Insulin was started in accordance with national guidelines in Ireland19 if 2 or more home glucose readings between clinic visits were outside the prespecified glucose targets (fasting level, ≤5.1 mmol/L; 1-hour postprandial level, ≤7 mmol/L) and could not be explained by transient factors such as sleep, stress, or infection. The dosing of insulin was based on maternal weight and gestational week of initiation. If insulin was initiated, study medication was continued.
Laboratory tests were performed (eg, hemoglobin A1C and fasting blood glucose) at gestational weeks 32 and 38 (plus or minus 1 week was permitted for testing time). The Diabetes Treatment Satisfaction Questionnaire was completed at study week 12,20 and study drug pill counting was completed every 4 weeks to measure adherence in the previous 4-week time period. If medication was discontinued, it was recorded as 0 for that time period. A birth visit occurred within 72 hours after delivery to determine maternal and fetal outcomes. Participants were contacted by phone at 4 weeks and in person at 12 weeks after delivery (eTable 1 in Supplement 2).
Outcomes
The primary outcome, determined at the time of infant delivery, was a composite of insulin initiation (before delivery) or a fasting laboratory blood glucose value of 5.1 mmol/L or greater at week 32 or week 38 of gestation. There were 12 prespecified secondary outcomes. Secondary maternal outcomes were time to insulin initiation, insulin dose required, development of pregnancy-induced hypertension or preeclampsia, antepartum and postpartum hemorrhage, any bleeding, mode and time of delivery with determination of numbers with preterm birth before 37 weeks of gestation, gestational weight gain from randomization to delivery and from randomization to 12 weeks’ postpartum, self-reported capillary glycemic control, and treatment satisfaction.
Secondary neonatal outcomes included infant birth weight, length, and head circumference with derived measures for large for gestational age above the 90th percentile, small for gestational age below the 10th percentile, macrosomia greater than 4 kg, low birth weight less than 2.5 kg, proportion of infants with neonatal morbidities including need for neonatal intensive care, respiratory distress requiring respiratory support, jaundice requiring phototherapy, major congenital anomalies, Apgar score less than 7 at 5 minutes, and neonatal hypoglycemia (<2.6 mmol/L on 1 or more occasions within 60 minutes postdelivery).
Statistical Analysis
Analyses were performed according to an intention-to-treat principle, with primary analysis including all participants who underwent randomization and including information through the end of the trial. The difference between placebo and metformin for the primary outcome was assessed for statistical significance at α less than .05 using a z test for 2 independently estimated proportions. Treatment effects were expressed as risk ratios (RRs) with 95% CIs calculated using the delta method.21 No imputations were used for missing data. We also completed a time-to-event analysis for insulin initiation; data were censored at date of delivery for participants who completed the planned treatment period without initiation of insulin. The statistical analyses were conducted in accordance with the prespecified outcomes in accordance with the statistical analysis plan (Supplement 3), which was finalized before unblinding. All analyses were completed using R-package, version 4.1.2.22
The sample size of 550 pregnancies was based on an ability to detect a minimally important difference of a 30% risk reduction in the primary outcome measure and on the informed assumptions that 40% of participants in the placebo group would require insulin (based on the Metformin vs Insulin for the treatment of gestational diabetes [MiG] trial14) at a significance level of .05 and 80% power, with a dropout rate of 5% or less and a nonadherence rate of 8% or less in the metformin group. There were no planned comparative interim analyses for this study. However, we reviewed the overall event rate in the trial to determine whether our initial assumption regarding expected event rate was consistent with the observed event rate, and the observed event rate (upon which size of the samples was based) was higher than initially assumed. Based on this information, it was established that a reduced sample of 535 participants would achieve the same 80% power to answer the primary research question. The COVID-19 pandemic also challenged recruitment during the study period, which was a factor in not achieving the original sample size, and together with the information on observed event rates, resulted in a decision to stop recruitment at 535 participants.
Results
Participants
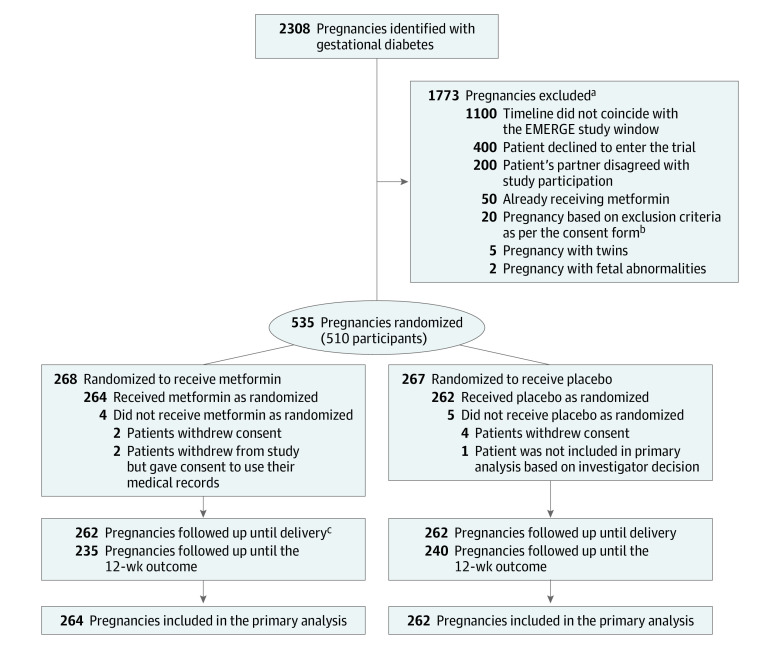
From June 2017 to September 2022, after screening 2308 pregnancies with gestational diabetes resulted, 535 pregnancies (in 510 participants) underwent randomization (268 to metformin and 267 to placebo; Figure 1). Information on 526 (98%) pregnant individuals and their infants was available for the primary outcome and participant and neonatal pregnancy outcomes. At randomization, maternal characteristics were similar between groups (Table 1).
