Abstract
A single intravenous dose of cefpirome, 50 mg/kg, was administered to 15 children with bacterial meningitis 24 to 48 h after initiation of standard antibiotic and steroid therapy. Cefpirome concentrations in serum and cerebrospinal fluid were determined at selected time intervals. The mean (standard deviation) peak concentration in cerebrospinal fluid (n = 5) was 10.8 (7.8) μg/ml. Drug concentrations in cerebrospinal fluid above the MIC for Streptococcus pneumoniae at which 90% of the isolates were inhibited were found 2, 4, and 8 h after the dose of cefpirome was given. The penetration of cefpirome into cerebrospinal fluid compares favorably with that of other extended-spectrum cephalosporins and suggests that this agent would be useful in the therapy of childhood meningitis, including cases caused by drug-resistant S. pneumoniae.
Cefotaxime and ceftriaxone are most often recommended in the United States for therapy of meningitis in children (3), but the recent emergence of pneumococcal strains resistant to these agents predicates the need for alternative agents. Cefpirome is a parenteral cephalosporin with a very broad spectrum of activity (12, 13). In adults with meningitis, cefpirome concentrations in cerebrospinal fluid (CSF) exceeding the MBCs for usual meningeal pathogens are readily achieved (15).
Cefpirome is highly active against common pediatric meningeal pathogens, including Streptococcus pneumoniae. Its in vitro activity against penicillin-resistant pneumococci is generally twice that of cefotaxime or ceftriaxone. For example, the MICs of cefotaxime, ceftriaxone, and cefpirome for the same penicillin-resistant pneumococcal strains at which 90% of the isolates are inhibited (MIC90s) were reported as 2.0, 2.0, and 1.0 μg/ml, respectively (1, 2). An MIC of cefpirome of >2 μg/ml for pneumococcal strains has rarely been reported (2). The aim of this study was to determine the concentrations of cefpirome in CSF after administration of a single 50-mg/kg intravenous dose to children with meningitis.
Sixteen children with clinical and laboratory features of bacterial meningitis were enrolled, but one child was subsequently excluded (see below). All children received cefotaxime (200 mg/kg of body weight/day) or ceftriaxone (100 mg/kg/day) therapy as well as dexamethasone (0.8 mg/kg/day) for 2 to 4 days. Dexamethasone therapy was started before or at the same time as antimicrobial therapy. Between 24 and 48 h after the start of standard therapy, a single 50-mg/kg dose of cefpirome was administered intravenously over 5 to 15 min. A lumbar tap was performed either 2, 4, or 8 h after the dose of cefpirome was given; five children were assigned to each time interval. Serum was obtained at the end of the cefpirome infusion and at the time of the spinal tap. Serum and aliquots of CSF specimens were immediately frozen at −20°C. The remainder of the CSF specimens were processed by routine methods in the hospital laboratory. Patients were closely observed for 24 h after the cefpirome infusion for possible adverse events. Blood counts, liver profiles, and electrolytes were measured before and within 48 h after the administration of cefpirome. Stored specimens were sent on dry ice to the Department of Pharmacokinetics and Metabolism, Hoechst Marion Roussel, Frankfurt, Germany. Assays were completed within 2 months after collection and in most cases within 4 weeks. The effect of this storage period on the sensitivity of the assay was not evaluated.
The concentrations of cefpirome in serum and CSF were measured by high-performance liquid chromatography (HPLC) (9). Serum and CSF specimens were deproteinized with 7% aqueous HClO4–methanol. After centrifugation, the supernatant was separated on a C18 reverse-phase column by using UV detection at 270 nm. The limit of quantification was 0.5 μg/ml. Concentrations of ceftriaxone, cefotaxime, and its metabolite, desacetyl-cefotaxime, were also measured in serum and CSF by HPLC (7, 16). Specimens were assayed in four batches. Control specimens for calibration were prepared and assayed twice for each batch. The coefficients of variation of the assays for each batch ranged from 0.7 to 4.6% for cefpirome, 0.3 to 6.7% for cefotaxime, 1.4 to 2.2% for desacetyl-cefotaxime, and 2.4 to 3.3% for ceftriaxone.
