Abstract
Background
Increased HIV testing is essential to ending the HIV epidemic. People who inject drugs (PWID) are among the highest risk for HIV infection. Previous research at Tufts Medical Center identified low HIV testing rates in hospitalized PWID. Our research team aimed to identify and overcome barriers to inpatient HIV screening of PWID using implementation science methods.
Methods
Stakeholders were engaged to gather perspectives on barriers and facilitators of HIV testing. A PWID care bundle was developed and implemented, which included (1) HIV screening; (2) hepatitis A, B, and C testing and vaccination; (3) medications for opioid use disorder; and (4) naloxone prescription. Strategies from all nine Expert Recommendations for Implementing Change (ERIC) clusters guided the implementation plan. Stakeholder feedback was gathered throughout implementation, and implementation outcomes of acceptability and feasibility were assessed.
Results
PWID overall felt comfortable with HIV testing being offered while hospitalized. Clinicians cited that the main barriers to HIV testing were discomfort and confusion around consenting requirements. Many resident physicians surveyed reported that, at times, they forgot HIV testing for PWID. Overall, though, resident physicians felt that the PWID bundle was useful and did not distract from other patient care responsibilities.
Conclusions
Engagement of key stakeholders to increase HIV testing in an inpatient setting led to the implementation of a PWID bundle, which was feasible and acceptable. Bundling evidence-informed care elements for inpatient PWID should be investigated further.
Keywords: HIV–AIDS, substance abuse treatment, implementation strategy, implementation
Plain Language Summary
People who inject drugs (PWID) are at an increased risk of contracting HIV. HIV testing is a key strategy to stop the spread of HIV. Our study created a bundle of services and tests to offer to all PWID who were admitted to the hospital. The bundle included HIV testing; hepatitis A, B, and C testing and vaccination; medications for opioid use disorder; and prescription for Narcan, a medication that can reverse opioid overdose. We then asked doctors and patients how they felt about the bundle and any barriers and facilitators that they predicted for expanding HIV testing to PWID while admitted to the hospital. Patients were accepting of expanding HIV testing, and resident physicians felt it was important as well and was a manageable addition to their list of responsibilities. However, the most likely part of the bundle to be forgotten was HIV testing. This study lays the groundwork for bundling services for PWID while they are hospitalized. We also highlight areas for future exploration.
Introduction
Ending the HIV epidemic requires increased diagnosis, treatment, prevention, and robust public health responses to HIV outbreaks (Explore CDC's Role in Ending the HIV Epidemic in the U.S., 2021). The Centers for Disease Control and Prevention guidelines have recommended HIV testing for all hospitalized patients since 1993 (Centers for Disease & Prevention, 1993). Hospitalization rates for management of drug use–associated infections have increased rapidly since 2009, with a marked increase in 2013 with the introduction of fentanyl to the illicit drug market and more recently with methamphetamines (See et al., 2020; Serota et al., 2021; Wurcel et al., 2016). Yet, in a 2018 report, people living with HIV, including people with a history of injection drug use, reported missed HIV testing opportunities with healthcare providers before their diagnosis (Wejnert et al., 2018). The COVID-19 pandemic has further hampered the process of linking people with substance use disorder to treatment and harm reduction tools, further increasing overdose rates, injection-related bacterial infections, and HIV (DiGennaro et al., 2021; Park et al., 2022; Vasylyeva et al., 2020).
Considerable work has been done to engage key stakeholders, including patients, clinicians, and healthcare administrators to improve HIV testing in inpatient settings using systems-based changes (Gustafson et al., 2020; Osorio et al., 2017). However, major gaps in HIV testing remain, especially for people with opioid use disorder and people who inject drugs (PWID) (Zhang et al., 2020). We previously reported data from our institution that about 10% of hospitalized people who used drugs received HIV testing, with Black and Hispanic patients being less likely to receive HIV testing (Zubiago et al., 2021; Hamdan et al., 2022). This low rate of testing occurred in the wake of persistent HIV outbreaks in the communities our hospital serves (Gonsalves et al., 2021; Hamdan et al., 2022). Although there has been recent improvement of including perspectives of nurses and physicians’ assistants in the development of improved care, their voices are often missing (Ober et al., 2021; Wilson et al., 2021; Zhang et al., 2020).
Knowing the importance of HIV testing during hospitalization for PWID, and the current low rates at our hospital, we used implementation science frameworks to guide the process of working with key stakeholders to discover the best way to improve HIV testing rates for PWID.
Method
Framework Selection
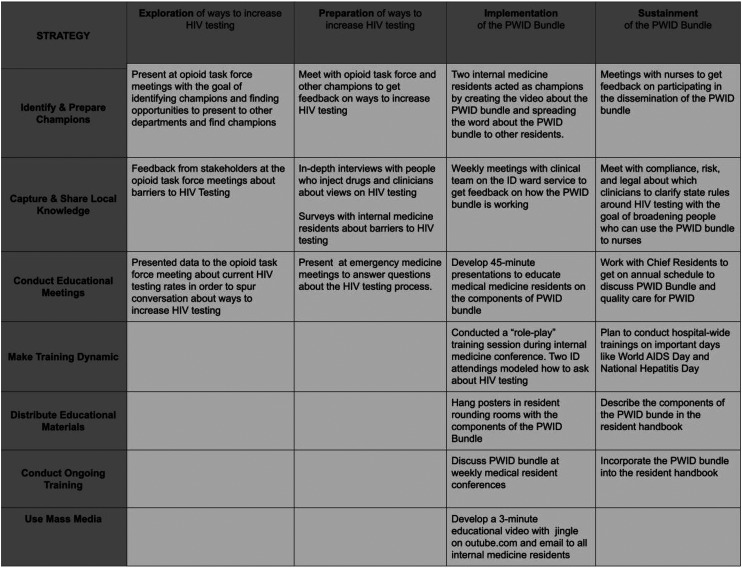
Several complementary implementation method frameworks guided the research. The Exploratory, Preparatory, Implementation, and Sustainment (EPIS) framework provided the scaffold for the research process (Aarons et al., 2011). Prior to implementation, we selected seven strategies from the Expert Recommendations for Implementing Change to further guide the process. The seven strategies included identifying and preparing champions; capturing and sharing local knowledge; conducting educational meetings; dynamic training; distributing educational materials; conducting ongoing training; and using mass media. These seven strategies were selected through joint review of the available strategies by the research team (AGW, ES, BP, and DS) and review of the literature, with a focus on implementation science and improving care for PWID (Bogan et al., 2020; Damschroder & Hagedorn, 2011; Patel et al., 2022). The team prioritized selecting feasible, synergistic strategies. The 2013 Proctor et al. framework was used to organize and present data on the implementation strategies, targets of action, timing, and justification (Proctor et al., 2013).
