Abstract
The agar dilution MIC method was used to test the activity of cefminox, a β-lactamase-stable cephamycin, compared with those of cefoxitin, cefotetan, moxalactam, ceftizoxime, cefotiam, cefamandole, cefoperazone, clindamycin, and metronidazole against 357 anaerobes. Overall, cefminox was the most active β-lactam, with an MIC at which 50% of isolates are inhibited (MIC50) of 1.0 μg/ml and an MIC90 of 16.0 μg/ml. Other β-lactams were less active, with respective MIC50s and MIC90s of 2.0 and 64.0 μg/ml for cefoxitin, 2.0 and 128.0 μg/ml for cefotetan, 2.0 and 64.0 μg/ml for moxalactam, 4.0 and >128.0 μg/ml for ceftizoxime, 16.0 and >128.0 μg/ml for cefotiam, 8.0 and >128.0 μg/ml for cefamandole, and 4.0 and 128.0 μg/ml for cefoperazone. The clindamycin MIC50 and MIC90 were 0.5 and 8.0 μg/ml, respectively, and the metronidazole MIC50 and MIC90 were 1.0 and 4.0 μg/ml, respectively. Cefminox was especially active against Bacteroides fragilis (MIC90, 2.0 μg/ml), Bacteroides thetaiotaomicron (MIC90, 4.0 μg/ml), fusobacteria (MIC90, 1.0 μg/ml), peptostreptococci (MIC90, 2.0 μg/ml), and clostridia, including Clostridium difficile (MIC90, 2.0 μg/ml). Time-kill studies performed with six representative anaerobic species revealed that at the MIC all compounds except ceftizoxime were bactericidal (99.9% killing) against all strains after 48 h. At 24 h, only cefminox and cefoxitin at 4× the MIC and cefoperazone at 8× the MIC were bactericidal against all strains. After 12 h, at the MIC all compounds except moxalactam, ceftizoxime, cefotiam, cefamandole, clindamycin, and metronidazole gave 90% killing of all strains. After 3 h, cefminox at 2× the MIC produced the most rapid effect, with 90% killing of all strains.
Anaerobes are established causes of serious human infections, especially in debilitated hosts. Although infections caused by members of the Bacteroides fragilis group occur most commonly, infections caused by other gram-negative anaerobic rods, as well as by gram-positive cocci and rods, are increasingly encountered (3, 25). The susceptibility spectrum of clinically isolated anaerobes is changing. Although β-lactamase production and the concomitant resistance to β-lactams are the rule in the B. fragilis group, both phenomena are increasingly encountered in non-B. fragilis group Bacteroides, Prevotella, Porphyromonas, and Fusobacterium species. β-Lactamase production has also been described in Clostridium butyricum, Clostridium ramosum, and Clostridium clostridioforme. Metronidazole resistance is the rule among gram-positive, non-spore-forming rods, but it has also been reported in peptostreptococci, non-Clostridium perfringens clostridia, and members of the B. fragilis group. Additionally, clindamycin resistance is not unusual among anaerobic gram-negative rods (4–11, 13, 17, 19).
With the exception of the cephamycin group, β-lactam antibiotics are generally inactive against β-lactamase-producing anaerobes (4–11). Among the cephamycins, the cefoxitin MICs for these organisms (especially for the B. fragilis group) often cluster around the breakpoint. Cefotetan MICs are generally 1 to 2 dilutions higher than those of cefoxitin (4–11). Cefminox is a β-lactamase-stable cephamycin; previous studies have documented that this compound has improved activity over those of other cephamycins against β-lactamase-positive and -negative aerobes and anaerobes (1, 12, 15, 16, 20–22, 26, 27). This study has compared the in vitro activity of cefminox with those of cefoxitin, cefotetan, moxalactam, ceftizoxime, cefotiam, cefamandole, cefoperazone, clindamycin, and metronidazole against 357 clinically isolated anaerobes. The activities of these compounds against six selected anaerobes were also studied by the time-kill method.
MATERIALS AND METHODS
Bacteria.
All anaerobic strains were recent clinical isolates (1990 to 1996) which were identified by standard procedures (14, 25) and which were kept frozen in double-strength skim milk (Difco Laboratories, Detroit, Mich.) at −70°C until use. Prior to testing, the strains were subcultured twice onto enriched sheep blood agar plates. Throughout the study, the strains were tested for purity by Gram staining and examining the colonial morphology. Agar dilution MIC studies were performed with all 357 strains, and time-kill studies were performed with 6 organisms, chosen to represent a spectrum of species encountered in clinical practice.
Antimicrobial agents.
Powders of antimicrobial agents with known potencies were obtained as follows: cefminox, Meiji Seika Pharma, International, Tokyo, Japan; cefoxitin, Merck & Co., Rahway, N.J.; cefotetan, Zeneca Pharmaceuticals, Wilmington, Del.; ceftizoxime, Fujisawa Laboratories, Deerfield Park, Ill.; cefamandole, Eli Lilly & Co., Indianapolis, Ind.; cefoperazone, Pfizer, Inc., New York, N.Y.; moxalactam, Sigma Chemical Co., St. Louis, Mo.; clindamycin, The Upjohn Co., Kalamazoo, Mich.; and metronidazole, Searle, Inc., Skokie, Ill. Cefotiam was a gift from Arne C. Rodloff, Institute for Medical Microbiology, University of Leipzig, Leipzig, Germany.
Susceptibility testing.
β-Lactamase testing was by the nitrocefin disk method (Cefinase; BBL Microbiology Systems, Cockeysville, Md.) (6). No attempt was made to differentiate between the type and amount of β-lactamase(s) produced by each enzyme-positive strain. Agar dilution susceptibility testing of 357 strains was performed by the method recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (18) with Wilkins-Chalgren agar (Difco Laboratories) and 5% sterile defibrinated sheep blood for non-B. fragilis group strains and inocula of 105 CFU/spot. For the six strains tested by the time-kill method (one strain each of B. fragilis, Bacteroides thetaiotaomicron, Prevotella bivia, Fusobacterium mortiferum, Peptostreptococcus anaerobius, and C. perfringens), microdilution MICs were determined according to the recommendations of NCCLS (18) with Anaerobe Broth (Difco Laboratories) supplemented with 5% sterile defibrinated sheep blood for non-B. fragilis group organisms. Trays were inoculated with 106 CFU/ml. Incubation of all plates and trays used for MIC testing was done in an anaerobic glove box (Coy Laboratory Products, Ann Arbor, Mich.) in an atmosphere of 85% N2, 5% H2, and 10% CO2. Standard quality control strains were included with each run.
Time-kill testing.
