Abstract
The opioid G-protein coupled receptors (GPCRs) strongly modulate many of the central nervous system structures that contribute to neurological and psychiatric disorders including pain, major depressive disorder, and substance use disorders. To better treat these and related diseases, it is essential to understand the signaling of their endogenous ligands. In this review, we focus on what is known and unknown about the regulation of the over two dozen endogenous peptides with high affinity for one or more of the opioid receptors. We briefly describe which peptides are produced, with a particular focus on the recently proposed possible synthesis pathways for the endomorphins. Next, we describe examples of endogenous opioid peptide expression organization in several neural circuits and how they appear to be released from specific neural compartments that vary across brain regions. We discuss current knowledge regarding the strength of neural activity required to drive endogenous opioid peptide release, clues about how far peptides diffuse from release sites, and their extracellular lifetime after release. Finally, as a translational example, we discuss the mechanisms of action of naltrexone (NTX), which is used clinically to treat alcohol use disorder. NTX is a synthetic morphine analog that non-specifically antagonizes the action of most endogenous opioid peptides developed in the 1960s and FDA approved in the 1980s. We review recent studies clarifying the precise endogenous activity that NTX prevents. Together, the works described here highlight the challenges and opportunities the complex opioid system presents as a therapeutic target.
1. Introduction
Drugs targeting opioid GPCRs remain the most effective treatments for acute intense pain (Bagley and Ingram, 2020; Corder et al., 2018; Lindsay et al., 2021) and alcohol use disorder (AUD), and are in clinical trials to treat major depressive disorder (MDD; Krystal et al., 2020; Pizzagalli et al., 2020). Yet after decades of “rational” development, available opioid-targeted therapies continue to have substantial liabilities. Mu opioid receptor (MOR) agonist drugs cause life threatening depression of respiration (Aghabiklooei et al., 2013; Gerak et al., 2018) and disruption of gut motility (Holzer, 2009). Continuous use can lead to rapid development of tolerance and risks development of opioid use disorder (OUD; Marie, 2019). OUD-related costs in the US exceed $1B/year (Luo et al., 2021) and accidental toxic opioid poisonings now contribute to over 70,000 deaths/year. A major scientific challenge in overcoming these liabilities is that in vitro systems, cell lines that overexpress the receptor of interest and a reporter signaling pathway, largely fail to predict clinical outcomes. For instance, full MOR agonists should produce reward and we expect all such compounds will be self-administered in animal models. Yet, a cyclized variant of endomorphin 1 (EM-1), ZH853, which has similar in vitro activities to other full MOR agonists, does not support self administration in rodent studies (Zadina et al., 2016). In vivo models also often lack predictive power. To explain this and other unexpected observations, a range of mechanistic hypotheses and ligand features have been proposed, including biased agonism, partial agonism, ion-dependence, heteromeric complexes, alternative splicing, G protein selectivity, and polypharmacology. However, fundamentally, a complete explanation must recognize how opioid drugs interact with the endogenous opioid system. In turn, this requires a thorough characterization of the regulation and function of endogenous opioid peptides. Understanding how the endogenous opioid peptides act at the opioid receptors may also improve ligand targeting because the endogenous systems have evolved together and are highly conserved throughout vertebrate evolution (Abbadie et al., 2002; Larhammar et al., 2015; Li et al., 1996; Stevens, 2009).
Here we summarize basic facts, recent developments, and knowledge gaps about how the at least 26 endogenous opioid peptides are regulated and function in the central nervous system. We present information on the synthesis of the endogenous opioid peptides and what is known regarding how they interact with the different members of the opioid receptor family. We describe examples of different modes of subcellular neuronal distributions of the peptide precursors and putative signaling peptides, and how this relates to potential release sites and receptor target localization. We then describe what these distributions might mean for how we conceptualize the spatiotemporal dynamics of opioid neuromodulation in the brain and thus highlight some remaining fundamental questions about the properties and functions of the endogenous opioid peptide system. Finally, to illustrate the clinical significance of understanding and targeting the endogenous opioid system, we describe how naltrexone’s (NTX) interference with endogenous opioid peptide signaling treats AUD. We review NTX’s clinical efficacy and some of the subsequent research aimed at learning more about the underlying biological phenomena.
2. Basics on the peptides
In 1975, Hughes and colleagues first identified endogenously produced peptides that activate opioid receptors, the pentapeptides met-enkephalin (Tyr-Gly-Gly-Phe-Met; YGGFM; MENK) and leu-enkephalin (Tyr-Gly-Gly-Phe-Leu; YGGFL; LENK; Hughes et al., 1975). Subsequent studies identified at least 28 additional endogenous peptides that activate the opioid receptors; many of these are longer peptide fragments that include the Tyr-Gly-Gly-Phe motif. Early pharmacology studies proposed three opioid receptors: “mu” for its response to morphine (MOR), “kappa” for its response to ketazocine (KOR), and “delta” for the physiological response of the mouse vas deferens assay (DOR; Lord et al., 1977; Martin et al., 1976). Agonist effects at these three receptors were all blocked by NTX, thus they were considered the family of opioid receptors (Takemori and Portoghese, 1984). Later gene sequencing studies identified a fourth receptor, opioid-like receptor 1/nociceptin receptor with approximately 60% amino acid sequence homology with the other 3 receptors (Mollereau et al., 1994, reviewed in Toll et al., 2016). Gene studies led to the identification of most of the precursors for the active endogenous opioid peptides even before the receptors were cloned, except for nociceptin (Cox, 1982; Meunier et al., 1995; Reinscheid et al., 1995). Here we provide a very brief overview of the known sources of the identified endogenous opioid peptides from a precursor perspective. For a detailed description of the biochemistry of how proenkephalin (PENK), prodynorphin (PDYN), and proopiomelanocortin (POMC) are cleaved and which enzymes are involved in their processing please see (Fricker et al., 2020).
For most of the identified endogenous ligands, the differences in their binding affinities to MOR, DOR, and KOR are not remarkably different (Fig. 1; Gomes et al., 2020; Kosterlitz, 1987). For signaling there is an added layer of complexity. Several recent studies demonstrate that although different peptides carry an identical opioid consensus sequence of Tyr-Gly-Gly-Phe (the ‘address’ terminus that determines binding), the additional amino acids on the non-address terminus (the ‘message’) determine the preferred signaling pathway of the peptide-receptor complex consistent with the signaling bias model (Gomes et al., 2020; Portoghese, 1989; Schwyzer, 1977; Thompson et al., 2015, 2016). Signaling bias may take the form of preference for a specific signaling pathway such as G protein versus β-arrestin dependent signaling (Fig. 1) or preference for trafficking of receptors for recycling or degradation. Thus, the identity of the released peptide is critical to understanding the downstream effects of opioid neurotransmission, beyond simply which receptor it is binding. For additional information on the translational potential of selective targeting of the different members of the opioid receptor family we recommend (Dalefield et al., 2022; Khan et al., 2022; Parker et al., 2020; Pradhan et al., 2011; Witkin et al., 2014). It is also important to note that non-opioid receptor activities have been identified for some opioid peptides as well (reviewed in Kaczyńska and Wojciechowski, 2021; Palmer et al., 2021). Next we describe the form and function for the five major classes of endogenous opioid peptides, focusing on knowledge gaps and recent developments.
Fig. 1.
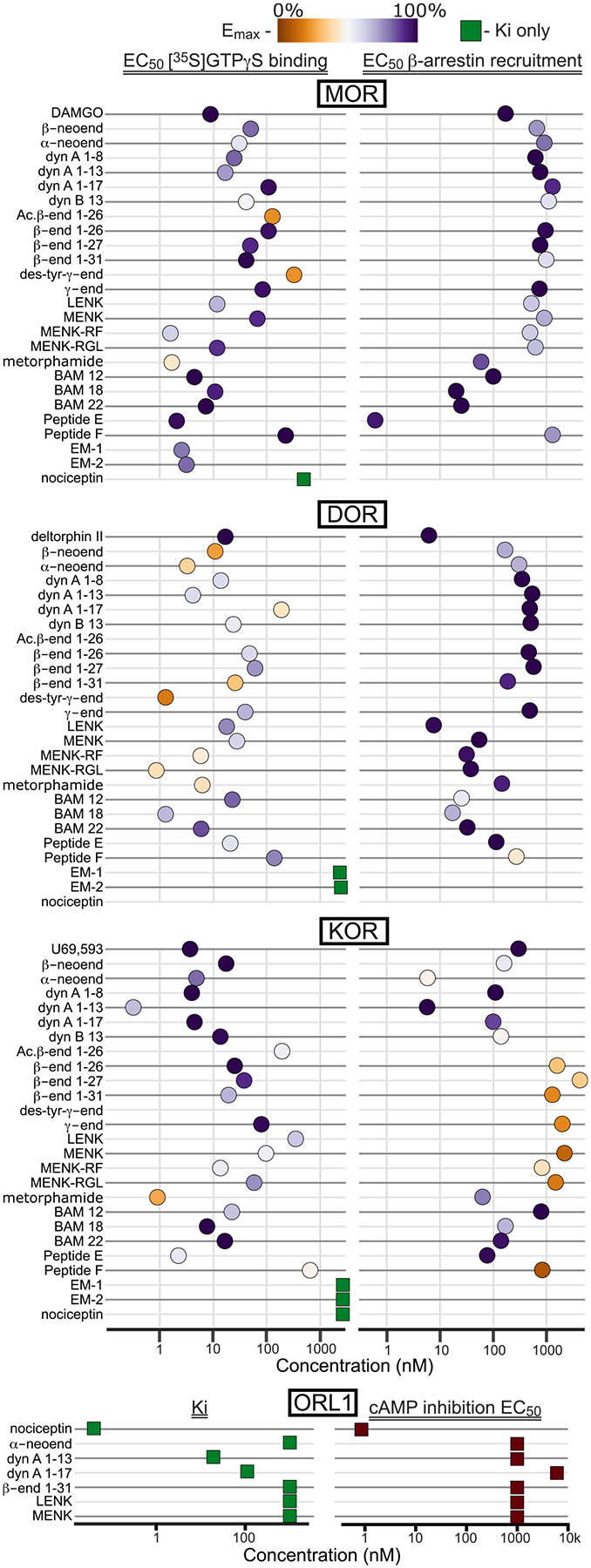
Binding and activity of endogenous opioid peptides at MOR, DOR, KOR, and ORL1. For MOR, DOR, and KOR, x-axis position is the EC50 concentration for G protein signaling (left) or β-arrestin signaling (right). Color indicates efficacy at 10 μM concentration compared to full agonists DAMGO (at MOR), deltorphin II (at DOR), or U69,593 (at KOR). Where signaling data are not available, Ki is graphed. For ORL1, only Ki and EC50 of cAMP inhibition were available. Data reported in (Butour et al., 1997; Gomes et al., 2020; Judd et al., 2003; Lapalu et al., 1998; Liu et al., 2017; Reinscheid et al., 1996; Sasaki et al., 2006; Wang et al., 2012).
2.1. Proenkephalin
Although it is often thought that the signaling response generated from PENK peptides occurs through MOR activation, for some of these peptides their highest affinities are at other opioid receptors. The PENK peptide encodes 6 copies of MENK, 4 of which are predicted to be cleaved, and 1 copy of LENK (Fig. 2; Yoshikawa et al., 1984). Longer peptides that contain the enkephalin sequence can be cleaved from PENK and also signal at opioid receptors, including MENK-arg-phe (MENK-RF/heptapeptide), MENK-arg-gly-leu (MENK-RGL/octapeptide), BAM-18, metorphamide, amidorphin, peptide E, and peptide F (Fig. 2; Fricker et al., 2020). It remains unknown whether MENK and LENK are the primary signaling molecules generated from PENK or if the longer peptide fragments also participate in neurotransmission.
Fig. 2.
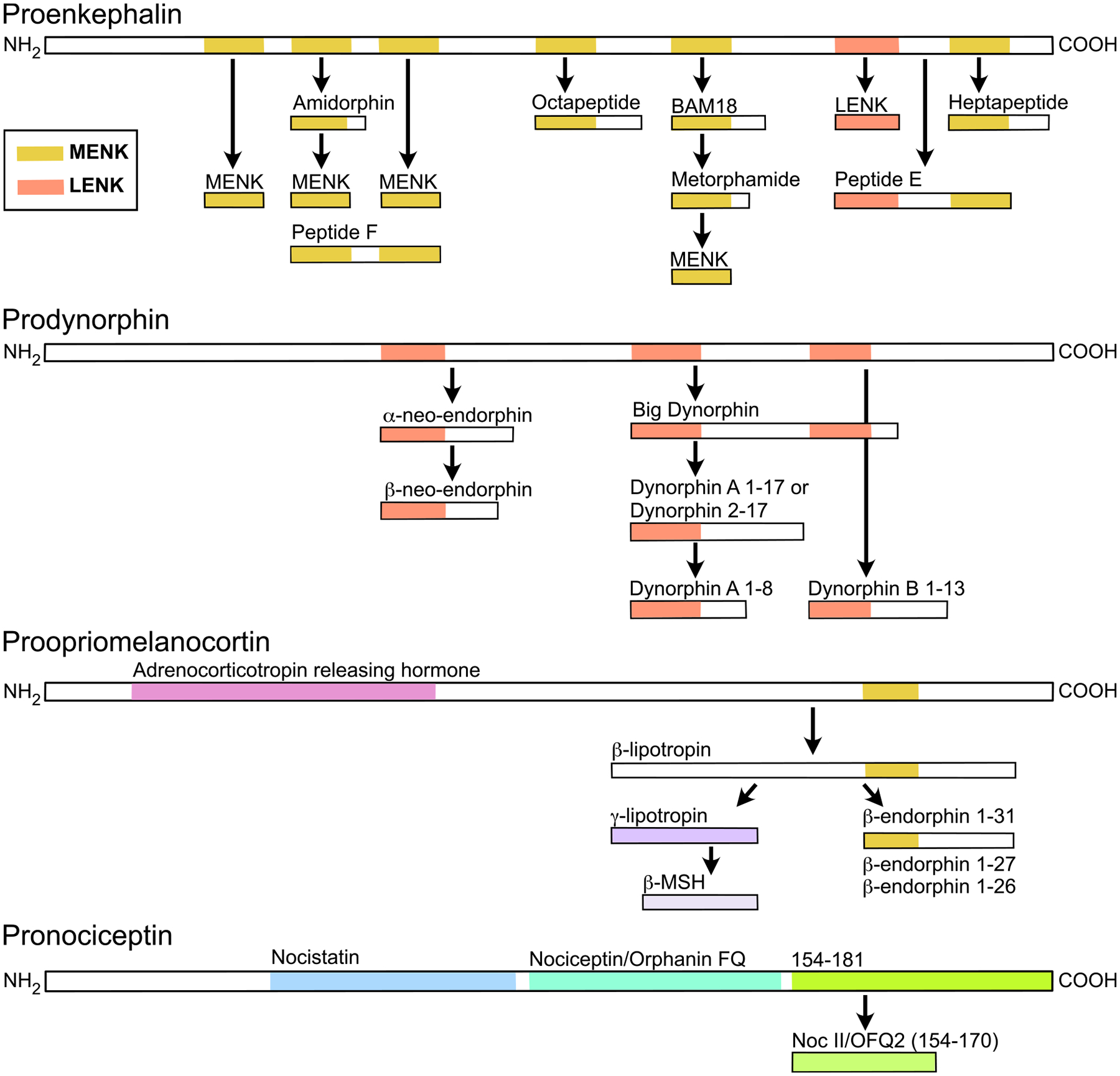
Peptide precursors and products of proenkephalin, prodynorphin, proopiomelanocortin, and pronociceptin. Organization of PENK is based on mouse and bovine studies (Fricker et al., 2020; Johanning et al., 1998; Jones et al., 1980). PDYN organization is based on human (reviewed in Cahill et al., 2021). POMC is based on mouse studies (reviewed in Fricker et al., 2020; Millington, 2007). PNOC is based on rat studies (Rossi et al., 2002). Illustration not to scale.
