Abstract
Background
Plant-based dietary patterns are gaining more attention due to their potential in reducing the risk of developing major chronic diseases, including type 2 diabetes (T2D), cardiovascular disease (CVD), cancer, and mortality, while an up-to-date comprehensive quantitative review is lacking. This study aimed to summarize the existing prospective observational evidence on associations between adherence to plant-based dietary patterns and chronic disease outcomes.
Methods
We conducted a systematic review and meta-analysis of evidence across prospective observational studies. The data sources used were PubMed and MEDLINE, Embase, Web of Science, and screening of references. We included all prospective observational studies that evaluated the association between adherence to plant-based dietary patterns and incidence of T2D, CVD, cancer, and mortality among adults (≥ 18 years).
Results
A total of 76 publications were identified, including 2,230,443 participants with 60,718 cases of incident T2D, 157,335 CVD cases, 57,759 cancer cases, and 174,435 deaths. An inverse association was observed between higher adherence to a plant-based dietary pattern and risks of T2D (RR, 0.82 [95% CI: 0.77–0.86]), CVD (0.90 [0.85–0.94]), cancer (0.88 [0.84–0.92]), and all-cause mortality (0.84 [0.78–0.92]) with moderate to high heterogeneity across studies (I2 ranged: 30.2–95.4%). The inverse associations with T2D, CVD and cancer were strengthened when healthy plant-based foods, such as vegetables, fruits, whole grains, and legumes, were emphasized in the definition of plant-based dietary patterns (T2D: 0.79 [0.72–0.87]; CVD: 0.85 [0.80–0.92]; cancer: 0.86 [0.80–0.92]; I2 ranged: 53.1–84.1%). Association for mortality was largely similar when the analyses were restricted to healthy plant-based diets (0.86 [0.80–0.92], I2 = 91.9%). In contrast, unhealthy plant-based diets were positively associated with these disease outcomes. Among four studies that examined changes in dietary patterns, increased adherence to plant-based dietary patterns was associated with a significantly reduced risk of T2D (0.83 [0.71–0.96]; I2 = 71.5%) and a marginally lower risk of mortality (0.95 [0.91–1.00]; I2 = 0%).
Conclusions
Better adherence to plant-based dietary patterns, especially those emphasizing healthy plant-based foods, is beneficial for lowering the risks of major chronic conditions, including T2D, CVD, cancer, as well as premature deaths.
Registration of review protocol
This review was registered at the PROSPERO International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/PROSPERO/) with the registration number CRD42022290202.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12937-023-00877-2.
Keywords: Systematic review, Meta-analysis, Plant-based dietary patterns, Type 2 diabetes, Cardiovascular disease, Cancer, Mortality
Introduction
According to the World Health Organization, type 2 diabetes (T2D), cardiovascular disease (CVD), and cancer account for nearly one of every two deaths globally – close to 30 million people in 2020 [1–3]. These diseases had significant clinical and public health implications by undermining health, shortening life expectancy, and causing enormous suffering, disability, and economic costs [4]. Habitual diet is known to play a critical role in modifying risks of these diseases. Plant-based dietary patterns that emphasize foods derived from plant sources and de-emphasize consumption of animal products have gained significant attention in recent years. They have been associated with lower risks of CVD, cancer, and T2D in prospective human studies [5–7], and thus have the potential to prevent and manage these diseases.
Although a few systematic reviews and meta-analyses have examined associations between long-term vegetarian versus non-vegetarian dietary patterns and risks of CVD [6, 8] and cancer [7, 9–11], much less has been examined systematically on whether plant-based diets that do not necessarily exclude animal products also lower risks of these chronic conditions. In comparison with vegan or vegetarian diets, plant-based diets may be more feasible for the general population to adopt. Moreover, more studies have been published since the last meta-analyses of plant-based diets in relation to T2D [12–20] and mortality [21–28]. Furthermore, no meta-analysis has quantitatively evaluated the impact of changes in plant-based dietary patterns on risks of T2D and mortality.
To summarize the current evidence regarding plant-based diets in relation to human health, we conducted an updated systematic review and meta-analysis of prospective studies to assess the association of adherence to plant-based dietary patterns and changes in plant-based dietary patterns with risks of major chronic diseases, including T2D, CVD, and cancer, as well as mortality. We hypothesized that higher adherence to plant-based dietary patterns is associated with lower risks of T2D, CVD, cancer, and mortality.
Methods
Data sources and searches
We systematically searched three databases, including PubMed and MEDLINE, Embase, and Web of Science through June 29, 2023. The search initially started in June 2021, and then updated in June 2023. Reference lists of prior systematic reviews were also checked to identify relevant original studies that were not found in three databases. Search terms included “plant-based diet”, “vegetarian”, “vegan”, “type 2 diabetes”, “cardiovascular disease”, “stroke”, “coronary artery disease”, “myocardial infarction”, “angina pectoris”, “cerebrovascular disorders”, “cancer”, and “mortality”. MESH and Emtree terms with explosion of narrower terms were used to broaden search results. The detailed search strategy for each database and the number of records retrieved was presented in Supplemental Table S1. We followed the recommended standards in the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) [29] guideline. To report the studies included, we used the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [30].
Study selection
The current analysis considered prospective studies (cohort studies, case-cohort studies, or nested case-control studies) that assessed associations between adherence to a priori-defined plant-based dietary patterns and the incidence of T2D, CVD, cancer, and mortality among adults (aged ≥ 18 years). Other types of studies (e.g., cross-sectional studies, reviews, commentaries, meeting abstracts), studies using a posteriori approach (e.g., principal component analysis, factor analysis) to derive dietary patterns, or studies reported outcomes other than T2D (e.g., type 1 diabetes, gestational diabetes), CVD, cancer, and mortality were excluded. The detailed inclusion and exclusion criteria were presented in Supplemental Table S2.
The primary exposure of interest was adherence to plant-based dietary patterns, defined as higher consumption of plant-based foods and lower consumption or exclusion of animal-based foods, or changes in plant-based dietary patterns. By this definition, vegetarian or vegan dietary patterns were also considered as plant-based dietary patterns. In studies that classified plant-based dietary patterns using overall, healthful or unhealthful plant-based dietary indices (PDI, hPDI, uPDI), the association for overall plant-based dietary index was included in the pooled risk estimate. We also summarized studies that explicitly examined healthful or unhealthful plant-based dietary patterns that further de-emphasize the intake of unhealthy plant-based foods, such as refined grains, starchy vegetables (e.g., white potatoes), or sugar (e.g., sweets, desserts, or sugar-sweetened beverages).
For studies that used dietary indices, we used the risk estimate that compared the highest with lowest quantiles, which represent the best (highest quantile) and poorest (lowest quantile) adherence to the plant-based dietary pattern. For studies that compared distinct dietary patterns (vegetarian, semi-vegetarian, non-vegetarian), we considered the study estimates comparing diets that are most restrictive of animal-based foods (vegan or vegetarian) with the least restrictive, such as omnivorous diets.
The primary outcomes were T2D, any CVD, any cancer, and all-cause mortality. In the secondary analysis, we also examined subtypes of CVD (coronary heart disease [CHD] including ischemic heart disease and myocardial infarction, stroke, and heart failure), cancer (e.g., breast cancer, colorectal cancer, lung cancer, and prostate cancer), and mortality (cancer mortality, and CVD mortality).
Data extraction and assessment of quality
Data from included studies were independently extracted by three authors (Y.W., B.L., and H.H.). Any discrepancies were evaluated by another author (Q.S.) and discussed among four authors. The following information was extracted from each study: author, year, study name, region, disease outcome, number of participants and cases of incident T2D, CVD, cancer, or deaths, mean age, mean body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), sex (percentage of men), follow-up duration, dietary assessment method, method of scoring adherence to plant-based diet, outcome ascertainment, and covariates that were adjusted for in the statistical models. The study quality was assessed using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies available on the National Heart, Lung, and Blood Institute website (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). This tool assigns a maximum score of 14 to each study where 0 indicates that the study does not meet any of the criteria for quality assessments and 14 indicates that the study meets all 14 criteria based on potential areas of bias such as duration of follow-up, assessment of exposure and outcomes, and rates of loss to follow-up. Studies identified with high risk of bias (quality score < 10) were excluded in a sensitivity analysis.