Setting
The project was conducted at Tufts Medical Center, a tertiary not-for-profit 415-bed academic center (Who We Are, 2022) located in Boston, Massachusetts, and a referral center for two local hospitals: Lowell General Hospital and Melrose-Wakefield Hospital. Previous data published by our research team using toxicology screening, billing codes, and medication review estimated that 200 people who use drugs (i.e., inject, snort, or consume) are admitted to Tufts Medical Center (Hamdan et al., 2022). Patients admitted to the Tufts Medical Center medical units are often cared for by various inpatient services, but many PWID are cared for by the infectious disease service because of the overlap between injection drug use and infections. HIV testing and hepatitis serologies can be drawn 24 h a day, 7 days a week. HIV testing in Massachusetts requires documentation of verbal consent. HIV test results return in 4 h, and positive results are emailed directly to specific HIV clinicians to ensure follow-up. Hepatitis A, B, and C results return in 24–48 h after blood draw. Vaccines and medications for opioid use disorder (M-OUD) can be ordered at any time during a patient's hospitalization stay.
Exportation/Preparation Phases
Origins of the Project. The impetus for this research came from a clinical interaction in Fall 2017. One of the research team members (AGW) diagnosed a patient with HIV during their hospitalization. Although this patient was diagnosed and linked to care, the experience fueled future research to identify barriers of testing people for HIV.
Implementation Team. The implementation team included an infectious disease physician/health service researcher (AGW), infectious disease fellow (AK), nurse/implementation scientist (DHD), research assistants (EDG, BP, ES, JZ, and YM), and Master's of Public Health students (VC, HN, and AJ). AGW had experience working with PWID, which influenced the key methods of centering the opinions of both PWID and those who provide care to them. Prior to conducting and analyzing pre-intervention, mid-intervention, and post-intervention assessments, research assistants were trained by AGW and DHD on qualitative and implementation research methods. Guided by AGW, members of the research team AK, BP, and ES worked on the institutional review board application.
Pre-Implementation Work. The two main implementation strategies used during the exploratory and preparatory phase were champion identification and capturing local knowledge with informal meetings, in-depth interviews, and surveys.
Meetings With Key Stakeholders. Key staff stakeholders were identified in April 2018 when AGW began attending Tufts Medical Center Opioid Task Force meetings. The main stakeholders were identified as medicine resident and attending physicians; nurses; nurse managers; hospital administrators; emergency medicine physicians; pharmacists; and addiction psychiatrists. AGW presented data about low HIV testing rates to the Opioid Task Force (Zubiago et al., 2021). The stakeholders at these meetings facilitated further presentations at departmental meetings (medicine, infectious diseases, orthopedics, and surgery), morbidity and mortality conferences, and nursing education sessions to present low HIV testing rates and get feedback on how to improve HIV testing. Field notes were taken at these meetings.
In-Depth Interviews. In-depth interviews were conducted with (1) hospitalized patients and (2) physician assistants (PAs). Hospitalized patients with substance use disorder were recruited for 45-min semistructured, in-depth interviews on ways to improve their healthcare, including their perspectives on antibiotics and experiences of inpatient HIV screening. An analysis of the themes related to PWID care has been published (Morales et al., 2022). Inclusion criteria included self-reported injection drug use within the past year. The potential participant was approached first by a medical team member and asked if they would be interested in participating in research. Monetary compensation was not permitted, so participants were given an insulated water bottle. Our research team decided to conduct in-depth interviews with PAs because they were identified as an under-engaged stakeholder group that was spread out across departments in the hospital. PAs were not present at the Opioid Task Meeting, and there was no joint PA-focused meeting, so in-depth interviews were felt to be the best method of gathering data from them. PAs were recruited for 45-min semistructured in-depth interviews on barriers and facilitators to HIV testing through email and word of mouth. They received $25 for participation. Research team members audio recorded the interviews, and then audio files were transcribed using Dedoose 6.1.18, (SocioCultural Research Consultants, UCLA). Inductive thematic analysis (ITA) methods was used to capture recurrent themes (Braun & Clarke, 2006). Codes were developed through an iterative process, until all recurring themes were captured. The interview guides are attached as Supplements 1 and 2.
Medicine Resident Surveys. Medicine residents were identified as an important group to survey about barriers to HIV testing. Following a review of the literature, DHD and AGW developed the survey that asked residents to rate the frequency with which they experience specific barriers to HIV testing in the inpatient setting using a five-point Likert scale (never, rarely, sometimes, often, and very often). Prior to distribution, the survey was piloted with three internal medicine residents from other local hospitals, and their feedback was incorporated. With the support of the internal medicine chief residents, an anonymous 13-question survey was emailed to all medicine residents (n = 63) in October 2021.