2.2. Prodynorphin
PDYN is the source of dynorphin peptides, which are broadly thought to underlie aversive signals through activation of the KOR. This precursor was first described as Proenkephalin B, due to its similarity to PENK (Chavkin et al., 1982; James et al., 1982). Peptides generated from PDYN include dynorphin 1–8, dynorphin-A 1–17, dynorphin-B 1–13, dynorphin 2–17, big dynorphin, and α- and β-neo-endorphin (Fig. 2). In addition to activating KORs, dynorphins do also appear to have relatively high affinity for MOR and DOR (Fig. 1; Gomes et al., 2020). For instance, the affinities of dynorphin A 1–8, 1–17, and dynorphin B 1–13 are within an order of magnitude of MENK at MOR (Gomes et al., 2020). For β-neo-endorphin, binding affinity and potency at KOR and DOR are not distinguishable (Gomes et al., 2020; Mansour et al., 1995). On the other hand, in recent studies the in vivo impacts of selective stimulation of Pdyn neurons have been blocked by selective KOR antagonists (e.g., Abraham et al., 2021; Al-Hasani et al., 2015). Such selectivity may also be facilitated by neural structural and organizational features described below.
Interestingly, the address end of these PDYN peptides is the LENK sequence; Pdyn contains 3 copies of the LENK sequence plus the appropriate cleavage sites to generate the pentapeptide (Fig. 2). It remains to be determined if PENK, PDYN, or an unidentified precursor gives rise to LENK destined for signaling. Evidence supporting the possibility that LENK is a product of PENK alone includes early ex vivo release studies measuring MENK and LENK release from acute striatal brain slice preparations, which contain both Penk and Pdyn neurons. Here the peptides were detected at ratios of between 3:1 and 4:1, consistent with the predicted ratio of MENK to LENK synthesized from PENK alone (Henderson et al., 1978). Mouse knockout studies are also consistent with the majority of LENK arising from PENK (Gupta et al., 2021). On the other hand, studies supporting PDYN also being a source of LENK include observations that enzymes extracted from the striatum, hippocampus, and substantia nigra metabolize longer dynorphin peptides into LENK (Sandin et al., 1997). The approach used in this study, however, did not limit detection to peptides that are likely released as signaling molecules. Further, destruction of the ventral striatal neurons that project to the substantia nigra that express Pdyn and not Penk decreased LENK abundance in the nigra (Christensson-Nylander et al., 1986). Together, while evidence is mostly against PDYN being directly upstream of LENK release, it remains plausible that it may occur in specific brain regions or under certain conditions.
Clear functional differences have been observed between dynorphin A and B. Kunselman and colleagues (2021) found that in PC12 cells and cultured striatal neurons, while dynorphin B leads to KOR internalization and then recycling to the plasma membrane, dynorphin A causes internalization with little recovery of receptor to the plasma membrane. They also found that when KOR is activated by dynorphin A, signaling continues after internalization to a much greater extent than when it is activated by dynorphin B. Together these observations indicate a lasting change in receptor signaling and availability that depends upon which dynorphin peptide is released.
Another interesting phenomenon related to PDYN products is that sometimes the cleavage between dynorphin A (1–17) and dynorphin B fails, leaving a much longer functional peptide, big dynorphin (Fig. 2; Merg et al., 2006). Rather than acting through opioid receptors, it appears that big dynorphin acts through glutamatergic NMDA receptors or acid-sensing ion channels type 1a (ASIC1a): using ex vivo electrophysiology in acutely dissociated trigeminal neurons, big dynorphin inhibited NMDA receptor mediated currents, and these effects were not blocked by the non-selective opioid receptor antagonist naloxone or the selective KOR antagonist norBNI (Chen et al., 1995a, 1995b). With in vivo pharmacology experiments, Kuzmin and colleagues (2006) found that intracerebroventricular injection of big dynorphin increased locomotion and anxiolysis; these effects were not blocked by norBNI, but were blocked by the NMDA receptor antagonist MK-801. They also showed that while dynorphin A and B increased the latency to escape in a hot plate test, big dynorphin did not, strengthening the possibility that there are separate signaling impacts of these peptides (Kuzmin et al., 2006; Tan-No et al., 2002). In cortical neurons, big dynorphin limits the steady-state desensitization of ASIC1a and acid-activated currents, and this effect is blocked by a peptide that binds directly to ASIC1a (Sherwood and Askwith, 2009). More recently it was found that big dynorphin binding to ASIC1a results in a closed resting conformation (Borg et al., 2020). This effect at ASIC1a appears to be mediated by electrostatic interactions of the basic amino acids at the N terminus of big dynorphin, binding in an acidic pocket of the extracellular domain (Borg et al., 2020). The biological conditions that preferentially generate big dynorphin over dynorphin A and B are not yet known.
Another non-opioid receptor mediated effect has also been reported for shorter PDYN fragments. Dynorphin A (1–13) and 2–13 work as antagonists at melanocortin receptors 1, 3, and 4 in the 10s of nM concentration range. However, dynorphin B, nociceptin, β-endorphin, LENK, and MENK did not show this activity (Quillan and Sadée, 1997). An in vivo functionality of this interaction has not been identified, but it is provocative in light of the fact that α-melanocyte stimulating hormone (α-MSH), which activates the melanocortin receptors, is one of the peptides encoded by another endogenous opioid peptide precursor, POMC.
2.3. Proopiomelanocortin
POMC encodes several opioid and non-opioid peptides, and is the only known precursor for β-endorphin (reviewed in detail in Cawley et al., 2016). It was first isolated from sheep pituitary extract in 1964 (Birk and Li, 1964). POMC is initially cleaved into two peptides, adrenocorticotropic hormone which gives rise to α-MSH, corticotropin-like intermediate lobe peptide, and, at the C-terminal end, β-lipotropin (Fig. 2). β-lipotropin can then be processed to generate the full-length β-endorphin 1–31. β-lipotropin can also produce two other signaling products, γ-lipotropin and β-MSH (Fig. 2). It is not known whether these are necessarily produced during processing to β-endorphin. There are slight differences in the fragments produced in humans compared to rodents (Fricker et al., 2020). While β-endorphin is often thought of as a MOR-favoring peptide, it binds and activates DOR at similar concentrations and KOR with just slightly lower potency (Fig. 1). While β-endorphin 1–31 is often considered to be the signaling peptide, there is evidence that shorter peptides 1–27 and 1–26 may be produced as well. These shorter peptides have recently been shown to have full agonist properties, including having higher efficacy than β-endorphin 1–31 in some signaling assays (Fig. 1; Gomes et al., 2020). As a further consideration, these peptides have been detected both with and without an N-terminal acetyl group, just one of the possible post-translational modifications that are catalyzed at these peptides. This modification prevents binding to opioid receptors (Akil et al., 1984). Interestingly, though MENK is encoded at the address end of the β-endorphins, current understanding is that the pentapeptide is not generated from this precursor.
2.4. Pronociceptin
PNOC encodes nociceptin/orphanin-FQ, the endogenous ligand for the opioid-like receptor 1/nociceptin receptor (Houtani et al., 1996; Wang et al., 1996). Interestingly, although there are some sequence similarities between PDYN and PNOC (Houtani et al., 1996), the peptides derived from PNOC have minimal affinity for the other opioid receptors (Fig. 1; Zhang et al., 1997). The propeptide is processed to form 3 primary signaling peptides (Fig. 2): nociceptin/orphanin-FQ, nocistatin (Okuda-Ashitaka et al., 1998), whose target receptor(s) is unclear (Avenali et al., 2016; Kuspiel et al., 2021; Osmakov et al., 2019), and NocII/NocIII that produce behavioral effects when administered intracerebroventricularly (Florin et al., 1997, 1999) but whose effector (s) remains unknown. Although in vivo experiments reported that nociceptin and nocistatin might work in opposition (Okuda-Ashitaka et al., 1998), in whole cell electrophysiology in locus coeruleus (LC) neurons nocistatin application did not activate a conductance and it also did not block responses to nociceptin (Connor et al., 1999).
2.5. Endomorphins
The tetrapeptides endomorphin-1 (Tyr-Pro-Trp-Phe-NH2, YPWF, EM-1) and endomorphin-2 (Tyr-Pro-Phe-Phe-NH2, YPFF, EM-2) are unique among the endogenous opioid peptides that bind to the MOR in that they are quite selective for this receptor (Fig. 1; McConalogue et al., 1999; Zadina et al., 1997), however their synthesis pathway remains unclear and their classification as “endogenous” has been in question over the years. The EMs were originally detected in brain and spinal cord principally with radioimmunoassays (RIAs) using antibodies raised against synthetic peptide antisera (Narita et al., 1998; Xie et al., 2008; Zadina et al., 1997). It is important to note that RIA detection of small peptides such as EM-1 and EM-2 can lack selectivity, and without EM knockout tissue to validate antibody selectivity some questions have remained in the field. Alternatively, the chemical identity of detected peptides can be definitively characterized through mass spectrometry or Edman degradation, techniques to sequence short peptides. Accurate detection methods are particularly critical for EMs because conventional precursor mRNA sources for the EMs have not been found. Three bioinformatic publications identify potential endogenous sources for EM-1 and EM-2, which we describe in detail.
First, Jessop and colleagues (Jessop et al., 2000), searched uniprot 21 for YPWFG as Gly is required for C-terminal amidation (Eipper et al., 1992), however this only gave hits in honeybee, dogfish, or influenza virus, while YPFFG only gave two hits in plants.
Second, Terskiy et al. (2007) expanded the search to known and predicted human sequences in NCBI RefSeq (9/2006), SwissProt (10/2006), and TrEMBL (10/2006) for EM-1 and EM-2 sequences with and without the terminal Gly and with and without a terminal basic residue, further extending the recognition motif for C-terminal amidation. They similarly found hits for sequences containing C-terminal Gly motifs, SUT-1 sodium/sulfate symporter (SLC13A4), and coiled-coil domain containing 100 (CCDC8). The expression pattern for these, however, do not match EM-1 or EM-2 RAI staining, as SLC13A4 is predominantly expressed in placenta and testis with intermediate levels in brain and lower levels in heart, thymus, and liver (Girard et al., 1999), and CCDC8 is widely expressed with low levels in spleen, skeletal muscle, small intestine, kidney, and liver (Hanson et al., 2011). They also found hits without any C-terminal amidation motif, which are likely not biologically relevant. For EM-1, the 12 hits included SH2 containing proteins, olfactory receptor 51V1, trace amine-associated GPCRs 1–5 and Gαqs, which are effectors of MOR among other GPCRs (Barreto et al., 2021; Minami et al., 2010; Psifogeorgou et al., 2011). For EM-2, the 20 the hits were more diverse, including Galacto-cerebrosidase, Ataxin 1, choline kinase, Insulin-like growth factor receptor 1 precursor, and secreted frizzled-related protein 5. Hanson et al. (2011) showed by size exclusion that EM-2 immunostaining can stain multiple fractions of rat brains ranging in size from 25 to 117, suggesting they may stain one or more of these proteins (Terskiy et al., 2007).
While most opioid ligands are cleaved, it is possible that the YPWF and YPFF sequences are not. Longer proteins with these sequences might bind and exert effects at MOR, either in an attached loop, attached at a single end, or free at both ends without the C-terminal amidation. Building on Schwyzer’s message-address concept (Schwyzer, 1977) for opioid receptors (Portoghese, 1989), and extensive structure activity relationship development and structural modeling (Pasternak and Pan, 2013; Yu et al., 2007), it is widely assumed that the N-terminal tyrosine buries deep into the GPCR extracellular facing active site, with the C-terminus pointing outwards. Thus it is unlikely that EM-1 could be recognized with N-terminal extensions. Since the C-terminus is pointing outwards, it is possible that it is attached to a longer polypeptide chain, as it is for example in endorphins. If there is no C-terminal extension, then C-terminal amidation has long been recognized as important for MOR selectivity (Rónai et al., 1979) and indeed, EM-1OH and EM-2OH are reported to be either 2–4x less potent (Al-Khrasani et al., 2001) or 200–300x less potent (In et al., 2005) than EM-1 or EM-2.
Third, Matsushima, Sese, and Koyanagi (2019) further expanded the bioinformatic EM search to the mammalian expressed sequence tag database (Boguski et al., 1993), containing reads from shotgun mRNA sequencing deposited into NCBI prior to assembling into transcriptome profiles. They obtained one hit RYPWFGR in AI352151.1, however the position in human chromosome 8 differs RYPGFGR, and they confirmed this substitution with cDNA cloning. While Matsushima and colleagues showed that YPGF-NH2 has no appreciable activity at MOR, they noticed that the codon for G (GGG) only differs from that of W (TGG) by a single G->T base substitution and that the relatively common oxidation of guanine at position 8 (8-oxoguanine) may cause G->T substitution (Kanvah et al., 2010). Further, triple oxidation GGG -> TTT would generate the F needed for EM-2. It is unclear whether the required high levels of oxidative stress would occur in vivo. Acute hard exercise or chronic Fibromyalgia generate oxidative stress and have been associated with pain and reward, potentially due to EM release (Cordero, 2011; Hendrix et al., 2020). However, highly oxidative conditions may be particularly exaggerated in certain ex vivo tissue preparations (Ravanat et al., 2002). Using BLASTn to search for the cDNA nucleotide sequence among human transcripts, Matushima and colleagues found a strong match that spans adjacent DDHD domain-containing protein 2 (DDHD2) and Pan troglodytes nuclear receptor binding SET domain protein 3 (NSD3) predicted genes (2019).
To complement the characterization of potential source(s) of EM-1 and EM-2, a crucial dimension of its endogenous regulation is understanding how it may be degraded. EM-1 and EM-2 are substrates for the broadly expressed serine proteinase dipeptidyl peptidase IV (DPP4), which removes xP- from amino terminal peptides (Sakurada et al., 2003; Shane et al., 1999). DPP4 is well studied for its degrading glucagon-like peptide 1 (GLP-1) and the treatment of type-2 diabetes (Demuth et al., 2005), as well as other signaling peptides including substance P (Mentlein, 1999), and there are several FDA approved drugs targeting DPP4. Modulation through DPP4 is an interesting strategy to probe EM function as a potential therapeutic strategy to treat pain. For example, Shane et al. (1999) showed that Ala-Pyrrolidonyl-2-nitrile (ala-Pyrr-2-CN) inhibits DPP IV competitively with a Ki of 0.2 μM, modulated EM-2 effects on the tail-flick pain assay. A range of experiments using DPP4 inhibitors and exogenous EM-2 in animal models suggest a link. For example, by using a DPP4 knockout mouse model, Komiya et al. (2022) showed that while DPP4 inhibition of substance P metabolism increases psoriatic itch, EMs drove mechanical allokinesis through expression in the squamous and basal layer keratinocytes of the epidermis. Beyond DPP4, the less studied DPP3 has also been shown to metabolize EMs at similar enzymatic efficiency (Baršun et al., 2007), though the in vivo function is less well characterized (Malovan et al., 2022).
While definitive characterization of EM-1 and EM-2 production and degradation remains to be clarified, these studies together constrain the hypothesis space. Given the high-potency and selectivity of EM-1 and EM-2 for MOR, the robust detection of EM-1 and EM-2 with RIA, and modulation of MOR signaling by DPP4 suggests they are biologically produced and form an important dimension of endogenous MOR signaling. Therefore, a deeper understanding potential regulation of these processes may open up new therapeutic strategies and liabilities for treating pain and substance abuse disorder.