Figure 1. Flow of Patients With Gestational Diabetes Randomized to Metformin vs Placebo.
aNumeric values indicating reasons for exclusion are approximate.
bSee eBox in Supplement 2 for more information regarding exclusion criteria.
cFollow-up information until delivery is not shown for 2 pregnancies because delivery took place outside of Ireland.
Table 1. Maternal Characteristics at Baseline.
| Variable | Early metformin (n = 268)a | Placebo (n = 267)a |
|---|---|---|
| Demographic characteristics | ||
| Age, mean (SD), y | 34.3 (4.9) | 34.3 (4.7) |
| Racial or ethnic groupb | ||
| African/Black | 7 (2.6) | 6 (2.2) |
| Asian | 17 (6.3) | 29 (10.9) |
| Irish Traveller | 11 (4.1) | 2 (0.7) |
| White | 219 (81.7) | 209 (78.3) |
| Other | 14 (5.2) | 21 (7.9) |
| Highest level of education | (n = 268) | (n = 266) |
| Tertiary | 214 (79.9) | 220 (82.7) |
| Secondary | 45 (16.8) | 43 (16.2) |
| Primary | 9 (3.4) | 3 (1.1) |
| In receipt of medical card, No./total (%)c | 63/268 (23.5) | 63/266 (23.7) |
| Private health insurance, No./total (%)c | 126/268 (47) | 116/266 (43.6) |
| Unemployed, No./total (%) | 19/268 (7.1) | 27/266 (10.2) |
| Past history | ||
| Smoking during pregnancy | 17 (6.3) | 17 (6.4) |
| Prior hypertension | 12 (4.5) | 4 (1.5) |
| Nulliparous | 63 (23.5) | 64 (24) |
| Previous obstetrical history available, No./total (%)d | 181/205 (88.3) [n = 181] | 172/203 (84.7) [n = 172] |
| Cesarean delivery | 70 (38.7) | 74 (43) |
| Gestational diabetes | 65 (35.9) | 65 (37.8) |
| Infant with birth weight >4000 g | 57 (31.5) | 44 (25.6) |
| Postpartum hemorrhage | 28 (15.5) | 20 (11.6) |
| Preeclampsia | 24 (13.3) | 23 (13.4) |
| Antepartum hemorrhage | 20 (11) | 15 (8.7) |
| Infant with congenital anomaly | 13 (7.2) | 13 (7.6) |
| Previous stillbirth | 2 (1.1) | 2 (1.2) |
| Physical findings | ||
| BMI at enrollment, median (IQR)e | 29.9 (25.6-33.7) | 30 (26.6-34.3) |
| BMI <30, No./total (%)e | 131/255 (51.4) | 121/251 (48.2) |
| BMI ≥30, No./total (%)e | 124/255 (48.6) | 130/251 (51.8) |
| Gestation at randomization, median (IQR), wk | 27 (25.7-28) | 27 (25.6-28) |
| Duration from randomization to delivery, mean (SD), wk | 13.7 (4.7) | 13.6 (4.3) |
| Duration from randomization to delivery, median (IQR), wk | 12.3 (11-14.1) | 12.4 (11.1-14.2) |
| Systolic blood pressure at randomization, mean (SD), mmHg | 114.9 (9.5) | 114.4 (9.2) |
| Diastolic blood pressure at randomization, mean (SD), mmHg | 68.9 (8) | 68.7 (8.8) |
| Laboratory findings | ||
| Results of 75 g oral glucose tolerance, mean (SD), mmol/L | ||
| Plasma glucose level after overnight fast | 5.2 (0.5) | 5.2 (0.5) |
| Postprandial plasma glucose level at 1 h | 9.4 (1.9) | 9.7 (1.9) |
| Postprandial plasma glucose level at 2 h | 7.1 (1.6) | 7.1 (1.6) |
| Hemoglobin A1c at randomization, mean (SD), mmol/molf | 33 (3.4) [n = 266] | 32.9 (3.5) [n = 265] |
Abbreviation: BMI, body mass index.
Conventional unit conversion factor: to convert glucose to mg/dL, divide by 0.0555.
Numeric values are reported as No. (%) unless otherwise indicated.
Ethnic groups were collected in a closed form. Individuals accounted under the category of Other include the following: Afghan, 3; Bangladeshi, 3; Brazilian, 8; Croatian, 1; Euroasian, 1; Indian, 7; Lithuanian, 1; Nigerian, 1, Pakistani, 3; Filipino, 1; Polish, 3; Romanian, 1; Spanish, 1; and Syrian, 1. Irish Travellers are a nomadic indigenous minority that was formally recognized as an ethnic group by the Irish State in 2017.
Having a medical card indicates that the participant receives all care free of charge; private health insurance indicates that the participant can choose to receive private health care billed to their insurance.