PWID Bundle Development. Although previous work included all people who use drugs, for this project, we wanted to focus more specifically on PWID. We made this decision because infectious disease risks are increased for people who inject drugs compared to people who snort or smoke drugs. Additionally, several of the harm reduction tools in the bundle are specific to opioid use, and people with severe opioid use disorder who are hospitalized most commonly report injection as their method of drug use. The concept of a bundle to improve PWID care was originally suggested by AGW at one of the Opioid Task Force meetings and gained support leading to development and refinement. A care “bundle” is a system-based strategy that includes a group of three to five evidence-informed practices, performed collectively, to improve care (Lavallée et al., 2017). Care bundles that group HIV testing with other types of testing and vaccination are evidence-based approaches for increasing HIV testing for PWID in emergency rooms and ambulatory care settings (Cowan et al., 2018; Galvan et al., 2006; Marks et al., 2021; Polonijo et al., 2022). Other services that should be offered during hospitalization are hepatitis A and B testing and vaccination; hepatitis C testing; M-OUD; and prescription of opioid overdose reversal medications (Beieler et al., 2021; Jones et al., 2019; Skolnick, 2018). The practices selected for inclusion in the bundle consisted of (1) HIV screening; (2) hepatitis A, B, and C testing and vaccination; (3) M-OUD; and (4) naloxone prescription. These elements were selected because they are included in treatment guidelines for PWID (Levitt et al., 2020; Thakarar et al., 2016; Yehia et al., 2014).. There was some feedback to include offering pre-exposure prophylaxis (PrEP) to prevent HIV in the bundle, but several stakeholders were concerned this would make the bundle too cumbersome and prevent clinicians from using it.
Implementation/Sustainment Phase
The PWID bundle was piloted April–June 2021 on the infectious disease ward service with weekly informal feedback assessments from the residents and attendings. The research team presented weekly to the infectious disease service during the 8-week pilot period. We asked the team if they knew about the PWID bundle and for feedback on how easy it was to use. Then in July 2021, the bundle was expanded to the other internal medicine services. Implementation was supported by continued engagement of stakeholders and capturing of local knowledge but then expanded to include other strategies. We created buttons that read, “Ask me about the #PWIDbundle” (see Appendix A), flyers about the PWID bundle components (see Appendix B), and a YouTube video by two of the medicine residents with a jingle about the PWID bundle components to the tune of “ABC” by Michael Jackson (see Appendix C). We used the protected lunch didactic time for residents to conduct two sessions related to the bundle. In the first session, we presented with addiction psychiatry about the importance of each of the bundle elements. In the second session, AGW and other infectious diseases clinicians did a “role play” session, acting out patient–clinician interactions about HIV testing. We provided educational sessions with attendings during department meetings to show results of the preliminary work and create a forum for answering questions about HIV testing. We used both venues to spread education that all clinicians could get consent for HIV testing, that HIV tests are returned within 4 h, and that all positive HIV test results are automatically sent to the infectious disease department to ensure follow-up.
To support sustaining the bundle, the information about the PWID bundle was incorporated into the resident handbook, which is distributed every July to the medicine residents. In efforts to sustain the PWID bundle, we met with old and new stakeholders. For the first time, the research team met with information technology champions to discuss building the PWID bundle into the electronic medical record using “smart phrases.” Nurses who cared for PWID had been involved throughout the exploration and preparation phases but were not directly involved in the implementation phase. So, the study team met with nursing leadership and nurse champions to discuss how the bundle implementation went and to elicit feedback on next steps and potential nursing involvement. One question that arose was if nurses could legally obtain consent for HIV testing, so the research team also met with the legal, compliance, and administrative teams at Tufts Medical Center to gather more information.
Acceptability and Feasibility Assessment. In October 2021, a post-implementation survey was sent to all internal medicine residents to assess the acceptability and feasibility of the implementation of the PWID bundle. Residents were recruited through emails from chief residents, in-person following didactic lectures, and via QR code displayed on a projector at weekly resident meetings. The survey included 12 questions and asked residents to rate their level of agreement with statements about the feasibility and acceptability of HIV testing using a five-point Likert scale. Since the survey was designed for medicine residents, the abbreviation “AMA” was used, which means “against medical advice.” We appreciate that “AMA” is a complex term often used in a stigmatizing way. However, in efforts to reflect the stakeholder group, it was included in the language of the survey. Medicine residents who completed the survey were enrolled in a raffle to win one of five $50 Amazon gift cards.
Effectiveness Assessment. To measure effectiveness of the PWID bundle on increasing HIV testing, we analyzed the number of HIV tests done per month in hospitalizations with ICD9 and ICD10 codes that indicated drug use pre-intervention (September 2020–March 2021), mid-intervention (April 2021–June 2021), and post-intervention (July 2020–March 2022). These data were gathered in conjunction with the Tufts Clinical Translational Science Institute and was approved by the Tufts Health Sciences Institutional Review Board.
Results
The work guides by the EPIS framework and Proctor framework are displayed with the seven implementation strategies in Figure 1 and Table 1.
Figure 1.
Exploration, Preparation, Implementation, and Sustainment (EPIS) Model Including the Seven Expert Recommendations for Implementing Change (ERIC) Strategies
Table 1.
The 2013 Proctor Framework Applied to Implementation Strategies
| Strategy | Target(s) of action | Temporality | Justification for strategy |
|---|---|---|---|
| Identify and prepare champions | Key staff shareholders | Prior to and during implementation of the bundle | Champions passionate about improving healthcare for people who inject drugs is one of the most successful strategies in the literature. |
| Capture and share local knowledge | Clinicians who care for PWID and hospitalized PWID | Prior to and during implementation of the bundle | Patients and clinicians have an incredible amount of knowledge about the multi-level barriers preventing HIV testing. We captured local knowledge through in-depth interviews, survey, and field notes to inform the development of the PWID bundle. |
| Conduct educational meetings | Clinicians | Prior to and during implementation of the bundle | We identified several knowledge gaps in understanding evidence-based care for PWID as well as the rules around HIV testing. Educational meetings allowed for a forum for clinicians to ask questions. Educational meetings were necessary to teach about the PWID bundle. Although details were emailed to all clinicians, the internal medicine residents were often too busy to read or meaningfully interact with information delivered through email. |
| Distribute educational materials | Medicine residents | Prior to and during implementation of the bundle | A common theme found in the pre-implementation medicine resident survey was forgetting to ask about HIV testing. We developed flyers to remind people about the PWID bundle and posted them in places where internal medicine residents work. |
| Make training dynamic | Medicine residents | Prior to and during implementation of the bundle | We noticed that in preliminary discussions with internal medicine residents, they seemed tired and disengaged. We created different systems of education, including “role-play” exercises and case-based presentations to encourage the internal medicine resident participation. |
| Use mass media | Medicine residents | During the implementation of the bundle | Champions and project team members developed a 3-min jingle to the tune of “ABC” by Michael Jackson to reinforce the components. To make this easily accessible, the residents recorded themselves singing the jingle and published it on YouTube, an accessible and popular website among the medicine residents. |
| Conduct ongoing training | Medicine residents | During and after implementation of the bundle | We included the details of the PWID bundle in the resident handbook that is distributed to new residents each July. |
Exploratory/Preparatory
Meetings with key stakeholders. Hospital staff and employees identified a variety of facilitators and barriers to HIV testing and PWID bundle implementation before implementation. Among attending physicians, general concerns included the relative priority of HIV testing during an inpatient admission; liability concerns if patients leave the hospital prior to addressing positive test results; the appropriateness of initiating inpatient hepatitis A and B vaccination; and the potential for missing subsequent vaccination doses upon discharge. Resident physicians described concerns about the consenting process and the effect that offering HIV testing would have on patient rapport. Both the addiction psychiatry and nursing staff voiced concerns that HIV consent was potentially outside of their scope of practice. Pharmacists described logistical concerns and time constraints that could affect providing naloxone medication and counseling to patients.