3. Endogenous opioid peptides are expressed in specific neural circuits
3.1. Introduction to peptide distribution studies
Opioid peptide and receptor distributions in the central nervous system (CNS) have attracted intense research efforts to identify the neural circuits that potentially contribute to both their beneficial and deleterious effects. Of note, opioid receptors are expressed in several non-neuronal organs including heart and immune cells as well. The highest concentrations of endogenous opioid peptides are found in the adrenal gland, where they are synthesized by adrenal medullary chromaffin cells that release the opioid peptides into the adrenal vein for systemic circulation (Livett et al., 1981; Viveros et al., 1979). In brain, early on the highest concentration of MENK was found in the globus pallidus (Kobayashi et al., 1978). Intense ENK immunoreactivity was detected in the periaqueductal gray (PAG), a key brain region associated with pain transmission and opioid analgesia (Moss et al., 1983; Moss and Basbaum, 1983). In many brain regions ENK and dynorphin expression patterns have been found to be complementary, most easily discerned through mRNA detection (See Section 3.4).
Much research has also focused on how levels of opioid precursor mRNA or peptide content in different brain regions change following a wide variety of in vivo experiences. The functional implications for peptide release of changes in tissue peptide content or mRNA levels, however, remain mostly unknown, as changes in mRNA levels do not necessarily correlate with changes in peptide expression levels (e.g., Greenbaum et al., 2003; Guo et al., 2008; Haque et al., 2017; Tang et al., 2009). Extracellular measurements to detect exocytosed peptide are critical for the field’s understanding, and more temporally precise methods are required to align observed peptide release with specific behavioral events (Abraham et al., 2021; Calhoun et al., 2018; Conway et al., 2022; DiFeliceantonio et al., 2012; Girven et al., 2022; Mabrouk et al., 2011).
More recently immunocytochemical labeling has revealed distinct expression patterns for EM-1 and EM-2. EM-1 is preferentially detected in brain in the parabrachial nucleus, the nucleus of the solitary tract (NTS), septum, diagonal band, bed nucleus of the stria terminalis (BNST), hypothalamus, PAG, nucleus accumbens (NAc), LC, and amygdala (summarized in Fichna et al., 2007; Martin-Schild et al., 1999, 1997). In contrast, EM-2 is preferentially expressed in the spinal cord. Importantly, chemically stimulated release of EM-2 was detected in the spinal cord using HPLC and electrochemical detection in samples collected with push-pull perfusion (Lisi and Sluka, 2006).
3.2. Regions of endogenous opioid peptide mRNA expression
Mappings of brain regions with high expression levels of endogenous opioid peptide precursor genes have been reviewed elsewhere, including patterns of changes in expression levels following different in vivo treatments (Merrer et al., 2009). Briefly, high expression levels of endogenous opioid peptide mRNA is detected in many neurons in the hypothalamus, striatum, pallidum, amygdala, extended amygdala, PAG, spinal cord, and dorsal root ganglia, especially for Penk and Pdyn (e.g., Fallon and Leslie, 1986; Manzanares et al., 1998; Neal and Newman, 1989; Olsen et al., 2008; Sapio et al., 2020; Schlussman et al., 2011; Solecki et al., 2009; Sukhov et al., 1995). The main populations of POMC expressing neurons reside in the arcuate nucleus of the hypothalamus and the commissural NTS. POMC is also expressed in cells of the anterior pituitary (Bloch et al., 1979; Bloom et al., 1978; Jacobowitz and O’Donohue, 1978; Sofroniew, 1979). Recently, mice have been engineered to express Cre recombinase under the peptide precursor genes, which enable more precise identification of the neurons that express the opioid peptide precursor mRNA and facilitate mapping of their neural circuits (e.g., Viden et al., 2022).
PNOC is expressed widely throughout the brain, overlapping with many regions that express other endogenous opioid peptides. Mice that express Cre recombinase under the Pnoc promoter, enabling identification and manipulation of these neurons, have been used to investigate the distribution of Pnoc expressing neurons (Hardaway et al., 2019). In these animals Pnoc expressing neurons have been studied in the central nucleus of the amygdala (CEA), BNST (Rodriguez-Romaguera et al., 2020), and in a subregion of the ventral tegmental area (VTA) adjacent to the interpeduncular nucleus (Parker et al., 2019).
The peptide distribution observations made using in situ hybridization of mRNA identify regions with relatively high expression. While these regions are a logical focus, it is also possible that important populations of neurons that express endogenous opioid peptides fall below detection limits or analysis thresholds in these studies. Further, neuropeptides are generally released from axon terminals, often projections to distant brain regions, therefore techniques that identify somata or nuclei that express mRNA for precursors do not reveal where the opioid peptides are released (see Section 4 below). That said, exciting advances in mRNA detection have enabled new, detailed studies of expression patterns and how they change with behavioral state, contributing to our understanding of how neural circuits change with in vivo experiences.
3.3. New possibilities with single cell and low abundance mRNA detection: case study in the VTA
Genetic sequencing technology has advanced significantly such that high-resolution, high-throughput detection is now possible, including identifying low abundance signals at the single cell level (Svensson et al., 2017). With appropriate controls, there is no practical limit to how many transcripts can be detected in each cell via RNAseq, providing a different, and potentially very exciting, kind of information compared to techniques that require higher abundance of target material for detection. By isolating a brain region, one can then determine the transcripted mRNAs from each isolated cell including neurons, glia, and epithelial cells. These techniques require careful gross dissection and isolation of the desired brain region. Some structures naturally lend themselves to accurate isolation, but others do not. This can make it challenging when attempting to interpret low-expression mRNAs or low-abundance clusters, as they may either represent small populations in the target brain region or contamination from neighboring brain regions. For higher abundance mRNAs or proteins, distributions can be subsequently confirmed with RNAscope or immunofluorescence. Spatial transcriptomics quality is also rapidly improving to provide topographical organization information (Beauchamp et al., 2022). When used appropriately, these techniques can be incredibly powerful, both for confirming earlier observations and for detecting previously unknown expression patterns, thereby enabling the generation of novel hypotheses.
For example, unbiased populations of VTA cells from male and female rats were recently profiled with single nucleus RNA sequencing by Phillips and colleagues (2022). In their analysis of the dataset they focused on the expression of opioid peptide related genes. With respect to the conventional neurotransmitters associated with VTA neurons, dopamine, glutamate, and GABA, the combinatorial expression patterns that were previously reported (reviewed in Morales and Margolis, 2017; Barker et al., 2016) were also detected in this study. That is, there is anatomical, physiological, immunocytochemical, and conventional in situ hybridization evidence for VTA neurons that have the capacity to synthesize and signal through more than one neurotransmitter including dopamine/glutamate, glutamate/GABA, and dopamine/glutamate/GABA cell populations in the VTA. These different types of VTA neurons participate in specific neural circuits (Morales and Margolis, 2017). One feature of this RNAseq dataset is that it expands the profiling of the genes that contribute to the synthesis, transport, and degradation of these transmitters, all of which provide additional evidence that neurons can in fact synthesize and release each neurotransmitter. For example, they examine several genes involved in the synthesis of tetrahydrobiopterin, which is essential in the first step of dopamine synthesis, generating L-DOPA from tyrosine (Phillips et al., 2022). With respect to genes underlying endogenous opioid peptide production and the opioid receptors, their clustering shows that Penk is relatively highly expressed in the VTA glutamate-only neuron subcluster. Penk expression was also detected in small subpopulations of glutamate/GABA neurons, and a subcluster of GABA only neurons. Message for Pomc or Pdyn was detected just sparsely in VTA neurons, consistent with prior studies. Pnoc was detected among several cluster phenotypes of GABA only neurons and one cluster of glutamate/GABA neurons, consistent with transgenic mouse results (Parker et al., 2019). It is possible that these patterns relate to the projection targets of the VTA neurons, as has been reported for clusters of gene expression detected when dopamine neurons have been studied in isolation (Poulin et al., 2018). Evaluating the expression of the enzymes required for synthesis of the signaling peptides from the propeptides will help determine whether these newly resolved populations of VTA neurons can indeed generate the endogenous opioid peptides. All four opioid receptors were detected in this VTA study, with Oprm1 being most common and detected in many neurophenotypic clusters, followed by Oprd1, with much lower detection frequencies of Oprk1 and Oprl1. These findings are in contrast to strong detection of Oprk1 expression in dopamine neurons using RNAscope (Liu et al., 2019) and KOR activity using physiological techniques (Ford et al., 2007; Margolis et al., 2003, reviewed in Margolis and Karkhanis, 2019), as well as the physiological responses to nociceptin reported in both dopamine and non-dopamine VTA neurons (Driscoll et al., 2020; Zheng et al., 2002). Together, this kind of analysis of endogenous opioid peptide precursor message as well as that for their receptors can provide new perspectives on expression patterns, but need to be interpreted in the context provided by other techniques.
3.4. Patterns of opioid peptide mRNAs in specific neural circuits
Importantly, peptide-releasing neurons participate in specific neural circuits. In several brain regions it is established that the particular endogenous opioid peptides synthesized by a subpopulation of neurons has a strong correlation with the projection target of that subpopulation. One clear example of this is the organization of the Penk- and Pdyn-expressing neurons in the dorsal striatum and NAc (ventral striatum). Here, projection neurons are medium spiny (MSN) in morphology, release GABA as a fast neurotransmitter, and express several neuropeptides. The “indirect” pathway neurons of the dorsal/ventral striatum send axons to the globus pallidus external/ventral pallidum but not the substantia nigra/VTA; these neurons express Penk and dopamine D2 receptors (Drd2; Gerfen et al., 1990). The “direct” pathway neurons send axons to the substantia nigra/VTA and express Pdyn and protachykinin (precursor to substance P; Anderson and Reiner, 1990; Gerfen and Young, 1988), as well as dopamine D1 receptors (Drd1; Gerfen et al., 1990; Vincent et al., 1982). In the NAc, Drd1 MSNs also project to the ventral pallidum, thus do not exclusively project “directly” to the VTA (Kupchik et al., 2015). For a detailed review of the organization of the NAc with a particular focus on neuropeptides we recommend (Castro and Bruchas, 2019).
In other subregions of the extended amygdala there is also clear organization of Penk and Pdyn neurons. For instance, there is a substantial population of Penk neurons that are glutamatergic (Poulin et al., 2008). In the CEA, PDYN is expressed in a subset of neurons, many of which also express corticotropin releasing factor (Marchant et al., 2007). Indirect evidence is consistent with PENK being expressed in a non-overlapping subpopulation of CEA neurons (Marchant et al., 2007). CEA Penk neurons innervate the basolateral amygdala (BLA), BNST, substantia innominata, ventral pallidum, many regions in the hypothalamus, pedunculopontine tegmental nucleus, retrorubral field, substantia nigra, VTA, NTS, and trigeminal nucleus (Viden et al., 2022). In the BNST, Pdyn is expressed specifically in the fusiform, oval and anterior lateral nuclei (Marchant et al., 2007; Poulin et al., 2009). Unlike in the CEA, corticotropin releasing factor is not expressed in Pdyn neurons in the BNST, thus coexpression of different peptides varies across brain regions (Marchant et al., 2007). Penk expression is more widespread in the BNST, but low or no expression was detected in the fusiform and rhomboid nuclei (Poulin et al., 2009). In the anterior BNST, all Penk neurons were GABAergic (Poulin et al., 2009), but in the posterior BNST a small percentage of Penk neurons were found to be glutamatergic, not GABAergic (Poulin et al., 2009). The CEA and BNST share reciprocal Penk connections (Viden et al., 2022). Another example of a brain region with separate PENK and PDYN cells is the NTS (Lee and Basbaum, 1984). Thus, even though Penk and Pdyn are often expressed in the same brain region, they are consistently expressed in different neural subpopulations.
Arcuate nucleus Pomc neurons project widely, including targeting the BNST, lateral septal nucleus, NAc, PAG, supraoptic nucleus, VTA, dorsal medial hypothalamus (DMH), paraventricular nucleus of the hypothalamus (PVH), lateral hypothalamus, periventricular nucleus, amygdala, dorsal vagal complex, and NTS (King and Hentges, 2011; Metz et al., 2021, reviewed in Cone, 2005). Recent work demonstrates that there are two populations of Pomc neurons in the arcuate nucleus, distinguished by their expression of either the leptin receptor or the glucagon-like peptide 1 receptor (Biglari et al., 2021). Both of these populations contribute to major Pomc projections including to the BNST, DMH, PAG, PVH, and NTS (Biglari et al., 2021). In spite of this, for the most part arcuate POMC axons do not collateralize to different brain regions (Metz et al., 2021). Pomc neurons in the NTS innervate the caudal mesencephalon and spinal cord. LC, parabrachial nucleus, rostral NTS, dorsal motor nucleus of the vagus and lateral reticular nucleus receive Pomc axons from both the arcuate and the NTS (reviewed in Cone, 2005). Together, these relatively small populations of Pomc neurons innervate a great deal of the CNS.
Studies like these that demonstrate patterns of expression and are very powerful for improving our understanding of the possible range of neurotransmitters and neuromodulators that may be used for communication between neurons in specific circuits. Yet they do not help us resolve where or when the specific neuromodulators are released. Neurons have highly specialized subcellular compartments and many molecules are trafficked to specific parts of the neuron, even to specific subsets of axon terminals or neurotransmitter release sites within a single axon (Scanziani et al., 1998; Silm et al., 2019; Zhang et al., 2015). Therefore it cannot be assumed, for instance, that a neuron that expresses Penk releases enkephalins at all of its synaptic sites or in local dendritic compartments.
4. Subcellular compartmental distribution of opioid peptides
It is generally thought that propeptides are processed into signaling neuropeptides in rough endoplasmic reticulum then transported through the Golgi apparatus to be packaged into the dense core vesicles from which the peptides are released to the extracellular space. Large dense core vesicles are 80–120 nm in diameter, compared to small clear vesicles that contain fast neurotransmitters and are on average 40 nm in diameter. Therefore putative peptide-containing vesicles can be visually identified by size with sufficient magnification. In ultrastructure studies, dense core vesicles containing endogenous opioid peptides have mostly been observed in axon varicosities and axon terminals, with much less localization to dendrites and rare localization in somata. These vesicles are usually present in low abundance in any of these compartments. Terminals are typically filled with tens to hundreds of small clear vesicles but often co-contain no more than 2–3 dense core vesicles, for example reported in dynorphin containing terminals (Pickel et al., 1993) and ENK containing terminals (Sesack and Pickel, 1992) in the VTA. The apparent scarcity of dense core vesicles is curious given that it is generally thought that neuropeptides signal through volume transmission (Fig. 3A), diffusing far from release sites. This would probably require multiple release events in order to achieve pharmacologically relevant extracellular concentrations over a large volume of tissue.
Fig. 3.