Ten males and six females with a mean age of 2.3 years (range, 4 months to 10.5 years) were enrolled in the study. Organisms recovered from CSF included Haemophilus influenzae (n = 7), S. pneumoniae (n = 3), Neisseria meningitidis (n = 2), Listeria monocytogenes (n = 1), and Escherichia coli (n = 1). The CSF from an additional child showed gram-negative bacilli on a stained smear, but the organism did not grow on culture. No organism was recovered from the CSF of one child, who was eventually diagnosed as having tuberculous meningitis based on a history of contact, a positive Mantoux skin test, and suggestive computerized tomography findings. In this child, the cefpirome concentration in CSF (41.9 μg/ml) was much greater than that in other children. Since tuberculous meningitis was an a priori exclusion criterion, data from this child were not included in further analyses, leaving 15 evaluable patients.
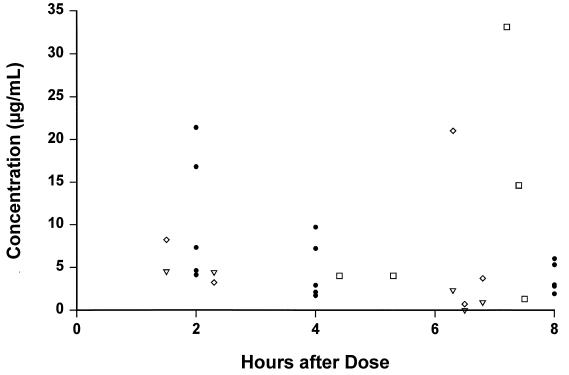
None of the CSF specimens analyzed for antibiotic concentrations was visibly blood stained. The initial and repeat CSF findings for the 15 children included in the analysis are shown in Table 1. Cefpirome concentrations in serum and CSF are shown in Table 2, and concentrations of all cephalosporins are shown in Fig. 1. Cefpirome was detected in all specimens, and the mean peak concentration in CSF was 10.8 μg/ml (range, 4.1 to 21.4 μg/ml). The cefpirome concentration was less than 2 μg/ml in only two CSF specimens. CSF penetration, assessed as the ratio of concentration in CSF/concentration in serum at 2 h, was 11 to 49%. There was no correlation between antibiotic concentrations in CSF and protein, glucose, or leukocyte concentrations in CSF.
TABLE 1.
Initial and repeat CSF values for 15 children with meningitis
| Characteristic | Value
|
|
|---|---|---|
| Initial CSF | Repeat CSFc | |
| Leukocytes (cells/mm3)a | 859 (0–10,000) | 517 (23–8808) |
| Protein (g/liter)b | 2.4 (3.1) | 1.6 (1.2) |
| Glucose (mmol/liter)b | 1.2 (1.2) | 3.3 (1.7) |
| Positive gram stain (n) | 13 | 3 |
| Positive culture (n) | 14 | 0 |
Values are medians, with ranges shown in parentheses.
Values are means, with standard deviations shown in parentheses.
CSF examination was repeated 24 to 48 h after the start of therapy for 13 children (sufficient CSF was not obtained for two children).
TABLE 2.
Cefpirome concentrations in serum and CSF after a single drug infusion in children with meningitisa
| Time after infusion | Drug concn (μg/ml)b
|
Concn ratio of CSF/serumb | |
|---|---|---|---|
| Serum | CSF | ||
| 5 min | 261.9 (86.6) | NDc | |
| 2 h | 37.2 (8.0) | 10.8 (7.8) | 0.28 (0.17) |
| 4 h | 10.8 (8.6) | 4.7 (3.5) | 0.46 (0.31) |
| 8 h | 4.7 (2.0) | 3.8 (1.8) | 1.10d (0.95) |
The cefpirome concentrations were measured for all 15 children at 5 min after infusion; concentrations were then measured for three groups of 5 children each at 2, 4, and 8 h after infusion.