Indicates that percentage values are calculated from 181 participants in the early metformin group and from 172 in the placebo group.
BMI was calculated as weight in kilograms divided by height in meters squared.
Reference range for hemoglobin A1c: 25 to 35 mmol/mol.
Adherence
Participants demonstrated greater than 90% adherence to fingerstick testing of blood glucose during the trial. The mean (SD) dose per day for metformin was 2087 (530) mg vs 2231 (378) mg for placebo, and the median (IQR) dose per day was 2214 (243) vs 2256 (240). The mean and median weeks for randomization to the trial and metformin initiation were similar and did not differ between groups.
Greater than 80% adherence to study medication at all trial visits was documented for 92% of participants. Discontinuation of metformin treatment occurred in 13 (4.9%) participants due to severe gastrointestinal adverse effects. In the metformin group, 65 participants (24.3%) experienced gastrointestinal adverse effects, all of whom required a dose reduction. In comparison, only 11 participants (4.1%) in the placebo group experienced both gastrointestinal adverse effects and required a dose reduction (difference, 20.2% [95% CI, 17.0%-25.0%]; P < .001). Study medications were not discontinued for any other reasons.
Primary Efficacy Outcome
Among 150 participants (56.8%) in the metformin group and 167 participants (63.7%) in the placebo group, there was no statistically significant difference in the primary composite outcome of insulin initiation or fasting glucose level of 5.1 mmol/L or greater at gestational weeks 32 or 38 (risk ratio, 0.89 [95% CI, 0.78-1.02]; P = .13) (Table 2).
Table 2. Maternal Outcomes.
| Outcome | No./total (%)a | Risk difference (95% CI) | Relative risk (95% CI) | P value | |
|---|---|---|---|---|---|
| Early metformin (n = 264) | Placebo (n = 262) | ||||
| Primary outcome b | |||||
| Primary composite outcome | 150/264 (56.8) | 167/262 (63.7) | −6.9% (−15.1% to 1.4%) | 0.89 (0.78 to 1.02) | .13 |
| Initiation of insulin only | 101/263 (38.4) | 134/262 (51.1) | −12.7% (−21.2% to −4.3%) | 0.75 (0.62 to 0.91) | .004 |
| Fasting glucose at wk 32, mean (SD), mmol/L | 4.9 (0.5) | 5.0 (0.5) | −0.1 (−0.19 to −0.01) | .03 | |
| Fasting glucose at wk 38, mean (SD), mmol/L | 4.5 (0.4) | 4.7 (0.5) | −0.2 (−0.28 to −0.09) | <.001 | |
| Secondary outcomes | |||||
| Time to insulin initiationc | .001 | ||||
| Mode of delivery | |||||
| Cesarean delivery | 114/262 (43.5) | 100/262 (38.2) | 5.2% (−3.1% to 13.7%) | 1.14 (0.93 to 1.4) | .40 |
| Induced delivery | 75/262 (28.6) | 88/262 (33.2) | −4.6% (−12.5% to 3.3%) | 0.86 (0.67 to 1.11) | |
| Spontaneous delivery | 73/262 (27.9) | 75/262 (28.6) | −0.7% (−8.5% to 6.9%) | 0.97 (0.74 to 1.28) | |
| Emergency cesarean | 53/114 (46.5) | 53/100 (53) | −6.5% (−13.9% to 12.9%) | 0.99 (0.74 to 1.32) | 1 |
| Elective cesarean | 61/114 (53.5) | 47/100 (47) | |||
| Preterm birth (<37 wk) | 24/261 (9.2) | 17/260 (6.5) | 2.8% (−2.0% to 7.3%) | 1.41 (0.78 to 2.56) | .33 |
| Hemoglobin A1C at wk 38, mean (SD), mmol/mol | 33.9 (3.4) | 35.0 (3.9) | −1.1% (−1.79 to −0.34) | .004 | |
| Insulin dose required, mean (SD), IU | 20.4 (19.8) | 24.2 (22.8) | −3.8 (−9.3 to 1.7) | .17 | |
| Maternal weight change randomization to delivery, mean (SD), kg | 0.8 (3.3) | 2.0 (3.6) | −1.2 (−1.99 to −0.42) | .003 | |
| Maternal weight change randomization to wk 12 postpartum, mean (SD), kg | −5.9 (4.4) | −5.1 (4.9) | −0.8 (−1.64 to 0.06) | .07 | |
| Treatment satisfaction | 173/227 (76.2) | 159/237 (67.1) | 9.1% (1.0% to 17.0%) | .04 | |
| Maternal morbidityd | |||||
| Gestational hypertension | 31/262 (11.8) | 28/262 (10.6) | 1.2% (−4.2% to 6.6%) | 1.11 (0.69 to 1.8) | .77 |
| Preeclampsia | 10/262 (3.8) | 5/262 (1.9) | 1.9% (−0.9% to 4.8%) | 2.01 (0.7 to 5.79) | .29 |
| Antepartum hemorrhage | 15/262 (5.7) | 27/262 (10.3) | −4.6% (−9.2% to 0.1%) | 0.56 (0.3 to 1.02) | .08 |
| Postpartum hemorrhage | 51/262 (19.5) | 63/262 (24.0) | −4.5% (−11.6% to 2.5%) | 0.81 (0.59 to 1.12) | .24 |
| Any bleeding | 60/262 (22.9) | 80/262 (30.5) | −7.6% (−15.2% to −0.1%) | 0.75 (0.56 to 1.0) | .06 |
Conventional unit conversion factor: to convert glucose to mg/dL, divide by 0.0555.