In-Depth Interviews. Fourteen patients were approached, 11 agreed to be interviewed, and 6 were female. Eight people identified as white, one person as Black, one as multi-racial, and one did not report their race/ethnicity. Most patients expressed that they felt comfortable being asked about HIV testing and most reported being tested regularly. One patient stated, when asked if she had ever been tested for HIV: “Oh yeah, yeah, ‘cause that was the first thing that they asked me when I got to the emergency room on that day. Sh’'s like, you mind if I test you for HIV and Hep C and all that, I was like no problem, go ahead… Test me for everything man, I want to know that.” In reference to a question about HIV testing, another patient said, “I know that they’re very pushy with that lately because I know the rise in HIV, drug use. So I’ll consider it. Yeah, it (testing) was good. So you don’t die of it, you know what I mean?” Another patient said, “I took the test and then it… it was the scariest shit that I ever been through in my life.” There was one person who said that they refused HIV testing when offered, “I haven’t been tested for HIV or anything. I’m not using drugs. I don’t need it.” It could be this person did not recently use drugs, so they did not feel like HIV testing was needed. Another explanation was that the participant did not want to discuss drug use openly with the research assistant.
Out of 46 physicians’ assistants, 7 (15%) participated in the research. Overall, PA participants felt HIV testing was important but did not believe it was within their scope of practice to offer HIV testing. One PA said, “Our protocols are very much, you know, managing kind of the big traumatic injuries and stabilizing, and moving on. You know long term management for these generally, HIV status and hepatitis status, often don’t matter as much…unless again there is some type of infection, it's [HIV testing] not a routine thing that we’re doing. Generally infectious disease service will ask.” Another theme that emerged was concern from the PA that testing might not be within the goals of the attending: “The surgeons are a bit more impatient. They’re a little bit less inclined to, you know, be entertaining kind of routine testing for certain things that may potentially be opening medical cans of worms that may not be appropriate for service.” PAs also expressed that they did not test for HIV or hepatitis because they were concerned that they would not know how to answer questions from the patients, “I find it …kind of challenging you know. Being in emergency medicine, I have this wide breadth of knowledge of a little bit of a lot of things, so knowing enough about hepatitis and the treatment options and what the next steps would be is still something I probably could use more education on. I find it pretty difficult and challenging too because people will have questions that I won’t know how to answer.”
Medicine Resident Pre-Implementation Survey
One-third (20/60) of medicine residents responded to the pre-implementation survey about potential barriers to HIV testing. About one-third (7/20) of residents sometimes worried about offending the patient when asking about HIV testing, and 60% (16/20) sometimes or often forgot to ask the patient about HIV testing (see Table 2). More than half of residents (55%; 11/20) believed that bringing up HIV testing would negatively impact patients' participation in other aspects of care, such as leaving AMA or refusal of other tests (Table 2).
Table 2.
Pre-Implementation Medicine Resident Survey Results
| Never | Rarely | Sometimes | Often | Very often | |
|---|---|---|---|---|---|
| I am unable to get consent from a patient about HIV testing | 0 | 12 | 7 | 0 | 1 |
| I worry about offending the patient by asking about HIV testing | 4 | 9 | 7 | 0 | 0 |
| I worry that the patient will get angry about HIV testing | 4 | 11 | 5 | 0 | 0 |
| I worry that bringing up HIV testing will negatively impact the patient’s participation in other aspects of care (leave AMA, refuse other tests) | 6 | 11 | 2 | 1 | 0 |
| I worry about telling a patient that they are positive for HIV | 6 | 6 | 7 | 1 | 0 |
| I worry about my responsibility for my patient if they test positive for HIV (connecting them to care) | 7 | 5 | 7 | 1 | 0 |
| I forget to ask the patient about HIV testing | 0 | 4 | 12 | 4 | 0 |
Note: PWID = People Who Inject Drugs; AMA = Against Medical Advice.