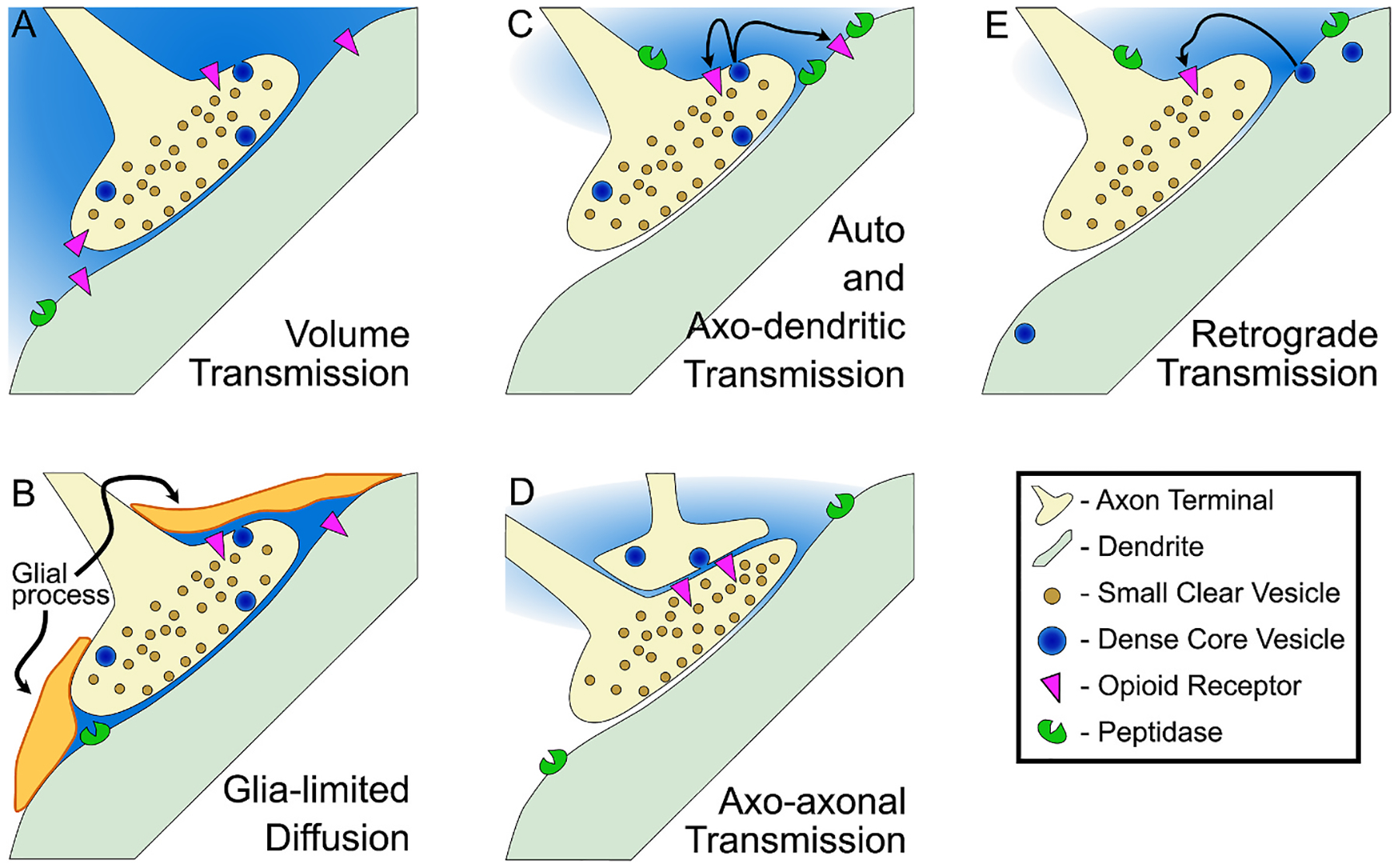
Arrangements of endogenous opioid peptide neurotransmission. (A) The features of the volume transmission model are that endogenous opioid peptides diffuse relatively freely from the release site, reaching opioid receptors that are 10s–100s of μm away from the release site, and acting on a slow time course, modulating neural activity on the scale of minutes or longer. Several biological mechanisms that oppose the volume transmission model have been observed. (B) In some brain regions glial processes seem to wrap axon terminals that have endogenous opioid-containing dense core vesicles, potentially limiting peptide diffusion. (C) Opioid peptide-containing vesicles have mostly been detected in axon terminals and varicosities, from which they may signal to opioid receptors on the same axon or on a nearby dendrite. (D) In some brain regions opioid-containing vesicles were found in axons that appose another axon terminal, and often these terminals express opioid receptors. (E) In a few cases endogenous opioid peptide-containing vesicles have been detected in dendrites, and it is postulated that the peptides released from these dendrites may signal retrogradely to opioid receptors on nearby axon terminals.
Many types of neurons express neuropeptides that are generally thought to have opposing physiological effects, such as lateral hypothalamic neurons that express both orexin (hypocretin) and PDYN (Chou et al., 2001), or striatal MSNs that express substance P and PDYN (Anderson and Reiner, 1990; Reiner, 1986). In a simple model where excitatory and inhibitory signals compete to control neural activity, such expression patterns are hard to interpret. One possibility is that the critical downstream intracellular signaling effects of the neuropeptides are as important, if not more important, than the modulation of contemporaneous action potential activity. Another is that the time course of the receptor responses differ. If something like these possibilities is the case, segregated vesicular loading would seem unnecessary (Fig. 4A). Sorting of peptides into different vesicles may also be unnecessary if neuromodulators signal through volume transmission (Fig. 3A; Agnati et al., 1995). Another possibility is that peptide transmission is more targeted, that the wiring diagram for neuropeptide control of neural circuits has higher spatial resolution than volume transmission (Fig. 3B–E). There is evidence that both of these arrangements are present in the brain.
Fig. 4.
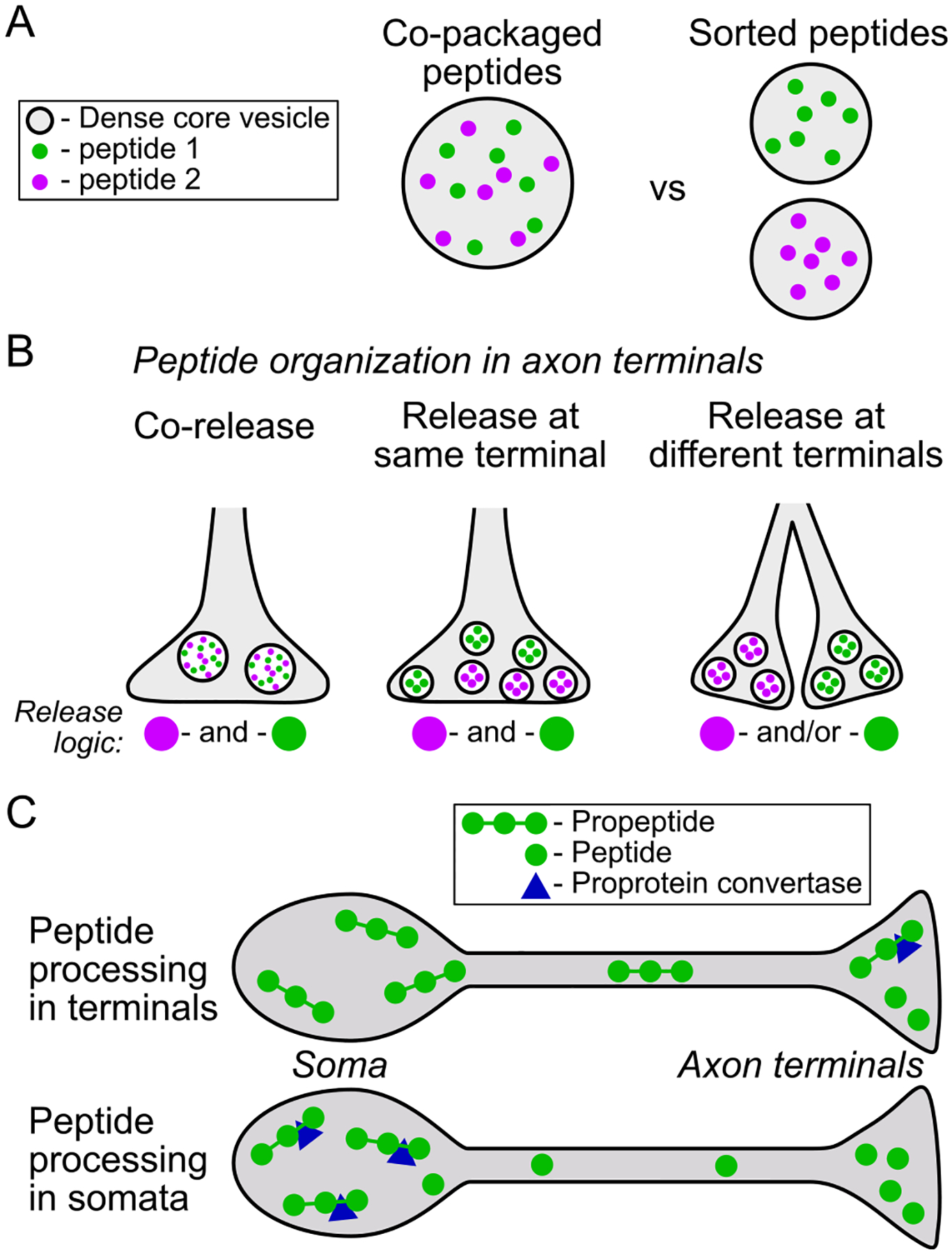
Neurons may sort peptides for co-release or independent release. (A) In different brain regions, neuromodulator peptides have been found to be packaged into the same vesicle resulting in simultaneous release or in separate vesicles potentially enabling independent release. (B) At the level of release sites, there is evidence that in some systems different signaling molecules, including different peptides, are segregated to different release sites. Various physiological phenomena may then independently control release events at the different synaptic boutons in the right hand case. (C, top) propeptides may be synthesized in the soma or axon terminals, and are mostly cleaved to shorter signaling molecules in axon terminals. This arrangement enables rapid changes in peptide production at the level of axon terminals. (C, bottom) in other neurons, propeptides appear to be delivered to axon terminals already processed and released without further modification.
There are several reports of detection of colocalization of different peptides in the same dense core vesicles (Fig. 4A and B). For opioid peptides, one example is in lateral hypothalamus neurons where the generally excitatory neuropeptide orexin is colocalized with dynorphin in dense core vesicles found in axon varicosities, axon terminals, and dendrites (Muschamp et al., 2014).
On the other hand, in neurons of the arcuate nucleus of the hypothalamus, neuropeptides generated from different precursor molecules are not co-packaged into the same dense core vesicles (Fig. 4A and B). In neurons that produce kisspeptin, neurokinin B, and dynorphin, neither kisspeptin nor neurokinin B was found to colocalize with dynorphin A in dense core vesicles in the somata or axons of these neurons (Murakawa et al., 2016).
The peptides synthesized in POMC neurons, including different peptides that are cleaved from POMC itself, may be aggregated at specific release sites (Fig. 4B). For instance, in the dorsal raphe nucleus (DRN) most axon terminals that are labeled for β-endorphin are in the dorsomedial DRN. These axon terminals tend to contain either a mix of dense core vesicles with small clear vesicles or mostly dense core vesicles, often synapsing onto dendritic spines of GABAergic profiles (Wang et al., 2001). However, in a separate study it was reported that axon terminals labeled for adrenocorticotropic hormone, also produced from POMC, contain an abundance of small clear vesicles with few dense core vesicles synapsing mostly onto dendritic shafts (Léger et al., 1997). While this is not direct evidence against colocalization, the differing arrangements are consistent with specific axon terminals releasing different peptides (Fig. 4). As proof-of-principle, separate packaging of the different peptides generated from the egg-laying hormone propeptide in Aplysia has been demonstrated to occur through sorting at the trans-Golgi (Fisher et al., 1988).
The extent of processing of propeptides prior to packaging into dense core vesicles and transporting of these vesicles to release sites may vary by neuron type. In some neurons there is evidence that propeptides are processed in axon terminals (Riesenberg and Nitsch, 1990), while in other neurons high concentrations of signaling peptides like MENK have been detected in somata (Fig. 4CB; e.g., Pickel et al., 1980). The processing enzymes prohormone convertases PC1 and PC2 have been detected at high levels in axon terminals throughout the brain (Winsky-Sommerer et al., 2000), suggesting that much propeptide processing occurs at terminals just prior to release. This has been investigated in detail in the VTA, where Pdyn mRNA has not been detected in somata. Therefore if present, both the longer and shorter peptides almost definitely reside in innervating axons. Using immuno-electron microscopy, both PDYN and dynorphin A were detected in axon terminals in the VTA (Yakovleva et al., 2006). Further, using western blotting, the concentration of PDYN was found to be much higher than dynorphin in VTA tissue samples (Yakovleva et al., 2006). C-terminal processing intermediates were also detected in the VTA (Yakovleva et al., 2006). This is consistent with much of the processing of PDYN into dynorphin A occurring in the axon terminals in the VTA, rather in the cell body region from which these axons originate such as the MSNs in the NAc (Yakovleva et al., 2006). Such an arrangement enables more rapid adaptation of peptide production and release than when peptide production is completed in somata and requires axonal transport to release sites.
Alternatively, there is evidence that EM-2 may be produced and packaged in the somatodendritic region of hypothalamic neurons before being trafficked axon terminals (Fig. 4C). By electron microscopy, EM-2 was detected in dense core vesicles in somatodendritic regions in the dorsomedial and ventromedial hypothalamic nuclei and the region near the third ventricle (Wang et al., 2003). EM-2 immunoreactivity was detected in granules in the Golgi apparatus, which likely give rise to EM-2-containing dense core vesicles (Wang et al., 2003). EM-2-labeled dense core vesicles were also detected in the axon terminals in these regions, therefore these dense core vesicles may be trafficked to these local terminals for release (Wang et al., 2003). The synaptic specializations where EM-2 dense core vesicles were detected contained mostly small clear vesicles and were predominantly asymmetric, consistent with excitatory glutamatergic type synapses (Wang et al., 2003). This difference in synthesis and packaging location may be related to the substantial differences in peptide generation for the endomorphins compared to the other endogenous opioid peptides.
5. Peptide release and volume of influence
In electron microscopy studies where synaptic appositions have been identified, most dense core vesicles are located somewhat distal from the synaptic specialization or active zone, the primary release site for small clear vesicles (e.g., Sesack and Pickel, 1992; Zhu et al., 1986). This observation has led to the proposal that dense core vesicles generally release their contents extrasynaptically (Fig. 3). One caveat to this idea is that in studies where samples were preserved with rapid freezing techniques indicate chemical fixation leads to some distortions of pre-synapse organization, including vesicle tethering and distributions (Bruckner et al., 2015). Fusion events of dense core vesicles can depend on some membrane proteins that are shared with small clear vesicles, such as the synaptosome-associated protein receptor (SNARE) proteins vesicle-associated membrane protein (VAMP), syntaxin, and synaptosome-associated protein [~25 kDa] (SNAP-25; Bruckner et al., 2015; Gormal and Meunier, 2022), but some are different such as calcium-dependent activator protein for secretion (CAPS; Berwin et al., 1998; Farina et al., 2015; Fujima et al., 2021; Kasai et al., 2012). Depending on the complement of fusion related proteins expressed on the plasma membrane of a population of dense core vesicles, different release sites for neuropeptides separate from the synaptic specialization may be enabled or required.
However, there is also some evidence that dense core vesicles do release their contents directly into synaptic specializations. For instance, dense core vesicle fusion directly in the presynapse at the neuromuscular junction has been detected (Jung et al., 2018). Where peptide release generally involves calcium dependent fusion machinery, in order to be exposed to adequate calcium concentrations to drive release the dense core vesicles likely need to be within tens of nm of calcium channels (Schneggenburger et al., 2012; Wang and Augustine, 2015). It is worth noting that calcium channels aggregate in the active zone, more so than in adjacent membrane, therefore dense core vesicles that require high concentrations of calcium for release may fuse in the active zone of the synaptic contact (Kaeser et al., 2011; Kusch et al., 2018).
