Abstract
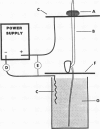
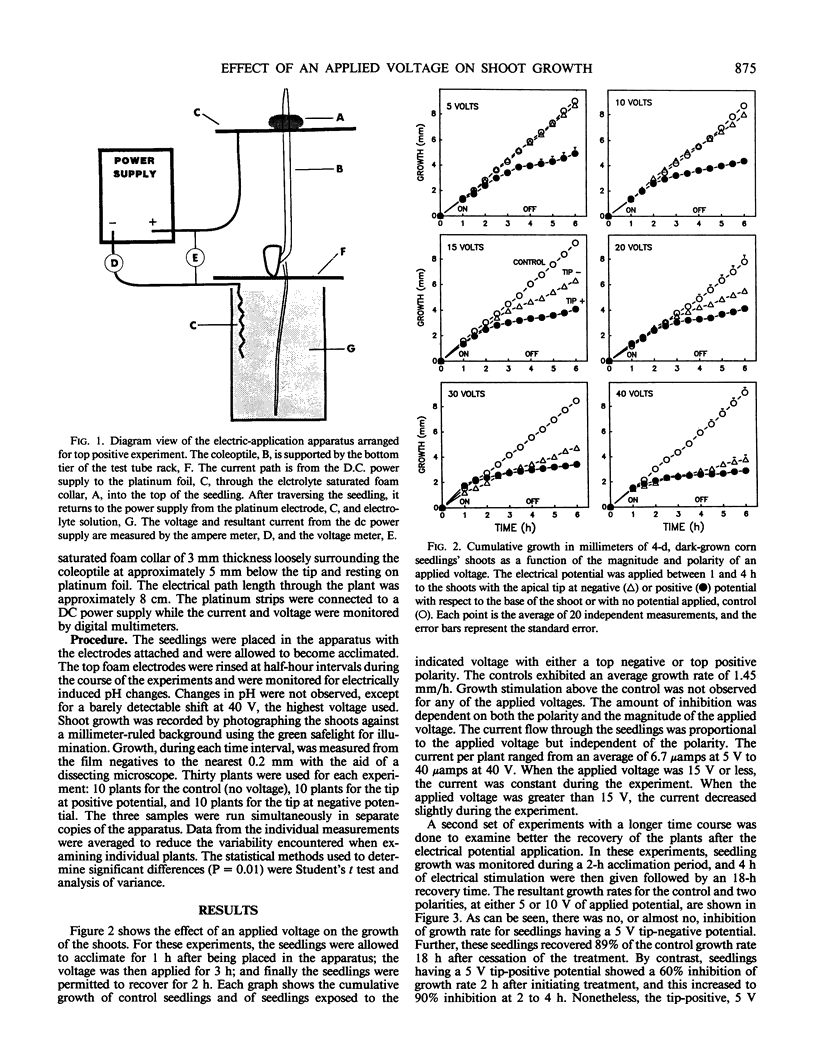
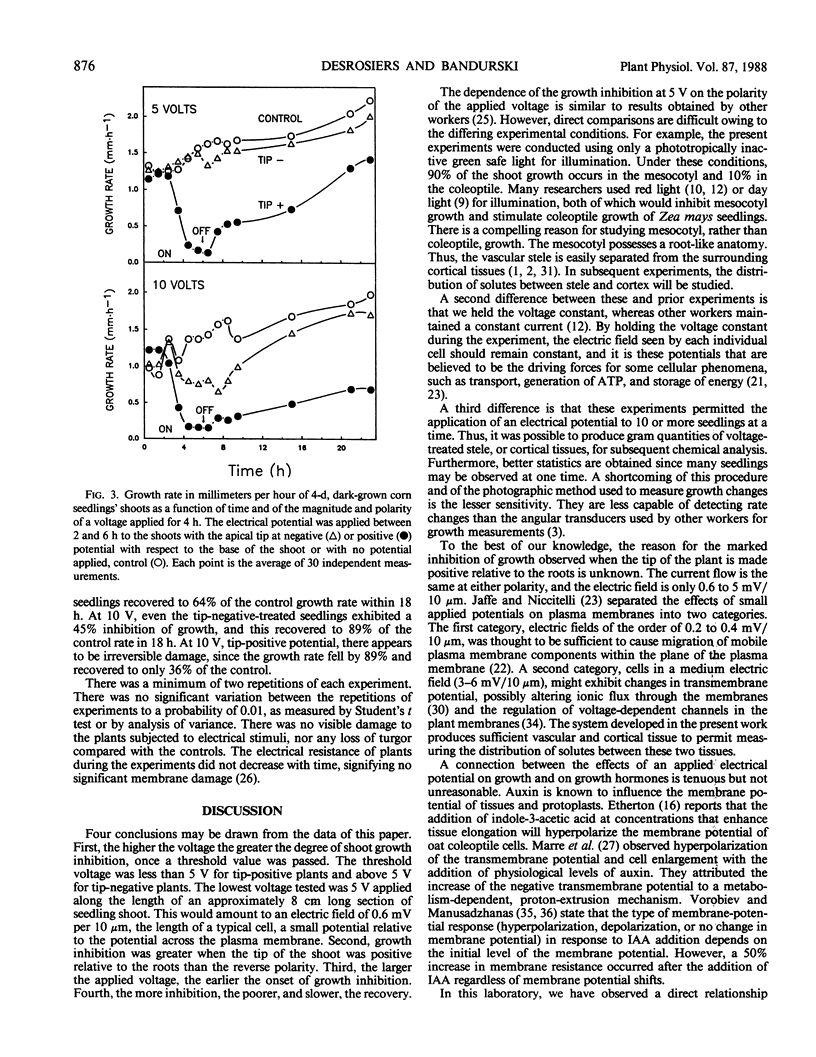
The electrical parameters that affect young seedling growth were investigated. Voltages ranging from 5 to 40 volts were applied longitudinally along the mesocotyl region of 4-day old Zea mays L. (cv Silver Queen) seedlings for periods of 3 or 4 hours. It was determined that: (a) making the tips of the seedlings electrically positive relative to the base strongly inhibited shoot growth at 5 volts, whereas the reverse polarity had no effect; (b) at higher voltages, making the tip of the seedlings negative caused less growth inhibition than the reverse polarity at each voltage level; (c) the higher the applied voltage the greater the degree of inhibition; and, (d) the more growth inhibition experienced by the plants the poorer, and slower, their recovery. Previous observations of a relationship between the amount of free indole-3-acetic acid in the mesocotyl cortex and the growth rate of the mesocotyl and of gravitropism-induced movement of labeled indole-3-acetic acid from the seed to the shoot lead to the prediction of a voltage-dependent gating of the movement of indole-3-acetic acid from the stele to the cortex. This provided the basis for attempting to alter the growth rate of seedlings by means of an applied voltage.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A., Dayanandan P., Kaufman P. B. Response to gravity by Zea mays seedlings. I. Time course of the response. Plant Physiol. 1984;74:284–288. doi: 10.1104/pp.74.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A., Domagalski W. Possible effects of organelle charge and density on cell metabolism. Adv Space Res. 1986;6(12):47–54. doi: 10.1016/0273-1177(86)90065-7. [DOI] [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A. Gravitational effects on plant growth hormone concentration. Adv Space Res. 1983;3(9):229–235. doi: 10.1016/0273-1177(83)90061-3. [DOI] [PubMed] [Google Scholar]
