Abstract
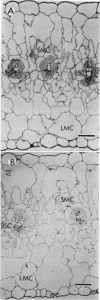
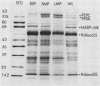
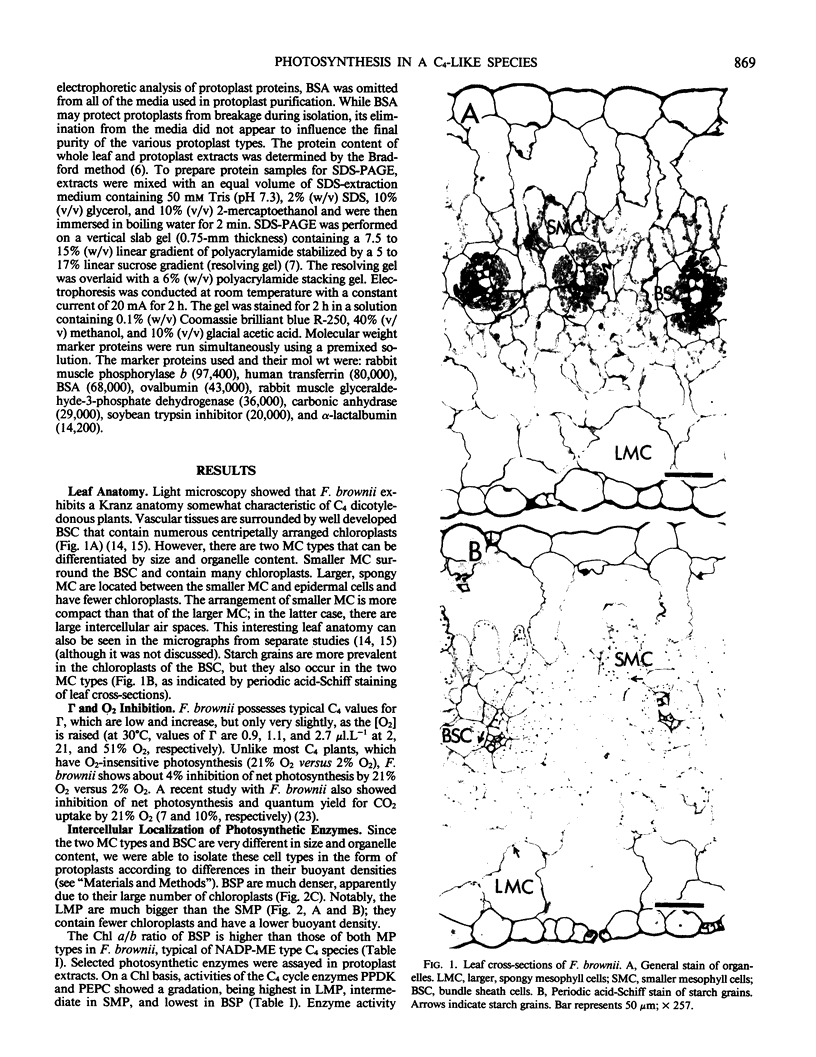
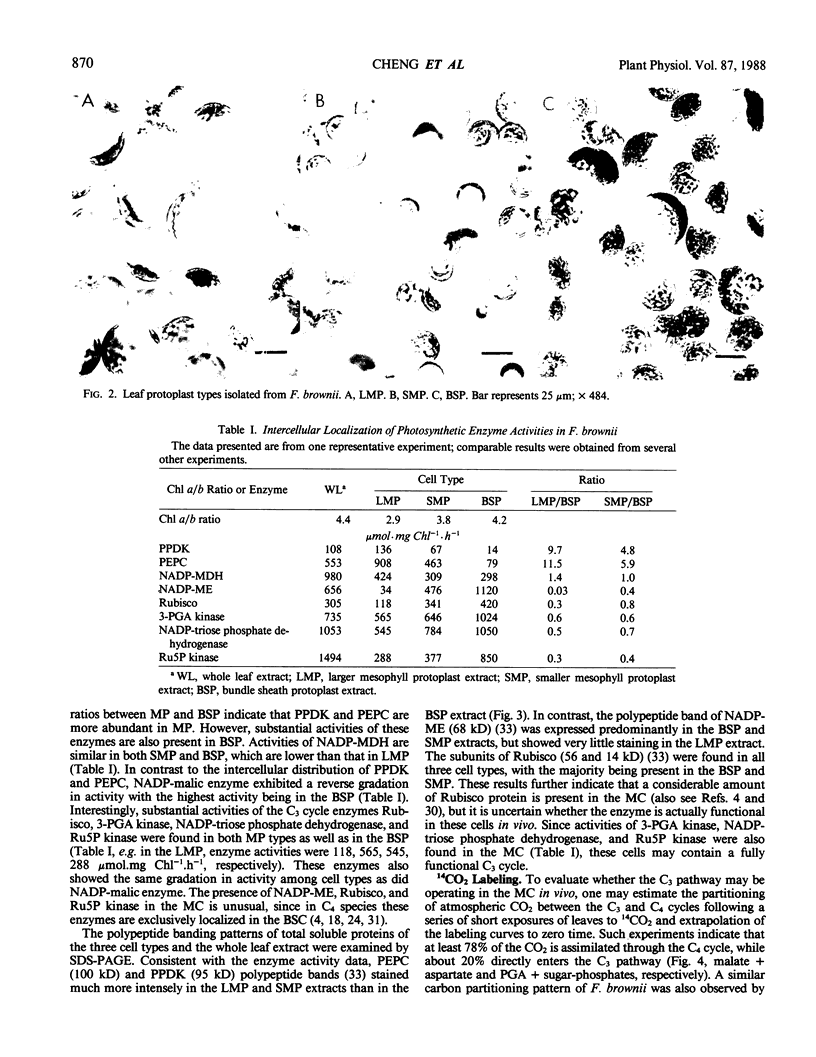
Light microscopic examination of leaf cross-sections showed that Flaveria brownii A. M. Powell exhibits Kranz anatomy, in which distinct, chloroplast-containing bundle sheath cells are surrounded by two types of mesophyll cells. Smaller mesophyll cells containing many chloroplasts are arranged around the bundle sheath cells. Larger, spongy mesophyll cells, having fewer chloroplasts, are located between the smaller mesophyll cells and the epidermis. F. brownii has very low CO2 compensation points at different O2 levels, which is typical of C4 plants, yet it does show about 4% inhibition of net photosynthesis by 21% O2 at 30°C. Protoplasts of the three photosynthetic leaf cell types were isolated according to relative differences in their buoyant densities. On a chlorophyll basis, the activities of phosphoenolpyruvate carboxylase and pyruvate, Pi dikinase (carboxylation phase of C4 pathway) were highest in the larger mesophyll protoplasts, intermediate in the smaller mesophyll protoplasts, and lowest, but still present, in the bundle sheath protoplasts. In contrast, activities of ribulose 1,5-bisphosphate carboxylase, other C3 cycle enzymes, and NADP-malic enzyme showed a reverse gradation, although there were significant activities of these enzymes in mesophyll cells. As indicated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the banding pattern of certain polypeptides of the total soluble proteins from the three cell types also supported the distribution pattern obtained by activity assays of these enzymes. Analysis of initial 14C products in whole leaves and extrapolation of pulse-labeling curves to zero time indicated that about 80% of the CO2 is fixed into C4 acids (malate and aspartate), whereas about 20% of the CO2 directly enters the C3 cycle. This is consistent with the high activity of enzymes for CO2 fixation by the C4 pathway and the substantial activity of enzymes of the C3 cycle in the mesophyll cells. Therefore, F. brownii appears to have some capacity for C3 photosynthesis in the mesophyll cells and should be considered a C4-like species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyagi K., Nakamoto H. Pyruvate, pi dikinase in bundle sheath strands as well as in mesophyll cells in maize leaves. Plant Physiol. 1985 Jul;78(3):661–664. doi: 10.1104/pp.78.