Numeric values are reported as No./total (%) unless otherwise indicated.
The composite primary outcome was achieved if a participant initiated insulin treatment during the period between randomization and delivery or had a measured fasting glucose level of at least 5.1 mmol/mol at 32 weeks’ gestation or at 38 weeks’ gestation.
Only a P value is shown for this row because it is not possible to calculate average or median survival times due to the censoring in the distribution (eg, the time to insulin would be censored at the delivery date for more than 50% of the metformin group).
Other outcomes related to maternal morbidity are detailed in eTable 2 in Supplement 2.
Secondary Maternal Outcomes
Insulin initiation occurred in 101 participants (38.4%) in the metformin and 134 (51.1%) in the placebo groups (relative risk, 0.75; 95% CI 0.62-0.91; P = .004). An alternative time-to-event analysis indicated a significant reduction in the hazard of initiating insulin in the metformin group (hazard ratio, 0.66 [95% CI, 0.51-0.85]; P = .001; Figure 2A). At the last assessment prior to delivery, the mean (SD) amount of insulin required was 20.4 (19.8) IU in the metformin group compared with 24.2 (22.8) IU in the placebo group (difference, −3.8 [95% CI, −9.3 to 1.7]; P = .17). This equated to a mean (SD) of 0.2 (0.2) units per kg body weight in participants receiving metformin vs 0.3 (0.2) units for those receiving placebo. Mean (SD) fasting glucose was significantly lower in the metformin group compared with the placebo group at gestational week 32 (4.9 [0.5] vs 5.0 [0.5] mmol/L; difference, −0.1 [95% CI, −0.19 to −0.01]; P = .03) and at gestational week 38 (4.5 [0.4] vs 4.7 [0.5] mmol/L; difference, −0.2 [95% CI, −0.28 to −0.09]; P < .001) (Figure 2B; Table 2). Mean (SD) self-reported capillary postprandial glucose was lower at lunch in the metformin group compared with the placebo group at gestational week 32 (5.5 [0.9] vs 5.7 [0.9] mmol/L; difference, −0.2 [95% CI, −0.47 to −0.04]; P = .02), and at week 38, it was lower in the metformin group at breakfast (5.4 [0.8] vs 5.7 [1.0] mmol/L; difference, −0.3 [95% CI, −0.59 to −0.04]; P = .02) and at dinner (5.5 [0.8] vs 6.0 [1.0] mmol/L; difference, −0.5 [95% CI, −0.75 to −0.25]; P < .001) (eTable 3 in Supplement 2). Participants in the metformin group gained less weight between time of randomization and delivery with a mean (SD) weight gain of (0.8 [3.3] kg vs 2.0 [3.6] kg; difference, −1.2 kg [95% CI, −1.99 to −0.42]; P = .003) (Table 2). Between-group outcomes were similar for the gestational week at delivery (metformin, 39.1 [1.5] weeks vs placebo, 39.1 [1.6] weeks; difference, 0.0 [95% CI, −0.3 to 0.2]; P = .65) and rate of preterm birth before 37 weeks’ gestation (metformin, 9.2% vs placebo, 6.5%; difference, 2.8% [95% CI, −2.0% to 7.3%]; P = .33). The rates of pregnancy-induced hypertension, preeclampsia, antepartum and postpartum hemorrhage, induction of labor, and cesarean birth did not differ between groups (Table 2; eTable 2 in Supplement 2). In the metformin group, 173 (76.2%) participants stated they would choose the same medication again in another pregnancy compared with 159 (67.1%) in the placebo group (difference, 9.1% [95% CI, 1% to 17%]; P = .04). During the treatment period, no participant deaths occurred. However, 1 birthing parent in the metformin group died at 12 weeks postpartum, due to a large pulmonary embolus.
Figure 2. Time to Insulin Initiation and Fasting Blood Glucose Between Metformin and Placebo Groups.