Data synthesis and analysis
We calculated a pooled relative risk (RR) using random-effects meta-analysis of hazard ratio, relative risk, rate ratio, or odds ratio from each study. The risk estimate from the statistical model with the highest degree of adjustment was included in the meta-analysis. For studies that only reported continuous plant-based dietary indices (per 25 percentile increment, per 5-unit increment, per 10-unit increment) [18, 21, 31–36], we converted the RRs to those comparing the extreme quintiles of dietary scores (riskconv package from STATA) [37, 38].
Statistical heterogeneity was assessed with the Cochran Q-test (P < 0.10) and I2 statistic. We performed subgroup analyses to characterize potential sources of heterogeneity, including analyses stratified by age, sex, BMI (< 25 vs. ≥25 kg/m2), global region (North America, Europe, Asia/Australia), definitions of plant-based diets (e.g., vegetarian or vegan diet vs. plant-based dietary scores), and follow-up duration (< 15 vs. ≥15 years). In addition, we performed meta-regression analyses using age, sex, BMI, and follow-up duration to explore potential sources of heterogeneity. Sensitivity analyses included those using inverse-variance fixed-effects meta-analysis as well as sequentially excluding individual studies to examine the effect on the overall risk estimate. Publication bias was assessed visually with funnel plots and with the Egger or Begg-Mazumdar regression tests [39]. All analyses were conducted with STATA, version 17.0 (STATA Corp). All P values were from 2-sided tests, and the results were statistically significant with a P < 0.05 unless stated otherwise.
Results
Included studies and baseline characteristics
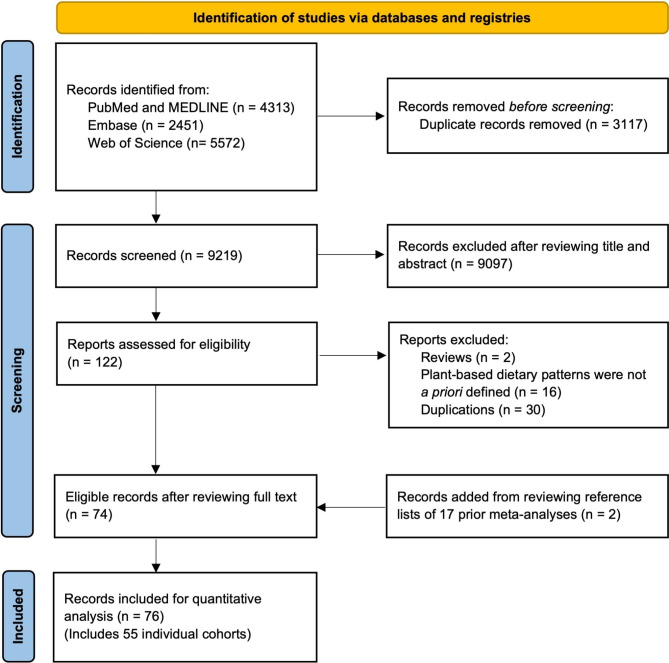
The PRISMA flow diagram of search and screening results from three databases were presented in Fig. 1. PRISMA checklist was presented in the Additional File 1. Our initial search identified 12,336 citations, of which, after excluding ineligible studies due to various reasons, 76 publications from 55 individual cohorts were included in the current analysis (Fig. 1). Of those, 17 publications examined T2D, 16 for CVD, 22 for cancer, and 27 for mortality. The characteristics of studies included in the meta-analysis were shown in Supplemental Table S3. The meta-analysis included 2,230,443 participants and 60,718 T2D cases, 157,335 CVD cases, 57,759 cancer cases, and 174,435 deaths. Studies had a follow-up duration between 2 and 36 years. All studies used a prospective cohort design except for one study that used a nested case-control study design [40]. The mean age ranged from 24.9 to 86.9 years, and the mean BMI ranged between 20.3 and 30.0 kg/m2 at baseline. Majority of studies used food frequency questionnaires (FFQs) to assess diet, while six studies used 24-hour recalls. Twenty-nine publications used repeated measurements of dietary intake, forty-seven publications used one-time dietary measurement at study baseline. A total of 49 publications characterized adherence to plant-based dietary patterns using pre-specified plant-based dietary indices (e.g., overall plant-based dietary index, healthful plant-based dietary index, pro plant-based dietary pattern, provegetarian food pattern, EAT-Lancet diet, Portfolio diet), and 27 publications compared vegetarian or vegan dietary patterns with those who consumed animal-based foods. Six studies examined the change of dietary patterns. Thirty-five publications examined healthful plant-based dietary index, and 29 publications examined unhealthful plant-based index. The study quality ranged between 6 and 14 (low to high), where most publications had high quality (n = 67, 88.2%) indicated by a score of 10 or higher.
Fig. 1.
PRISMA Flowchart of Prospective Observational Studies of Plant-Based Dietary Patterns and Incident Type 2 Diabetes, Cardiovascular Disease, Cancer, and Mortality
Plant-based diet and risk of T2D, CVD, cancer, and mortality
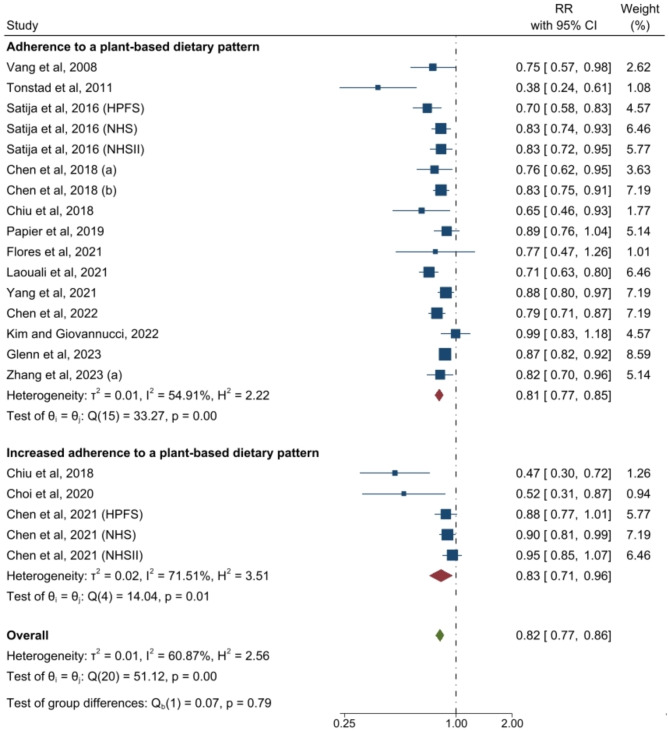
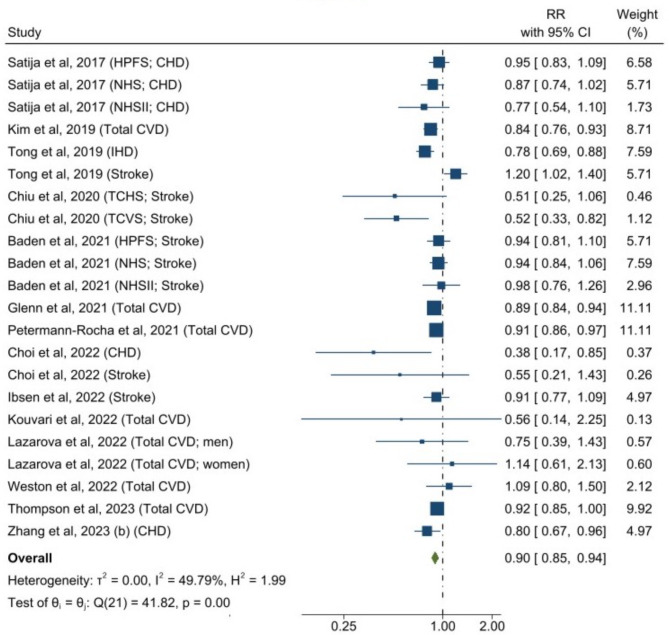
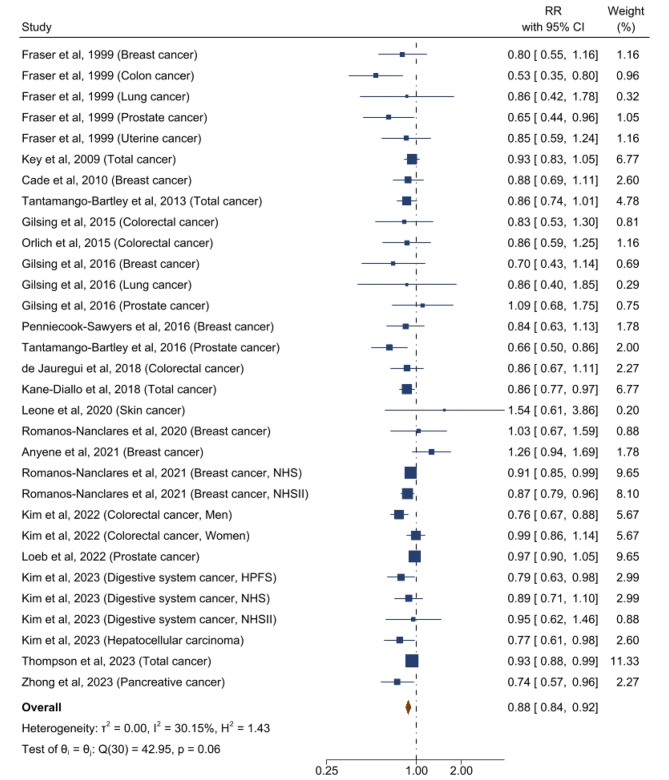
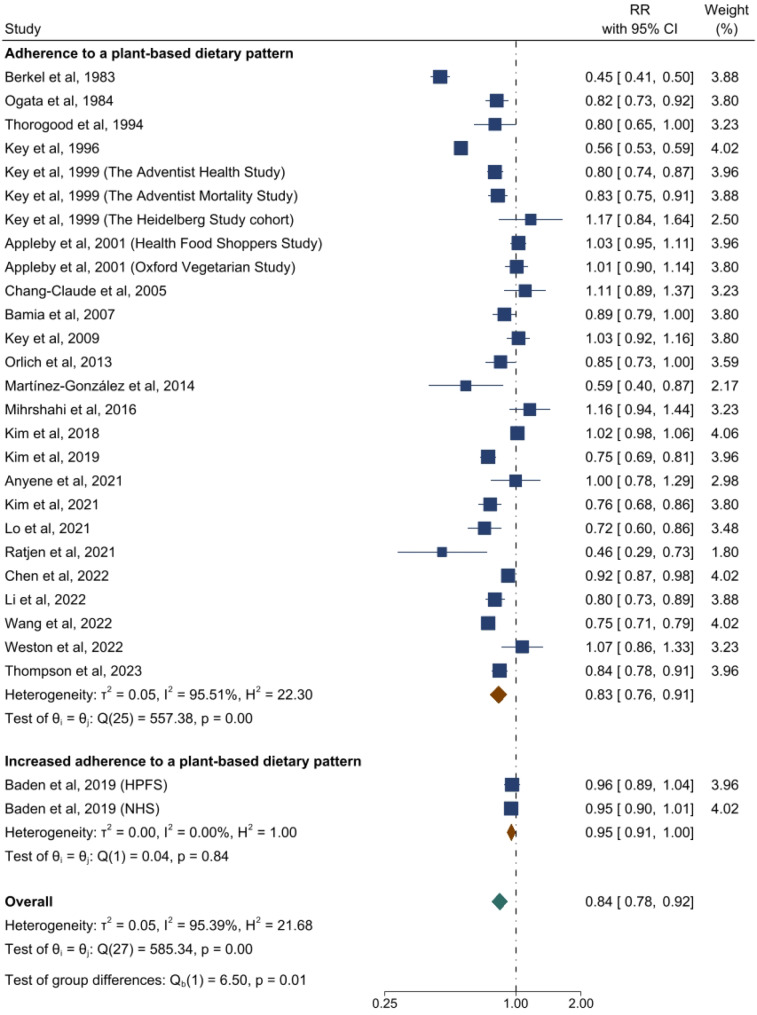