A major question is what is the volume of tissue whose function is impacted by endogenous opioid peptide release from a given site. The dominant model is that neuromodulators, including peptides, act through volume transmission (Fig. 3A). However, some studies lend support to a model where neuropeptide diffusion, including of endogenous opioid peptides, is limited by several factors. For instance, it is not unusual for glial processes to be detected covering the extrasynaptic areas that are inferred to be opioid peptide release sites in ultrastructure studies, such as for MENK terminals in the LC (Bockstaele et al., 1995) and VTA (Sesack and Pickel, 1992). Similar astrocytic processes were reported in the LC near putative release sites for β-endorphin (Bockstaele et al., 1995; Reyes et al., 2006), in the VTA near putative release sites for LENK (Sesack and Pickel, 1992, 1995), and in the substantia nigra pars reticulata near putative release sites for dynorphin (Pickel et al., 1995). It is possible that the function of this astrocytic arrangement is parallel to the regulation they provide of fast neurotransmitter synaptic connections, akin to a peptide active zone, guiding or limiting the diffusion of the peptides towards structures that contain the relevant receptors (Fig. 3B).
Enzymatic breakdown of extracellular endogenous opioid peptides is also quite rapid and likely limits diffusion (Fig. 3C–E). Aminopeptidase B and endopeptidase-24.11 (metalloendopeptidase/neprilysin) are membrane bound enzymes (Larrinaga et al., 2011; Mumford et al., 1981). The localization of these peptidases to specific neural and cellular compartments is not broadly established. Distributions of two endopeptidases were studied pig substantia nigra, endopeptidase-24.11 and angiotensin converting enzyme (Barnes et al., 1992). Endopeptidase-24.11 was detected in a subset of dendrites, and in axons was localized specifically to boutons, with a tendency for labeling to be more intense at or adjacent to synaptic contacts. Although measures were indirect, this study further concluded that angiotensin converting enzyme was more likely to be expressed on postsynaptic membranes rather than on axons. Axons, dendrites, and glial processes contained endopeptidase-24.11 in the NTS (Barnes et al., 1993; Lasher et al., 1990). Bouton expression of endopeptidase-24.11 was observed in the globus pallidus, localized to axonal plasma membranes as well as synaptic boutons, but not dendrites or glial membranes with electron microscopy (Barnes et al., 1988b). Expression levels of these enzymes vary markedly across brain regions; for instance there is very low abundance of angiotensin converting enzyme in globus pallidus and olfactory tubercle (Barnes et al., 1988a). We discuss how blocking opioid peptide catabolism enables the detection of endogenous opioid peptide release effects ex vivo below (Section 7), consistent with a strong regulatory role of these enzymes to limit peptide diffusion.
6. Receptor proximity to peptide release sites
Terminals that contain endogenous opioid peptides have mostly been detected to synapse onto dendrites and other axon terminals in ultrastructural studies (Fig. 3C and D). Far less frequently axon terminals containing opioid peptides have been detected synapsing onto somata, such as dynorphin A terminals synapsing onto cell bodies in the substantia nigra pars compacta (Riesenberg and Nitsch, 1990). An important question is: how close are opioid receptors to the release sites of endogenous opioid peptides? Similar to the ultrastructural localization of opioid peptide containing dense core vesicles, opioid receptors are also generally localized extrasynaptically, near the expected release sites for opioid peptide containing dense core vesicles (e.g., Aicher et al., 2003; Bockstaele et al., 1996, 1997). Are opioid receptors more likely to be near peptide release sites compared to more distant neuronal profiles?
There is some evidence that subpopulations of GPCRs are anchored to specific locations on the plasma membrane (Thurner et al., 2014; Yanagawa et al., 2018) including MORs (Metz et al., 2019; Suzuki et al., 2005). These single receptor tracking techniques suggest that individual receptors move within plasma membrane areas on the order of <10 μm2, consistent with some compartmentalization. Another clue for targeted localization of GPCRs to specific neural compartments is the distribution of G proteins. A recent study reported the distribution of Gαo proteins in Purkinje neurons in mouse cerebellum (Roldán-Sastre et al., 2021). Using immunocytochemical labeling of the G protein and localization with electron microscopy, Roldán-Sastre and colleagues reported that for Purkinje neurons most labeling was within dendritic spines, virtually no labeling was localized to somata, with low labeling in main/proximal dendrites and moderate labeling in spiny branchlet dendrites. Further, frequency of Gαo particle detection peaked at 180 nm from the synaptic specialization, with much lower density reported at distances ≥300 nm. Gαo particles were also located within the postsynaptic density, closer to the peripheral edges than to the center (see also Fernández et al., 2009). It is unknown whether these might be associated with neuropeptide receptors like opioid receptors or GPCRs that respond to fast neurotransmitters such as GABAB receptors or metabotropic glutamate receptors. While we do not know from this report the distribution of specific GPCRs relative to the Gαo, it does suggest some overall organization to the distribution of GPCRs within neural compartments.
As discussed above, another important component impacting the physiologically plausible distance(s) between endogenous opioid peptide release location and responding receptor site is enzymatic degradation of the peptides. It is clear that aminopeptidase B and endopeptidase-24.11 have strong regulatory control over peptide signaling because in experiments where these enzymes are blocked not only is the manipulation sufficient to change behavior in vivo but also to facilitate the detection of electrophysiological responses to endogenous opioid peptide release ex vivo (e.g., Bourgoin et al., 1986; Fournie-Zaluski et al., 1984; Waksman et al., 1985), described in the next Section. Thus it seems the distribution and organization of several elements of the endogenous opioid system at the subcellular level may be more consistent with models of relatively limited peptide diffusion away from release sites rather than broad diffusion events generating larger volumes of transmission (Fig. 3A).
There may be other cellular structures that target diffusion to nearby opioid receptors as well. For example, in the LC axon terminals labeled for LENK contain both small clear and large dense core vesicles along the periphery of the synaptic terminal. MORs were detected on dendrites lateral to the postsynaptic density, and astrocytic processes surround these synapses in an arrangement that might limit diffusion of LENK to nearby MOR containing dendrites (Bockstaele et al., 1996). Overall, though, patterns of proximity of opioid receptors to peptide release sites remain to be resolved.
7. Functional endogenous opioid peptide release ex vivo
A few researchers have been able to observe physiological neural responses that are blocked by opioid receptor antagonists, an indication that endogenous opioid peptides were released in these brain slice preparations. That said, in most ex vivo preparations detecting endogenous peptide release has been elusive. Successful studies provide some clues about the neural activity that drives release and the time course of the impact of this peptide release.
Early ex vivo extracellular recordings in the dentate gyrus of the hippocampus provided evidence for functional release of endogenous opioid peptides acting at the KOR (Drake et al., 1994; Simmons et al., 1995). Several seconds of high frequency electrical stimulus trains delivered to the hilus resulted in a depression of population spikes detected by field recordings in the granule cell layer. This effect reversed within 10 min of the stimulation and was mostly blocked by the selective KOR antagonist norBNI. The prolonged, high frequency stimulation applied in these experiments is consistent with the hypothesis that release of endogenous opioid peptides depends on extended burst firing.
Studies in other brain regions indicate that more moderate stimulation may be sufficient to drive release of endogenous opioid peptides. One neural population that seems to readily release endogenous opioid peptides is the magnocellular neurosecretory cells of the hypothalamus, where dynorphin is co-packaged into dense core vesicles with vasopressin (Watson et al., 1982). Ex vivo electrophysiology studies show that stimulus trains delivered to these neurons drive release of an endogenous opioid peptide that inhibits depolarizing after-polarizations (Brown et al., 2004; Brown and Bourque, 2004). The effect was seen with all train lengths tested, 10–100 pulses, although longer trains did produce stronger inhibitions. This effect is blocked by norBNI, but not the selective MOR antagonist CTOP, making it likely that dynorphin is being released and acting at KORs. The physiological response was augmented in the presence of peptidase inhibitors, demonstrating how strongly peptidases control endogenous opioid peptide signaling. Interestingly, the dynorphin induced inhibition recovers within 20 s of the stimulus train, a faster time course of effect than is usually conceptualized for neuromodulators.
A more recent finding of a somatodendritic effect of endogenous opioid peptides detected with slice electrophysiology was reported by Winters and colleagues (2017) in the intercalated neurons of the amygdala. Bath application of the peptidase inhibitors bestatin (leucine aminopeptidase, aminopeptidase B, and triamino peptidase inhibitor), captopril (angiotensin-converting enzyme inhibitor), and thiorphan (endopeptidase-24.11/neprilysin, CD10 inhibitor) was sufficient to drive an outward current that was reversed by either the selective MOR antagonist CTAP or the non-selective opioid receptor antagonist naloxone. This was consistent with ultrastructure evidence in the same study that MENK-containing terminals synapse onto the dendrites of the intercalated cells. The magnitude of these responses was augmented by electrical stimulation, intended to produce additional neural activity.
Endogenous opioid peptide effects on presynaptic terminals have also been observed with ex vivo whole cell electrophysiology. For instance, a study of presynaptic control of glutamate terminals in the dorsal striatum from Atwood et al. (2014) supports the model of rapid and limiting endopeptidase catabolism of released endogenous opioid peptides. In these experiments glutamatergic excitatory postsynaptic currents (EPSCs) were electrically evoked once every 20s, a relatively infrequent stimulation pattern. When bestatin, captopril, and thiorphan were applied to the slice for 5 min, evoked EPSC amplitudes decreased for tens of minutes. This effect was blocked by the non-selective opioid receptor antagonist naloxone. The effect of the peptidase inhibitors was also prevented by metabotropic glutamate receptor type 5 (mGluR5) blockade, suggesting the endogenous opioid peptide release that leads to this synaptic modulation requires activation of mGluR5. In separate experiments, bath application of MENK, LENK, or dynorphin A each inhibited evoked EPSC amplitudes, showing that the opioid receptors on these glutamate terminals are responsive to each of these peptides.
In a subsequent study in the NAc, Trieu and colleagues (2022) showed that captopril application was sufficient to cause an inhibition of electrically evoked EPSCs, specifically in Drd1 MSNs. This effect was blocked by naloxone. They also directly measured endogenous opioid peptide released from brain slices by bath application of high external KCl for 20 min to depolarize cells then collecting perfusate for peptide detection with LC-MS/MS. This broad excitation increased the perfusate concentrations of MENK-RF, dynorphin A 1–8, dynorphin B, MENK, and LENK. Captopril selectively increased the concentration of MENK-RF. When Drd2 MSNs that express Penk were stimulated with channelrhodopsin-2 (20 Hz, 10 min) MENK and LENK concentrations in the perfusate increased, but when captopril was applied concomitantly with the light stimulation, MENK-RF concentrations, but not MENK or LENK concentrations, increased. These observations are consistent with MENK-RF being released and subsequently cleaved into MENK by angiotensin-converting enzyme.
Several groups have pinpointed a role for endogenous opioid peptide release in a form of long term depression (LTD) of feedforward inhibition in the hippocampus (Leroy et al., 2017, 2021; Piskorowski and Chevaleyre, 2013). In CA2, vasoactive intestinal peptide-expressing (VIP) GABAergic interneurons synapse onto parvalbumin-expressing (PV) GABAergic interneurons that innervate pyramidal cells. Specific patterns of tetanic stimulation excite VIP neurons and drive depression of the output of PV neurons resulting in a potentiation of postsynaptic potentials in CA2 pyramidal neurons. Most VIP neurons in CA2 express Penk and vice versa (Blasco-Ibáñez et al., 1998), and DOR is mostly expressed in interneurons in the hippocampus (Commons and Milner, 1997). Application of the DOR antagonists naltrindole or ICI174864 during the tetanic stimulation prevented the depression of the GABAergic inputs as well as the overall potentiation of the postsynaptic potentials recorded in pyramidal neurons. Short hairpin mRNA knockdown of Penk broadly in CA2 or specifically in VIP neurons also eliminated this LTD, thus identifying the source of the endogenous opioid peptide driving these synaptic changes (Leroy et al., 2021). Behaviorally, blocking DOR or knocking down Penk in CA2 interferes with formation of social memory in mice (Leroy et al., 2017, 2021). While the exact identity of the released Penk-derived peptide is not known, these studies elegantly identified the neurons releasing the peptide, the identity of the target receptor on PV interneurons, and related this function to a key behavior.
Together these observations demonstrate some dynamic features of the endogenous opioid peptide system. Endogenous opioid peptides can be released with action potential activity patterns well within the range of those that have been observed with in vivo extracellular electrophysiology during common behavior assays. In fact, ex vivo experiments indicate that in at least some circumstances minimal action potential activity is required to drive peptide release. Enzymatic degradation strongly controls the diffusion of opioid peptides away from release sites, spatially limiting the population of opioid receptors they can activate. And, endogenously released peptides can have relatively dynamic actions in brain slices, with rapid onset and reversal of effect on the order of 10s of seconds after the termination of stimulation. One limitation to interpreting ex vivo electrophysiological observations of responses is that the perfusion bath is essentially an infinite sink for any peptides or other molecules that are released; this likely limits the maximum extracellular peptide concentration and accelerates the drop in peptide concentration after release in this preparation. Another consideration is that the electrical or optogenetic stimulation utilized in ex vivo techniques generate a great deal of synchrony across many separate axons or cell bodies. In many brain regions smaller subpopulations of neurons are active simultaneously during a given behaviorally relevant epoch, making this type of broad stimulation supraphysiological. Therefore these model experiments may generate an overestimate of peptide release and its physiological impact. Still, these ex vivo studies provide an important framework for thinking about what kinds of neural activity patterns are needed to produce endogenous opioid peptide release and the time course of receptor signaling resulting from such release events.
8. Treating alcohol use disorder by blocking endogenous opioid activity with Naltrexone
8.1. Clinical development and experience
Can this mechanistic neurobiological research inform our understanding of neuropsychiatric disorders or maladaptive behaviors? Because the opioid receptor antagonist NTX decreases alcohol consumption in humans and animals (Gonzales and Weiss, 1998; Herz, 1997; Mitchell et al., 2009) it is strongly hypothesized that a key contributor to alcohol’s rewarding effects is activation of the endogenous opioid system. As an antagonist, NTX has an effect when endogenous opioid peptides are released and activating opioid receptors. Although NTX was originally approved in 1984 to treat heroin addiction, studies do not support its use for this indication (Coffa and Snyder, 2019; Greenstein et al., 1981; Julius and Renault, 1976 (eds); Lee et al., 2018; Minozzi et al., 2011; NRC, 1978; Rawson and Tennant, 1984). Oral NTX was approved for treatment of AUD in 1994 (Center for Substance Abuse Treatment, 2009).
Early studies investigating the efficacy of NTX were performed in conjunction with psychotherapy (Balldin et al., 2003; O’Brien et al., 1996; O’Malley et al., 1992; Oslin et al., 2008; Volpicelli et al., 1992) and found that NTX decreased alcohol craving, the frequency of return to alcohol consumption, and Addiction Severity Index (ASI) scores compared to placebo (O’Malley et al., 1992; Volpicelli et al., 1992). ASI evaluates the severity of the subject’s alcohol use, family, legal, social, psychological, and medical problems and was assessed at the beginning and end of the 12 week study (O’Malley et al., 1992). It is important to note that there was also an interaction between type of therapy and frequency of relapse: providing coping skills to patients in the treatment group proved best for relapse prevention (O’Malley et al., 1992). This is consistent with studies in which cognitive behavioral therapy improved AUD treatment outcomes (Ray et al., 2020). A six-month follow-up study showed that the NTX treated subjects were still less likely to drink heavily, suggesting ongoing efficacy (O’Malley et al., 1996).
A clue to how endogenous opioids contribute to the motivation to drink alcohol is in an early study where subjects reported that their alcohol induced “high” was diminished when on NTX (Volpicelli et al., 1992). This effect should decrease the motivation to continue drinking because it makes consumption less rewarding. In fact, and consistent with this, studies indicate that rather than promoting abstinence, NTX reduces heavy drinking episodes (O’Malley et al., 1992; Rohsenow, 2004).