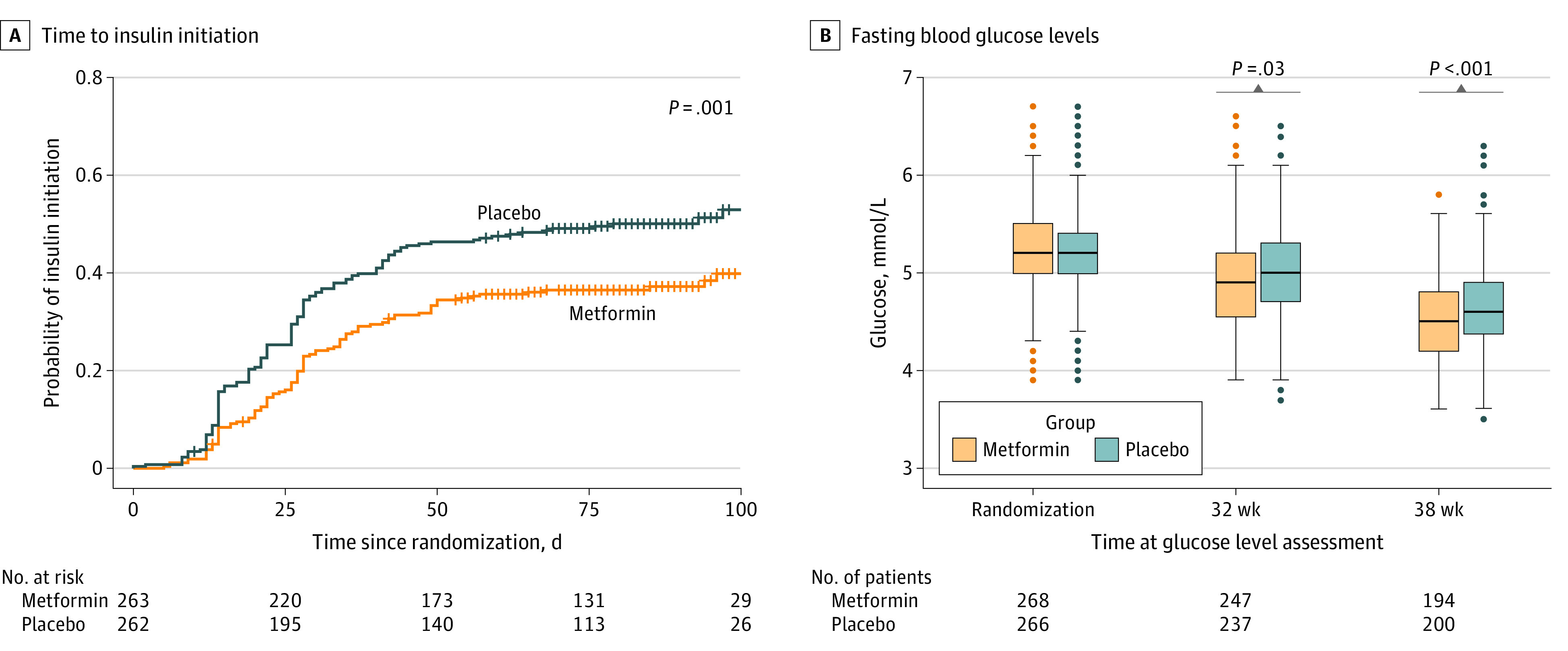
A, Time to first insulin was computed for each patient as the difference in days between the date of randomization and the earlier of the dates of initiation and delivery. Patients who did not receive insulin over this time period were censored at their date of delivery. Significantly fewer patients in the metformin group required insulin over the course of the study (P value calculated using the log-rank test). B, Fasting glucose (approximate distribution) levels were compared between the metformin and placebo groups at randomization, 32 weeks’ gestation, 38 weeks’ gestation, and 12 weeks’ postpartum. The horizontal lines in the boxes indicate the mean, lower and upper ends of the boxes indicate the first and third quartiles, whiskers indicate values within 1.5× the IQR from the upper or lower quartile, and data more extreme than the whiskers are plotted individually as circles. Mean fasting glucose at randomization was very similar between groups. However, the mean fasting glucose level decreased faster in the metformin group than the placebo group resulting in significant differences at 32 and 38 weeks. These differences were small compared with the standard deviation/IQR of fasting glucose at each time point. To convert glucose levels to mg/dL, divide values by 0.0555.
Secondary Neonatal Outcomes
The mean (SD) birth weight of infants was lower in the metformin group compared with the placebo group (3393 [527] g vs 3506 [510] g; difference, −113 g [95% CI, −201 to −24]; P = .005) with a lower proportion of infants weighing more than 4000 g (7.6% vs 14.8%; difference, −7.2% [95% CI, −12.6% to −1.8%]; P = .02) or being large for gestational age (>90th percentile) (6.5% in the metformin group vs 14.9% in the placebo group; difference, −8.4% [95% CI, −13.7% to −3.2%]; P = .003) (Table 3). In the metformin group, there were more cases of infants weighing less than 2500 g (6.1% [16 infants] vs 3.4% [9 infants]; difference, 2.7% [95% CI, −1% to 6.3%]; P = .12) or born small for gestational age (<10th percentile) (5.7% [15 infants] vs 2.7% [7 infants]; difference, 3.0% [95% CI, −0.4% to 6.5%]; P = .13). Mean (SD) crown-heel length was significantly shorter in metformin-exposed infants (51.0 [3.2] cm vs 51.7 [3.3] cm; difference, −0.7 cm [95% CI, −1.3 to −0.2]; P = .02) while head circumference was similar between groups (Table 3). Outcomes were not significantly different between the groups (metformin vs placebo) for the following variables: he need for neonatal intensive care unit admission (15.6% vs 12.5%), respiratory distress requiring support (9.2% vs 6.9%), jaundice requiring phototherapy (0.4% vs 0%), Apgar below 7 at 5 minutes (0.4% vs 0.4%), hypoglycemia less than 2.6 mmol/L (13.7% vs 13.0%), and major congenital anomalies (3.8% vs 2.7%) (Table 3; eTable 2 in Supplement 2). There was 1 neonatal death due to trisomy 18 in the metformin group and 1 intrauterine death due to antepartum hemorrhage in the placebo group.