Implementation/Sustainment Phase
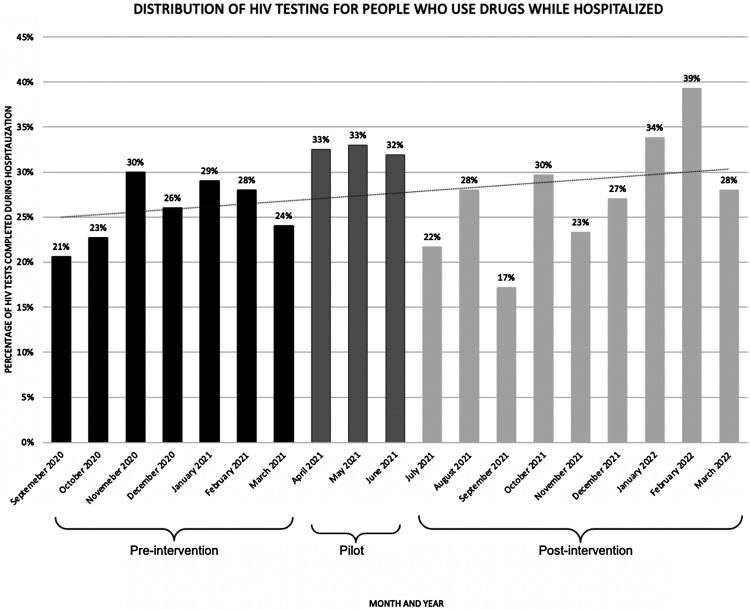
Among internal medicine residents, 56% (43/77) responded to the post-implementation survey. More than half (59%; 23/39) were familiar with the PWID bundle (Table 3). All respondents felt the bundle was either moderately, very, or extremely effective and important for improving healthcare delivery to PWID (Table 3). Of the residents, 57% (20/35) said that the bundle was not disruptive to workflow, and 71% (25/35) strongly or somewhat agreed that they felt sufficiently skilled to carry out and apply the components of the PWID bundle (Table 3). Although the rate of hospitalizations that included an HIV test increased overtime, there were no significant changes in the rate of tests completed (see Figure 2).
Table 3.
Post-Implementation Medicine Resident Survey Results
| Never | Rarely | Sometimes | Often | Very often | |
|---|---|---|---|---|---|
| I feel uncomfortable offering an HIV test to my patients | 23 | 6 | 0 | 0 | 6 |
| I feel comfortable ordering hepatitis A and B vaccination based on the result of testing | 0 | 6 | 6 | 16 | 7 |
| I feel comfortable prescribing Naloxone to my patients | 1 | 2 | 5 | 9 | 18 |
| I feel sufficiently skilled to carry out and apply the components of the PWID bundle | 0 | 5 | 5 | 17 | 8 |
| The PWID bundle can be performed by any healthcare provider on an inpatient service (including interns, residents, fellows, attendings, physician assistants, and nurse practitioners) | 0 | 0 | 6 | 7 | 22 |
Note. PWID = People Who Inject Drugs.
Figure 2.
HIV Testing Rates for People Who Inject Drugs (PWID) Before, During, and After Implementation of the PWID Bundle at Tufts Medical Center
Discussion
In this paper, we shared how we used implementation science methodology to engage stakeholders in the process of improving healthcare for PWID. The PWID bundle was an acceptable and feasible way to incorporate HIV testing into inpatient care and improve quality of PWID care but did not lead to increases in HIV testing rates for hospitalized PWID.
We believe the PWID bundle did not lead to increased HIV testing because of the absences of systematic implementation and limited systems of outcome assessment. The PWID bundle, as a pilot project, was not integrated into the electronic medical record. We plan to build from this research with continued engagement of key stakeholders and decision-makers to identify systems-based changes to increase PWID bundle uptake. Members of our research team have successfully implemented systematic interventions to increase HCV testing (Wurcel et al., 2017). We began working with informational technology champions to build “smart phrases” that can bring the PWID bundle into clinical notes during the sustainment phase and plan to continue to do this. These smart phrases can be queried, and using them would allow us to track bundle use. To help us track the acceptability of the bundle among PWID, we are working with information technology and research experts to develop a natural language processing algorithm to assess methods of early identification of PWID. This is how we developed Figure 2, but we would like to continue to refine our natural language processing algorithm. Using natural language processing and smart phrase querying together, we hope to develop better systems of evaluating if the PWID bundle was used, and if it translated into more HIV testing.
Another reason we hypothesize the PWID bundle did not work is the highly sensitized nature of HIV preventing discussions between clinicians and patients. “HIV exceptionalism” is the movement that advocated for unique policies and procedures for HIV (Blain et al., 2021). With this exceptional care came documented consent, and consent requirements vary across the United States (Blain et al., 2021). However, cornering HIV as different from every other illness has hampered work to end the HIV epidemic (Blain et al., 2021). As shown in the interviews with PAs, HIV testing was seen as something that was best done by infectious disease doctors. As seen in our sustainment phase, we are working with champions across the health system to define the role of nurses and PAs in the PWID bundle. We specifically hope to understand nurses’ and PAs’ perspectives about expanding their scope of practice to include HIV consent. We hope to build upon the existing infrastructure of nursing involvement in bundles, including bundles for the prevention of ventilator-acquired pneumonias, deep vein thromboses, wounds, and falls (Kennedy, 2022), and incorporate nurses as champion to improve HIV testing.
Prior to intervention, two of the main barriers to inpatient HIV testing were residents forgetting to ask patients and attendings not knowing HIV testing should be completed during inpatient hospitalization. Clinicians often felt HIV testing and other components of the PWID bundle should be deferred to the outpatient setting. PAs, representing surgical and medical subspecialties, felt that HIV was outside of the scope of subspecialty care. Hospitalization is a critical opportunity for testing and linkage to care, as many PWID are frequently reluctant to seek outpatient medical care due to significant barriers, including stigma and concerns about receiving substandard medical care (Dion et al., 2020; Motavalli et al., 2021; Paquette et al., 2018). Many of the inpatient providers’ main hesitation toward the PWID bundle was the potential for loss to follow-up and not completing the hepatitis A virus or hepatitis B virus vaccination series. However, research has shown that among people who are non-immune to hepatitis A or hepatitis B, even the first vaccine doses are known to offer some protection (Ahmed & Gray, 1996; Mayorga et al., 2016). Concern of PWID missing subsequent doses should not be a reason to postpone the first doses of hepatitis A and B vaccines.