The forest plots for associations between plant-based dietary patterns and T2D, CVD, cancer, and mortality are shown in Figs. 2, 3, 4 and 5. A greater adherence to plant-based dietary patterns was consistently associated with lower risks of T2D, CVD, cancer, and mortality. The random-effects pooled RR was 0.82 (95% CI: 0.77–0.86), 0.90 (95% CI: 0.85–0.94), 0.88 (95% CI: 0.84–0.92), and 0.84 (95% CI: 0.78–0.92), respectively. A moderate heterogeneity was observed among studies for T2D (60.9%), CVD (49.8%), cancer (30.2%), and a high heterogeneity was observed for studies reporting mortality (95.4%).
Fig. 2.
Forest Plot of Studies Examining the Association Between Plant-Based Dietary Patterns and Risks of Type 2 Diabetes using Random-Effects Meta-Analysis
Abbreviations: HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II
Fig. 3.
Forest Plot of Studies Examining the Association Between Plant-Based Dietary Patterns and Risks of Cardiovascular Disease using Random-Effects Meta-Analysis
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; IHD, ischemic heart disease; TCHS, The Tzu Chi Health Study; TCVS, The Tzu Chi Vegetarian Study; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II
Fig. 4.
Forest Plot of Studies Examining the Association Between Plant-Based Dietary Patterns and Risks of Cancer using Random-Effects Meta-Analysis
Abbreviations: NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; HPFS, Health Professionals Follow-up Study
Fig. 5.
Forest Plot of Studies Examining the Association Between Plant-Based Dietary Patterns and Risks of Mortality using Random-Effects Meta-Analysis
Abbreviations: HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study
For individual CVD outcomes, a greater adherence to plant-based dietary pattern was significantly associated with a lower CHD risk (0.86 [95% CI: 0.81–0.91; I2 = 34.5%]), but not with risks of stroke (0.92 [95% CI: 0.85–1.00; I2 = 51.6%]) and heart failure (0.89 [95% CI: 0.75–1.06; I2 = 42.9%]) (Supplemental Figure S1). For various cancer types, plant-based dietary patterns were significantly associated with lower risk of breast cancer (0.91 [95% CI: 0.86–0.95; I2 = 0%]), digestive system cancer (0.82 [95% CI: 0.72–0.94; I2 = 0%]), pancreatic cancer (0.68 [95% CI: 0.55–0.84; I2 = 0%]), and prostate cancer (0.87 [95% CI: 0.77–0.99; I2 = 53.3%]), but not with risks of colorectal cancer (0.90 [95% CI: 0.79–1.02; I2 = 61.8%]), liver cancer (0.51 [95% CI: 0.22–1.21; I2 = 57.7%]), lung cancer (0.82 [95% CI: 0.54–1.26; I2 = 36.6%]), or stomach cancer (1.73 [95% CI: 0.90–3.31; I2 = 0%]) (Supplemental Figure S2). For specific causes of mortality, plant-based dietary pattern was associated with lower risks of CVD mortality (0.79 [95% CI: 0.72–0.87; I2 = 77.0%]) and cancer mortality (0.82 [95% CI: 0.71–0.93; I2 = 88.4%]) (Supplemental Figure S3).
The associations between plant-based dietary pattern and T2D, CVD, cancer, and mortality remained similar when using an inverse-variance fixed-effects model (Supplemental Figures S4-S7) and when excluding any single study (Supplemental Figure S8). When using the “healthful plant-based dietary index” instead of the “overall plant-based dietary index”, results were strengthened for T2D (0.79 [95% CI: 0.72–0.87; I2 = 84.1%]) (Supplemental Figure S9 panel A), CVD (0.85 [95% CI: 0.80–0.92; I2 = 62.1%]) (Supplemental Figure S9 panel B) and cancer (0.87 [95% CI: 0.82–0.92; I2 = 53.1%]) (Supplemental Figure S9 panel C); while results were similar for mortality (0.86 [95% CI: 0.80–0.92; I2 = 91.9%]) (Supplemental Figure S9 panels D). Of note, unlike results summarized for “overall plant-based dietary index”, there were significant associations between the healthful plant-based dietary pattern with stroke (0.89 [95% CI: 0.83–0.95; I2 = 0%]) (Supplemental Figure S9 panel B) and colorectal cancer (0.85 [95% CI: 0.79–0.92; I2 = 0%]) (Supplemental Figure S9 panel C). On the contrary, adherence to unhealthful plant-based dietary patterns was positively associated with the risks of major chronic diseases (Supplemental Figure S10). Specifically, higher adherence to an unhealthful plant-based dietary patterns was associated with an increased risk of these outcomes by 8% for T2D (95% CI: 2–13%; I2 = 15.4%); 14% for CVD (95% CI: 4–26%; I2 = 71.1%); 7% for cancer (95% CI: 2–12%; I2 = 46.3%); and 16% for mortality (95% CI: 9–25%; I2 = 84.8%).
