Abstract
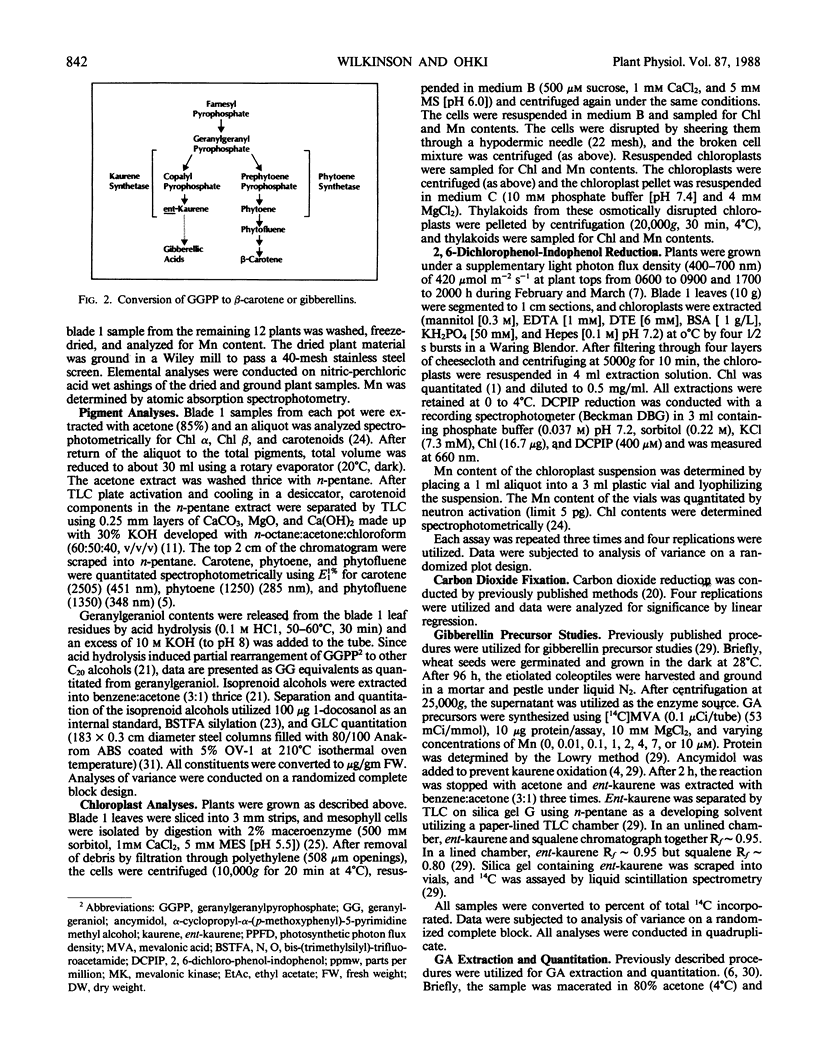
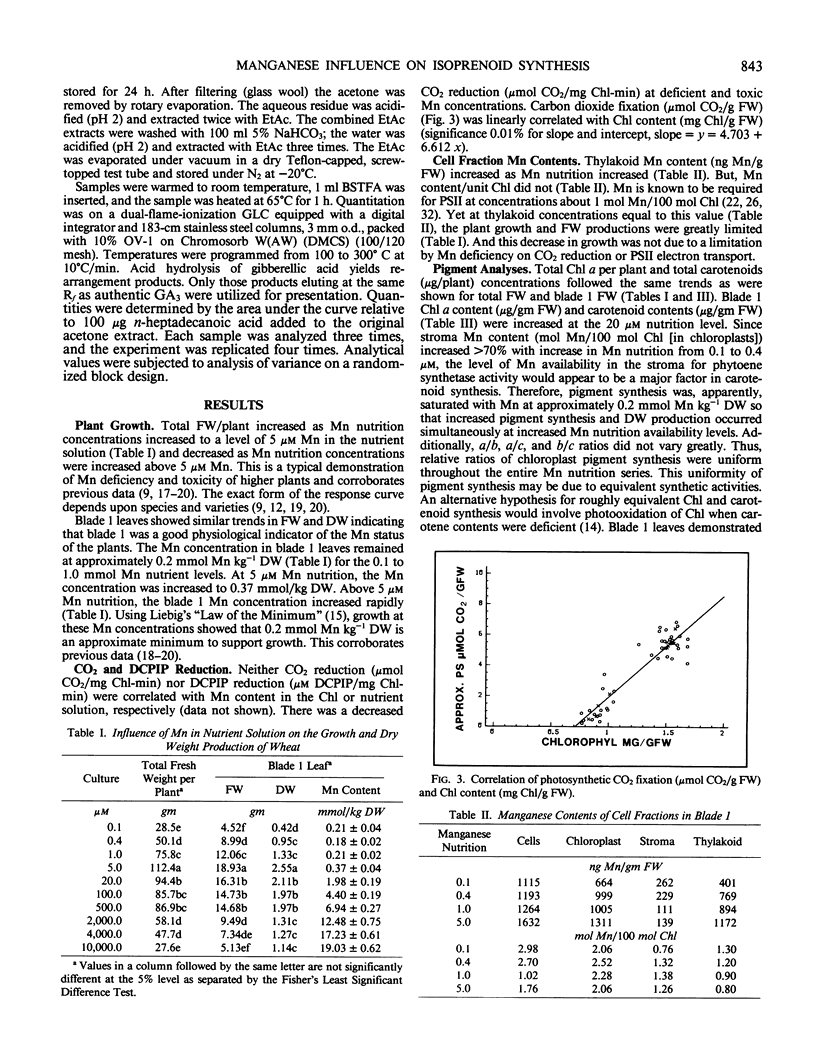
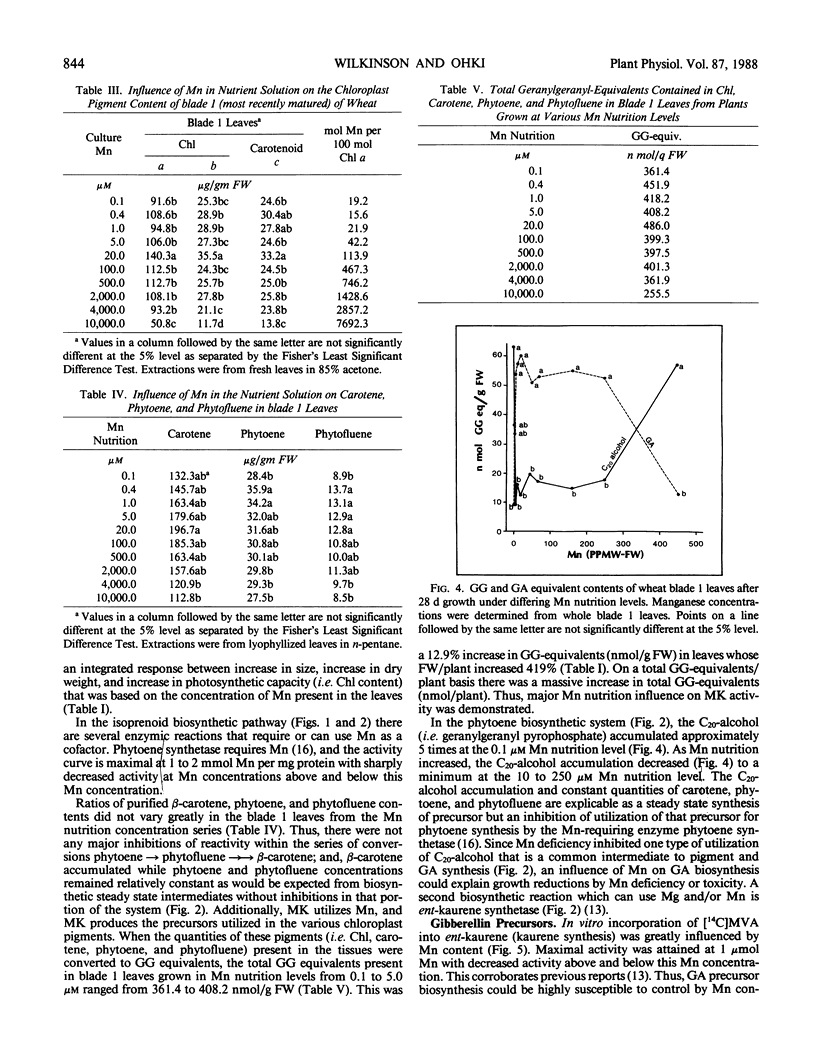
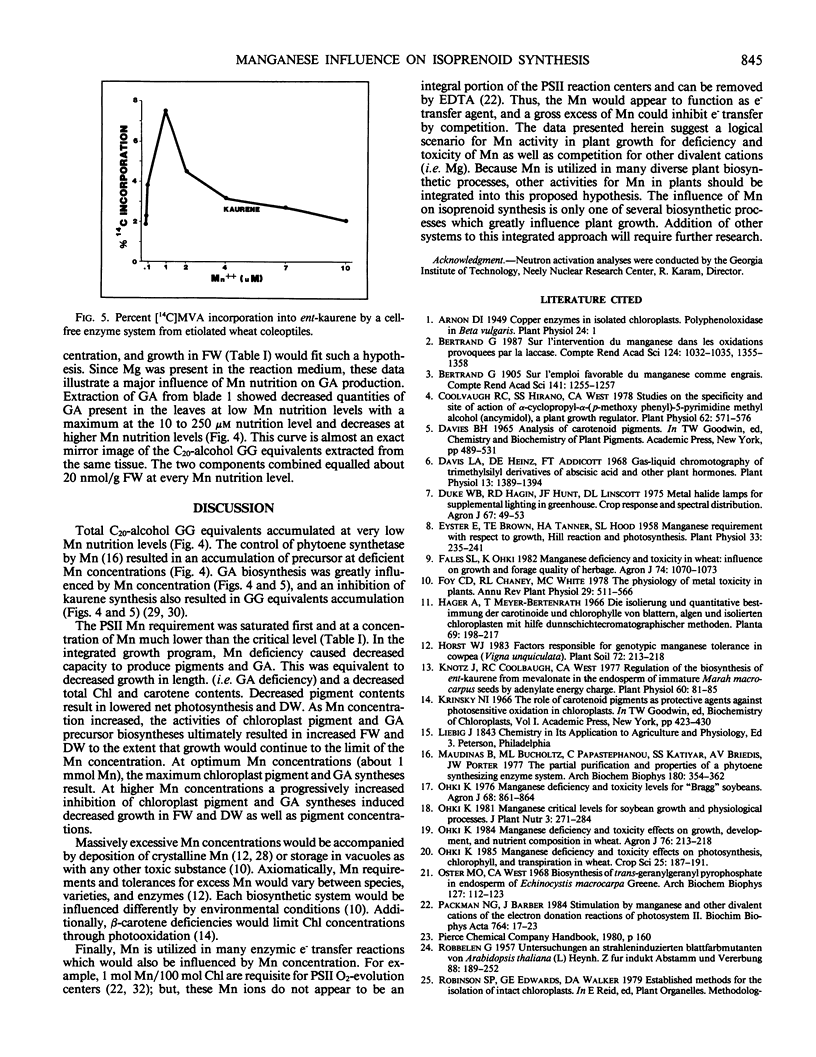
Twenty-eight day old wheat (Triticum aestivum L. cv Stacy) response to varying Mn concentration (10.1-10,000 micromolar) in nutrient solution was measured. Manganese concentrations in the most recently matured leaves (blade 1) were 0.21 to 19.03 mmol Mn per kilogram dry weight, respectively. Fresh and dry weights increased to a maximum at the 5 micromolar Mn nutritional level (0.37 millimole Mn per kilogram dry weight) and were decreased at Mn above and below this concentration. Blade 1 chloroplast pigment concentrations increased up to the 20 micromolar Mn nutritional level (1.98 millimole Mn per kilogram dry weight) and decreased at higher Mn concentrations. Thylakoid Mn content was above 1 mole Mn/100 mole chloroplast at Mn nutrition levels which resulted in greatly decreased plant growth. Total phytoene biosynthesis was decreased by Mn deficiency and toxicity. In vitro ent- kaurene synthesis was greatly influenced by Mn concentration