Testing is crucial to ending the HIV epidemic, but the data from our research show that, even 40 years after the first case of HIV, many clinicians feel uncomfortable discussing HIV in the scope of inpatient medical care. In Massachusetts, providers are required to document verbal consent. Obtaining verbal consent for an HIV test was noted to be uncomfortable by the medicine residents. While we should encourage providers’ confidence in obtaining consent for HIV testing, it is hard to ignore the fact that some PWID are not being offered HIV testing because the consent process is cumbersome and a barrier itself. Removing the verbal consent for HIV will take engagement of key stakeholders, including public health champions, policy experts, and people living with HIV. We strongly recommend changing the HIV consent process and believe it will have an immense impact on increasing HIV testing. We believe that HIV testing consent should be covered by consent to treatment, which is an agreement to receive indicated medical care (this includes every blood test besides HIV) that every hospitalized person must complete upon admission.
There are several limitations to this study. First, this intervention occurred in a single tertiary care facility in Boston, Massachusetts. The perspectives of providers and PWID interviewed in this intervention may not reflect those of all hospitals. The nature of implementation work often makes it necessary to pilot strategies in contained settings; however, we would like to reflect that our experience of implementation may be different than other centers. Second, the pre- and post-intervention medicine resident surveys were different. Although it would have been ideal to have the same survey for the sake of comparison, we had specific questions about the intervention that were only suited for the post-intervention survey. Third, stipends were introduced for the post-intervention survey, which affected participant response rates. The pre-intervention survey response rate was 26%, and post-intervention survey response rate was 55%. However, total response rates remained relatively low across both pre-intervention and post-intervention surveys, hopefully minimizing any significant differences. Fourth, stakeholders may have been reluctant to share honest and real opinions with coworkers during small group discussion. For this reason, research assistants were often the conveyors and gatherers of information to reduce the risk of likeability bias. Fifth, we collected post-intervention data from hospital employees but not patient stakeholders since pre-intervention data suggested that the PWID were overall supportive of HIV testing. However, it would have been interesting to hear the thoughts’ of PWID post-intervention. Sixth, the data used to calculate HIV testing rates was for all drug use, not specifically injection drug use. However, we believe that people who use drugs via injection are most likely to be tested for HIV, so we believe the difference in testing rates between people who use drugs and PWID is likely negligible. Lastly, as consent is necessary, we do not know if more clinicians asked for HIV testing consent but patients refused. We did not have any implementation strategies aimed directly at increasing the acceptability of HIV testing for PWID.
Conclusion
Implementation science–rooted methodology can guide the process of PWID care improvement. We hope that our experience encourages clinicians at other hospitals to work towards improved systems of care, particularly for PWID. High-quality healthcare for hospitalized PWID is an opportunity to foster trust, a necessary part of the equation to end the HIV epidemic.
Supplemental Material
Supplemental material, sj-docx-1-irp-10.1177_26334895231203410 for Implementation of a bundle to improve HIV testing during hospitalization for people who inject drugs by Emily D. Grussing, Bridget Pickard, Ayesha Khalid, Emma Smyth, Victoria Childs, Julia Zubiago, Hector Nunez, Amanda Jung, Yoelkys Morales, Denise H. Daudelin and Alysse G. Wurcel in Implementation Research and Practice
Supplemental material, sj-docx-2-irp-10.1177_26334895231203410 for Implementation of a bundle to improve HIV testing during hospitalization for people who inject drugs by Emily D. Grussing, Bridget Pickard, Ayesha Khalid, Emma Smyth, Victoria Childs, Julia Zubiago, Hector Nunez, Amanda Jung, Yoelkys Morales, Denise H. Daudelin and Alysse G. Wurcel in Implementation Research and Practice
Acknowledgments
The authors gratefully acknowledge Maura Macdonald for her help in creating the #PWID Bundle jingle video. We would like to thank Dr. Sami Hamdan and Dr. Anne Dowton for their support of this project. This research was supported in part by generous donations to the Tupper Research Fund at Tufts Medical Center.
Appendix A. Button to Promote the Bundle Throughout REDACTED FOR PEER REVIEW

Appendix B. Poster Hung Up in the Hospital to Advertise the PWID Bundle.
Note. This advertisement uses PWUD instead of PWID. This was an oversight on the research team's part. We do not think this impacts the intervention heavily.

Appendix C. #PWID Bundle Jingle YouTube Video Made to Promote the Bundle
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Tufts University Substance Use Disorder Initiative Institutional Grant (AGW), National Institutes of Health (K08HS026008-01A; AGW), and the Infectious Diseases Society of America GERM Seed Grant.
ORCID iD: Emily D. Grussing https://orcid.org/0000-0001-9596-2197
Supplemental Material: Supplemental material for this article is available online.
References
- Aarons G. A., Hurlburt M., Horwitz S. M. (2011). Advancing a conceptual model of evidence-based practice implementation in public service sectors. Administration and Policy in Mental Health and Mental Health Services Research, 38(1), 4–23. 10.1007/s10488-010-0327-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Gray D. (1996). Immunological memory and protective immunity: Understanding their relation. Science, 272(5258), 54–60. 10.1126/science.272.5258.54 [DOI] [PubMed] [Google Scholar]
- Beieler A. M., Klein J. W., Bhatraju E., Iles-Shih M., Enzian L., Dhanireddy S. (2021). Evaluation of bundled interventions for patients with opioid use disorder experiencing homelessness receiving extended antibiotics for severe infection. Open Forum Infectious Diseases, 8(6), Article ofab285. 10.1093/ofid/ofab285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain M., Wallace S. E., Tuegel C. (2021). Shadow of HIV exceptionalism 40 years later. Journal of Medical Ethics, 47(11), 727–728. 10.1136/medethics-2020-106908 [DOI] [PubMed] [Google Scholar]
- Bogan C., Jennings L., Haynes L., Barth K., Moreland A., Oros M., Goldsby S., Lane S., Funcell C., Brady K. (2020). Implementation of emergency department–initiated buprenorphine for opioid use disorder in a rural southern state. Journal of Substance Abuse Treatment, 112, 73–78. 10.1016/j.jsat.2020.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Clarke V. (2006). Using thematic analysis in psychology. Qualitative Research in Psychology, 3(Camplain et al.), 77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- Centers for Disease & Prevention. (1993). Recommendations for HIV testing services for inpatients and outpatients in acute-care hospital settings. MMWR. Morbidity and Mortality Weekly Report, 42(8), 157–158. https://www.ncbi.nlm.nih.gov/pubmed/8437547 [PubMed] [Google Scholar]
- Cowan E., Herman H., Rahman S., Zahn J., Leider J., Calderon Y. (2018). Bundled HIV and Hepatitis C testing in the emergency department: A randomized controlled trial. Western Journal of Emergency Medicine, 19(6), 1049–1056. 10.5811/westjem [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damschroder L. J., Hagedorn H. J. (2011). A guiding framework and approach for implementation research in substance use disorders treatment. Psychology of Addictive Behaviors, 25(2), 194–205. 10.1037/a0022284 [DOI] [PubMed] [Google Scholar]
- DiGennaro C., Garcia G. P., Stringfellow E. J., Wakeman S., Jalali M. S. (2021). Changes in characteristics of drug overdose death trends during the COVID-19 pandemic. The International Journal on Drug Policy, 98(2021), 103392. 10.1016/j.drugpo.2021.103392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion K., Chiodo L., Whynott L., Loftus B., Alvarez P., Shanahan J., Quaglia G., Roman A., Sorbi E., Wilkins-Carmody D. (2020). Exploration of the unmet health care needs of people who inject drugs. Journal of the American Association of Nurse Practitioners, 32(1), 60–69. 10.1097/jxx.0000000000000201 [DOI] [PubMed] [Google Scholar]
- Explore CDC’s Role in Ending the HIV Epidemic in the U.S. (2021). Centers for Disease Control. https://www.cdc.gov/endhiv/index.html.