For outcomes of T2D and mortality, data were available to evaluate the impact of change of dietary patterns. Increased adherence to a plant-based dietary pattern was associated with lower risks of T2D (0.83 [95% CI: 0.71–0.96; I2 = 71.5%]) and a marginally lower risk of mortality (0.95 [95% CI: 0.91–1.00; I2 = 0%]) (Figs. 2 and 5). Among these studies, only two studies examined changes of hPDI (healthy plant-based diet index) and uPDI (unhealthy plant-based diet index) for T2D and mortality (Supplemental Figure S9–S10) [16, 32].
Subgroup results and meta-regression
The stratified analyses by study characteristics are shown in Table 1. Overall, we did not observe significant heterogeneity by study region, dietary classification, age, BMI, sex, and follow-up duration for T2D and mortality. The association between plant-based dietary patterns and CVD was stronger in studies conducted in Asia and Australia compared to those conducted in North America and Europe (P-heterogeneity = 0.02). The association for T2D was stronger in studies that examined vegan or vegetarian diets compared to studies reporting on a priori defined plant-based diets (P-heterogeneity = 0.048). No other sources of heterogeneity were observed for CVD and cancer associations.
Table 1.
Subgroup Analysis of Plant-Based Dietary Patterns and Risks of Incident Type 2 Diabetes, Cardiovascular Disease, Cancer, and Mortality
| Characteristics | Study estimates, No. | Relative Risk (95% CI) | |||
|---|---|---|---|---|---|
| Inverse-Variance Fixed-Effects Meta-analysis |
Random-Effects Meta-analysis |
I2, % | P value for Heterogeneity Between Subgroups | ||
| Type 2 diabetes | |||||
| Main estimate | 22 | 0.84 (0.81–0.86) | 0.81 (0.77–0.86) | 59.7 | |
| Age, y | 0.56 | ||||
| <55 | 11 | 0.81 (0.78–0.85) | 0.79 (0.73–0.87) | 68.7 | |
| ≥55 | 11 | 0.85 (0.82–0.89) | 0.84 (0.79–0.89) | 42.0 | |
| Sex | 0.48 | ||||
| Studies among males only | 2 | 0.81 (0.72–0.90) | 0.79 (0.63–0.99) | 75.4 | |
| Studies among females only | 6 | 0.86 (0.82–0.89) | 0.85 (0.79–0.91) | 64.8 | |
| Studies among males and females | 14 | 0.82 (0.78–0.86) | 0.81 (0.77–0.86) | 58.3 | |
| BMI, kg/m2 | 0.90 | ||||
| <25 | 11 | 0.83 (0.79–0.86) | 0.81 (0.75–0.88) | 67.8 | |
| ≥25 | 11 | 0.84 (0.81–0.88) | 0.82 (0.76–0.87) | 51.8 | |
| Region | 0.87 | ||||
| North America | 12 | 0.85 (0.82–0.89) | 0.82 (0.76–0.88) | 60.1 | |
| Europe | 4 | 0.78 (0.72–0.84) | 0.79 (0.71–0.87) | 43.5 | |
| Asia/Australia | 6 | 0.83 (0.79–0.88) | 0.82 (0.73–0.91) | 66.4 | |
| Dietary pattern classification | 0.048 | ||||
| Vegan or vegetarian diets | 5 | 0.75 (0.67–0.84) | 0.63 (0.47–0.84) | 77.4 | |
| A priori-defined PDI | 17 | 0.84 (0.82–0.87) | 0.83 (0.80–0.87) | 48.3 | |
| Follow-up duration, y | 0.22 | ||||
| <15 | 11 | 0.81 (0.77–0.86) | 0.76 (0.68–0.84) | 66.3 | |
| ≥15 | 11 | 0.84 (0.81–0.86) | 0.84 (0.79–0.88) | 51.5 | |
| Cardiovascular disease | |||||
| Main estimate | 23 | 0.90 (0.87–0.92) | 0.89 (0.85–0.94) | 51.0 | |
| Age, y | 0.88 | ||||
| <55 | 16 | 0.89 (0.85–0.93) | 0.87 (0.80–0.96) | 63.8 | |
| ≥55 | 7 | 0.90 (0.87–0.93) | 0.89 (0.85–0.93) | 0 | |
| Sex | 0.68 | ||||
| Studies among males only | 3 | 0.94 (0.85–1.04) | 0.94 (0.85–1.04) | 0 | |
| Studies among females only | 6 | 0.90 (0.85–0.94) | 0.90 (0.85–0.94) | 0 | |
| Studies among males and females | 14 | 0.89 (0.86–0.93) | 0.86 (0.79–0.97) | 68.1 | |
| BMI, kg/m2 | 0.76 | ||||
| <25 | 7 | 0.91 (0.85–0.98) | 0.87 (0.72–1.05) | 77.9 | |
| ≥25 | 12 | 0.90 (0.87–0.93) | 0.90 (0.87–0.93) | 0 | |
| Region | 0.02 | ||||
| North America | 14 | 0.89 (0.86–0.93) | 0.89 (0.85–0.94) | 16.2 | |
| Europe | 7 | 0.91 (0.87–0.95) | 0.91 (0.83-1.00) | 71.5 | |
| Asia/Australia | 2 | 0.52 (0.35–0.76) | 0.52 (0.35–0.76) | 0 | |
| Dietary classification | 0.99 | ||||
| Vegan or vegetarian diets | 5 | 0.90 (0.86–0.95) | 0.85 (0.70–1.03) | 85.0 | |
| A priori-defined PDI | 18 | 0.90 (0.87–0.93) | 0.90 (0.86–0.93) | 6.1 | |
| Follow-up duration, y | 0.45 | ||||
| <15 | 8 | 0.91 (0.87–0.95) | 0.86 (0.77–0.96) | 46.0 | |
| ≥15 | 15 | 0.89 (0.86–0.93) | 0.90 (0.85–0.96) | 55.9 | |
| Cancer | |||||
| Main estimate | 32 | 0.90 (0.87–0.92) | 0.88 (0.85–0.92) | 29.0 | |
| Age, y | 0.86 | ||||
| <55 | 14 | 0.88 (0.85–0.92) | 0.88 (0.85–0.92) | 0 | |
| ≥55 | 16 | 0.91 (0.87–0.94) | 0.88 (0.82–0.94) | 50.2 | |
| Sex | 0.40 | ||||
| Studies among males only | 5 | 0.89 (0.84–0.95) | 0.83 (0.70–0.98) | 75.3 | |
| Studies among females only | 12 | 0.90 (0.86–0.94) | 0.90 (0.86–0.94) | 0 | |
| Studies among males and females | 15 | 0.89 (0.86–0.93) | 0.87 (0.82–0.92) | 18.5 | |
| BMI, kg/m2 | 0.51 | ||||
| <25 | 12 | 0.90 (0.87–0.94) | 0.90 (0.87–0.94) | 0 | |
| ≥25 | 19 | 0.89 (0.86–0.93) | 0.87 (0.81–0.92) | 41.0 | |
| Region | 0.39 | ||||
| North America | 20 | 0.89 (0.85–0.92) | 0.86 (0.81–0.91) | 47.7 | |
| Europe | 12 | 0.91 (0.87–0.95) | 0.91 (0.86–0.95) | 0 | |
| Asia/Australia | |||||
| Dietary classification | 0.14 | ||||
| Vegan or vegetarian diets | 16 | 0.85 (0.80–0.91) | 0.85 (0.80–0.91) | 0 | |
| A priori-defined PDI | 16 | 0.91 (0.88–0.93) | 0.90 (0.85–0.94) | 43.0 | |
| Follow-up duration, y | 0.98 | ||||
| <15 | 15 | 0.90 (0.86–0.94) | 0.87 (0.81–0.94) | 38.2 | |
| ≥15 | 17 | 0.89 (0.86–0.93) | 0.88 (0.84–0.93) | 23.4 | |
| Mortality | |||||
| Main estimate | 32 | 0.86 (0.84–0.87) | 0.85 (0.79–0.91) | 94.6 | |
| Age, y | 0.77 | ||||
| <55 | 17 | 0.87 (0.85–0.88) | 0.88 (0.80–0.96) | 95.9 | |
| ≥55 | 12 | 0.88 (0.85–0.90) | 0.86 (0.80–0.94) | 83.9 | |
| Sex | 0.66 | ||||
| Studies among males only | 3 | 0.94 (0.91–0.97) | 0.92 (0.86–0.99) | 66.4 | |
| Studies among females only | 3 | 0.85 (0.82–0.88) | 0.90 (0.77–1.04) | 91.6 | |
| Studies among males and females | 26 | 0.83 (0.82–0.85) | 0.83 (0.76–0.91) | 95.5 | |
| BMI, kg/m2 | 0.24 | ||||
| <25 | 12 | 0.89 (0.87–0.91) | 0.90 (0.84–0.95) | 86.6 | |
| ≥25 | 11 | 0.83 (0.80–0.86) | 0.84 (0.77–0.92) | 77.0 | |
| Region | 0.56 | ||||
| North America | 13 | 0.89 (0.87–0.90) | 0.87 (0.81–0.94) | 92.6 | |