The time-kill testing methodology was that which we have described previously (23, 24). Inocula for time-kill studies were prepared inside the anaerobe chamber as follows. Five colonies from plates were suspended in 4 ml of prereduced brucella broth (Difco Laboratories), and the suspension was vortexed. A 100-μl aliquot of this suspension was added to 3.6 ml of prereduced brucella broth and 400 μl of laked horse erythrocytes. For metronidazole, for which thorough prereduction is necessary, 200 μl of Oxyrase solution (Oxyrase, Inc., Mansfield, Ohio) was added. Oxyrase is an enzyme prepared from Escherichia coli cell membranes which, in the presence of formate, lactate, and succinate, binds O2. The suspensions were placed into borosilicate screw-cap tubes (15 by 45 mm) with 13-425 screw-thread, open-top screw caps and 13-mm Teflon-faced rubber septa. The tubes were removed from the chamber and were incubated for 24 h in a shaking water bath at 35°C.
After the preincubation described above, the antibiotics were prepared to a volume of 3.6 ml as follows. Syringes containing laked horse blood, brucella broth, and antibiotics were drawn up separately inside the chamber and appropriate volumes were mixed in screw-cap tubes with septa, avoiding the introduction of air. Ranges included 3 dilutions above and 3 dilutions below the broth microdilution MICs. One antibiotic-free growth control was used in each experiment. Aliquots containing 200 μl of an appropriately diluted inoculum were added, with the final inoculum being 106 to 107 CFU/ml (23, 24). The suspensions were incubated at 35°C in a shaking water bath, and viability counts were determined at 0, 3, 12, 24, and 48 h (23, 24). The plates were incubated inside the chamber for 48 h; plates yielding 30 to 300 colonies were used to determine viability counts. Each experiment was done in duplicate, and the mean was used. The data were analyzed by expressing growth as the log10 CFU per milliliter higher or lower than the count for the original inoculum at 0 h. Bacteriostatic activity was defined as 0 to 3 Δlog10 CFU/ml and bactericidal activity was defined as >3 Δlog10 CFU/ml at each of the time periods compared to the counts at 0 h.
Drug carryover was minimized as described previously (23, 24). We believe that the spreading of 0.1 ml of undiluted broth onto a plate containing 25 ml of medium would dilute the drug 1:250; further 10-fold dilutions would dilute the drug 1:2,500, 1:25,000, etc. With the concentrations of drugs used, only undiluted inocula would have had any potential for drug carryover, and only plates with low counts (<1,000 CFU/ml) would be likely to be affected. We therefore feel that drug carryover was not a confounding factor in data generation.
RESULTS
β-Lactamase was detected in 91 of 99 (91.9%) of the B. fragilis group, 33 of 67 (49.3%) of the Prevotella and Porphyromonas isolates, and 4 of 59 (6.8%) of the fusobacteria. All gram-positive strains were β-lactamase negative. The results of agar dilution MIC testing are presented in Table 1.Overall, cefminox was the most active β-lactam, with an MIC at which 50% of strains are inhibited (MIC50) of 1.0 μg/ml and an MIC90 of 16.0 μg/ml. The other β-lactams were less active, with respective MIC50s and MIC90s of 2.0 and 64.0 μg/ml for cefoxitin, 2.0 and 128.0 μg/ml for cefotetan, 2.0 and 64.0 μg/ml for moxalactam, 4.0 and >128.0 μg/ml for ceftizoxime, 16.0 and >128.0 μg/ml for cefotiam, 8.0 and >128.0 μg/ml for cefamandole, and 4.0 and 128.0 μg/ml for cefoperazone. The clindamycin MIC50 and MIC90 were 0.5 and 8.0 μg/ml, respectively, and the metronidazole MIC50 and MIC90 were 1.0 and 4.0 μg/ml, respectively. Cefminox was especially active against B. fragilis (MIC90, 2.0 μg/ml), B. thetaiotaomicron (MIC90, 4.0 μg/ml), fusobacteria (MIC90, 1.0 μg/ml), peptostreptococci (MIC90, 2.0 μg/ml), and clostridia, including Clostridium difficile (MIC90, 2.0 μg/ml). Although cefminox was generally more active, especially against Bacteroides species, fusobacteria, and C. difficile, this cephamycin was frequently twofold less active than cefoxitin against Prevotella, Porphyromonas and Peptostreptococcus spp. and had activity similar to that of cefoxitin against the other species tested.
TABLE 1.
Agar dilution MICs for 358 strains
| Strain and drug | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Bacteroides fragilis (33/33)a | |||
| Cefminox | 0.25–>128.0 | 1.0 | 2.0 |
| Cefoxitin | 4.0–64.0 | 8.0 | 32.0 |