- Galvan F. H., Bluthenthal R. N., Ani C., Bing E. G. (2006). Increasing HIV testing among Latinos by bundling HIV testing with other tests. Journal of Urban Health, 83(5), 849–859. 10.1007/s11524-006-9072-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves G. S., David Paltiel A., Thornhill T., Iloglu S., DeMaria A., Jr., Cranston K., Monina Klevens R., Walensky R. P., Warren J. L. (2021). The dynamics of infectious diseases associated with injection drug use in Lawrence and Lowell, Massachusetts. Open Forum Infectious Diseases, 8(6), Article ofab128. 10.1093/ofid/ofab128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson R., Demlow S. E., Nathoo A., McKee G., MacDonald L. E., Chu T., Sandhu J., Grafstein E., Hull M., Chittock D., Carere R., Krajden M., Sherlock C. H., Harrison S., Buchner C. S., Montaner J. S. G., Daly P. (2020). Routine HIV testing in acute care hospitals: Changing practice to curb a local HIV epidemic in Vancouver, BC. Preventive Medicine, 137(August 2020), 106132. 10.1016/j.ypmed.2020.106132 [DOI] [PubMed] [Google Scholar]
- Hamdan S., Smyth E., Murphy M. E., Grussing E. D., Wei M., Guardado R., Wurcel A. (2022). Racial and ethnic disparities in HIV testing in people who use drugs admitted to a tertiary care hospital. AIDS Patient Care and STDs, 36(11), 425–430. 10.1089/apc.2022.0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. M., Byrd D. J., Clarke T. J., Campbell T. B., Ohuoha C., McCance-Katz E. F. (2019). Characteristics and current clinical practices of opioid treatment programs in the United States. Drug and Alcohol Dependence, 205, Article 107616. 10.1016/j.drugalcdep.2019.107616 [DOI] [PubMed] [Google Scholar]
- Kennedy E. (2022). Bundling your way to quality care. J Dr Nurs Pract, 15(1), 11–17. 10.1891/jdnp-2021-0039 [DOI] [PubMed] [Google Scholar]
- Lavallée J. F., Gray T. A., Dumville J., Russell W., Cullum N. (2017). The effects of care bundles on patient outcomes: A systematic review and meta-analysis. Implementation Science, 12(1), 142. 10.1186/s13012-017-0670-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt A., Mermin J., Jones C. M., See I., Butler J. C. (2020). Infectious diseases and injection drug use: Public health burden and response. Journal of Infectious Diseases, 222(Suppl 5), S213–S217. 10.1093/infdis/jiaa432 [DOI] [PubMed] [Google Scholar]
- Marks L. R., Reno H., Liang S. Y., Schwarz E. S., Liss D. B., Jiang L., Nolan N. S., Durkin M. J. (2021). Value of packaged testing for sexually transmitted infections for persons who inject drugs hospitalized with serious injection-related infections. Open Forum Infectious Diseases, 8(11), e1192. 10.1093/ofid/ofab489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga O., Bühler S., Jaeger V. K., Bally S., Hatz C., Frösner G., Protzer U., Van Damme P., Egger M., Herzog C. (2016). Single-dose hepatitis A immunization: 7.5-year observational pilot study in Nicaraguan children to assess protective effectiveness and humoral immune memory response. Journal of Infectious Diseases, 214(10), 1498–1506. 10.1093/infdis/jiw411 [DOI] [PubMed] [Google Scholar]
- Morales Y., Smyth E., Zubiago J., Bearnot B., Wurcel A. G. (2022). “They just assume that we’re all going to do the wrong thing with it. It’s just not true.” Stakeholders perspectives about PICCs in people who inject drugs. Open Forum infectious Diseases, Accepted July 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motavalli D., Taylor J. L., Childs E., Valente P. K., Salhaney P., Olson J., Biancarelli D. L., Edeza A., Earlywine J. J., Marshall B. D. L., Drainoni M. L., Mimiaga M. J., Biello K. B., Bazzi A. R. (2021). “Health is on the back burner:” Multilevel barriers and facilitators to primary care among people who inject drugs. Journal of General Internal Medicine, 36(1), 129–137. 10.1007/s11606-020-06201-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober A. J., Takada S., Zajdman D., Todd I., Horwich T., Anderson A., Wali S., Ladapo J. A. (2021). Factors affecting statin uptake among people living with HIV: Primary care provider perspectives. BMC Family Practice, 22(1), 215. 10.1186/s12875-021-01563-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio G., Hoenigl M., Quartarolo J., Barger K., Morris S. R., Reed S. L., Lee J., Little S. J. (2017). Evaluation of opt-out inpatient HIV screening at an urban teaching hospital. AIDS Care, 29(8), 1014–1018. 10.1080/09540121.2017.1282106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette C. E., Syvertsen J. L., Pollini R. A. (2018). Stigma at every turn: Health services experiences among people who inject drugs. The International Journal on Drug Policy, 57, 104–110. 10.1016/j.drugpo.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. N., Owczarzak J., Urquhart G., Morris M., Weicker N. P., Rouhani S., Sherman S. G. (2022). HIV risk among urban and suburban people who inject drugs: Elevated risk among fentanyl and cocaine injectors in Maryland. AIDS and Behavior, 26(1), 277–283. 10.1007/s10461-021-03381-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel E., Solomon K., Saleem H., Saloner B., Pugh T., Hulsey E., Leontsini E. (2022). Implementation of buprenorphine initiation and warm handoff protocols in emergency departments: A qualitative study of Pennsylvania hospitals. Journal of Substance Abuse Treatment, 136, 108658. 10.1016/j.jsat.2021.108658 [DOI] [PubMed] [Google Scholar]