| Europe | 14 | 0.75 (0.73–0.78) | 0.80 (0.67–0.96) | 96.4 | |
| Asia/Australia | 5 | 0.88 (0.84–0.92) | 0.86 (0.76–0.97) | 80.9 | |
| Dietary classification | 0.90 | ||||
| Vegan or vegetarian diets | 14 | 0.76 (0.74–0.78) | 0.86 (0.73–1.01) | 96.4 | |
| A priori-defined PDI | 18 | 0.89 (0.87–0.90) | 0.85 (0.80–0.91) | 90.6 | |
| Follow-up duration, y | 0.16 | ||||
| <15 | 20 | 0.80 (0.78–0.82) | 0.81 (0.74–0.89) | 91.2 | |
| ≥15 | 12 | 0.88 (0.87–0.90) | 0.90 (0.80-1.00) | 97.1 | |
Assessment of publication bias and risk of bias in individual studies
Visual inspection of the funnel plot suggests some degree of publication bias. Four studies lay outside of the pseudo 95% CI of the funnel plot for T2D and CVD, five for cancer, while 16 studies lay outside for mortality (Supplemental Figure S11). Furthermore, Egger regression tests and Begg-Mazumdar regression tests suggested potential publication bias for T2D (P = 0.0001 and P = 0.01, respectively) and CVD (P = 0.04 and P = 0.21); but tests did not detect significant publication bias for cancer (P = 0.12 and P = 0.96) and mortality (P = 0.83 and P = 0.52). After performing the trim-and-fill analysis to evaluate the robustness of associations after accounting for potential publication bias, our results remained largely unchanged. The random-effects pooled RR was 0.84 (95% CI: 0.80–0.90) for T2D, 0.91 (95% CI: 0.86–0.96) for CVD, 0.91 (95% CI: 0.87–0.95) for cancer and 0.83 (95% CI: 0.76–0.90) for mortality (Supplemental Figure S12).
Common problems affecting the study quality included the lack of sample size justification, the absence of repetitive measurement of exposure measures, and not having information on the loss of follow-up. The detailed study quality assessment is shown in Supplemental Table S4. One publication for T2D risk (5.9%) and 7 publications for mortality (25.9%) had low study quality (< 10). After excluding these studies, the associations remained significant. The random-effects pooled RRs were 0.82 (95% CI: 0.77–0.86) for T2D and 0.87 (95% CI: 0.82–0.92) for mortality. All publications for CVD and cancer were deemed to have high study quality (≥ 10).
Discussion
In this meta-analysis of 55 prospective cohort studies with 2,230,443 participants, we found that greater adherence to a plant-based dietary pattern was inversely associated with risks of T2D, CVD, cancer and all-cause mortality. Associations for T2D, CVD, and cancer were strengthened when the plant-based diets further emphasized healthful plant-based foods, such as vegetables, fruits, whole grains, and legumes. These findings were largely consistent across subgroups of study characteristics and robust in sensitivity analyses. For specific disease outcomes, the inverse association for CVD was mainly driven by CHD, and for cancer by breast cancer, pancreatic cancer, and prostate cancer. In contrast, the inverse association for mortality was observed for both CVD mortality and cancer mortality. Among studies with measurements of changes in dietary patterns, increased adherence to plant-based dietary patterns was inversely associated with lower risks of T2D and mortality.
The current analysis was consistent with previous systematic reviews and meta-analyses on vegetarian diets and together suggest that plant-based dietary patterns, including the vegan or vegetarian diets, were inversely associated with CVD risk, which was mainly driven by CHD but not stroke [6, 8]. A prospective cohort study in the U.S. found that plant-based diets wre inversely associated with ischemic stroke risk but not with hemorrhagic stroke [41], suggesting that the null association between plant-based diets and total stroke might be due to the heterogenous pathophysiology of stroke subtypes and the relatively smaller number of cases compared to CHD. In terms of heart failure, since only two studies examined the association with plant-based diets and had mixed results [23, 42], the non-significant association is likely to be underpowered and inconclusive and needs further assessment in future studies. For T2D and mortality, our results were consistent with previous meta-analyses that higher adherence to plant-based dietary patterns is associated with lower risks of T2D, all-cause mortality, cancer mortality, and CVD mortality [5, 43], although earlier meta-analyses with fewer studies failed to find significant associations with all-cause mortality or cancer mortality [7, 9, 44]. We further extended the evidence to changes in dietary patterns and showed that increased adherence to plant-based dietary patterns is also associated with lower risks of T2D and mortality. Furthermore, previous meta-analyses on vegetarian diets have also found an inverse association between plant-based dietary patterns with total cancer [7, 9–11]. While we found that the inverse association with cancer is mainly driven by breast, pancreatic, and prostate cancer, but not by colorectal, liver, lung, or stomach cancer. Since the study points for the latter four cancers (3–8 studies) were fewer than those for breast cancer (11 studies), the observed null association could also be due to the limited statistical power, and future studies are needed to investigate associations between plant-based diets and different types of cancer.
In the current study, the inverse association of plant-based diet with T2D, CVD, and cancer were strengthened when we summarized data of studies that examined healthy plant-based patterns. Similarly, previous prospective cohort studies also found stronger associations with healthy plant-based dietary patterns compared to overall plant-based patterns on T2D, CVD and cancer outcomes [5, 25, 45]. In light of existing evidence suggesting that unhealthy plant-based dietary patterns that are rich in refined carbohydrates are detrimental for T2D and CHD risk, the current evidence provides strong evidence to support increased consumption of high-quality plant-based diets, including whole grains, legumes, nuts, fruits, and vegetables.