| Cefotetan | 4.0–>128.0 | 8.0 | 64.0 |
| Moxalactam | 0.5–>128.0 | 1.0 | 4.0 |
| Ceftizoxime | 8.0–>128.0 | 64.0 | >128.0 |
| Cefotiam | 64.0–>128.0 | >128.0 | >128.0 |
| Cefamandole | 64.0–>128.0 | 128.0 | >128.0 |
| Cefoperazone | 32.0–>128.0 | 128.0 | >128.0 |
| Clindamycin | 0.125–8.0 | 1.0 | 4.0 |
| Metronidazole | 0.125–2.0 | 1.0 | 1.0 |
| Bacteroides thetaiotaomicron (14/14) | |||
| Cefminox | 2.0–128.0 | 4.0 | 4.0 |
| Cefoxitin | 32.0–128.0 | 32.0 | 128.0 |
| Cefotetan | 8.0–>128.0 | >128.0 | >128.0 |
| Moxalactam | 1.0–>128.0 | 64.0 | 128.0 |
| Ceftizoxime | 16.0–128.0 | 64.0 | 128.0 |
| Cefotiam | 128.0–>128.0 | >128.0 | >128.0 |
| Cefamandole | 128.0–>128.0 | >128.0 | >128.0 |
| Cefoperazone | 32.0–>128.0 | 128.0 | >128.0 |
| Clindamycin | 2.0–8.0 | 4.0 | 8.0 |
| Metronidazole | 0.25–2.0 | 1.0 | 2.0 |
| Bacteroides ovatus (12/12) | |||
| Cefminox | 2.0–>128.0 | 16.0 | 128.0 |
| Cefoxitin | 16.0–>128.0 | 32.0 | >128.0 |
| Cefotetan | 64.0–>128.0 | >128.0 | >128.0 |
| Moxalactam | 32.0–>128.0 | 128.0 | >128.0 |
| Ceftizoxime | 16.0–>128.0 | 64.0 | >128.0 |
| Cefotiam | 128.0–>128.0 | >128.0 | >128.0 |
| Cefamandole | >128.0 | >128.0 | >128.0 |
| Cefoperazone | 64.0–>128.0 | 64.0 | >128.0 |
| Clindamycin | 2.0–>64.0 | 4.0 | >64.0 |
| Metronidazole | 0.5–1.0 | 1.0 | 1.0 |
| Bacteroides distasonis (20/12) | |||
| Cefminox | 1.0–>128.0 | 4.0 | >128.0 |
| Cefoxitin | 8.0–128.0 | 32.0 | 128.0 |
| Cefotetan | 32–>128.0 | 128.0 | >128.0 |
| Moxalactam | 16.0–>128.0 | 64.0 | >128.0 |
| Ceftizoxime | 0.25–>128.0 | 64.0 | >128.0 |
| Cefotiam | 32.0–>128.0 | >128.0 | >128.0 |
| Cefamandole | >128.0 | >128.0 | >128.0 |
| Cefoperazone | 8.0–>128.0 | 64.0 | >128.0 |
| Clindamycin | 0.5–>64.0 | 8.0 | 16.0 |
| Metronidazole | 0.5–2.0 | 1.0 | 1.0 |
| Bacteroides vulgatus (10/10) | |||
| Cefminox | 1.0–8.0 | 1.0 | 8.0 |
| Cefoxitin | 2.0–32.0 | 4.0 | 16.0 |
| Cefotetan | 2.0–16.0 | 4.0 | 8.0 |
| Moxalactam | 1.0–16.0 | 1.0 | 8.0 |
| Ceftizoxime | 2.0–>128.0 | 8.0 | >128.0 |
| Cefotiam | 16.0–>128.0 | 32.0 | >128.0 |
| Cefamandole | 32.0–>128.0 | 64.0 | >128.0 |
| Cefoperazone | 16.0–>128.0 | 32.0 | >128.0 |
| Clindamycin | <0.008–>64.0 | 0.25 | >64.0 |
| Metronidazole | 0.5–1.0 | 1.0 | 1.0 |
| Bacteroides uniformis (10/10) | |||
| Cefminox | 0.5–128.0 | 0.5 | 64.0 |
| Cefoxitin | 1.0–64.0 | 2.0 | 16.0 |
| Cefotetan | 2.0–128.0 | 16.0 | 64.0 |
| Moxalactam | 0.5–128.0 | 1.0 | 32.0 |
| Ceftizoxime | 16.0–>128.0 | 32.0 | >128.0 |
| Cefotiam | 64.0–>128.0 | 128.0 | >128.0 |
| Cefamandole | 64.0–>128.0 | 128.0 | >128.0 |
| Cefoperazone | 16.0–>128.0 | 32.0 | >128.0 |
| Clindamycin | 0.03–4.0 | 2.0 | 4.0 |
| Metronidazole | 0.5–1.0 | 0.5 | 1.0 |
| Bacteroides fragilis group (99/91) | |||
| Cefminox | 0.25–>128.0 | 2.0 | 128.0 |
| Cefoxitin | 1.0–>128.0 | 16.0 | 128.0 |
| Cefotetan | 2.0–>128.0 | 64.0 | >128.0 |
| Moxalactam | 0.5–>128.0 | 16.0 | >128.0 |
| Ceftizoxime | 0.25–>128.0 | 64.0 | >128.0 |
| Cefotiam | 16.0–>128.0 | >128.0 | >128.0 |
| Cefamandole | 32.0–>128.0 | >128.0 | >128.0 |
| Cefoperazone | 8.0–>128.0 | 128.0 | >128.0 |
| Clindamycin | 0.008–>64.0 | 4.0 | 16.0 |
| Metronidazole | 0.125–2.0 | 1.0 | 1.0 |
| Prevotella bivia (20/10) | |||
| Cefminox | 0.5–16.0 | 2.0 | 8.0 |
| Cefoxitin | 0.5–8.0 | 1.0 | 4.0 |
| Cefotetan | 2.0–16.0 | 2.0 | 16.0 |
| Moxalactam | 0.25–32.0 | 1.0 | 16.0 |
| Ceftizoxime | 0.06–32.0 | 0.125 | 4.0 |
| Cefotiam | 1.0–>128.0 | 4.0 | 128.0 |
| Cefamandole | 2.0–>128.0 | 8.0 | 32.0 |
| Cefoperazone | 2.0–64.0 | 4.0 | 8.0 |
| Clindamycin | 0.016–0.06 | 0.03 | 0.03 |
| Metronidazole | 0.25–4.0 | 1.0 | 4.0 |
| Prevotella disiens (9/8) | |||
| Cefminox | 0.5–64.0 | 32.0 | |
| Cefoxitin | 0.25–16.0 | 16.0 | |
| Cefotetan | 0.5–64.0 | 32.0 | |
| Moxalactam | 0.5–64.0 | 32.0 | |
| Ceftizoxime | 0.25–64.0 | 8.0 | |
| Cefotiam | 0.5–>128.0 | >128.0 | |
| Cefamandole | 1.0–128.0 | 128.0 | |
| Cefoperazone | 1.0–64.0 | 32.0 | |
| Clindamycin | 0.016 | 0.016 | |
| Metronidazole | 0.5–8.0 | 1.0 | |
| Prevotella melaninogenica (9/6) | |||
| Cefminox | 1.0–16.0 | 4.0 | |
| Cefoxitin | 0.5–4.0 | 1.0 | |
| Cefotetan | 0.5–16.0 | 2.0 | |
| Moxalactam | 0.5–16.0 | 4.0 | |
| Ceftizoxime | 0.25–32.0 | 2.0 | |
| Cefotiam | 1.0–>128.0 | 16.0 | |
| Cefamandole | 2.0–>128.0 | 4.0 | |
| Cefoperazone | 2.0–32.0 | 8.0 | |
| Clindamycin | 0.016–0.03 | 0.03 | |
| Metronidazole | 0.5 | 0.5 | |
| Prevotella intermedia (10/5) | |||
| Cefminox | 0.5–16.0 | 1.0 | 16.0 |
| Cefoxitin | 0.25–4.0 | 0.5 | 4.0 |
| Cefotetan | 0.5–8.0 | 1.0 | 8.0 |