Table 3. Neonatal Weight, Height, and Size.
| Outcome | No./total (%)a | Unadjusted risk difference (95% CI) | Unadjusted relative risk (95% CI) | P valueb | P valuec | |
|---|---|---|---|---|---|---|
| Metformin (n = 262) | Placebo (n = 262) | |||||
| Neonatal size | ||||||
| Gestational age at birth, mean (SD), wkd | 39.1 (1.5) | 39.1 (1.6) | 0 (−0.3 to 0.2) | .66 | ||
| Birth weight, mean (SD), g | 3393 (527) | 3506 (510) | −113 (−201 to −24) | .01 | .005 | |
| Birth weight >4000 g | 20/262 (7.6) | 39/262 (14.8) | −7.2% (−12.6% to −1.8%) | 0.5 (0.3 to 0.9) | .02 | .02 |
| Birth weight >90th percentile | 17/261 (6.5) | 39/261 (14.9) | −8.4% (−13.7% to −3.2%) | 0.4 (0.3 to 0.8) | .003 | |
| Birth weight <2500 g | 16/262 (6.1) | 9/262 (3.4) | 2.7% (−1% to 6.3%) | 1.8 (0.8 to 4.0) | .12 | .12 |
| Birth weight <10th percentile | 15/261 (5.7) | 7/261 (2.7) | 3.0% (−0.4% to 6.5%) | 2.1 (0.9 to 5.2) | .13 | |
| Head circumference, mean (SD) [No.], cm | 34.7 (1.6) [253] | 34.7 (1.8) [253] | 0 (−0.3 to 0.3) | .82 | .60 | |
| Crown-heel length, mean (SD) [No.], cm | 51.0 (3.2) [253] | 51.7 (3.3) [253] | −0.7 (−1.3 to −0.2) | .02 | .02 | |
| Abdominal circumference, mean (SD) [No.], cm | 33.4(2.4) [154] | 33.3 (2.8) [157] | 0.1 (−0.5 to 0.7) | .72 | .81 | |
| Mid-upper-arm circumference, mean (SD) [No.], cm | 11.0 (1.0) [154] | 11.1 (1.1) [156] | −0.1 (−0.3 to 0.1) | .47 | .33 | |
| Ponderal index mean, mean (SD) [No.]e | 2.6 (0.4) [253] | 2.6 (0.5) [253] | 0 (−0.1 to 0.1) | .68 | ||
| Neonatal morbiditiesf | ||||||
| Need for NICU care | 41/262 (15.6) | 33/262 (12.6) | 3% (−2.9% to 9%) | (0.8 to 1.9) | .38 | |
| Respiratory distress requiring respiratory support | 24/262 (9.2) | 18/262 (6.9) | 2.3% (−2.4% to 6.9%) | 1.3 (0.7 to 2.4) | .42 | |
| Jaundice requiring phototherapy | 1/262 (0.4) | 0 | 0.4% (−.4% to 1.1%) | >.99 | ||
| Major congenital anomalies | 10/262 (3.8) | 7/262 (2.7) | 1.1% (−1.9% to 4.2%) | 1.5 (0.6 to 3.7) | .62 | |
| Apgar score <7 at 5 min | 1/262 (0.4) | 1/260 (0.4) | 0% (−1% to 1%) | 1 (0.1 to 15.8) | >.99 | |
| Neonatal hypoglycemia (<2.6 mmol/L on ≥1 occasion <60 min postdelivery) | 36/262 (13.7) | 34/262 (13) | 0.7% (−5.1% to 6.6%) | 1.1 (0.7 to 1.6) | .90 | |
Abbreviation: NICU, neonatal intensive care unit.
Numeric values are reported as No./total (%) unless otherwise indicated.
P values for birthweight (in grams), birth weight >4000 g, birth weight <2500 g, head circumference, crown-heel length, abdominal circumference, and mid-upper-arm circumference were derived from a statistical model that adjusts for the infant’s sex and maternal height and weight at randomization. The other P values in this column pertain to unadjusted comparisons.
P values were derived from a model that adjusts for gestational age at birth, in addition to the infant’s sex and maternal height and weight at randomization.
Although gestational age at birth is determined postrandomization, no evidence was found to support an effect of metformin on gestational age at birth.
Ponderal index is calculated as 100 × weight (grams)/(crown-heel length)^3. This index is a measure of relative body size (similar to measuring body mass index), with larger values indicating larger body size relative to height (range, 1.5 to 7.3).
A more complete listing of neonatal morbidities is given in eTable 2 in Supplement 2.
Discussion
This randomized clinical trial did not confirm statistical superiority of early metformin over placebo for the primary outcome, a composite of insulin initiation or a fasting blood glucose level of 5.1 mmol/L or greater at gestational week 32 or 38 between groups. This trial was not powered to establish safety of metformin for individual secondary outcome analyses, and therefore, lack of statistical significance should not be interpreted as evidence of safety.