Several mechanisms for the favorable effect of plant-based dietary patterns on CVD, cancer, and T2D have been proposed previously. As obesity is the common risk factor for CVD, T2D, and certain types of cancer (4), a healthful plant-based diet that emphasizes intakes of fruits, vegetables, whole grains, nuts and legumes, and healthy vegetable oils is likely to be low in energy density due to its low saturated fat and high fiber content, which could help with weight loss and long-term weight maintenance (46). Evidence from clinical trials and observational studies showed that higher intakes of plant-based diets could lead to short-term weight loss or prevention of long-term weight gain (47–50), which may mitigate risks of CVD, cancer, and T2D. In addition, CVD and T2D share some common pathophysiology (51) such as insulin resistance (52), inflammation (53, 54), oxidative stress (55), high blood pressure (56), and dyslipidemia (57, 58). A meta-analysis of randomized clinical trials suggested that higher fiber content has potential cholesterol-lowering effect (59). Similarly, high unsaturated fat and low saturated fat content may also improve the blood lipid profile (60), and replacing saturated fats with unsaturated fat has shown to enhance insulin sensitivity (61) and activate anti-inflammatory pathways (62). Moreover, plant foods are rich in polyphenols (e.g., flavonoids, lignans, phenolic acids), which may lower the risk of CVD (63). Other nutrients such as vitamin C, vitamin E, beta-carotene, potassium and magnesium have shown effects in reducing blood pressure (64), and improving glucose metabolism and insulin sensitivity (65). In addition, recent studies have shed light on the relationship between plant-based dietary patterns and specific circulating metabolites as well as a healthy composition of gut microbiome, which were associated with decreased risks of T2D and CVD. Specifically, Wang and colleagues constructed multi-metabolite profiles for plant-based dietary patterns using data of 10,684 participants from three prospective cohorts (NHS, NHSII, HPFS), where key metabolites in constructing the scores included trigonelline, hippurate, isoleucine and a subset of triacylglycerols (66). They identified that higher scores of multi-metabolite profiles for PDI and hPDI were significantly associated with lower risk of incident T2D (PDI: HR per 1 SD higher = 0.81 [0.75–0.88]), hPDI: (0.77 [0.71–0.84]) (66). The associations between plant-based dietary patterns and metabolic risks might also be modified by gut microbiome. In the Men’s Lifestyle Validation Study (MLVS), individuals with higher hPDI had a distinct gut microbiome signature, which was primarily marked by a higher presence of Bacteroides cellulosilyticus and Eubacterium eligens, as well as enriched pathways for branched-chain amino acid (BCAA) biosynthesis and depleted pathways for processing animal-derived components [67]. Both the overall microbial profile and individual species play a role in enhancing the favorable associations between hPDI and metabolic health.
In addition, plant-based diets contain no or less consumption of red and processed meat, the intake of which has been associated with higher risks of CVD, T2D, and various cancer types such as breast, colorectal, and lung cancers [5, 68, 69]. Red meat may cause disrupted insulin signaling and increased inflammation and oxidative stress through several components including heme iron [70], nitrates [71], advanced glycation end products and trimethylamine N-oxide [72], and inflammatory mediators [73]. In terms of cancer, plant-based food have well-known anticarcinogenic properties, and thus likely to exert anti-inflammatory and anti-oxidative effects that are against the development of cancer [74].
Our findings have important clinical and public health implications. First, our results suggest that plant-based dietary patterns, especially those based on healthy plant food sources, may be beneficial for the primary prevention of T2D, CVD, cancer, and mortality. A comparative analysis of the health and climate change benefits of global dietary changes projected that moving to diets with fewer animal-sourced foods would result in around 5–8 million avoidable deaths and ~ 200 million life years saved in 2050 [75]. In addition, it would also have environmental benefits by easing pressure on land use and reducing greenhouse gas emissions by 29–70% [75]. Second, our findings corroborated various dietary guidelines, such as those from American Heart Association [76], American Diabetes Association [77], and American Cancer Society [78], which recommend eating an overall healthy dietary pattern that emphasizes a wide variety of fruits and vegetables, whole grains and healthy sources of proteins to prevent CVD, T2D, and cancer. In the current study, we stratified the analysis by dietary classification, and found that people who consumed strict vegetarian patterns and those who consumed plant-based dietary patterns including some animal foods had similar protective effect for CVD, T2D, and mortality. Plant-based diets that do not completely exclude animal products can be easier to adopt than strict vegan or vegetarian diets which are practiced by a small proportion of the total population [79].
The main strength of the current study was the comprehensive assessment of all available prospective studies on various outcomes including CVD, cancer, T2D, and mortality. We evaluated adherence to and changes in plant-based dietary patterns and examined the effect of vegetarian diets and plant-based dietary indices. We estimated effects from both overall and healthy plant-based dietary patterns and evaluated overall disease outcomes and specific disease subtypes. We assessed the robustness of the results by using different statistical methods and observed consistent results across various subgroups.
Nevertheless, some limitations merit consideration. First, since dietary intake was self-reported using food frequency questionnaire or 24-hour recall in included studies, and majority of the studies did not have repeated measurements of the dietary intake, measurement error and misclassification may exist. However, it is likely to be nondifferential among people who developed CVD, cancer, T2D, or mortality and those who did not, which may result in an underestimation of the true effect size of associations between plant-based patterns and chronic diseases. Second, all included studies were prospective cohort studies; therefore, unmeasured or residual confounding factors cannot be ruled out. In addition, included studies adjusted for different covariates in the multivariable models, had different socio-demographic and clinical characteristics, and thus may contribute to the observed moderate-to-high heterogeneity across studies. However, results from random-effects models were similar to those from fix-effects models, and subgroup results were largely similar. Third, study points on some diseases are limited (e.g., heart failure, lung cancer, liver cancer, stomach cancer), and therefore it is unknown whether the observed non-significant association is true or due to the lack of statistical power. Future studies with larger sample sizes are warranted to examine associations of these diseases and plant-based dietary patterns.
Conclusions
In conclusion, higher adherence to plant-based dietary patterns, especially from healthy sources, may be universally beneficial for the primary prevention of T2D, CVD, cancer, and mortality. The current analysis provides further evidence in support of the current recommendations that emphasize consuming high-quality plant-based foods for achieving an optimal health. Future studies are needed to elucidate associations for certain individual health outcomes, as well as mechanistic pathways linking plant-based diets with multiple disease outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
List of abbreviations
- CHD
Coronary heart disease
- CI
Confidence interval
- CVD
Cardiovascular disease
- hPDI
Healthful plant-based diet index
- MOOSE
Meta-analysis Of Observational Studies in Epidemiology
- PDI
Plant-based diet index
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- RR
Relative risk
- T2D
Type 2 diabetes
- uPDI
Unhealthful plant-based diet index
Authors’ contributions
YW and BL conducted the literature search. YW, BL, HH, YH and LZ conducted screening of publications. YW, BL and HH extracted the data. YW and BL did the analysis. YW wrote the first draft of the manuscript. QS supervised the project. All authors reviewed the manuscript critically and approved the content of the manuscript.
Funding
The current analysis is supported by National Institutes of Health grants DK126698, DK127601, DK119268, DK120870, HL035464, HL060712, and T32 HL 098048. The funding sources did not participate in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Data Availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yeli Wang and Binkai Liu contributed equally to the manuscript.