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwe H., Chollet R. Kinetic properties of phosphoenolpyruvate carboxylase from c(3), c(4), and c(3)-c(4) intermediate species of flaveria (asteraceae). Plant Physiol. 1986 Nov;82(3):695–699. doi: 10.1104/pp.82.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Holaday A. S., Chollet R. Photosynthetic/Photorespiratory Carbon Metabolism in the C(3)-C(4) Intermediate Species, Moricandia arvensis and Panicum milioides. Plant Physiol. 1983 Nov;73(3):740–745. doi: 10.1104/pp.73.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobza J., Edwards G. E. Influences of leaf temperature on photosynthetic carbon metabolism in wheat. Plant Physiol. 1987 Jan;83(1):69–74. doi: 10.1104/pp.83.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M. S., Monson R. K., Littlejohn R. O., Nakamoto H., Fisher D. B., Edwards G. E. Photosynthetic Characteristics of C(3)-C(4) Intermediate Flaveria Species : I. Leaf Anatomy, Photosynthetic Responses to O(2) and CO(2), and Activities of Key Enzymes in the C(3) and C(4) Pathways. Plant Physiol. 1983 Apr;71(4):944–948. doi: 10.1104/pp.71.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Enzyme activities of the carbon reduction cycle in some photosynthetic organisms. Plant Physiol. 1969 Feb;44(2):295–300. doi: 10.1104/pp.44.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Monson R. K., Schuster W. S., Ku M. S. Photosynthesis in Flaveria brownii A.M. Powell : A C(4)-Like C(3)-C(4) Intermediate. Plant Physiol. 1987 Dec;85(4):1063–1067. doi: 10.1104/pp.85.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. D., Edwards G. E. Photosynthetic Induction in a C(4) Dicot, Flaveria trinervia: II. Metabolism of Products of CO(2) Fixation after Different Illumination Times. Plant Physiol. 1986 Jun;81(2):669–673. doi: 10.1104/pp.81.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muss H. B., Bundy B. N., DiSaia P. J., Ehrlich C. E. Mitoxantrone for carcinoma of the endometrium: a phase II trial of the Gynecologic Oncology Group. Cancer Treat Rep. 1987 Feb;71(2):217–218. [PubMed] [Google Scholar]
- Rumpho M. E., Ku M. S., Cheng S. H., Edwards G. E. Photosynthetic Characteristics of C(3)-C(4) Intermediate Flaveria Species : III. Reduction of Photorespiration by a Limited C(4) Pathway of Photosynthesis in Flaveria ramosissima. Plant Physiol. 1984 Aug;75(4):993–996. doi: 10.1104/pp.75.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Wittenbach V. A. Effect of pod removal on leaf senescence in soybeans. Plant Physiol. 1982 Nov;70(5):1544–1548. doi: 10.1104/pp.70.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]