| Moxalactam | 0.5–16.0 | 0.5 | 8.0 |
| Ceftizoxime | 0.5–16.0 | 1.0 | 16.0 |
| Cefotiam | 0.25–128.0 | 0.5 | 128.0 |
| Cefamandole | 0.5–16.0 | 0.5 | 16.0 |
| Cefoperazone | 0.5–2.0 | 1.0 | 2.0 |
| Clindamycin | 0.008–0.03 | 0.016 | 0.016 |
| Metronidazole | 0.5–1.0 | 0.5 | 1.0 |
| Prevotella corporis (9/1) | |||
| Cefminox | 0.25–4.0 | 0.5 | |
| Cefoxitin | 0.25–2.0 | 0.5 | |
| Cefotetan | 0.25–8.0 | 0.5 | |
| Moxalactam | 0.5–4.0 | 0.5 | |
| Ceftizoxime | 0.06–2.0 | 0.5 | |
| Cefotiam | 0.06–128.0 | 0.5 | |
| Cefamandole | 0.125–8.0 | 0.5 | |
| Cefoperazone | 0.5–2.0 | 1.0 | |
| Clindamycin | 0.016–0.03 | 0.016 | |
| Metronidazole | 0.125–0.25 | 0.25 | 0.5 |
| Miscellaneous strainsb (10/3) | |||
| Cefminox | 0.125–64.0 | 0.5 | 64.0 |
| Cefoxitin | 0.125–16.0 | 1.0 | 16.0 |
| Cefotetan | 0.125–64.0 | 1.0 | 64.0 |
| Moxalactam | 0.125–64.0 | 0.5 | 64.0 |
| Ceftizoxime | 0.125–16.0 | 0.5 | 16.0 |
| Cefotiam | 0.125–>128.0 | 1.0 | >128.0 |
| Cefamandole | 0.125–128.0 | 4.0 | 128.0 |
| Cefoperazone | 0.125–16.0 | 2.0 | 16.0 |
| Clindamycin | 0.008–0.03 | 0.016 | 0.016 |
| Metronidazole | 0.25–1.0 | 0.5 | 1.0 |
| Prevotella and Porphyromonas (67/33) | |||
| Cefminox | 0.125–64.0 | 2.0 | 32.0 |
| Cefoxitin | 0.125–16.0 | 1.0 | 16.0 |
| Cefotetan | 0.125–64.0 | 2.0 | 32.0 |
| Moxalactam | 0.125–64.0 | 1.0 | 32.0 |
| Ceftizoxime | 0.06–64.0 | 0.5 | 16.0 |
| Cefotiam | 0.06–>128.0 | 4.0 | >128.0 |
| Cefamandole | 0.125–>128.0 | 4.0 | 128.0 |
| Cefoperazone | 0.125–64.0 | 2.0 | 32.0 |
| Clindamycin | 0.008–0.06 | 0.016 | 0.03 |
| Metronidazole | 0.125–8.0 | 1.0 | 2.0 |
| Fusobacterium necrophorum (15/0) | |||
| Cefminox | 0.06–0.25 | 0.25 | 0.25 |
| Cefoxitin | 0.125–4.0 | 0.125 | 0.25 |
| Cefotetan | 0.06–0.125 | 0.125 | 0.125 |
| Moxalactam | 0.125–0.25 | 0.25 | 0.25 |
| Ceftizoxime | 0.06–0.125 | 0.06 | 0.125 |
| Cefotiam | 0.125–0.25 | 0.125 | 0.25 |
| Cefamandole | 0.06–0.125 | 0.125 | 0.125 |
| Cefoperazone | 0.06–0.125 | 0.125 | 0.125 |
| Clindamycin | 0.016–0.06 | 0.03 | 0.06 |
| Metronidazole | 0.125–0.5 | 0.125 | 0.25 |
| Fusobacterium nucleatum (15/2) | |||
| Cefminox | 0.06–1.0 | 0.5 | 1.0 |
| Cefoxitin | 0.25–1.0 | 0.25 | 1.0 |
| Cefotetan | 0.125–0.5 | 0.25 | 0.5 |
| Moxalactam | 1.0–4.0 | 1.0 | 2.0 |
| Ceftizoxime | 0.125–1.0 | 0.25 | 1.0 |
| Cefotiam | 0.125–2.0 | 0.5 | 2.0 |
| Cefamandole | 0.125–1.0 | 0.25 | 1.0 |
| Cefoperazone | 0.125–2.0 | 0.25 | 0.25 |
| Clindamycin | 0.06–0.125 | 0.06 | 0.125 |
| Metronidazole | 0.125–0.5 | 0.125 | 0.25 |
| Fusobacterium mortiferum (13/2) | |||
| Cefminox | 0.5–1.0 | 0.5 | 1.0 |
| Cefoxitin | 1.0–8.0 | 4.0 | 8.0 |
| Cefotetan | 2.0–8.0 | 4.0 | 8.0 |
| Moxalactam | 4.0–16.0 | 8.0 | 8.0 |
| Ceftizoxime | >128.0 | >128.0 | >128.0 |
| Cefotiam | 4.0–>128.0 | 128.0 | 128.0 |
| Cefamandole | 0.5–128.0 | 64.0 | 128.0 |
| Cefoperazone | 1.0–32.0 | 4.0 | 8.0 |
| Clindamycin | 0.125–4.0 | 0.25 | 0.5 |
| Metronidazole | 0.125–0.5 | 0.25 | 0.5 |
| Fusobacterium varium (16/0) | |||
| Cefminox | 0.25–2.0 | 0.5 | 1.0 |
| Cefoxitin | 2.0–8.0 | 8.0 | 8.0 |
| Cefotetan | 0.5–4.0 | 2.0 | 4.0 |
| Moxalactam | 4.0–16.0 | 8.0 | 8.0 |
| Ceftizoxime | 4.0–64.0 | 32.0 | 32.0 |
| Cefotiam | 4.0–32.0 | 16.0 | 16.0 |
| Cefamandole | 8.0–32.0 | 16.0 | 32.0 |
| Cefoperazone | 2.0–16.0 | 8.0 | 16.0 |
| Clindamycin | 2.0–64.0 | 8.0 | 64.0 |
| Metronidazole | 0.125–0.25 | 0.125 | 0.25 |
| Fusobacteria (59/4) | |||
| Cefminox | 0.06–2.0 | 0.5 | 1.0 |
| Cefoxitin | 0.125–8.0 | 1 | 8.0 |
| Cefotetan | 0.06–8.0 | 0.5 | 4.0 |
| Moxalactam | 0.125–16.0 | 4.0 | 8.0 |
| Ceftizoxime | 0.06–>128.0 | 1.0 | >128.0 |
| Cefotiam | 0.125–>128.0 | 2.0 | 128.0 |
| Cefamandole | 0.06–128.0 | 1.0 | 64.0 |
| Cefoperazone | 0.06–32.0 | 1.0 | 8.0 |
| Clindamycin | 0.016–64.0 | 0.125 | 32.0 |
| Metronidazole | 0.125–0.5 | 0.125 | 0.25 |
| Peptostreptococcic (47/0) | |||
| Cefminox | 0.125–4.0 | 1.0 | 2.0 |
| Cefoxitin | 0.06–2.0 | 0.5 | 1.0 |
| Cefotetan | 0.25–2.0 | 0.5 | 2.0 |
| Moxalactam | 0.06–16.0 | 2.0 | 8.0 |
| Ceftizoxime | 0.06–16.0 | 1.0 | 8.0 |
| Cefotiam | 0.5–16.0 | 2.0 | 4.0 |
| Cefamandole | 0.125–64.0 | 2.0 | 16.0 |
| Cefoperazone | 0.125–4.0 | 1.0 | 4.0 |
| Clindamycin | 0.06–8.0 | 1.0 | 2.0 |
| Metronidazole | 0.125–4.0 | 1.0 | 2.0 |
| Propionibacterium spp.d (25/0) | |||
| Cefminox | 0.5–4.0 | 0.5 | 1.0 |
| Cefoxitin | 0.125–1.0 | 0.25 | 1.0 |
| Cefotetan | 0.25–1.0 | 0.5 | 1.0 |
| Moxalactam | 1.0–2.0 | 1.0 | 2.0 |
| Ceftizoxime | 0.06–0.25 | 0.125 | 0.25 |
| Cefotiam | 0.125–4.0 | 0.5 | 0.5 |