Change history
1/4/2024
A Correction to this paper has been published: 10.1186/s12937-023-00891-4
References
- 1.World Health Organization. Diabetes. [Internet]. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes
- 2.World Health Organization. Cardiovascular diseases (CVDs). [Internet]. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
- 3.World Health Organization. Cancer. [Internet]. 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer
- 4.Eyre H, Kahn R, Robertson RM, Clark NG, Doyle C, Hong Y, et al. Preventing Cancer, Cardiovascular Disease, and diabetes. Circulation. 2004;109(25):3244–55. doi: 10.1161/01.CIR.0000133321.00456.00. [DOI] [PubMed] [Google Scholar]
- 5.Qian F, Liu G, Hu FB, Bhupathiraju SN, Sun Q. Association between Plant-Based dietary patterns and risk of type 2 diabetes: a systematic review and Meta-analysis. JAMA Intern Med. 2019;179(10):1335–44. doi: 10.1001/jamainternmed.2019.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glenn AJ, Viguiliouk E, Seider M, Boucher BA, Khan TA, Blanco Mejia S et al. Relation of Vegetarian Dietary Patterns With Major Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Front Nutr. 20190613th ed. 2019;6:80. [DOI] [PMC free article] [PubMed]
- 7.Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57(17):3640–9. doi: 10.1080/10408398.2016.1138447. [DOI] [PubMed] [Google Scholar]
- 8.Lu JW, Yu LH, Tu YK, Cheng HY, Chen LY, Loh CH et al. Risk of Incident Stroke among Vegetarians Compared to Nonvegetarians: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutrients. 20210829th ed. 2021;13(9). [DOI] [PMC free article] [PubMed]
- 9.Huang T, Yang B, Zheng J, Li G, Wahlqvist ML, Li D. Cardiovascular disease mortality and cancer incidence in vegetarians: a meta-analysis and systematic review. Ann Nutr Metab. 2012;60(4):233–40. doi: 10.1159/000337301. [DOI] [PubMed] [Google Scholar]
- 10.Molina-Montes E, Salamanca-Fernández E, Garcia-Villanova B, Sánchez MJ. The impact of plant-based dietary patterns on Cancer-Related outcomes: a Rapid Review and Meta-Analysis. Nutrients 20200706th ed. 2020;12(7). [DOI] [PMC free article] [PubMed]
- 11.Godos J, Bella F, Sciacca S, Galvano F, Grosso G. Vegetarianism and breast, colorectal and prostate cancer risk: an overview and meta-analysis of cohort studies. J Hum Nutr Diet. 2017;30(3):349–59. doi: 10.1111/jhn.12426. [DOI] [PubMed] [Google Scholar]
- 12.Papier K, Appleby PN, Fensom GK, Knuppel A, Perez-Cornago A, Schmidt JA et al. Vegetarian diets and risk of hospitalisation or death with diabetes in British adults: results from the EPIC-Oxford study. Nutr Diabetes. 2019/02/26 ed. 2019;9(1):7. [DOI] [PMC free article] [PubMed]
- 13.Laouali N, Shah S, MacDonald CJ, Mahamat-Saleh Y, El Fatouhi D, Mancini F et al. BMI in the Associations of Plant-Based Diets with Type 2 Diabetes and Hypertension Risks in Women: The E3N Prospective Cohort Study. J Nutr. 2021/07/09 ed. 2021;151(9):2731–40. [DOI] [PubMed]
- 14.Yang X, Li Y, Wang C, Mao Z, Chen Y, Ren P et al. Association of plant-based diet and type 2 diabetes mellitus in Chinese rural adults: The Henan Rural Cohort Study. J Diabetes Investig. 2021/02/10 ed. 2021;12(9):1569–76. [DOI] [PMC free article] [PubMed]
- 15.Choi Y, Larson N, Gallaher DD, Odegaard AO, Rana JS, Shikany JM et al. A Shift Toward a Plant-Centered Diet From Young to Middle Adulthood and Subsequent Risk of Type 2 Diabetes and Weight Gain: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes Care. 2020/08/28 ed. 2020;43(11):2796–803. [DOI] [PMC free article] [PubMed]
- 16.Chen Z, Drouin-Chartier JP, Li Y, Baden MY, Manson JE, Willett WC et al. Changes in Plant-Based Diet Indices and Subsequent Risk of Type 2 Diabetes in Women and Men: Three U.S. Prospective Cohorts. Diabetes Care. 2021/01/15 ed. 2021;44(3):663–71. [DOI] [PMC free article] [PubMed]
- 17.Chen B, Zeng J, Qin M, Xu W, Zhang Z, Li X, et al. The Association between Plant-Based Diet indices and obesity and metabolic Diseases in Chinese adults: longitudinal analyses from the China Health and Nutrition Survey. Front Nutr. 2022;9:881901. doi: 10.3389/fnut.2022.881901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Giovannucci E. Healthful Plant-Based Diet and incidence of type 2 diabetes in Asian Population. Nutrients. 2022;14(15):3078. doi: 10.3390/nu14153078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glenn AJ, Li J, Lo K, Jenkins DJA, Boucher BA, Hanley AJ, et al. The Portfolio Diet and Incident Type 2 diabetes: findings from the women’s Health Initiative prospective cohort study. Diabetes Care. 2022;46(1):28–37. doi: 10.2337/dc22-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Stubbendorff A, Olsson K, Ericson U, Niu K, Qi L et al. Adherence to the EAT-Lancet diet, genetic susceptibility, and risk of type 2 diabetes in Swedish adults. Metabolism - Clinical and Experimental [Internet]. 2023 Apr 1 [cited 2023 Jul 29];141. Available from: https://www.metabolismjournal.com/article/S0026-0495(23)00004-5/fulltext. [DOI] [PubMed]
- 21.Anyene IC, Ergas IJ, Kwan ML, Roh JM, Ambrosone CB, Kushi LH et al. Plant-Based Dietary Patterns and Breast Cancer Recurrence and Survival in the Pathways Study. Nutrients. 2021/10/24 ed. 2021;13(10). [DOI] [PMC free article] [PubMed]
- 22.Ratjen I, Enderle J, Burmeister G, Koch M, Nöthlings U, Hampe J, et al. Post-diagnostic reliance on plant-compared with animal-based foods and all-cause mortality in omnivorous long-term colorectal cancer survivors. Am J Clin Nutr. 2021;114(2):441–9. doi: 10.1093/ajcn/nqab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.PeTermann-Rocha F, Parra-Soto S, Gray S, Anderson J, Welsh P, Gill J et al. Vegetarians, fish, poultry, and meat-eaters: who has higher risk of cardiovascular disease incidence and mortality? A prospective study from UK Biobank. Eur Heart J. 2020/12/15 ed. 2021;42(12):1136–43. [DOI] [PMC free article] [PubMed]
- 24.Li H, Zeng X, Wang Y, Zhang Z, Zhu Y, Li X et al. A prospective study of healthful and unhealthful plant-based diet and risk of overall and cause-specific mortality. Eur J Nutr. 2021/08/12 ed. 2022;61(1):387–98. [DOI] [PubMed]
- 25.Thompson AS, Tresserra-Rimbau A, Karavasiloglou N, Jennings A, Cantwell M, Hill C, et al. Association of Healthful Plant-based Diet Adherence with Risk of Mortality and Major Chronic Diseases among adults in the UK. JAMA Netw Open. 2023;6(3):e234714. doi: 10.1001/jamanetworkopen.2023.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang DD, Li Y, Nguyen XMT, Song RJ, Ho YL, Hu FB, et al. Degree of adherence to plant-based diet and total and cause-specific mortality: prospective cohort study in the million veteran program. Public Health Nutr. 2023;26(2):381–92. doi: 10.1017/S1368980022000659. [DOI] [PubMed] [Google Scholar]
- 27.Weston LJ, Kim H, Talegawkar SA, Tucker KL, Correa A, Rebholz CM. Plant-based diets and incident cardiovascular disease and all-cause mortality in African Americans: a cohort study. PLoS Med. 2022;19(1):e1003863. doi: 10.1371/journal.pmed.1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Shen J, Xuan J, Zhu A, Ji JS, Liu X, et al. Plant-based dietary patterns in relation to mortality among older adults in China. Nat Aging. 2022;2(3):224–30. doi: 10.1038/s43587-022-00180-5. [DOI] [PubMed] [Google Scholar]
- 29.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000/05/02 ed. 2000;283(15):2008–12. [DOI] [PubMed]
- 30.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Reviews. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shan Z, Li Y, Baden MY, Bhupathiraju SN, Wang DD, Sun Q et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern Med. 2020/06/17 ed. 2020;180(8):1090–100. [DOI] [PMC free article] [PubMed]
- 32.Baden MY, Liu G, Satija A, Li Y, Sun Q, Fung TT, et al. Changes in plant-based Diet Quality and Total and cause-specific mortality. Circulation. 2019;140(12):979–91. doi: 10.1161/CIRCULATIONAHA.119.041014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H, Caulfield LE, Rebholz CM. Healthy Plant-Based Diets Are Associated with Lower Risk of All-Cause Mortality in US Adults. J Nutr. 2018/04/17 ed. 2018;148(4):624–31. [DOI] [PMC free article] [PubMed]
- 34.Chen Z, Zuurmond MG, van der Schaft N, Nano J, Wijnhoven HAH, Ikram MA et al. Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: the Rotterdam Study. Eur J Epidemiol. 2018/06/28 ed. 2018;33(9):883–93. [DOI] [PMC free article] [PubMed]