One issue with oral NTX is subject non-compliance (Volpicelli et al., 1997), a common problem for substance use disorder treatments (Herbeck et al., 2005). To mitigate this, a long-acting intramuscular injection that lasts 4 weeks, brand named Vivitrol®, was developed (Center for Substance Abuse Treatment, 2009; Jarvis et al., 2018; O’Brien et al., 1996). In addition to compliance, another advantage of the injectable is more consistent blood levels of NTX compared to daily oral dosing (Johnson, 2007; Mannelli et al., 2014). Clinical trials for the long-acting injectable showed a 25% reduction in heavy drinking episodes compared to placebo (Garbutt et al., 2005). For patients that were abstinent before treatment began, the time to first drink with extended-release treatment was 41 days versus 12 days for those who received placebo (O’Malley et al., 2007). Importantly, only 14% of subjects discontinued treatment (Garbutt et al., 2005).
While duration of abstinence had long been used as the main clinical endpoint for AUD treatment studies, there is a mismatch between this endpoint and the model of NTX target action (Garbutt et al., 2005; Leighty and Ansara, 2019; O’Malley et al., 2007; Pettinati et al., 2011). That is, if the hypothesized mechanism is correct, NTX should affect signaling during drinking that causes endogenous opioid peptide release, not decrease the desire to drink while abstinent. Consistent with this model, oral NTX decreased total drinking days per month, decreased heavy drinking days per month, and increased days until the first heavy drinking event (O’Malley et al., 2007; Pettinati et al., 2011). Extended release NTX also decreased the number of heavy drinking days for patients with high severity AUD (Espiridion, 2019; Pettinati et al., 2011). Fewer heavy drinking days can improve mental and physical health, mitigating many of the problems associated with AUD.
Several side effects of oral and intramuscular NTX have been recorded, including drowsiness (30%), nausea (26%), vomiting (17%), decreased appetite (18%), abdominal pain (16%), insomnia (16%), dizziness (12%), and injection site reactions for intramuscular NTX (Jonas et al., 2014; Kranzler and Soyka, 2018). Nausea was less prevalent in patients with lower severity drinking episodes and longer abstinence before treatment initiation, consistent with NTX producing some opioid withdrawal by reversing endogenous opioid peptide actions (O’Malley et al., 2000).
It is important to note that most of these clinical data were collected in men (Schick et al., 2020), especially because there are known sex differences in opioid systems (Becker and Chartoff, 2019) and in motivation(s) to drink (Peltier et al., 2019). One study reported that NTX was not efficacious in heavy drinking women (Hernandez-Avila et al., 2006), but other studies report conflicting results (Baros et al., 2008; Peltier et al., 2019; Yoon et al., 2016). The COMBINE study, which is the largest study of pharmacotherapy treatment for AUD, did not detect any sex differences in NTX efficacy (Greenfield et al., 2010). Given data available now, it seems that sex differences do not limit NTX efficacy in treating AUD.
8.2. Current understanding of naltrexone function
A great deal of preclinical work has been done to determine how NTX works at the biological level to decrease alcohol consumption. Like with humans, NTX decreases alcohol consumption in other species (Bienkowski et al., 1999; Egli, 2005; Mitchell et al., 2009; Stromberg et al., 1998). Some differences in efficacy have been noted, such as sex differences in rats (Matzeu et al., 2018), and the pattern of alcohol access and route of NTX administration matters (Goodwin et al., 2001; Korpi et al., 2017). Animal studies suggest that consistent use of NTX changes the endogenous opioid system to make it less effective (Yoburn et al., 1989; Zukin et al., 1982), a potential consideration for intramuscular NTX.
Animal studies have also identified some of the brain regions that likely contribute to NTX’s efficacy. For instance, microinjection of NTX into the NAc decreases EtOH self administration and prevents the consumption induced increase in extracellular dopamine in the NAc (Gonzales and Weiss, 1998). Microinjection of the selective MOR antagonist CTOP into the VTA decreases EtOH consumption in rats (Margolis et al., 2008). NTX’s blockade of KOR actions may also contribute to its ability to decrease alcohol consumption. Systemic administration of selective KOR antagonists decreases EtOH consumption in rodents that are EtOH dependent or that have been subjected to an aversive stressor (Anderson et al., 2016; Rose et al., 2016; Sperling et al., 2010), although not in rodents that are neither dependent nor stressed (Mitchell et al., 2005). In EtOH dependent rodents, microinjecting a selective KOR antagonist specifically into the BNST (Erikson et al., 2018) or CEA (Kissler and Walker, 2016) decreases EtOH consumption. The contribution specifically of the KOR system to the motivation to consume alcohol is reviewed in (Anderson and Becker, 2017).
Microdialysis has been used to investigate the identities of the endogenous opioid peptides released in response to EtOH, the actions of which NTX would reverse. For instance, acute i.p. injection of EtOH leads to an increase in β-endorphin, MENK, and dynorphin A 1–8 in the NAc (Marinelli et al., 2003, 2005, 2006). A similar study in the VTA found an increase in β-endorphin, but not MENK following i.p. EtOH (Jarjour et al., 2009). One limitation of these studies that prevents making a link between endogenous opioid peptide release and the motivation to drink is the animals were not voluntarily consuming EtOH. Another is that i.p. EtOH injections generate discomfort. These were also first EtOH exposures for the study animals, and future studies are required to determine if similar changes in peptide levels occur with experienced drinkers or animals that have learned to associate the i.p. injection with the subsequent rewarding effects of EtOH.
Human imaging studies have also identified changes in brain activity induced by NTX in alcohol-using and abstinent individuals. Changes in fMRI BOLD signals do not indicate where NTX is binding, but provide important information on which brain regions might be contributing to human experience and behavior. BOLD signals often interact with the personality trait locus of control, a measure of an individual’s conceptualization of how much control one has over his/her life, with internal locus of control indicating a feeling of strong control. In alcohol using males, NTX modulated the brain-wide functional connectivity of rostral anterior cingulate cortex/ventromedial prefrontal cortex, enhancing connectivity of a left frontoparietal network and decreasing connectivity with visual and motor regions (Elton et al., 2019). The strongest changes with NTX were in individuals who indicated they consumed alcohol to cope (Elton et al., 2019). On placebo, those who showed stronger functional connectivity between these regions also had a more external locus of control (Elton et al., 2019). These studies start to build an understanding of the relationships between NTX effect, personality traits, and functional connectivity that may help predict who will respond to NTX and what kinds of changes in brain activity may be important for therapeutic effect.
A positron emission tomography (PET) study in humans does indicate endogenous opioid peptides are released in the NAc following alcohol consumption (Mitchell et al., 2012). Using PET detection of the highly selective MOR agonist [11C]carfentanil, Mitchell et al. (2012) found a decrease in radioligand binding in the NAc and orbitofrontal cortex after subjects consumed an alcoholic beverage. This was observed both in heavy drinkers and controls. There was also some indication that the magnitude of orbital frontal endogenous opioid peptide release relates to heavy drinking as the change in binding correlated with alcohol induced subjective high and several measures of problem drinking.
In humans, plasma levels of β-endorphin are increased during acute heavy intoxication, but in chronic drinkers the levels are lower than average (Zalewska-Kaszubska and Czarnecka, 2005). This low β-endorphin in people with AUD persists for more than ten years during abstinence (Arbol et al., 1995). Thus heavy or chronic alcohol consumption produces long term endogenous opioid alterations.
Risk of elevated alcohol use is a partially heritable trait in both humans and animals, and the endogenous opioid system can contribute (Edwards et al., 2018; Mitchell et al., 2012; Palmer et al., 2019; Schuckit et al., 2005; Verhulst et al., 2015; Walters et al., 2018). Humans with a family history of AUD tend to require higher blood alcohol levels to reach intoxication (Eng et al., 2005; Schuckit et al., 2004). Family history also interacts with NTX effects. In college students with a positive family history for alcohol abuse, there is a strong relationship between locus of control and the change in impulsive choice ratio in a delay discounting task, such that individuals with a strong internal locus of control showed an increase in impulsive choice on NTX while those with a strong external locus of control showed a decrease in impulsive choice on NTX (Altamirano et al., 2011). A similar pattern was found in abstinent alcoholics (Mitchell et al., 2007). Decreasing impulsive choice should have a beneficial effect with respect to decreasing alcohol consumption, and this work indicates a link to family history for this specific effect.
One interesting genetic polymorphism is A118G in OPRM1. This polymorphism is not associated with AUD, but is associated with NTX’s effectiveness (Anton et al., 2008; Bergen et al., 1997; Chamorro et al., 2012; Franke et al., 2001; Oslin et al., 2008); patients carrying the G allele had lower relapse rates and a longer time before returning to heavy drinking on NTX than those homozygous for the A allele (Chamorro et al., 2012; Oslin et al., 2008). Subjects with at least one copy of the G allele also had a significant reduction in the self-reported “high” due to alcohol intake while taking NTX compared to those lacking this allele (Ray and Hutchison, 2007). A transgenic mouse model containing this OPRM1 polymorphism was generated (Bilbao et al., 2015). Consistent with observations in humans, NTX selectively suppressed both home cage EtOH drinking and operant self-administration of EtOH in h/mOPRM1–118GG mouse line compared to the h/mOPRM1–118AA line (Bilbao et al., 2015). MORs with this polymorphism have an increase in binding affinity for β-endorphin 1–31, but no change in binding affinity for MENK, dynorphin A 1–17, or EM-1 (Bond et al., 1998). This SNP also appears to greatly decrease the number of MORs translated in a heterologous system (Zhang et al., 2005). The polymorphism reduces synaptic modulation by DAMGO and morphine (Popova et al., 2019; Robinson et al., 2015). While this polymorphism could lead to other kinds of biological differences in the endogenous opioid system in vivo such as changes in receptor distribution or signaling, so far findings suggest that β-endorphin 1–31 plays a particularly dominant role in the interaction between this SNP and NTX’s effects.
At least some of the differences that develop in rats bred for high and low EtOH consumption also reside in the opioid system. While NTX attenuates EtOH seeking in both alcohol preferring (P) and standard rats, P rats showed more sensitivity to low doses of NTX (Henderson-Redmond and Czachowski, 2014). A systemic EtOH injection increases β-endorphin release in the NAc in alcohol preferring but not alcohol-avoiding rats (Lam et al., 2009). In mice that consume high levels of EtOH (C57BL/6) a similar phenomenon has also been observed. There was more β-endorphin release in the hypothalamus after EtOH injection in C57BL/6 mice compared to alcohol averse DBA/2 mice (Waele and Gianoulakis, 1993).
Animal studies have also revealed some possible limitations to the efficacy of systemic administration of a non-selective opioid receptor antagonist like NTX. For instance, Kemppainen et al. (2012) showed that microinjecting the selective MOR antagonist CTOP into the ventral pallidum increases EtOH consumption and microinjection of the selective MOR agonist DAMGO decreases EtOH consumption, thus there is at least one brain site where blocking MOR opposes the desired effect. Also, microinjecting the selective DOR antagonist TIPP-Ψ into the VTA increases EtOH consumption (Margolis et al., 2008). DOR blockade is also anxiogenic (Balerio et al., 2005; Narita et al., 2006; Solati, 2011; Solati et al., 2010), which could be problematic, especially in individuals who drink alcohol to relieve anxiety. Therefore an opioid receptor antagonist that does not bind to DOR might have improved efficacy for AUD.
NTX does not bind to the nociceptin receptor, however in preclinical studies there is evidence that endogenous nociceptin release also contributes to the motivation to consume EtOH. The nociceptin receptor antagonist LY2940094/BTRX-246040 has proven safe in humans and in a small proof-of-concept clinical study decreased MDD symptoms, reduced heavy alcohol drinking, and increased abstinence (Witkin et al., 2019). In samples from humans with AUD, Pnoc mRNA expression levels in the hippocampus were 1.7 fold lower compared to controls (Kuzmin et al., 2009). In rodents, systemic blockade of nociceptin receptors decreases EtOH drinking (Brunori et al., 2019) and stress-induced reinstatement of EtOH seeking (Borruto et al., 2021). Microinjections of a nociceptin receptor antagonist into the VTA or the CEA, but not in the NAc, also decreases stress induced EtOH seeking (Borruto et al., 2019, 2021; Rorick-Kehn et al., 2016). Together these observations make blockade of the nociceptin receptor a provocative therapeutic target for AUD.
These are just some of the observations that help to understand how NTX decreases alcohol consumption. While NTX remains the most effective treatment for AUD, side effects are not rare and not everyone shows improvement. Another drawback of NTX is its ability to inhibit Cytochrome P450 (CYP), which is an important enzyme in the metabolism of opioids and other drugs, including those that treat other mental health disorders (AlRabiah et al., 2018; Smith, 2009). There is also evidence that the inactive NTX isomer, (+)-naltrexone, is a toll-like receptor 4 (TLR4) antagonist (Zhang et al., 2018). TLR4 antagonism could potentially have anti-inflammatory effects, but the benefits or drawbacks of this interaction are not known. Together these studies also identify several ways NTX might be improved upon to increase efficacy and decrease side effects.
9. Critical questions and conclusions
There are many remaining questions about endogenous opioid peptide function. Very little is known regarding when and where endogenous opioid peptides are released. Additional questions that require study include: how is peptide release driven, how far do the peptides diffuse from a release site, what is the extracellular concentration of peptide produced by release, how long does released peptide impact preand postsynaptic function, which signaling pathways are activated by the different endogenous opioid peptides at each opioid receptor, and do these phenomena vary with brain region? Recent reviews describe advances and ongoing challenges in detecting endogenous opioid peptide fluctuations (Conway et al., 2022; Girven et al., 2022). New transgenic rodents continue to be created that enable careful investigations of endogenous opioid peptide circuits. Improved resolution and sensitivity of sequencing based assays enable detection of lower abundance RNA message identifying previously unknown expression patterns for opioid peptides and receptors. For recent and future single cell transcriptome studies, when the datasets are made public researchers can probe them for opioid system expression patterns. Recent advances in sequential re-staining and co-registration of cleared tissue that enable labeling of a dozen or more proteins in the same sample promise the opportunity to integrate detection of elements of the endogenous opioid system into a broader context (Hickey et al., 2022; Kim et al., 2022; Murray et al., 2015). Advances in electron microscopy and reconstruction techniques provide improved understanding of the 3 dimensional structures such as where the peptides are released and the available extracellular space for diffusion to their target receptors. New tools for imaging fluctuations in neuropeptide levels in vivo are being developed (e.g. Ding et al., 2019). At the systems level, many questions remain regarding the contributions of the endogenous opioid system to sex differences including in pain, AUD, and SUD, as well as in treatment efficacies for these and other disorders.
Still, much progress has been made in our understanding of endogenous opioid peptide generation and how the different peptides bind and activate each of the opioid receptors. The opioid field has a wealth of tool compounds as well that have been used to investigate the behavioral impact of activating or blocking different elements of this system, and the subsequent observations have generated many hypotheses not only for how to improve on NTX efficacy for treating AUD, but also how to interact with the endogenous opioid system in novel ways to treat other indications. In addition to applying recent discoveries to design better analgesics that activate MOR and MDD treatments that block KOR, one exciting possibility is to boost the activity of endogenous opioid peptides with a positive allosteric modulator of MOR. Blockade of endogenous opioid peptide catabolism in vivo does not cause respiratory depression, raising the possibility that a positive allosteric modulator at MOR will generate analgesia without causing respiratory depression (Boudinot et al., 2001; Fournie-Zaluski et al., 1984; Kayser et al., 1989). Preclinical studies indicate potential for this approach (Kandasamy et al., 2021). Provocatively, positive allosteric activity at MORs might be one of the mechanisms by which ketamine produces analgesia (Gupta et al., 2011; Petrocchi et al., 2019). The efficacy of a positive allosteric modulator requires endogenous opioid peptide activity at the orthosteric binding site of the receptor. Thus, continuing to investigate the conditions when different endogenous opioid peptides are released with new and exciting tools, along with new advances in understanding structure-activity relationships in the opioid systems, has the potential to not only generate exciting biological observations but also new therapeutic strategies for various clinical indications.
