Abstract
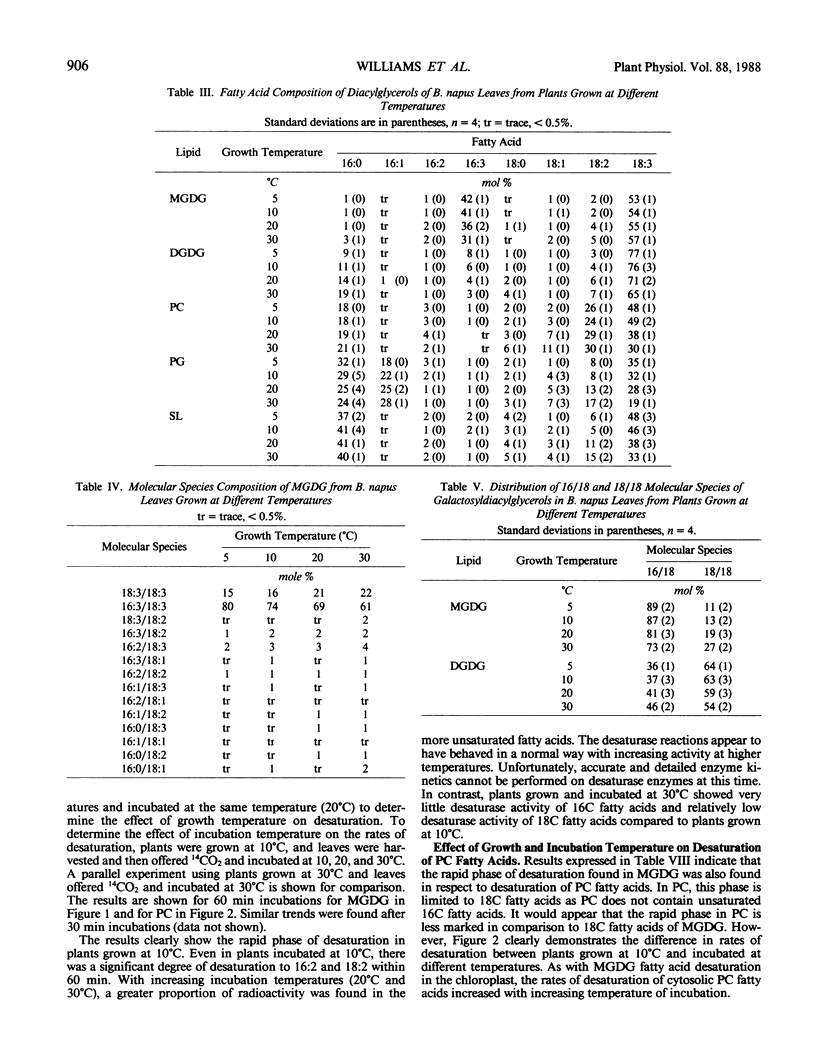
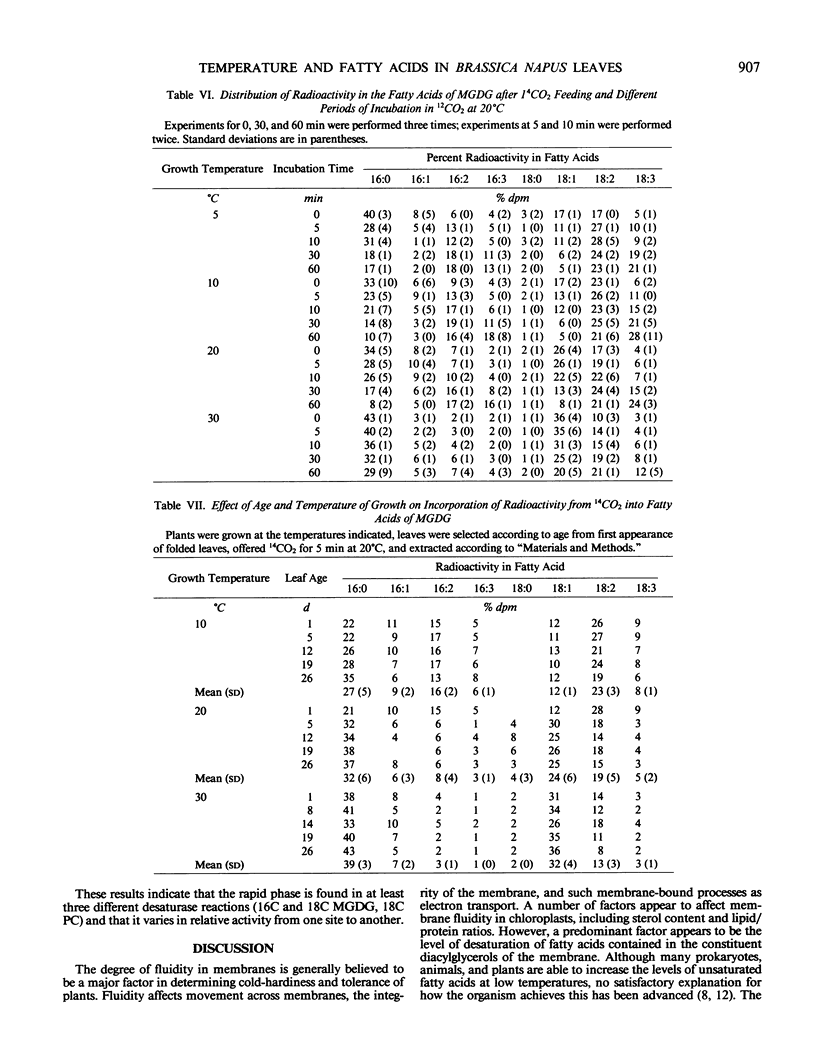
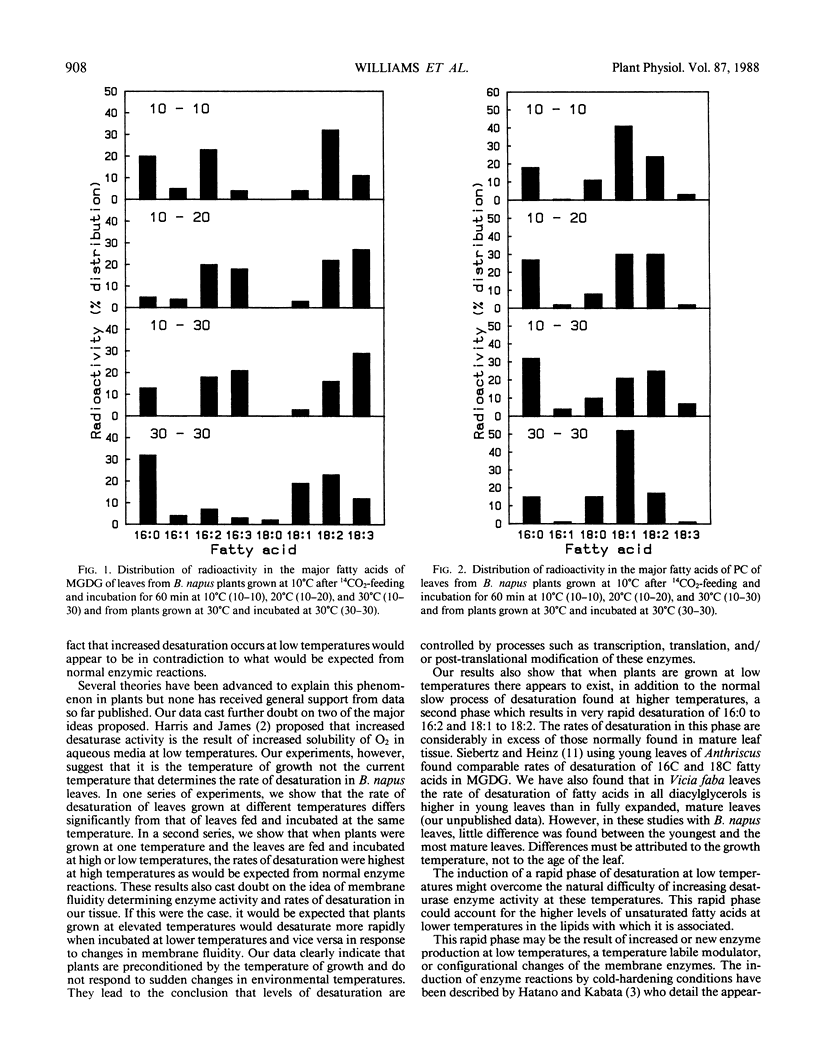
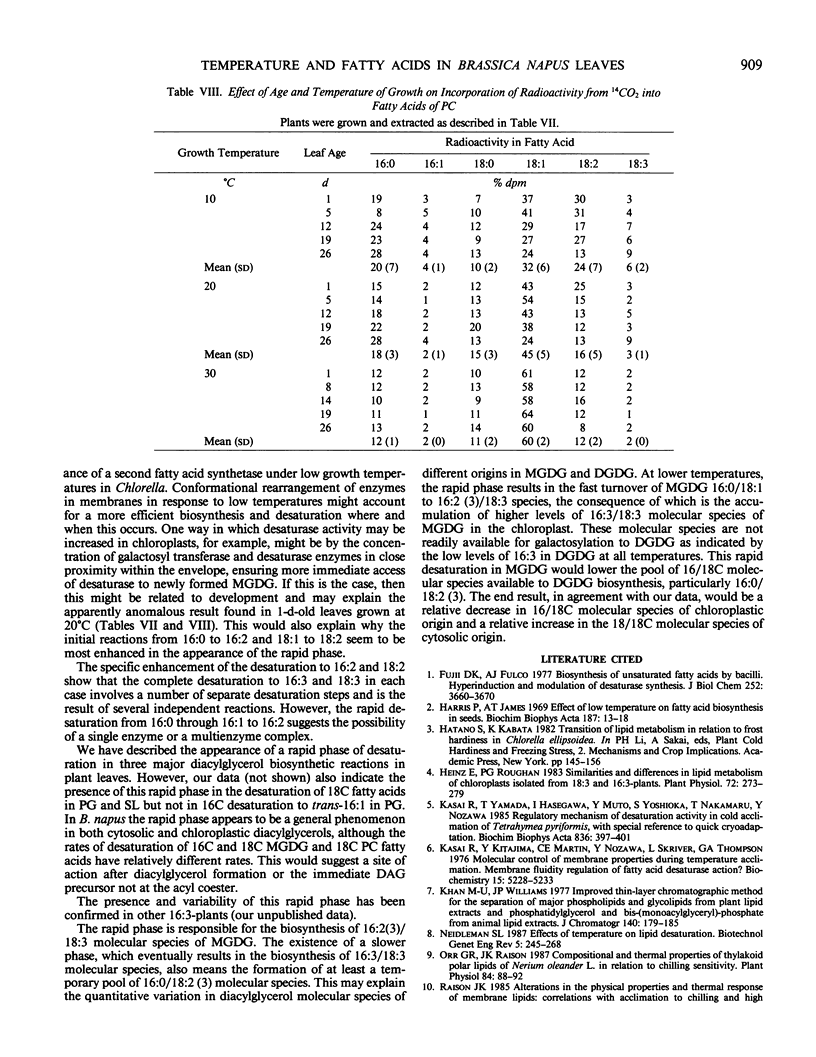
Experiments on the effects of temperature on the levels of unsaturated fatty acids and their rates of desaturation in Brassica napus leaf lipids have shown that significant differences occur in the composition of all diacylglycerols in the leaf between plants grown at high and low temperatures. In the major thylakoid diacylglycerols, monogalactosyl-diacylglycerol and digalactosyldiacylglycerol, not only is there an increase in the level of unsaturation at low temperatures, but there is a change in the balance between molecular species of chloroplastic origin (16/18C) and cytosolic origin (18/18C). Radioactivity tracer data indicate that at low temperatures there are two distinct phases of desaturation in the fatty acids of the major diacylglycerols of these leaves. A rapid phase, which appears in plants grown at low temperatures and results in the desaturation of palmitic acid to hexadecadienoic acid and oleic acid to