- Behrens H. M., Weisenseel M. H., Sievers A. Rapid Changes in the Pattern of Electric Current around the Root Tip of Lepidium sativum L. following Gravistimulation. Plant Physiol. 1982 Oct;70(4):1079–1083. doi: 10.1104/pp.70.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholodny N. G., Sankewitsch E. C. INFLUENCE OF WEAK ELECTRIC CURRENTS UPON THE GROWTH OF THE COLEOPTILE. Plant Physiol. 1937 Apr;12(2):385–408. doi: 10.1104/pp.12.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D., Baldi B. G., Slovin J. P. C(6)-[benzene ring]-indole-3-acetic Acid: a new internal standard for quantitative mass spectral analysis of indole-3-acetic Acid in plants. Plant Physiol. 1986 Jan;80(1):14–19. doi: 10.1104/pp.80.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D., Meudt W. J. Investigations on the Mechanism of the Brassinosteroid Response: I. Indole-3-acetic Acid Metabolism and Transport. Plant Physiol. 1983 Jul;72(3):691–694. doi: 10.1104/pp.72.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Effect of Indole-3-acetic Acid on Membrane Potentials of Oat Coleoptile Cells. Plant Physiol. 1970 Apr;45(4):527–528. doi: 10.1104/pp.45.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F. Electrophoresis along cell membranes. Nature. 1977 Feb 17;265(5595):600–602. doi: 10.1038/265600a0. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F., Nuccitelli R. Electrical controls of development. Annu Rev Biophys Bioeng. 1977;6:445–476. doi: 10.1146/annurev.bb.06.060177.002305. [DOI] [PubMed] [Google Scholar]
- Meiners S., Schindler M. Immunological evidence for gap junction polypeptide in plant cells. J Biol Chem. 1987 Jan 25;262(3):951–953. [PubMed] [Google Scholar]
- Moran N., Ehrenstein G., Iwasa K., Bare C., Mischke C. Ion channels in plasmalemma of wheat protoplasts. Science. 1984 Nov 16;226(4676):835–838. doi: 10.1126/science.6093255. [DOI] [PubMed] [Google Scholar]
- Peng H. B., Jaffe L. F. Polarization of fucoid eggs by steady electrical fields. Dev Biol. 1976 Oct 15;53(2):277–284. doi: 10.1016/0012-1606(76)90229-3. [DOI] [PubMed] [Google Scholar]
- Pengelly W. L., Hall P. J., Schulze A., Bandurski R. S. Distribution of Free and Ester Indole-3-Acetic Acid in the Cortex and Stele of the Zea mays Mesocotyl. Plant Physiol. 1982 Jun;69(6):1304–1307. doi: 10.1104/pp.69.6.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smack D. P., Colombini M. Voltage-dependent channels found in the membrane fraction of corn mitochondria. Plant Physiol. 1985 Dec;79(4):1094–1097. doi: 10.1104/pp.79.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]