Acknowledgements
This work was supported by National Institutes of Health grant R01AA026609 (to E.B.M.)
Abbreviations:
- CYP
Cytochrome P450
- DOR
Delta opioid receptor
- KOR
Kappa opioid receptor
- MOR
Mu opioid receptor
- ORL1
Opioid receptor-like 1
- Nociceptin receptor
- PENK
proenkephalin
- PDYN
prodynorphin
- POMC
proopiomelanocortin
- PNOC
pronociceptin
- LENK
leu-enkephalin
- MENK
met-enkephalin
- EM
endomorphin
- PAG
periaqueductal gray
- NTS
nucleus of the solitary tract
- BNST
bed nucleus of the stria terminalis
- NAc
nucleus accumbens
- LC
locus coeruleus
- CEA
central nucleus of the amygdala
- VTA
ventral tegmental area
- DMH
dorsal medial hypothalamus
- PVH
paraventricular nucleus of the hypothalamus
- VIP
vasoactive intestinal peptide
- DRN
dorsal raphe nucleus
Data availability
No data was used for the research described in the article.
References
- Abbadie C, Lombard M-C, Besson J-M, Trafton JA, Basbaum AI, 2002. Mu and delta opioid receptor-like immunoreactivity in the cervical spinal cord of the rat after dorsal rhizotomy or neonatal capsaicin: an analysis of pre- and postsynaptic receptor distributions. Brain Res. 930, 150–162. 10.1016/s0006-8993(02)02242-4. [DOI] [PubMed] [Google Scholar]
- Abraham AD, Casello SM, Schattauer SS, Wong BA, Mizuno GO, Mahe K, Tian L, Land BB, Chavkin C, 2021. Release of endogenous dynorphin opioids in the prefrontal cortex disrupts cognition. Neuropsychopharmacology 46, 2330–2339. 10.1038/s41386-021-01168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghabiklooei A, Hassanian-Moghaddam H, Zamani N, Shadnia S, Mashayekhian M, Rahimi M, Nasouhi S, Ghoochani A, 2013. Effectiveness of naltrexone in the prevention of delayed respiratory arrest in opioid-naive methadone-intoxicated patients. BioMed Res. Int 2013, 903172 10.1155/2013/903172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati LF, Bjelke B, Fuxe K, 1995. Volume versus wiring transmission in the brain: a new theoretical frame for neuropsychopharmacology. Med. Res. Rev 15, 33–45. 10.1002/med.2610150104. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Mitchell JL, Swanson KC, Zadina JE, 2003. Endomorphin-2 axon terminals contact mu-opioid receptor-containing dendrites in trigeminal dorsal horn. Brain Res. 977, 190–198. 10.1016/s0006-8993(03)02678-7. [DOI] [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM, 1984. Endogenous opioids: biology and function. Annu. Rev. Neurosci 7, 223–255. 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo C-O, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, Bruchas MR, 2015. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87, 1063–1077. 10.1016/j.neuron.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khrasani M, Orosz G, Kocsis L, Farkas V, Magyar A, Lengyel I, Benyhe S, Borsodi A, Rónai AZ, 2001. Receptor constants for endomorphin-1 and endomorphin-1-ol indicate differences in efficacy and receptor occupancy. Eur. J. Pharmacol 421, 61–67. 10.1016/s0014-2999(01)01014-7. [DOI] [PubMed] [Google Scholar]
- AlRabiah H, Ahad A, Mostafa GAE, Al-Jenoobi FI, 2018. Effect of naltrexone hydrochloride on Cytochrome P450 1A2, 2C9, 2D6, and 3A4 activity in human liver microsomes. Eur. J. Drug Metab. Pharmokinet 43, 707–713. 10.1007/s13318-018-0482-x. [DOI] [PubMed] [Google Scholar]
- Altamirano LJ, Fields HL, D’Esposito M, Boettiger CA, 2011. Interaction between family history of alcoholism and locus of control in the opioid regulation of impulsive responding under the influence of alcohol. Alcohol Clin. Exp. Res 35, 1905–1914. 10.1111/j.1530-0277.2011.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KD, Reiner A, 1990. Extensive co-occurrence of substance P and dynorphin in striatal projection neurons: an evolutionarily conserved feature of basal ganglia organization. J. Comp. Neurol 295, 339–369. 10.1002/cne.902950302. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Becker HC, 2017. Role of the dynorphin/kappa opioid receptor system in the motivational effects of ethanol. Alcohol Clin. Exp. Res 41, 1402–1418. 10.1111/acer.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, Becker HC, 2016. Stress-induced enhancement of ethanol intake in C57bl/6J mice with a history of chronic ethanol exposure: involvement of kappa opioid receptors. Front. Cell. Neurosci 10, 45. 10.3389/fncel.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D, 2008. An evaluation of μ-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the combined pharmacotherapies and behavioral interventions for alcohol dependence (COMBINE) study. Arch. Gen. Psychiatr 65, 135–144. 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Arbol JL, Aguirre JC, Raya J, Rico J, Ruiz-Requena ME, Miranda MT, 1995. Plasma concentrations of β-endorphin, adrenocorticotropic hormone, and cortisol in drinking and abstinent chronic alcoholics. Alcohol 12, 525–529. 10.1016/0741-8329(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Lovinger DM, Mathur BN, 2014. Presynaptic long-term depression mediated by Gi/o-coupled receptors. Trends Neurosci. 37, 663–673. 10.1016/j.tins.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenali L, Fulas OA, Sondermann J, Narayanan P, Gomez-Varela D, Schmidt M, 2016. Nocistatin sensitizes TRPA1 channels in peripheral sensory neurons. Channels 11. 10.1080/19336950.2016.1207025, 00–00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley EE, Ingram SL, 2020. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology 173, 108131. 10.1016/j.neuropharm.2020.108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balerio GN, Aso E, Maldonado R, 2005. Involvement of the opioid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology 181, 260–269. 10.1007/s00213-005-2238-y. [DOI] [PubMed] [Google Scholar]
- Balldin J, Berglund M, Borg S, Månsson M, Bendtsen P, Franck J, Gustafsson L, Halldin J, Nilsson L-H, Stolt G, Willander A, 2003. A 6-month controlled naltrexone study: combined effect with cognitive behavioral therapy in outpatient treatment of alcohol dependence. Alcohol Clin. Exp. Res 27, 1142–1149. 10.1097/01.alc.0000075548.83053.a9. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Zhang S, Morales M, 2016. Multiplexed neurochemical signaling by neurons of the ventral tegmental area. J. Chem. Neuroanat 73, 33–42. 10.1016/j.jchemneu.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes K, Matsas R, Hooper NM, Turner AJ, Kenny AJ, 1988a. Endopeptidase-24.11 is striosomally ordered in pig brain and, in contrast to aminopeptidase n and peptidyl dipeptidase a (‘angiotensin converting enzyme’), is a marker for a set of striatal efferent fibres. Neuroscience 27, 799–817. 10.1016/0306-4522(88)90184-4. [DOI] [PubMed] [Google Scholar]
- Barnes K, Turner AJ, Kenny AJ, 1993. An immunoelectron microscopic study of pig substantia nigra shows co-localization of endopeptidase-24.11 with substance P. Neuroscience 53, 1073–1082. 10.1016/0306-4522(93)90490-7. [DOI] [PubMed] [Google Scholar]
- Barnes K, Turner AJ, Kenny AJ, 1992. Membrane localization of endopeptidase-24.11 and peptidyl dipeptidase A (angiotensin converting enzyme) in the pig brain: a study using subcellular fractionation and electron microscopic immunocytochemistry. J. Neurochem 58, 2088–2096. 10.1111/j.1471-4159.1992.tb10950.x. [DOI] [PubMed] [Google Scholar]
- Barnes K, Turner AJ, Kenny AJ, 1988b. Electronmicroscopic immunocytochemistry of pig brain shows that endopeptidase-24.11 is localized in neuronal membranes. Neurosci. Lett 94, 64–69. 10.1016/0304-3940(88)90271-6. [DOI] [PubMed] [Google Scholar]
- Baros AM, Latham PK, Anton RF, 2008. Naltrexone and cognitive behavioral therapy for the treatment of alcohol dependence: do sex differences exist? Alcohol Clin. Exp. Res 32, 771–776. 10.1111/j.1530-0277.2008.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto CAV, Baptista SJ, Preto AJ, Silvério D, Melo R, Moreira IS, 2021. Decoding partner specificity of opioid receptor family. Front. Mol. Biosci 8, 715215 10.3389/fmolb.2021.715215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baršun M, Jajčanin N, Vukelíc B, Špoljarić J, Abramić M, 2007. Human dipeptidyl peptidase III acts as a post-proline-cleaving enzyme on endomorphins. Biol. Chem 388, 343–348. 10.1515/bc.2007.039. [DOI] [PubMed] [Google Scholar]
- Beauchamp A, Yee Y, Darwin BC, Raznahan A, Mars RB, Lerch JP, 2022. Whole-brain comparison of rodent and human brains using spatial transcriptomics. Elife 11. 10.7554/elife.79418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Chartoff E, 2019. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44, 166–183. 10.1038/s41386-018-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Kokoszka J, Peterson R, Long JC, Virkkunen M, Linnoila M, Goldman D, 1997. μ opioid receptor gene variants: lack of association with alcohol dependence. Mol. Psychiatr 2, 490–494. 10.1038/sj.mp.4000331. [DOI] [PubMed] [Google Scholar]
- Berwin B, Floor E, Martin TFJ, 1998. CAPS (mammalian UNC-31) protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron 21, 137–145. 10.1016/s0896-6273(00)80521-8. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kostowski W, Koros E, 1999. Ethanol-reinforced behaviour in the rat: effects of naltrexone. Eur. J. Pharmacol 374, 321–327. 10.1016/s0014-2999(99)00245-9. [DOI] [PubMed] [Google Scholar]
- Biglari N, Gaziano I, Schumacher J, Radermacher J, Paeger L, Klemm P, Chen W, Corneliussen S, Wunderlich CM, Sue M, Vollmar S, Klöckener T, Sotelo-Hitschfeld T, Abbasloo A, Edenhofer F, Reimann F, Gribble FM, Fenselau H, Kloppenburg P, Wunderlich FT, Brüning JC, 2021. Functionally distinct POMC-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting. Nat. Neurosci 24, 913–929. 10.1038/s41593-021-00854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbao A, Robinson JE, Heilig M, Malanga CJ, Spanagel R, Sommer WH, Thorsell A, 2015. A pharmacogenetic determinant of mu-opioid receptor antagonist effects on alcohol reward and consumption: evidence from humanized mice. Biol. Psychiatr 77, 850–858. 10.1016/j.biopsych.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Birk Y, Li CH, 1964. Isolation and properties of a new, biologically active peptide from sheep pituitary glands. J. Biol. Chem 239, 1048–1052. [PubMed] [Google Scholar]
- Blasco-Ibáñez JM, Martínez-Guijarro FJ, Freund TF, 1998. Enkephalin-containing interneurons are specialized to innervate other interneurons in the hippocampal CA1 region of the rat and Guinea-pig. Eur. J. Neurosci 10, 1784–1795. 10.1046/j.1460-9568.1998.00190.x. [DOI] [PubMed] [Google Scholar]
- Bloch B, Bugnon C, Fellmann D, Lenys D, Gouget A, 1979. Neurons of the rat hypothalamus reactive with antisera against endorphins, ACTH, MSH and β-LPH. Cell Tissue Res. 204, 1–15. 10.1007/bf00235160. [DOI] [PubMed] [Google Scholar]
- Bloom F, Battenberg E, Rossier J, Ling N, Guillemin R, 1978. Neurons containing beta-endorphin in rat brain exist separately from those containing enkephalin: immunocytochemical studies. Proc. Natl. Acad. Sci. USA 75, 1591–1595. 10.1073/pnas.75.3.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockstaele EJV, Branchereau P, Pickel VM, 1995. Morphologically heterogeneous met-enkephalin terminals form synapses with tyrosine hydroxylase-containing dendrites in the rat nucleus locus coeruleus. J. Comp. Neurol 363, 423–438. 10.1002/cne.903630307. [DOI] [PubMed] [Google Scholar]
- Bockstaele EJV, Colago EEO, Moriwaki A, Uhl GR, 1996. Mu-opioid receptor is located on the plasma membrane of dendrites that receive asymmetric synapses from axon terminals containing leucine-enkephalin in the rat nucleus locus coeruleus. J. Comp. Neurol 376, 65–74. . [DOI] [PubMed] [Google Scholar]
- van Bockstaele EJ, Commons K, Pickel VM, 1997. δ-opioid receptor is present in presynaptic axon terminals in the rat nucleus locus coeruleus: relationships with methionine5-enkephalin. J. Comp. Neurol 388, 575–586. . [DOI] [PubMed] [Google Scholar]
- Boguski MS, Lowe TMJ, Tolstoshev CM, 1993. dbEST — database for “expressed sequence tags. Nat. Genet 4, 332–333. 10.1038/ng0893-332. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L, 1998. Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: possible implications for opiate addiction. Proc. Natl. Acad. Sci. USA 95, 9608–9613. 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg CB, Braun N, Heusser SA, Bay Y, Weis D, Galleano I, Lund C, Tian W, Haugaard-Kedström LM, Bennett EP, Lynagh T, Strømgaard K, Andersen J, Pless SA, 2020. Mechanism and site of action of big dynorphin on ASIC1a. Proc. Natl. Acad. Sci. USA 117, 7447–7454. 10.1073/pnas.1919323117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borruto A, Fotio Y, Stopponi S, Brunori G, Petrella M, Caputi F, Romualdi P, Candeletti S, Narendran R, Rorick-Kehn LM, Ubaldi M, Weiss F, Ciccocioppo R, 2019. NOP receptor antagonism reduces alcohol drinking in male and female rats through mechanisms involving the central amygdala and ventral tegmental area. Br. J. Pharmacol 10.1111/bph.14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borruto AM, Fotio Y, Stopponi S, Petrella M, Carlo SD, Domi A, Ubaldi M, Weiss F, Ciccocioppo R, 2021. NOP receptor antagonism attenuates reinstatement of alcohol-seeking through modulation of the mesolimbic circuitry in male and female alcohol-preferring rats. Neuropsychopharmacology 46, 2121–2131. 10.1038/s41386-021-01096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudinot E, MorinSurun M, Foutz AS, FourniéZaluski M, Roques BP, DenavitSaubié M, 2001. Effects of the potent analgesic enkephalin-catabolizing enzyme inhibitors RB101 and kelatorphan on respiration. Pain 90, 7–13. 10.1016/s0304-3959(00)00382-1. [DOI] [PubMed] [Google Scholar]
- Bourgoin S, Bars DL, Artaud F, Clot AM, Bouboutou R, Fournie-Zaluski MC, Roques BP, Hamon M, Cesselin F, 1986. Effects of kelatorphan and other peptidase inhibitors on the in vitro and in vivo release of methionine-enkephalin-like material from the rat spinal cord. J. Pharmacol. Exp. Therapeut 238, 360–366. [PubMed] [Google Scholar]
- Brown CH, Bourque CW, 2004. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J. Physiol. (London) 557, 949–960. 10.1113/jphysiol.2004.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Bull PM, Bourque CW, 2004. Phasic bursts in rat magnocellular neurosecretory cells are not intrinsically regenerative in vivo. Eur. J. Neurosci 19, 2977–2983. 10.1111/j.0953-816x.2004.03408.x. [DOI] [PubMed] [Google Scholar]
- Bruckner JJ, Zhan H, O’Connor-Giles KM, 2015. Advances in imaging ultrastructure yield new insights into presynaptic biology. Front. Cell. Neurosci 9, 196. 10.3389/fncel.2015.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori G, Weger M, Schoch J, Targowska-Duda K, Barnes M, Borruto AM, Rorick-Kehn LM, Zaveri NT, Pintar JE, Ciccocioppo R, Toll L, Cippitelli A, 2019. NOP receptor antagonists decrease alcohol drinking in the dark in C57bl/6J mice. Alcohol Clin. Exp. Res 43, 2167–2178. 10.1111/acer.14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butour J-L, Moisand C, Mazarguil H, Mollereau C, Meunier J-C, 1997. Recognition and activation of the opioid receptor-like ORL1 receptor by nociceptin, nociceptin analogs and opioids. Eur. J. Pharmacol 321, 97–103. 10.1016/s0014-2999(96)00919-3. [DOI] [PubMed] [Google Scholar]