linoleic acid may explain the high levels of unsaturated fatty acids found in the leaf diacylglycerols from plants grown at low temperatures. The appearance of this rapid phase is controlled by the temperature at which the plant is grown and is not subject to rapid variations in environmental temperature.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujii D. K., Fulco A. J. Biosynthesis of unsaturated fatty acids by bacilli. Hyperinduction and modulation of desaturase synthesis. J Biol Chem. 1977 Jun 10;252(11):3660–3670. [PubMed] [Google Scholar]
- Harris P., James A. T. Effect of low temperature on fatty acid biosynthesis in seeds. Biochim Biophys Acta. 1969 Jul 29;187(1):13–18. doi: 10.1016/0005-2760(69)90127-1. [DOI] [PubMed] [Google Scholar]
- Heinz E., Roughan P. G. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983 Jun;72(2):273–279. doi: 10.1104/pp.72.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai R., Kitajima Y., Martin C. E., Nozawa Y., Skriver L., Thompson G. A., Jr Molecular control of membrane properties during temperature acclimation. Membrane fluidity regulation of fatty acid desaturase action? Biochemistry. 1976 Nov 30;15(24):5228–5233. doi: 10.1021/bi00669a005. [DOI] [PubMed] [Google Scholar]
- Khan M. U., Williams J. P. Improved thin-layer chromatographic method for the separation of major phospholipids and glycolipids from plant lipid extracts and phosphatidyl glycerol and bis(monoacylglyceryl) phosphate from animal lipid extracts. J Chromatogr. 1977 Oct 11;140(2):179–185. doi: 10.1016/s0021-9673(00)88412-5. [DOI] [PubMed] [Google Scholar]
- Neidleman S. L. Effects of temperature on lipid unsaturation. Biotechnol Genet Eng Rev. 1987;5:245–268. doi: 10.1080/02648725.1987.10647839. [DOI] [PubMed] [Google Scholar]
- Orr G. R., Raison J. K. Compositional and Thermal Properties of Thylakoid Polar Lipids of Nerium oleander L. in Relation to Chilling Sensitivity. Plant Physiol. 1987 May;84(1):88–92. doi: 10.1104/pp.84.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. P., Merrilees P. A. The removal of water and nonlipid contaminants from lipid extracts. Lipids. 1970 Apr;5(4):367–370. doi: 10.1007/BF02532100. [DOI] [PubMed] [Google Scholar]