| Cefamandole | 0.25–2.0 | 0.5 | 1.0 |
| Cefoperazone | 0.25–2.0 | 1.0 | 2.0 |
| Clindamycin | 0.06–0.5 | 0.06 | 0.25 |
| Metronidazole | >16.0 | >16.0 | >16.0 |
| Other gram-positive, non-spore-forming rodse (11/0) | |||
| Cefminox | 2.0–>128.0 | >128.0 | >128.0 |
| Cefoxitin | 0.25–>128.0 | >128.0 | >128.0 |
| Cefotetan | 0.5–>128.0 | >128.0 | >128.0 |
| Moxalactam | 1.0–>128.0 | >128.0 | >128.0 |
| Ceftizoxime | 0.06–32.0 | 32.0 | 32.0 |
| Cefotiam | 2.0–64.0 | 64.0 | 64.0 |
| Cefamandole | 1.0–64.0 | 64.0 | 64.0 |
| Cefoperazone | 0.25–128.0 | 16.0 | 64.0 |
| Clindamycin | 0.03–1.0 | 0.5 | 1.0 |
| Metronidazole | 0.5–>16.0 | >16.0 | >16.0 |
| Clostridium perfringens (20/0) | |||
| Cefminox | 0.5–2.0 | 1.0 | 2.0 |
| Cefoxitin | 1.0–4.0 | 2.0 | 2.0 |
| Cefotetan | 1.0–4.0 | 2.0 | 4.0 |
| Moxalactam | 0.5–8.0 | 2.0 | 2.0 |
| Ceftizoxime | 0.5–8.0 | 4.0 | 4.0 |
| Cefotiam | 2.0–16.0 | 8.0 | 8.0 |
| Cefamandole | 2.0–8.0 | 4.0 | 8.0 |
| Cefoperazone | 1.0–4.0 | 4.0 | 4.0 |
| Clindamycin | 0.06–4.0 | 1.0 | 4.0 |
| Metronidazole | 0.5–2.0 | 1.0 | 2.0 |
| Clostridium difficile (10/0) | |||
| Cefminox | 2.0–4.0 | 2.0 | 2.0 |
| Cefoxitin | 64.0–128.0 | 128.0 | 128.0 |
| Cefotetan | 8.0–32.0 | 16.0 | 16.0 |
| Moxalactam | 64.0–128.0 | 64.0 | 128.0 |
| Ceftizoxime | >128.0 | >128.0 | >128.0 |
| Cefotiam | >128.0 | >128.0 | >128.0 |
| Cefamandole | 32.0–128.0 | 64.0 | 128.0 |
| Cefoperazone | 32.0–64.0 | 64.0 | 64.0 |
| Clindamycin | 4.0–>64.0 | 8.0 | >64.0 |
| Metronidazole | 0.25–0.5 | 0.25 | 0.5 |
| Other clostridiaf (19/0) | |||
| Cefminox | 0.25–2.0 | 0.5 | 2.0 |
| Cefoxitin | 0.5–4.0 | 2.0 | 4.0 |
| Cefotetan | 0.25–4.0 | 2.0 | 4.0 |
| Moxalactam | 0.5–8.0 | 4.0 | 8.0 |
| Ceftizoxime | 0.125–>128.0 | 64.0 | >128.0 |
| Cefotiam | 1.0–128.0 | 16.0 | 128.0 |
| Cefamandole | 1.0–16.0 | 4.0 | 16.0 |
| Cefoperazone | 0.25–128.0 | 4.0 | 128.0 |
| Clindamycin | 0.016–>64.0 | 2.0 | 16.0 |
| Metronidazole | 0.25–1.0 | 0.5 | 1.0 |
| All strains (357/128) | |||
| Cefminox | 0.06–>128.0 | 1.0 | 16.0 |
| Cefoxitin | 0.06–>128.0 | 2.0 | 64.0 |
| Cefotetan | 0.06–>128.0 | 2.0 | 128.0 |
| Moxalactam | 0.06–>128.0 | 2.0 | 64.0 |
| Ceftizoxime | 0.06–>128.0 | 4.0 | >128.0 |
| Cefotiam | 0.06–>128.0 | 16.0 | >128.0 |
| Cefamandole | 0.06–>128.0 | 8.0 | >128.0 |
| Cefoperazone | 0.06–>128.0 | 4.0 | 128.0 |
| Clindamycin | 0.008–>64.0 | 0.5 | 8.0 |
| Metronidazole | 0.125–>16.0 | 1.0 | 4.0 |
Values in parentheses are numbers of strains tested/numbers of strains β-lactamase positive.
Prevotella oris (n = 1), Prevotella oralis (n = 1), Prevotella buccae (n = 5), and Porphyromonas asaccharolytica (n = 3).
Peptostreptococcus asaccharolyticus (n = 15), Peptostreptococcus magnus (n = 13), Peptostreptococcus anaerobius (n = 6), and Peptostreptococcus tetradius (n = 13).
Propionibacterium acnes (n = 24) and Propionibacterium sp. (n = 1).
Actinomyces sp. (n = 1), Eubacterium lentum (n = 2); Lactobacillus spp. (n = 6), and Bifidobacterium spp. (n = 2).
Clostridium tertium (n = 5), Clostridium bifermentans (n = 3), Clostridium sordellii (n = 5), Clostridium cadaveris (n = 2), and Clostridium spp. (n = 4).
β-Lactam MICs for Bacteroides ovatus and Bacteroides distasonis strains were uniformly high; some strains were also resistant to clindamycin (≥4.0 μg/ml). The highest β-lactam MICs for any of the members of the Prevotella and Porphyromonas group were for Prevotella disiens. Although β-lactam MICs for all strains of Propionibacterium acnes were ≤2.0 μg/ml, for the other gram-positive, non-spore-forming rods tested, β-lactam MICs were much higher.
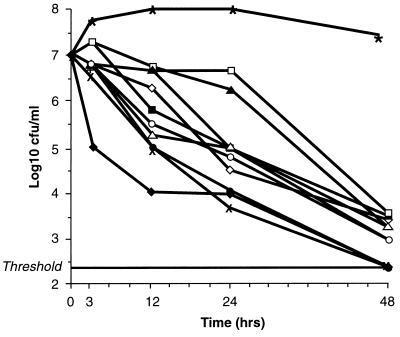
The microdilution MIC results for the six strains used for time-kill experiments are listed in Table 2. The MICs were within 1 doubling dilution of those obtained by the agar dilution method. The results of the time-kill experiments (Table 3) revealed that at and above the MIC all compounds except ceftizoxime were bactericidal (99.9% killing) against all strains after 48 h. At 24 h, only cefminox and cefoxitin at 4× the MIC and cefoperazone at 8× the MIC were bactericidal against all strains. After 12 h, at the MIC all compounds except moxalactam, ceftizoxime, cefotiam, cefamandole, clindamycin, and metronidazole produced 90% killing of all strains. After 3 h, cefminox at 2× the MIC produced the most rapid effect, giving 90% killing of all strains. The killing kinetics of all compounds at the MIC against the B. fragilis strain (Table 2) are presented in Fig. 1.