with a maximal biosynthesis at 1 micromolar Mn and decreases at Mn levels above and below this concentration. In vivo blade 1 gibberellic acid equivalent concentrations were maximal at 20 parts per million Mn nutrition solution levels (1.98 millimole Mn per kilogram dry weight) and decreased at Mn tissue concentrations above and below this value; additionally, gibberellic acid concentrations were reciprocal to extracted C20 alcohol concentrations. Mn influence on gibberellin and chloroplast pigment biosyntheses exactly matched the measured changes in growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolbaugh R. C., Hirano S. S., West C. A. Studies on the Specificity and Site of Action of alpha-Cyclopropyl-alpha-[p-methoxyphenyl]-5-pyrimidine Methyl Alcohol (Ancymidol), a Plant Growth Regulator. Plant Physiol. 1978 Oct;62(4):571–576. doi: 10.1104/pp.62.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. A., Heinz D. E., Addicott F. T. Gas-liquid chromatography of trimethylsilyl derivatives of abscisic Acid and other plant hormones. Plant Physiol. 1968 Sep;43(9):1389–1394. doi: 10.1104/pp.43.9.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyster C., Brown T. E., Tanner H. A., Hood S. L. Manganese Requirement with Respect to Growth, Hill Reaction and Photosynthesis. Plant Physiol. 1958 Jul;33(4):235–241. doi: 10.1104/pp.33.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knotz J., Coolbaugh R. C., West C. A. Regulation of the Biosynthesis of Ent-Kaurene from Mevalonate in the Endosperm of Immature Marah macrocarpus Seeds by Adenylate Energy Charge. Plant Physiol. 1977 Jul;60(1):81–85. doi: 10.1104/pp.60.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudinas B., Bucholtz M. L., Papastephanou C., Katiyar S. S., Briedis A. V., Porter J. W. The partial purification and properties of a phytoene synthesizing enzyme system. Arch Biochem Biophys. 1977 Apr 30;180(2):354–362. doi: 10.1016/0003-9861(77)90049-2. [DOI] [PubMed] [Google Scholar]
- Oster M. O., West C. A. Biosynthesis of trans-geranylgeranyl pyrophosphate in endosperm of Echinocystis macrocarpa Greene. Arch Biochem Biophys. 1968 Sep 20;127(1):112–123. doi: 10.1016/0003-9861(68)90207-5. [DOI] [PubMed] [Google Scholar]