The mixed results of the prespecified secondary outcomes suggest areas of focus for future research, including some of the secondary neonatal outcomes. The decreased proportion of large for gestational age infants or infants weighing more than 4000 g in participants randomized to early metformin is consistent with previous clinical trials.13,14,16 Minimizing excessive intrauterine growth reduces increased risks of diabetes, obesity, and hypertension in adulthood.7,23,24 However, there was an increase in the proportion of infants weighing less than 2500 g or small for gestational age (<10th percentile). This is important in view of the effect of metformin on the mammalian target of rapamycin pathway, which regulates placenta amino acid transport and may contribute to a reduction in lean body mass. In addition, the decrease in crown-heel length in the metformin group (although within the normal range), which remained significant after adjustment for infant sex and maternal height and weight, requires ongoing careful evaluation. Future longitudinal follow-up is required as this observation in crown-heel length may reflect an adverse effect on lean body mass, which may have a potential for long-term adverse consequence in terms of future adiposity. Follow-up in the medium and long term is essential to document ongoing implications for child weight and size of metformin-exposed offspring as a previous systematic review has identified overweight or obesity in metformin-exposed children.25
In addition, the findings of this study support benefits of metformin on maternal weight gain, which has been reported in previous clinical trials.13,14,16 Lower weight gain in the metformin group is likely to be due to less insulin use and a direct effect of metformin on food intake. At randomization, half of participants in this trial had a baseline BMI greater than 30, a level at which minimization of excess weight gain is desirable. Prior studies report significant associations of gestational glycemic control and weight gain with future risk of diabetes and cardiovascular disease.5,6,26,27 Medium- and long-term maternal follow-up of this cohort has begun to assess if early use of metformin has long-term maternal cardio-metabolic benefits.
The proportion of participants requiring insulin in the metformin group was lower than observed in metformin-exposed women in the MiG trial (46.3%)14; this likely reflects the ethnic and BMI differences between the trials as MiG enrolled a broader ethnic group with a greater mean BMI than EMERGE.14 In addition, participants randomized in EMERGE had a gestational diabetes diagnosis based on World Health Organization (WHO) 2013 criteria, which are lower than the criteria used in the MiG trial.
Although metformin is considered a suitable first-line therapy by National Institute for Health and Care Excellence guideline recommendations,24 the American Diabetes Association does not consider metformin as first-line therapy, particularly in pregnant individuals with hypertension or preeclampsia or those at risk for intrauterine growth restriction.10
This trial has several strengths as a large double-blind placebo-controlled trial of early metformin in pregnant individuals with gestational diabetes based on WHO 2013 criteria. Participants across all BMI categories were included, and 50% of participants had a BMI of less than 30, a participant category that has not been well-represented in other gestational diabetes clinical trials. Based on this spread of BMI across all categories, the results of this trial may have greater generalizability. Despite the COVID-19 pandemic, there was low attrition and high adherence to treatment allocation, and 526 (98%) pregnancies are available for the primary outcome.
Limitations
This study has several limitations. First, the trial took longer to complete than originally planned due to the COVID-19 pandemic, and trial duration was also affected by 2 cyber attacks (one on the University of Galway and one on the Health Services Executive [HSE] Saolta Hospital Group in which 2 hospitals conducting this trial were located), which created logistical challenges but did not impact, influence, or compromise any EMERGE data).
Second, 80% of the participants were White European individuals, reflective of the Irish population. The lack of a larger ethnic mix may have limited broad generalizability for other races or ethnicities.
Third, we employed the WHO 2013 gestational diabetes diagnostic criteria, which are widely used in Europe, Australia, and Asia but not in other regions of the world. For example the 2-step Carpenter and Coustan criteria are more widely used in the US, potentially limiting generalizability of this study’s findings.
Fourth, our management approach included implementation of tight glucose control (fasting glucose <5.1 mmol/L) and strict insulin initiation rules. While our approach was based on national guidelines, it may have limited generalizability of findings to clinical practices where a more conservative approach to glycemic control is practiced.
Fifth, the 2 individual components of our primary outcome were not formally listed as prespecified secondary outcomes.
Conclusions
Early treatment with metformin was not superior to placebo for the composite primary outcome. Prespecified secondary outcome data support further investigation of metformin in larger clinical trials.
Trial Protocol, Version 6.0
eAppendix 1. Investigators and Corresponding Author; Trial Sites and Institutions of Authors; EMERGE Steering Group; Data and Safety Monitoring Committee
eBox. Contraindications to the Use of Metformin Excluding Women From EMERGE Trial
eAppendix 2. Usual Care of Women With GDM
eTable 1. List of Variables Collected at Delivery and at 4 and 12 Weeks Postpartum
eTable 2. Neonatal and Maternal Morbidity
eFigure 1. Subgroup Analysis of the Primary Outcome
eFigure 2. Subgroup Analysis of Insulin Initiation Only
eTable 3. Fasting (Laboratory) and Postprandial (Capillary) Glucose Values at Weeks 32 and 38 Between Groups
Statistical Analysis Plan
Data Sharing Statement
References
- 1.World Health Organization . Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Published January 1, 2013. Accessed July 1, 2023. https://www.who.int/publications/i/item/WHO-NMH-MND-13.2 [PubMed]