- Polonijo A. N., Sein S., Maldonado R., Delos Santos J., Brown B. (2022). Promoting vaccination during rapid HIV testing: Recommendations from men who have sex with men in California. Health & Social Care in the Community, 30(5). 10.1111/hsc.v30.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor E. K., Powell B. J., McMillen J. C. (2013). Implementation strategies: recommendations for specifying and reporting. Implementation Science, 8(1), 226. 10.1186/1748-5908-8-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- See I., Gokhale R. H., Geller A., Lovegrove M., Schranz A., Fleischauer A., McCarthy N., Baggs J., Fiore A. (2020). National public health burden estimates of endocarditis and skin and soft-tissue infections related to injection drug use. A Review. J Infect Dis, 222(Suppl 5), S429–s436. 10.1093/infdis/jiaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serota D. P., Bartholomew T. S., Tookes H. E. (2021). Evaluating differences in opioid and stimulant use-associated infectious disease hospitalizations in Florida, 2016-2017. Journal of Biochemistry and Molecular Biology, 73(7), e1649–e1657. 10.1093/cid/ciaa1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P. (2018). The opioid epidemic: Crisis and solutions. Annual Review of Pharmacology and Toxicology, 58, 143–159. 10.1146/annurev-pharmtox-010617-052534 [DOI] [PubMed] [Google Scholar]
- Thakarar K., Weinstein Z. M., Walley A. Y. (2016). Optimising health and safety of people who inject drugs during transition from acute to outpatient care: Narrative review with clinical checklist. Postgraduate Medical Journal, 92(1088), 356–363. 10.1136/postgradmedj-2015-133720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasylyeva T. I., Smyrnov P., Strathdee S., Friedman S. R. (2020). Challenges posed by COVID-19 to people who inject drugs and lessons from other outbreaks. Journal of The International Aids Society, 23(7), Article e25583. 10.1002/jia2.25583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wejnert C., Prejean J., Hoots B., Hall H. I., McCray E., Mermin J., Group N. S. (2018). Prevalence of missed opportunities for HIV testing among persons unaware of their infection. JAMA, 319(24), 2555–2557. 10.1001/jama.2018.7611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Who We Are. (2022). Tufts Medical Center. https://www.tuftsmedicalcenter.org/about-us/overview#:∼:text=Tufts%20Medical%20Center%20is%20a,and%20children%20throughout%20New%20England.
- Wilson K., Bleasdale J., Przybyla S. M. (2021). Provider-patient communication on pre-exposure prophylaxis (Prep) for HIV prevention: An exploration of healthcare provider challenges. Health Communication, 36(13), 1677–1686. 10.1080/10410236.2020.1787927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurcel A. G., Anderson J. E., Chui K. K., Skinner S., Knox T. A., Snydman D. R., Stopka T. J. (2016). Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infectious Diseases, 3, Article ofw157. 10.1093/ofid/ofw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurcel A. G., Chen D. D., Chui K. K. H., Knox T. A. (2017). “Tweak your order set!” Implementation of modified laboratory order set improves Hepatitis C virus screening rates in people living with human immunodeficiency virus. Open Forum Infectious Diseases, 4(2), 395. 10.1093/ofid/ofx098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia B. R., Schranz A. J., Umscheid C. A., Lo Re V., Rizza S. A. (2014). The treatment cascade for chronic Hepatitis C virus infection in the United States: A systematic review and meta-analysis. PLoS ONE, 9(7), e101554. 10.1371/journal.pone.0101554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Mitchell W., Xue Y., LeBlanc N., Liu Y. (2020). Understanding the role of nurse practitioners, physician assistants and other nursing staff in HIV pre-exposure prophylaxis care in the United States: A systematic review and meta-analysis. BMC Nursing, 19(1). 10.1186/s12912-020-00503-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubiago J., Murphy M., Guardado R., Daudelin D., Patil D., Wurcel A. (2021). Increased HIV testing in people who use drugs hospitalized in the first wave of the COVID-19 pandemic. Journal of Substance Abuse Treatment, 124, Article 108266. 10.1016/j.jsat.2020.108266 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-irp-10.1177_26334895231203410 for Implementation of a bundle to improve HIV testing during hospitalization for people who inject drugs by Emily D. Grussing, Bridget Pickard, Ayesha Khalid, Emma Smyth, Victoria Childs, Julia Zubiago, Hector Nunez, Amanda Jung, Yoelkys Morales, Denise H. Daudelin and Alysse G. Wurcel in Implementation Research and Practice
Supplemental material, sj-docx-2-irp-10.1177_26334895231203410 for Implementation of a bundle to improve HIV testing during hospitalization for people who inject drugs by Emily D. Grussing, Bridget Pickard, Ayesha Khalid, Emma Smyth, Victoria Childs, Julia Zubiago, Hector Nunez, Amanda Jung, Yoelkys Morales, Denise H. Daudelin and Alysse G. Wurcel in Implementation Research and Practice