TABLE 2.
Microdilution MICs of the tested agents for the six strains tested by the time-kill method
| Drug | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| B. fragilis | B. thetaiotaomicron | P. bivia | F. mortiferum | P. anaerobius | C. perfringens | |
| Cefminox | 0.5 | 2.0 | 2.0 | 1.0 | 1.0 | 2.0 |
| Cefoxitin | 8.0 | 128.0 | 2.0 | 8.0 | 1.0 | 1.0 |
| Cefotetan | 8.0 | 256.0 | 2.0 | 1.0 | 2.0 | 2.0 |
| Moxalactam | 0.5 | 64.0 | 1.0 | 8.0 | 4.0 | 2.0 |
| Ceftizoxime | 32.0 | 128.0 | 0.06 | 128.0 | 0.125 | 4.0 |
| Cefotiam | 8.0 | 512.0 | 0.5 | 256.0 | 0.5 | 2.0 |
| Cefamandole | 64.0 | 256.0 | 4.0 | 32.0 | 2.0 | 4.0 |
| Cefoperazone | 64.0 | 256.0 | 4.0 | 4.0 | 2.0 | 4.0 |
| Clindamycin | 4.0 | 4.0 | 0.03 | 0.06 | 0.5 | 4.0 |
| Metronidazole | 0.5 | 0.5 | 4.0 | 0.06 | 1.0 | 1.0 |
TABLE 3.
Time-kill study results for the six strains described in Table 2
| Drug and concn | No. of strains with the indicated log10 CFU/ml decrease in colony count at the following timesa:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 h
|
12 h
|
24 h
|
48 h
|
|||||||||
| −1 | −2 | −3 | −1 | −2 | −3 | −1 | −2 | −3 | −1 | −2 | −3 | |
| Cefminox | ||||||||||||
| 4× MIC | 6 | 5 | 1 | 6 | 6 | 3 | 6 | 6 | 6 | 6 | 6 | 6 |
| 2× MIC | 6 | 1 | 0 | 6 | 4 | 3 | 6 | 6 | 4 | 6 | 6 | 6 |
| MIC | 4 | 1 | 0 | 6 | 3 | 0 | 6 | 5 | 2 | 6 | 6 | 0 |
| Cefoxitin | ||||||||||||
| 4× MIC | 4 | 1 | 0 | 6 | 5 | 0 | 6 | 6 | 6 | 6 | 6 | 6 |
| 2× MIC | 1 | 0 | 0 | 6 | 5 | 0 | 6 | 6 | 5 | 6 | 6 | 6 |
| MIC | 1 | 0 | 0 | 6 | 0 | 0 | 6 | 4 | 0 | 6 | 6 | 6 |
| Cefotetan | ||||||||||||
| 4× MIC | 1 | 0 | 0 | 6 | 4 | 0 | 6 | 6 | 4 | 6 | 6 | 6 |
| 2× MIC | 1 | 0 | 0 | 6 | 4 | 0 | 6 | 6 | 4 | 6 | 6 | 6 |
| MIC | 1 | 0 | 0 | 6 | 2 | 0 | 6 | 6 | 1 | 6 | 6 | 6 |
| Moxalactam | ||||||||||||
| 4× MIC | 2 | 2 | 0 | 6 | 5 | 1 | 6 | 6 | 5 | 6 | 6 | 6 |
| 2× MIC | 2 | 0 | 0 | 6 | 4 | 1 | 6 | 6 | 5 | 6 | 6 | 6 |
| MIC | 2 | 0 | 0 | 5 | 1 | 0 | 6 | 6 | 3 | 6 | 6 | 6 |
| Ceftizoxime | ||||||||||||
| 4× MIC | 4 | 1 | 0 | 6 | 4 | 2 | 6 | 6 | 4 | 6 | 6 | 6 |
| 2× MIC | 1 | 1 | 0 | 6 | 3 | 1 | 6 | 6 | 3 | 6 | 6 | 6 |
| MIC | 0 | 0 | 0 | 5 | 2 | 1 | 0 | 5 | 3 | 6 | 0 | 0 |
| Cefotiam | ||||||||||||
| 4× MIC | 4 | 1 | 1 | 5 | 3 | 2 | 6 | 6 | 4 | 6 | 6 | 6 |
| 2× MIC | 2 | 1 | 0 | 5 | 3 | 1 | 6 | 5 | 3 | 6 | 6 | 6 |
| MIC | 2 | 0 | 0 | 5 | 2 | 1 | 6 | 4 | 2 | 6 | 6 | 6 |
| Cefamandole | ||||||||||||
| 4× MIC | 1 | 1 | 0 | 6 | 6 | 0 | 6 | 6 | 5 | 6 | 6 | 6 |
| 2× MIC | 1 | 1 | 0 | 6 | 1 | 0 | 6 | 6 | 3 | 6 | 6 | 6 |
| MIC | 1 | 1 | 0 | 5 | 0 | 0 | 6 | 6 | 0 | 6 | 6 | 6 |
| Cefoperazone | ||||||||||||
| 4× MIC | 1 | 1 | 0 | 6 | 4 | 1 | 6 | 6 | 5 | 6 | 6 | 6 |
| 2× MIC | 1 | 1 | 0 | 6 | 3 | 1 | 6 | 6 | 4 | 6 | 6 | 6 |
| MIC | 1 | 1 | 0 | 6 | 0 | 0 | 6 | 6 | 3 | 6 | 6 | 6 |
| Clindamycin | ||||||||||||
| 4× MIC | 3 | 0 | 0 | 6 | 4 | 2 | 6 | 6 | 4 | 6 | 6 | 6 |
| 2× MIC | 2 | 0 | 0 | 6 | 4 | 0 | 6 | 4 | 3 | 6 | 6 | 6 |
| MIC | 1 | 0 | 0 | 5 | 2 | 0 | 6 | 4 | 2 | 6 | 6 | 6 |
| Metronidazole | ||||||||||||
| 4× MIC | 4 | 1 | 0 | 6 | 4 | 3 | 6 | 6 | 4 | 6 | 6 | 6 |
| 2× MIC | 3 | 0 | 0 | 6 | 2 | 0 | 6 | 4 | 2 | 6 | 6 | 6 |
| MIC | 3 | 0 | 0 | 5 | 1 | 0 | 6 | 4 | 2 | 6 | 6 | 6 |
Log10 CFU per milliliter decrease in colony count compared to that at time 0 h. −1, 90% killing; −2, 99% killing; −3, 99.9% killing.
FIG. 1.
Killing kinetics of a strain of B. fragilis (Table 2) by drugs used at the MIC. ⧫, cefminox; ◊, metronidazole; ▪, ceftizoxime; □, clindamycin; ▴, cefotiam; ▵, cefotetan; •, cefoperazone; ○, cefmandole; ×, cefmitin; +, moxalactam; ____, threshold; ∗, positive controls.