- 35.Bhupathiraju SN, Sawicki CM, Goon S, Gujral UP, Hu FB, Kandula NR, et al. A healthy plant–based diet is favorably associated with cardiometabolic risk factors among participants of south asian ancestry. Am J Clin Nutr. 2022;116(4):1078–90. doi: 10.1093/ajcn/nqac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J, Khil J, Kim H, Keum N, Zhang X, Giovannucci E. Plant-based dietary patterns and the risk of digestive system cancers in 3 large prospective cohort studies. Eur J Epidemiol. 2023;38(6):617–27. doi: 10.1007/s10654-023-01007-2. [DOI] [PubMed] [Google Scholar]
- 37.Chene G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. American journal of epidemiology. 1996/09/15 ed. 1996;144(6):610–21. [DOI] [PubMed]
- 38.University of Cambridge Department of Public Health and Primary Care. Programs [Internet]. Available from: https://www.phpc.cam.ac.uk/ceu/erfc/programs/
- 39.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 40.Leone A, Martínez-González M, Martin-Gorgojo A, Sánchez-Bayona R, De Amicis R, Bertoli S et al. Mediterranean diet, Dietary Approaches to Stop Hypertension, and Pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020/06/04 ed. 2020;112(2):364–72. [DOI] [PubMed]
- 41.Baden MY, Shan Z, Wang F, Li Y, Manson JE, Rimm EB, et al. Quality of plant-based Diet and Risk of Total, ischemic, and hemorrhagic stroke. Neurology. 2021;96(15):e1940–53. doi: 10.1212/WNL.0000000000011713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glenn AJ, Lo K, Jenkins DJA, Boucher BA, Hanley AJ, Kendall CWC et al. Relationship Between a Plant-Based Dietary Portfolio and Risk of Cardiovascular Disease: Findings From the Women’s Health Initiative Prospective Cohort Study. J Am Heart Assoc. 2021/08/05 ed. 2021;10(16):e021515. [DOI] [PMC free article] [PubMed]
- 43.Jafari S, Hezaveh E, Jalilpiran Y, Jayedi A, Wong A, Safaiyan A et al. Plant-based diets and risk of disease mortality: a systematic review and meta-analysis of cohort studies. Crit Rev Food Sci Nutr. 20210506th ed. 2021;1–13. [DOI] [PubMed]
- 44.Kwok CS, Umar S, Myint PK, Mamas MA, Loke YK. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;176(3):680–6. doi: 10.1016/j.ijcard.2014.07.080. [DOI] [PubMed] [Google Scholar]
- 45.Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W et al. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J Am Coll Cardiol. 2017/07/22 ed. 2017;70(4):411–22. [DOI] [PMC free article] [PubMed]
- 46.Satija A, Hu FB. Plant-based diets and cardiovascular health. Trends Cardiovasc Med. 2018;28(7):437–41. doi: 10.1016/j.tcm.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnard ND, Levin SM, Yokoyama Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J Acad Nutr Diet. 2015;115(6):954–69. doi: 10.1016/j.jand.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 48.Huang RY, Huang CC, Hu FB, Chavarro JE. Vegetarian diets and weight reduction: a Meta-Analysis of Randomized controlled trials. J Gen Intern Med. 2016;31(1):109–16. doi: 10.1007/s11606-015-3390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. N Engl J Med. 2011;364(25):2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith JD, Hou T, Ludwig DS, Rimm EB, Willett W, Hu FB, et al. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: results from 3 prospective cohorts. Am J Clin Nutr. 2015;101(6):1216–24. doi: 10.3945/ajcn.114.100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front Endocrinol (Lausanne). 20180117th ed. 2018;9:2. [DOI] [PMC free article] [PubMed]
- 52.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263(21):2893–8. doi: 10.1001/jama.1990.03440210043030. [DOI] [PubMed] [Google Scholar]
- 53.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 54.Festa A, D’Agostino R, Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the insulin resistance atherosclerosis study (IRAS) Circulation. 2000;102(1):42–7. doi: 10.1161/01.CIR.102.1.42. [DOI] [PubMed] [Google Scholar]
- 55.Otani H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid Redox Signal. 2011;15(7):1911–26. doi: 10.1089/ars.2010.3739. [DOI] [PubMed] [Google Scholar]
- 56.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37(4):1053–9. doi: 10.1161/01.HYP.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 57.Stern MP. Diabetes and cardiovascular disease. The common soil hypothesis. Diabetes. 1995;44(4):369–74. doi: 10.2337/diab.44.4.369. [DOI] [PubMed] [Google Scholar]
- 58.Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 20140829th ed. 2014;63(12):1469–79. [DOI] [PubMed]
- 59.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69(1):30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- 60.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146–55. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 61.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48(1):44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petersson H, Basu S, Cederholm T, Risérus U. Serum fatty acid composition and indices of stearoyl-CoA desaturase activity are associated with systemic inflammation: longitudinal analyses in middle-aged men. Br J Nutr. 2008;99(6):1186–9. doi: 10.1017/S0007114507871674. [DOI] [PubMed] [Google Scholar]
- 63.Tangney CC, Rasmussen HE. Polyphenols, inflammation, and cardiovascular disease. Curr Atheroscler Rep. 2013;15(5):324. doi: 10.1007/s11883-013-0324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. 2013;346:f1378. doi: 10.1136/bmj.f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr. 2013;4(3):378s–83s. doi: 10.3945/an.112.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang F, Baden MY, Guasch-Ferré M, Wittenbecher C, Li J, Li Y, et al. Plasma metabolite profiles related to plant-based diets and the risk of type 2 diabetes. Diabetologia. 2022;65(7):1119–32. doi: 10.1007/s00125-022-05692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Wang DD, Satija A, Ivey KL, Li J, Wilkinson JE, et al. Plant-based Diet Index and metabolic risk in men: exploring the role of the gut Microbiome. J Nutr. 2021;151(9):2780–9. doi: 10.1093/jn/nxab175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes–an updated review of the evidence. Curr Atheroscler Rep. 2012;14(6):515–24. doi: 10.1007/s11883-012-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farvid MS, Sidahmed E, Spence ND, Mante Angua K, Rosner BA, Barnett JB. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2021;36(9):937–51. doi: 10.1007/s10654-021-00741-9. [DOI] [PubMed] [Google Scholar]
- 70.Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB. The role of iron in type 2 diabetes in humans. Biochimica et biophysica acta. 2008/05/27 ed. 2009;1790(7):671–81. [DOI] [PubMed]
- 71.Tong M, Neusner A, Longato L, Lawton M, Wands JR, de la Monte SM. Nitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer’s disease. J Alzheimers Dis. 2009;17(4):827–44. [PMC free article] [PubMed] [Google Scholar]
- 72.Peppa M, Goldberg T, Cai W, Rayfield E, Vlassara H. Glycotoxins: a missing link in the relationship of dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care. 2002;25(10):1898–9. doi: 10.2337/diacare.25.10.1898. [DOI] [PubMed] [Google Scholar]
- 73.Azadbakht L, Esmaillzadeh A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J Nutr. 2009;139(2):335–9. doi: 10.3945/jn.108.096297. [DOI] [PubMed] [Google Scholar]
- 74.Subramaniam S, Selvaduray KR, Radhakrishnan AK. Bioactive Compounds: Natural Defense Against Cancer? Biomolecules. 2019/11/27 ed. 2019;9(12). [DOI] [PMC free article] [PubMed]
- 75.Springmann M, Godfray HCJ, Rayner M, Scarborough P. Analysis and valuation of the health and climate change cobenefits of dietary change. Proceedings of the National Academy of Sciences. 2016;113(15):4146–51. [DOI] [PMC free article] [PubMed]
- 76.American Heart Association. The American Heart Association Diet and Lifestyle Recommendations. [Internet]. 2021. Available from: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/aha-diet-and-lifestyle-recommendations
- 77.American Diabetes Association. 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2020;44(Supplement_1):S34–9. [DOI] [PubMed]
- 78.American Cancer Society. American Cancer Society Guideline for Diet and Physical Activity. [Internet]. 2020. Available from: https://www.cancer.org/healthy/eat-healthy-get-active/acs-guidelines-nutrition-physical-activity-cancer-prevention/guidelines.html [DOI] [PubMed]
- 79.Top Trends in Prepared Foods. 2017: Exploring trends in meat, fish and seafood; pasta, noodles and rice; prepared meals; savory deli food; soup; and meat substitutes. [Internet]. Available from: https://www.reportbuyer.com/product/4959853/top-trends-in-prepared-foods-exploring-trends-in-meat-fish-and-seafood-pasta-noodles-and-rice-prepared-meals-savory-deli-food-soup-and-meat-substitutes.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.