Values are means, with standard deviations shown in parentheses.
ND, not done.
Median value, 0.76.
FIG. 1.
Antibiotic concentrations in CSF related to timing of dose administration in children with bacterial meningitis. Symbols: •, cefpirome, 50 mg/kg; ▿, cefotaxime, 67 to 75 mg/kg; ◊, desacetyl-cefotaxime; □, ceftriaxone, 100 mg/kg.
Five patients developed seizures at some time after the administration of cefpirome. Two of these children had had at least one seizure before cefpirome administration. Only one patient had a seizure documented for the first time in the 24 h after the cefpirome infusion. In this child, the concentrations of cefpirome and cefotaxime in CSF 8 h after dosing were 1.9 and 0.9 μg/ml, respectively. The seizures were focal, and a cerebral infarct was visible on a cranial computerized tomogram. Although it is likely that the seizures were related to a brain injury, it is possible that the combination of two cephalosporins given in high doses could have predisposed the patient to seizures. One additional patient developed a temporary rise in the serum glutamic oxalacetic transaminase concentration (to 106 U/ml), which returned to 14 U/ml within 48 h. No unexpected changes in leukocyte, hematocrit, platelet, or electrolyte values occurred after the cefpirome infusion.
In this study, the penetration of cefpirome into CSF of children with meningitis was similar to or better than that of other extended-spectrum cephalosporins. The mean 2-h concentration in CSF of 10.8 μg/ml compares favorably with reported peak concentrations of ceftriaxone and cefotaxime in CSF (5). Concentrations in CSF and serum 4 and 8 h after the cefpirome dose were very similar to those reported in adults with meningitis, whereas concentrations in CSF after 2 h were much higher in children than in adults (10.8 μg/ml versus 2.7 μg/ml [15]). This suggests that the early penetration of cefpirome into CSF is greater in children than in adults. Although little is known about competitive inhibition of clearance of cephalosporins from the CSF, it is possible that cefpirome clearance was diminished by the coadministration of cefotaxime or ceftriaxone. The mean 2-h concentration of cefpirome in CSF was 10-fold greater than the MIC90 for penicillin-resistant pneumococci and more than 10- to 100-fold greater than the MIC90 for penicillin-susceptible pneumococci (14) and other pediatric meningeal pathogens, including H. influenzae and N. meningitidis (4). Experimental data suggest that for β-lactam antibiotics, bactericidal activity in the CSF is directly related to the duration that antibiotic concentrations exceed the MBC for the infecting organism (10). Even 8 h after a single dose of cefpirome, concentrations in CSF exceeded the MIC90 for common meningeal pathogens, indicating that 50 mg of cefpirome per kg given every 8 h is likely to be effective for the common meningeal pathogens, including penicillin-resistant pneumococci. Although we did not measure the drug concentration in CSF 12 h after the dose of cefpirome was given, it seems likely that a 12-h dosing interval would also be effective (6).
Experimental animal data show that dexamethasone therapy can result in diminished penetration of some antibiotics into CSF (11). This has not been confirmed in human studies (8), and in the present study, satisfactory antibiotic concentrations in CSF were achieved with the coadministration of dexamethasone. However, because control subjects were not enrolled, it is not known whether even greater concentrations are achievable in the absence of steroid therapy.
In conclusion, cefpirome is a potentially useful agent for therapy of childhood bacterial meningitis because of its enhanced activity against resistant pneumococci and good penetration into CSF.
Acknowledgments
This study was supported by Hoechst Marion Roussel, Romainville, France.