DISCUSSION
Cefminox is a cephamycin antibiotic with antibacterial activity against a variety of aerobic gram-positive and -negative bacteria. Its activity is especially higher than those of conventional cephamycins against E. coli, Klebsiella pneumoniae, Proteus mirabilis, Proteus vulgaris, Serratia marcescens, Yersinia enterocolitica, and Burkholderia cepacia. Moreover, cefminox is as stable as most other cephamycins to the actions of the β-lactamases produced by these bacteria. Although cefminox MICs are higher than those of the other cephamycins for some species, it displays an earlier onset of activity and a higher rate of bactericidal activity than those compounds. In contrast to ceftizoxime, cefminox and cefoxitin have negligible inoculum effects against E. coli (12, 15, 22).
The results of our in vitro MIC and time-kill experiments, in which we tested the largest number and widest spectrum of anaerobes of which we are aware, support the results of clinical studies showing that cefminox is clinically effective for the treatment of infections of the respiratory, biliary, and gynecological tracts and of peritonitis caused by gram-negative and -positive aerobes and anaerobes (20).
Cefminox MIC and kinetic data should be considered together with pharmacokinetic data. In human volunteers, single intravenous doses of 1 and 2 g of cefminox resulted in maximum concentrations in serum of 56.6 ± 16.1 and 117.3 ± 7.6 μg/ml, respectively with area under the curve values of 140.9 ± 5.8 and 260.0 ± 10.4 mg · h/ml/1.73 m2, respectively (1). Against three strains of the family Enterobacteriaceae and one B. fragilis strain, the increase in the area under the bactericidal curve observed with the 2-g dose was at least 3.5 times that seen with the 1-g dose and was larger than that predicted by the corresponding increase (1.84 times) in the area under the serum concentration-versus-time curve (AUC). The minimal bactericidal concentrations (MBCs) at 6 h showed a better association with the bactericidal titer in serum than did standard MICs or MBCs (1). After the administration of a 1-g dose, the maximal cefminox concentration in the pelvic retroperitoneal space was 37.9 μg/ml compared to the maximal concentrations of ≤30.3 μg/ml for the other cephalosporins tested (16).
A few published studies of relatively few anaerobic strains have shown that the MICs of cefminox for B. fragilis are lower than those cefoxitin and cefotetan, and the cefminox MICs for peptostreptococci, C. perfringens, and C. difficile are low (15, 26). Watanabe and coworkers (26) have confirmed our findings that the antibacterial activity of cefminox is comparable to that of moxalactam but superior to that of cefoxitin against B. fragilis, with the activity of cefminox against peptostreptococci being slightly inferior to that of cefoxitin. Cefminox was active against a wide variety of anaerobes, excluding Clostridium innocuum (26). Soriano and coworkers (21) have demonstrated that the susceptibility of cefminox and cefoxitin to hydrolysis by crude extracts of β-lactamases from B. fragilis group strains does not correlate with the results of conventional susceptibility testing. Both compounds were found to have equivalent activities against strains that produced enhanced levels of β-lactamase. The cefminox MICs in our study were similar to those described by previous investigators (15, 26); however, the MIC90s for C. difficile (2.0 μg/ml) were lower than those described previously by Inoue et al. (15) (12.5 μg/ml) but were similar to those reported by Watanabe et al. (26) (3.13 μg/ml). In confirmation of our findings, Watanabe and coworkers (26) have published information indicating that the cefminox MIC for B. ovatus ATCC 8483 is 6.25 μg/ml, whereas the MICs for other members of the B. fragilis group are lower, but they did not report the MICs for any other B. ovatus strains. Other anaerobe species for which cefminox MICs are high (P. disiens, non-Propionibacterium acnes gram-positive, non-spore-forming rods) either have not been studied or too few species have been examined to permit valid comparisons (15, 26). Because both of the studies reported above (15, 26) examined relatively few numbers of anaerobes, valid comparisons with the results obtained in the current study must await testing of larger numbers of strains by other workers.
The only members of the B. fragilis group for which cefminox MICs are high were B. ovatus and B. distasonis. The last two species could have been strong β-lactamase producers or could have produced enzymes which differed from those produced by other gram-negative rods. This was not examined in the current study. None of the gram-positive, non-spore-forming rods for which cefminox MIC90s were >128.0 μg/ml produced β-lactamase.
The time-kill studies confirmed the activity of cefminox against anaerobic bacteria compared to the activities of other compounds and confirmed its higher rate of killing, especially at earlier time periods. The results obtained for other β-lactam and non-β-lactam compounds reflect the findings of our group and those of other investigators, i.e., higher MICs of cefotetan compared to those of cefoxitin; the lack of activity of cefotiam, cefamandole, and cefoperazone against β-lactamase-producing strains; and the good activities of clindamycin and metronidazole against all anaerobic groups except clostridia and gram-positive, non-spore-forming rods (7–9, 15, 17, 26, 28). Of the cephalosporins tested, cefoxitin, cefotetan, and, in some cases, ceftizoxime had lower MICs for β-lactamase-positive strains compared to the MICs of the other β-lactams tested, with cefoxitin MICs being a few dilutions lower than those of cefotetan. The higher ceftizoxime MICs compared to those found by other investigators probably reflect the difficulty in standardizing testing of the susceptibility of this drug against anaerobes due to trailing endpoints and a marked inoculum effect, especially in broth (2).
In summary, the results of the MIC and time-kill investigations support previously published studies showing the clinical efficacy of cefminox. Further studies will determine whether cefminox has a place in the treatment of mixed infections with aerobic and anaerobic organisms.
ACKNOWLEDGMENT
This study was sponsored by Meiji Seika Kaisha, Ltd., Tokyo, Japan.
REFERENCES
- 1.Aguilar L, Esteban C, Frias J, Pérez-Balcabao I, Carcas A J, Dal-Ré R. Cefminox: correlation between in-vitro susceptibility and pharmacokinetics and serum bactericidal activity in healthy volunteers. J Antimicrob Chemother. 1994;33:91–101. doi: 10.1093/jac/33.1.91. [DOI] [PubMed] [Google Scholar]
- 2.Aldridge K E, Wexler H M, Sanders C V, Finegold S M. Comparison of in vitro antibiograms of Bacteroides fragilis group isolates: differences in resistance rates in two institutions because of differences in susceptibility testing methodology. Antimicrob Agents Chemother. 1990;34:179–181. doi: 10.1128/aac.34.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelbaum, P. C. 1987. Anaerobic infections: nonsporeformers, p. 45–109. In B. B. Wentworth (coordinating ed.), Diagnostic procedures for bacterial infections. American Public Health Association, Washington, D.C.