- 2.Yuen L, Saeedi P, Riaz M, et al. Projections of the prevalence of hyperglycaemia in pregnancy in 2019 and beyond: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. Published online September 10, 2019. doi: 10.1016/j.diabres.2019.107841 [DOI] [PubMed] [Google Scholar]
- 3.O’Sullivan EP, Avalos G, O’Reilly M, Dennedy MC, Gaffney G, Dunne F; Atlantic DIP Collaborators . Atlantic Diabetes in Pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia. 2011;54(7):1670-1675. doi: 10.1007/s00125-011-2150-4 [DOI] [PubMed] [Google Scholar]
- 4.Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002. doi: 10.1056/NEJMoa0707943 [DOI] [PubMed] [Google Scholar]
- 5.Fu J, Retnakaran R. The life course perspective of gestational diabetes: an opportunity for the prevention of diabetes and heart disease in women. EClinicalMedicine. 2022;45:101294. doi: 10.1016/j.eclinm.2022.101294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773-1779. doi: 10.1016/S0140-6736(09)60731-5 [DOI] [PubMed] [Google Scholar]
- 7.Lowe WL Jr, Scholtens DM, Kuang A, et al. ; HAPO Follow-up Study Cooperative Research Group . Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42(3):372-380. doi: 10.2337/dc18-1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landon MB, Spong CY, Thom E, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network . A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348. doi: 10.1056/NEJMoa0902430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group . Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477-2486. doi: 10.1056/NEJMoa042973 [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association Professional Practice Committee . 15. Management of diabetes in pregnancy: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(suppl 1):S232-S243. doi: 10.2337/dc22-S015 [DOI] [PubMed] [Google Scholar]
- 11.Brown J, Grzeskowiak L, Williamson K, Downie MR, Crowther CA. Insulin for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;11(11):CD012037. doi: 10.1002/14651858.CD012037.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogdanet D, Egan A, Reddin C, Kirwan B, Carmody L, Dunne F. ATLANTIC DIP: despite insulin therapy in women with IADPSG diagnosed GDM, desired pregnancy outcomes are still not achieved: what are we missing? Diabetes Res Clin Pract. 2018;136:116-123. doi: 10.1016/j.diabres.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 13.He K, Guo Q, Ge J, Li J, Li C, Jing Z. The efficacy and safety of metformin alone or as an add-on therapy to insulin in pregnancy with GDM or T2DM: a systematic review and meta-analysis of 21 randomized controlled trials. J Clin Pharm Ther. 2022;47(2):168-177. doi: 10.1111/jcpt.13503 [DOI] [PubMed] [Google Scholar]
- 14.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP; MiG Trial Investigators . Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358(19):2003-2015. doi: 10.1056/NEJMoa0707193 [DOI] [PubMed] [Google Scholar]
- 15.Spaulonci CP, Bernardes LS, Trindade TC, Zugaib M, Francisco RP. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol. 2013;209(1):34.e1-34.e7. doi: 10.1016/j.ajog.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 16.Feig DS, Donovan LE, Zinman B, et al. ; MiTy Collaborative Group . Metformin in women with type 2 diabetes in pregnancy (MiTy): a multicentre, international, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2020;8(10):834-844. doi: 10.1016/S2213-8587(20)30310-7 [DOI] [PubMed] [Google Scholar]
- 17.Nguyen L, Chan SY, Teo AKK. Metformin from mother to unborn child—are there unwarranted effects? EBioMedicine. 2018;35:394-404. doi: 10.1016/j.ebiom.2018.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunne F, Newman C, Devane D, et al. A randomised placebo-controlled trial of the effectiveness of early metformin in addition to usual care in the reduction of gestational diabetes mellitus effects (EMERGE): study protocol. Trials. 2022;23(1):795. doi: 10.1186/s13063-022-06694-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Health Service Executive Office of the Nursing and Midwifery Services Director . Lenus Irish Health Repository website. Guidelines for the management of pregestational and gestational diabetes mellitus from pre-conception to the postnatal period. Published July 2010. Accessed August 30, 2023. http://hdl.handle.net/10147/112890
- 20.Sampson MJ, Singh H, Dhatariya KK, Jones C, Walden E, Bradley C. Psychometric validation and use of a novel diabetes in-patient treatment satisfaction questionnaire. Diabet Med. 2009;26(7):729-735. doi: 10.1111/j.1464-5491.2009.02754.x [DOI] [PubMed] [Google Scholar]
- 21.van der Vaart AW. Asymptotic Statistics. Cambridge University Press; 1998. [Google Scholar]
- 22.R Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing. Published 2021. Accessed March 3, 2023. https://www.R-project.org
- 23.National Institute for Health and Care Excellence . Diabetes in Pregnancy: Management of Diabetes and its Complications From Preconception to the Postnatal Period. Version 2.1. National Collaborating Centre for Women’s and Children’s Health; 2015. [PubMed] [Google Scholar]
- 24.Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21(3):149-157. doi: 10.1080/14767050801929430 [DOI] [PubMed] [Google Scholar]
- 25.Tarry-Adkins JL, Aiken CE, Ozanne SE. Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: a systematic review and meta-analysis. PLoS Med. 2019;16(8):e1002848. doi: 10.1371/journal.pmed.1002848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905-914. doi: 10.1007/s00125-019-4840-2 [DOI] [PubMed] [Google Scholar]
- 27.Lowe WL Jr, Scholtens DM, Lowe LP, et al. ; HAPO Follow-up Study Cooperative Research Group . Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320(10):1005-1016. doi: 10.1001/jama.2018.11628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol, Version 6.0
eAppendix 1. Investigators and Corresponding Author; Trial Sites and Institutions of Authors; EMERGE Steering Group; Data and Safety Monitoring Committee
eBox. Contraindications to the Use of Metformin Excluding Women From EMERGE Trial
eAppendix 2. Usual Care of Women With GDM
eTable 1. List of Variables Collected at Delivery and at 4 and 12 Weeks Postpartum
eTable 2. Neonatal and Maternal Morbidity
eFigure 1. Subgroup Analysis of the Primary Outcome
eFigure 2. Subgroup Analysis of Insulin Initiation Only
eTable 3. Fasting (Laboratory) and Postprandial (Capillary) Glucose Values at Weeks 32 and 38 Between Groups
Statistical Analysis Plan
Data Sharing Statement