REFERENCES
- 1.Bajaksouzian S, Visalli M A, Jacobs M R, Appelbaum P C. Antipneumococcal activities of cefpirome and cefotaxime, alone and in combination with vancomycin and teicoplanin, determined by checkerboard and time-kill methods. Antimicrob Agents Chemother. 1996;40:1973–1976. doi: 10.1128/aac.40.9.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry A L, Brown S D, Novick W J. In vitro activities of cefotaxime, ceftriaxone, ceftazidime, cefpirome, and penicillin against Streptococcus pneumoniae isolates. Antimicrob Agents Chemother. 1995;39:2193–2196. doi: 10.1128/aac.39.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley J S, Kaplan S L, Klugman K P, Leggiadro R J. Consensus: management of infections in children caused by Streptococcus pneumoniae with decreased susceptibility to penicillin. Pediatr Infect Dis J. 1995;14:1037–1041. [PubMed] [Google Scholar]
- 4.Cheng A F, Ling T K, Lam A W, Fung K S, Wise R. The antimicrobial activity and beta-lactamase stability of cefpirome, a new fourth-generation cephalosporin, in comparison with other agents. J Antimicrob Chemother. 1993;31:699–709. doi: 10.1093/jac/31.5.699. [DOI] [PubMed] [Google Scholar]
- 5.Cherubin C E, Eng R H K, Norrby R, Modai J, Humbert G, Overturf G. Penetration of newer cephalosporins into cerebrospinal fluid. Rev Infect Dis. 1989;11:526–548. doi: 10.1093/clinids/11.4.526. [DOI] [PubMed] [Google Scholar]
- 6.Craig, W. A. 1993. The pharmacokinetics of cefpirome: rationale for a twelve-hour dosing regimen. Scand. J. Infect. Dis. 91(Suppl.):33–40. [PubMed]
- 7.Jehl F, Birckel P, Monteil H. Hospital routine analysis of penicillins, third generation cephalosporins and aztreonam by conventional and high-speed high-performance liquid chromatography. J Chromatogr. 1987;413:109–119. doi: 10.1016/0378-4347(87)80218-9. [DOI] [PubMed] [Google Scholar]
- 8.Klugman K P, Friedland I R, Bradley J S. Bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis. Antimicrob Agents Chemother. 1995;39:1988–1992. doi: 10.1128/aac.39.9.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehr K H, Weil D. Determination of HR 810 in serum and plasma by HPLC. Report Hoechst AG 012145. Romainville, France: Hoechst; 1993. [Google Scholar]
- 10.Lutsar I, Ahmed A, Friedland I R, Trujillo M, Wubbel L, Olsen K, McCracken G H., Jr Pharmacodynamics and bactericidal activity of ceftriaxone therapy in experimental cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1997;41:2414–2417. doi: 10.1128/aac.41.11.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paris M M, Hickey S M, Usher M I, Shelton S, Olsen K D, McCracken G H., Jr Effect of dexamethasone on therapy of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1994;38:1320–1324. doi: 10.1128/aac.38.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves D S, Bywater M J, Holt H A. The activity of cefpirome and ten other antibacterial agents against 2858 clinical isolates collected from 20 centres. J Antimicrob Chemother. 1993;31:345–362. doi: 10.1093/jac/31.3.345. [DOI] [PubMed] [Google Scholar]
- 13.Sanders C C. In vitro activity of fourth-generation cephalosporins against enterobacteriaceae producing extended-spectrum beta-lactamases. J Chemother. 1996;8:57–62. [PubMed] [Google Scholar]
- 14.Spangler S K, Jacobs M R, Appelbaum P C. Susceptibilities of 177 penicillin-susceptible and -resistant pneumococci to FK 037, cefpirome, cefepime, ceftriaxone, cefotaxime, ceftazidime, imipenem, biapenem, meropenem, and vancomycin. Antimicrob Agents Chemother. 1994;38:898–900. doi: 10.1128/aac.38.4.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff, M., P. Chavanet, A. Kazmierczak, A. Pechinot, C. Demtons, H. Portier, and B. Lenfant. 1992. Diffusion of cefpirome into the cerebrospinal fluid of patients with purulent meningitis. J. Antimicrob. Chemother. 29(Suppl. A):59–62. [DOI] [PubMed]
- 16.Yost R L, Derendorf H. Rapid chromatographic determination of cefotaxime and its metabolite in biological fluid. J Chromatogr. 1985;341:131–138. doi: 10.1016/s0378-4347(00)84017-7. [DOI] [PubMed] [Google Scholar]