- 4.Appelbaum P C, Jacobs M R, Spangler S K, Yamabe S. Comparative activity of β-lactamase inhibitors YTR 830, clavulanate, and sulbactam combined with β-lactams against β-lactamase-producing anaerobes. Antimicrob Agents Chemother. 1986;30:789–791. doi: 10.1128/aac.30.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appelbaum P C, Philippon A, Jacobs M R, Spangler S K, Gutmann L. Characterization of β-lactamases from non-Bacteroides fragilis group Bacteroides spp. belonging to seven species and their role in β-lactam resistance. Antimicrob Agents Chemother. 1990;34:2169–2176. doi: 10.1128/aac.34.11.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appelbaum P C, Spangler S K, Jacobs M R. Evaluation of two methods for rapid testing for beta-lactamase production in Bacteroides and Fusobacterium. Eur J Clin Microbiol Infect Dis. 1990;9:47–50. doi: 10.1007/BF01969535. [DOI] [PubMed] [Google Scholar]
- 7.Appelbaum P C, Spangler S K, Jacobs M R. β-Lactamase production and susceptibilities to amoxicillin, amoxicillin-clavulanate, ticarcillin, ticarcillin-clavulanate, cefoxitin, imipenem, and metronidazole of 320 non-Bacteroides fragilis Bacteroides and 129 fusobacteria from 28 U.S. centers. Antimicrob Agents Chemother. 1990;34:1546–1550. doi: 10.1128/aac.34.8.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appelbaum P C, Spangler S K, Jacobs M R. Susceptibilities of 394 Bacteroides fragilis, non-B. fragilis group Bacteroides species, and Fusobacterium species to newer antimicrobial agents. Antimicrobial Agents Chemother. 1991;35:1214–1218. doi: 10.1128/aac.35.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appelbaum P C, Spangler S K, Jacobs M R. Susceptibility of 539 gram-positive and -negative anaerobes to new agents, including RP 59500, biapenem, trospectomycin and piperacillin/tazobactam. J Antimicrob Chemother. 1993;32:223–231. doi: 10.1093/jac/32.2.223. [DOI] [PubMed] [Google Scholar]
- 10.Appelbaum P C, Spangler S K, Pankuch G A, Philippon A, Jacobs M R, Shiman R, Goldstein E J C, Citron D. Characterization of a β-lactamase from Clostridium clostridioforme. J Antimicrob Chemother. 1994;33:33–40. doi: 10.1093/jac/33.1.33. [DOI] [PubMed] [Google Scholar]
- 11.Appelbaum P C, Spangler S K, Shiman R, Jacobs M R. Susceptibilities of 540 anaerobic gram-negative bacilli to amoxicillin, amoxicillin-BRL 42715, amoxicillin-clavulanate, temafloxacin, and clindamycin. Antimicrob Agents Chemother. 1992;36:1140–1143. doi: 10.1128/aac.36.5.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goi H, Watanabe T, Miyauchi K, Kazuno Y, Inouye S. Comparative bactericidal and morphological effects of five cephamycins on cells of three gram-negative bacilli at decreasing drug concentrations. Drugs Exp Clin Res. 1985;11:771–780. [PubMed] [Google Scholar]
- 13.Haggoud A, Reysset G V, Sebald M. Cloning of a Bacteroides fragilis chromosomal determinant coding for 5-nitroimidazole resistance. FEMS Microbiol Lett. 1992;95:1–6. doi: 10.1016/0378-1097(92)90728-7. [DOI] [PubMed] [Google Scholar]
- 14.Holdeman L V, Moore W E C, editors. Anaerobic laboratory manual. 4th ed. Blacksburg: Virginia Polytechnic Institute and State University; 1977. [Google Scholar]
- 15.Inoue S, Goi H, Watanabe T, Hara T, Miyauchi K, Yoshida T, Kazuno Y, Kadosawa H, Hirano F, Kawaharajo K, Orikasa Y, Nishino T. In vitro and in vivo antibacterial activities of MT-141, a new semisynthetic cephamycin, compared with those of five cephalosporins. Antimicrob Agents Chemother. 1984;26:722–729. doi: 10.1128/aac.26.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito K, Hayasaki M, Tamaya T. Pharmacokinetics of cephem antibiotics in exudate of pelvic retroperitoneal space after radical hysterectomy and pelvic lymphadenectomy. Antimicrob Agents Chemother. 1990;34:1160–1164. doi: 10.1128/aac.34.6.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs M R, Spangler S K, Appelbaum P C. β-Lactamase production, β-lactam sensitivity and resistance to synergy with clavulanate of 737 Bacteroides fragilis group organisms from thirty-three US centres. J Antimicrob Chemother. 1990;26:361–370. doi: 10.1093/jac/26.3.361. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 3rd ed. Approved standard. NCCLS publication M11-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 19.Nord, C. E. 1986. Mechanisms of β-lactam resistance in anaerobic bacteria. Rev. Infect. Dis. 8(Suppl. 5):S543–S548. [DOI] [PubMed]
- 20.Omoto S, Watanabe S. Efficacy of cefminox in the treatment of bacterial infections. Int J Clin Pharm Res. 1990;10:361–368. [PubMed] [Google Scholar]
- 21.Soriano F, Edwards R, Greenwood D. Comparative susceptibility of cefminox and cefoxitin to β-lactamases of Bacteroides spp. J Antimicrob Chemother. 1991;28:55–60. doi: 10.1093/jac/28.1.55. [DOI] [PubMed] [Google Scholar]
- 22.Soriano F, Edwards R, Greenwood D. Effect of inoculum size on bacteriolytic activity of cefminox and four other β-lactam antibiotics against Escherichia coli. Antimicrob Agents Chemother. 1991;36:223–226. doi: 10.1128/aac.36.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spangler, S. K., M. R. Jacobs, and P. C. Appelbaum. 1997. Time-kill study of the activity of trovafloxacin compared with ciprofloxacin, sparfloxacin, metronidazole, cefoxitin, piperacillin and piperacillin/tazobactam against six anaerobes. J. Antimicrob. Chemother. 39(Suppl. B):23–27. [DOI] [PubMed]
- 24.Spangler S K, Jacobs M R, Appelbaum P C. Bactericidal activity of DU-6859a compared to activities of three quinolones, three β-lactams, clindamycin, and metronidazole against anaerobes as determined by time-kill methodology. Antimicrob Agents Chemother. 1997;41:847–849. doi: 10.1128/aac.41.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Summanen P, Baron E J, Citron D M, Strong C A, Wexler H M, Finegold S M. Wadsworth anaerobic bacteriology manual. 5th ed. Belmont, Calif: Star Publishing Co.; 1993. [Google Scholar]
- 26.Watanabe K, Sawa K, Bunai M, Ueno K. Antibacterial activity of cefminox against anaerobes. J Antibiot (Tokyo) 1985;38:649–660. doi: 10.7164/antibiotics.38.649. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe T, Kazuno Y, Kawaharajo K, Goi H, Inouye S, Nishino T. In vivo antibacterial activity of a novel cephamycin, MT-141, on gram-negative infections in mice. Drugs Exp Clin Res. 1984;10:293–302. [Google Scholar]
- 28.Watt B, Brown F V. In-vitro activity of cefotiam against bacteria of clinical interest. J Antimicrob Chemother. 1982;10:391–395. doi: 10.1093/jac/10.5.391. [DOI] [PubMed] [Google Scholar]