- Cahill C, Tejeda HA, Spetea M, Chen C, Liu-Chen L-Y, 2021. The kappa opioid receptor. Handb. Exp. Pharmacol 271, 3–21. 10.1007/164_2021_433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun SE, Meunier CJ, Lee CA, McCarty GS, Sombers LA, 2018. Characterization of a multiple-scan-rate voltammetric waveform for real-time detection of met-enkephalin. ACS Chem. Neurosci 10, 2022–2032. 10.1021/acschemneuro.8b00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Bruchas MR, 2019. A motivational and neuropeptidergic hub: anatomical and functional diversity within the nucleus accumbens shell. Neuron 102, 529–552. 10.1016/j.neuron.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley NX, Li Z, Loh YP, 2016. 60 years of POMC: biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J. Mol. Endocrinol 56, T77–T97. 10.1530/jme-15-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A, Marcos M, Mirón-Canelo J, Pastor I, González-Sarmiento R, Laso F, 2012. Association of μ-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis. Addiction Biol. 17, 505–512. 10.1111/j.1369-1600.2012.00442.x. [DOI] [PubMed] [Google Scholar]
- Chavkin C, James I, Goldstein A, 1982. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215, 413–415. 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chen L, Gu Y, Huang LY, 1995a. The opioid peptide dynorphin directly blocks NMDA receptor channels in the rat. J. Physiol. (London) 482, 575–581. 10.1113/jphysiol.1995.sp020541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Gu Y, Huang LY, 1995b. The mechanism of action for the block of NMDA receptor channels by the opioid peptide dynorphin. J Neurosci Official J Soc Neurosci 15, 4602–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE, 2001. Orexin (hypocretin) neurons contain dynorphin. J Neurosci Official J Soc Neurosci 21, RC168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensson-Nylander I, Herrera-Marschitz M, Staines W, Hökfelt T, Terenius L, Ungerstedt U, Cuello C, Oertel WH, Goldstein M, 1986. Striato-nigral dynorphin and substance P pathways in the rat. Exp. Brain Res 64, 169–192. 10.1007/bf00238213. [DOI] [PubMed] [Google Scholar]
- Coffa D, Snyder H, 2019. Opioid use disorder: medical treatment options. Am. Fam. Physician 100, 416–425. [PubMed] [Google Scholar]
- Commons KG, Milner TA, 1997. Localization of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. J. Comp. Neurol 381, 373–387. . [DOI] [PubMed] [Google Scholar]
- Cone RD, 2005. Anatomy and regulation of the central melanocortin system. Nat. Neurosci 8, 571–578. 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Connor M, Vaughan CW, Jennings EA, Allen RG, Christie MJ, 1999. Nociceptin, Phe1ψ-nociceptin1-13, nocistatin and prepronociceptin154–181 effects on calcium channel currents and a potassium current in rat locus coeruleus in vitro. Br. J. Pharmacol 128, 1779–1787. 10.1038/sj.bjp.0702971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway SM, Mikati MO, Al-Hasani R, 2022. Challenges and new opportunities for detecting endogenous opioid peptides in reward. Addict Neurosci 2, 100016. 10.1016/j.addicn.2022.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Castro DC, Bruchas MR, Scherrer G, 2018. Endogenous and exogenous opioids in pain. Annu. Rev. Neurosci 41, 1–21. 10.1146/annurevneuro-080317-061522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero MD, 2011. Oxidative stress in Fibromyalgia: pathophysiology and clinical implications. Reumatología Clínica Engl Ed 7, 281–283. 10.1016/j.reumae.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Cox BM, 1982. Endogenous opioid peptides: a guide to structures and terminology. Life Sci. 31, 1645–1658. 10.1016/0024-3205(82)90179-5. [DOI] [PubMed] [Google Scholar]
- Dalefield ML, Scouller B, Bibi R, Kivell BM, 2022. The kappa opioid receptor: a promising therapeutic target for multiple pathologies. Front. Pharmacol 13, 837671 10.3389/fphar.2022.837671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth H-U, McIntosh CHS, Pederson RA, 2005. Type 2 diabetes—therapy with dipeptidyl peptidase IV inhibitors. Biochimica Et Biophysica Acta Bba - Proteins Proteom 1751, 33–44. 10.1016/j.bbapap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- DiFeliceantonio AG, Mabrouk OS, Kennedy RT, Berridge KC, 2012. Enkephalin surges in dorsal neostriatum as a signal to eat. Curr. Biol 22, 1918–1924. 10.1016/j.cub.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K, Han Y, Seid TW, Buser C, Karigo T, Zhang S, Dickman DK, Anderson DJ, 2019. Imaging neuropeptide release at synapses with a genetically engineered reporter. Elife 8, e46421. 10.7554/elife.46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Terman GW, Simmons ML, Milner TA, Kunkel DD, Schwartzkroin PA, Chavkin C, 1994. Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. J Neurosci Official J Soc Neurosci 14, 3736–3750. 10.1523/jneurosci.14-06-03736.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll JR, Wallace TL, Mansourian KA, Martin WJ, Margolis EB, 2020. Differential modulation of ventral tegmental area circuits by the nociceptin/orphanin FQ system. Eneuro 7. 10.1523/eneuro.0376-19.2020. ENEURO.0376–19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Deak JD, Gizer IR, Lai D, Chatzinakos C, Wilhelmsen KP, Lindsay J, Heron J, Hickman M, Webb BT, Bacanu S, Foroud TM, Kendler KS, Dick DM, Schuckit MA, 2018. Meta-analysis of genetic influences on initial alcohol sensitivity. Alcohol Clin. Exp. Res 42, 2349–2359. 10.1111/acer.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, 2005. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addiction Biol. 10, 309–319. 10.1080/13556210500314550. [DOI] [PubMed] [Google Scholar]
- Eipper BA, Stoffers DA, Mains RE, 1992. The biosynthesis of neuropeptides: peptide alpha-amidation. Annu. Rev. Neurosci 15, 57–85. 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- Elton A, Dove S, Spencer CN, Robinson DL, Boettiger CA, 2019. Naltrexone acutely enhances connectivity between the ventromedial prefrontal cortex and a left frontoparietal network. Alcohol Clin. Exp. Res 43, 965–978. 10.1111/acer.13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng MY, Schuckit MA, Smith TL, 2005. The level of response to alcohol in daughters of alcoholics and controls. Drug Alcohol Depend. 79, 83–93. 10.1016/j.drugalcdep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Erikson CM, Wei G, Walker BM, 2018. Maladaptive behavioral regulation in alcohol dependence: role of kappa-opioid receptors in the bed nucleus of the stria terminalis. Neuropharmacology 140, 162–173. 10.1016/j.neuropharm.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espiridion ED, 2019. A retrospective study of hospital recidivism among patients with alcohol use disorders treated with intramuscular naltrexone. Cureus 11, e6287. 10.7759/cureus.6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Leslie FM, 1986. Distribution of dynorphin and enkephalin peptides in the rat brain. J. Comp. Neurol 249, 293–336. 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- Farina M, van de Bospoort R, He E, Persoon CM, van Weering JR, Broeke JH, Verhage M, Toonen RF, 2015. Correction: CAPS-1 promotes fusion competence of stationary dense-core vesicles in presynaptic terminals of mammalian neurons. Elife 4, e12968. 10.7554/elife.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández E, Collins MO, Uren RT, Kopanitsa MV, Komiyama NH, Croning MDR, Zografos L, Armstrong JD, Choudhary JS, Grant SGN, 2009. Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Mol. Syst. Biol 5 10.1038/msb.2009.27, 269–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichna J, Janecka A, Costentin J, Rego J-CD, 2007. The endomorphin system and its evolving neurophysiological role. Pharmacol. Rev 59, 88–123. 10.1124/pr.59.1.3. [DOI] [PubMed] [Google Scholar]
- Fisher JM, Sossin W, Newcomb R, Scheller RH, 1988. Multiple neuropeptides derived from a common precursor are differentially packaged and transported. Cell 54, 813–822. 10.1016/s0092-8674(88)91131-2. [DOI] [PubMed] [Google Scholar]
- Florin S, Leblond F, Suaudeau C, Meunier J-C, Costentin J, 1999. Comparison of behavioural effects of NocII or NocIII, two related pronociceptin-derived peptides. Life Sci. 65, 2727–2733. 10.1016/s0024-3205(99)00541-x. [DOI] [PubMed] [Google Scholar]
- Florin S, Suaudeau C, Meunier J-C, Costentin J, 1997. Orphan neuropeptide NocII, a putative pronociceptin maturation product, stimulates locomotion in mice. Neuroreport 8, 705–707. 10.1097/00001756-199702100-00025. [DOI] [PubMed] [Google Scholar]
- Ford CP, Beckstead MJ, Williams JT, 2007. Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. J. Neurophysiol 97, 883–891. 10.1152/jn.00963.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournie-Zaluski M-C, Chaillet P, Bouboutou R, Coulaud A, Cherot P, Waksman G, Costentin J, Roques BP, 1984. Analgesic effects of kelatorphan, a new highly potent inhibitor of multiple enkephalin degrading enzymes. Eur. J. Pharmacol 102, 525–528. 10.1016/0014-2999(84)90575-2. [DOI] [PubMed] [Google Scholar]
- Franke P, Wang T, Nöthen MM, Knapp M, Neidt H, Albrecht S, Jahnes E, Propping P, Maier W, 2001. Nonreplication of association between μ-opioid-receptor gene (OPRM1) A118G polymorphism and substance dependence. Am. J. Med. Genet 105, 114–119. . [DOI] [PubMed] [Google Scholar]
- Fricker LD, Margolis E, Gomes I, Devi LA, 2020. Five decades of research on opioid peptides: current knowledge and unanswered questions. Mol. Pharmacol 98 10.1124/mol.120.119388 mol.120.119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujima S, Yamaga R, Minami H, Mizuno S, Shinoda Y, Sadakata T, Abe M, Sakimura K, Sano Y, Furuichi T, 2021. CAPS2 deficiency impairs the release of the social peptide oxytocin, as well as oxytocin-associated social behavior. J. Neurosci 41, 4524–4535. 10.1523/jneurosci.3240-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW, Group, for the V.S., 2005. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA 293, 1617–1625. 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Maguire DR, Woods JH, Husbands SM, Disney A, France CP, 2018. Reversal and prevention of the respiratory-depressant effects of heroin by the novel μ opioid receptor antagonist methocinnamox in rhesus monkeys. J. Pharmacol. Exp. Therapeut 368 10.1124/jpet.118.253286 jpet.118.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, M FJ Jr., Sibley DR, 1990. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432. 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Young WS, 1988. Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 460, 161–167. 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Girard J-P, Baekkevold ES, Feliu J, Brandtzaeg P, Amalric F, 1999. Molecular cloning and functional analysis of SUT-1, a sulfate transporter from human high endothelial venules. Proc. Natl. Acad. Sci. USA 96, 12772–12777. 10.1073/pnas.96.22.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girven KS, Mangieri L, Bruchas MR, 2022. Emerging approaches for decoding neuropeptide transmission. Trends Neurosci. 10.1016/j.tins.2022.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Sierra S, Lueptow L, Gupta A, Gouty S, Margolis EB, Cox BM, Devi LA, 2020. Biased signaling by endogenous opioid peptides. Proc. Natl. Acad. Sci. USA 117, 11820–11828. 10.1073/pnas.2000712117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F, 1998. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J. Neurosci 18, 10663–10671. 10.1523/jneurosci.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin FLW, Campisi M, Babinska I, Amit Z, 2001. Effects of naltrexone on the intake of ethanol and flavored solutions in rats. Alcohol 25, 9–19. 10.1016/s0741-8329(01)00163-x. [DOI] [PubMed] [Google Scholar]
- Gormal RS, Meunier FA, 2022. Nanoscale organization of the pre-synapse: tracking the neurotransmitter release machinery. Curr. Opin. Neurobiol 75, 102576 10.1016/j.conb.2022.102576. [DOI] [PubMed] [Google Scholar]
- Greenbaum D, Colangelo C, Williams K, Gerstein M, 2003. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 4, 117. 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Pettinati HM, O’Malley S, Randall PK, Randall CL, 2010. Gender differences in alcohol treatment: an analysis of outcome from the COMBINE study. Alcohol Clin. Exp. Res 34, 1803–1812. 10.1111/j.1530-0277.2010.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein RA, O’Brien CP, McLellan AT, Woody GE, Grabowski J, Long M, Coyle-Perkins G, Vittor A, 1981. Naltrexone: a short-term treatment for opiate dependence. Am. J. Drug Alcohol Abuse 8, 291–300. 10.3109/00952998109009554. [DOI] [PubMed] [Google Scholar]
- Guo Y, Xiao P, Lei S, Deng F, Xiao GG, Liu Y, Chen X, Li L, Wu S, Chen Y, Jiang H, Tan L, Xie J, Zhu X, Liang S, Deng H, 2008. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim. Biophys. Sin 40, 426–436. 10.1111/j.1745-7270.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- Gupta A, Devi LA, Gomes I, 2011. Potentiation of μ-opioid receptor-mediated signaling by ketamine. J Neurochem, Journal of Neurochemistry 119, 294–302. 10.1111/j.1471-4159.2011.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Gullapalli S, Pan H, Ramos-Ortolaza DL, Hayward MD, Low MJ, Pintar JE, Devi LA, Gomes I, 2021. Regulation of opioid receptors by their endogenous opioid peptides. Cell. Mol. Neurobiol 41, 1103–1118. 10.1007/s10571-020-01015-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D, Murray PG, O’Sullivan J, Urquhart J, Daly S, Bhaskar SS, Biesecker LG, Skae M, Smith C, Cole T, Kirk J, Chandler K, Kingston H, Donnai D, Clayton PE, Black GCM, 2011. Exome sequencing identifies CCDC8 mutations in 3-M syndrome, suggesting that CCDC8 contributes in a pathway with CUL7 and OBSL1 to control human growth. Am. J. Hum. Genet 89, 148–153. 10.1016/j.ajhg.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Engel J, Teichmann SA, Lönnberg T, 2017. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 9, 75. 10.1186/s13073-017-0467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway AJ, Halladay LR, Mazzone CM, Pati D, Bloodgood DW, Kim M, Jensen J, DiBerto JF, Boyt KM, Shiddapur A, Erfani A, Hon OJ, Neira S, Stanhope CM, Sugam JA, Saddoris MP, Tipton G, McElligott Z, Jhou TC, Stuber GD, Bruchas MR, Bulik CM, Holmes A, Kash TL, 2019. Central amygdala prepronociceptin-expressing neurons mediate palatable food consumption and reward. Neuron. 10.1016/j.neuron.2019.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G, Hughes J, Kosterlitz HW, 1978. In vitro release of Leu- and Met-enkephalin from the corpus striatum. Nature 271, 677–679. 10.1038/271677a0. [DOI] [PubMed] [Google Scholar]
- Henderson-Redmond A, Czachowski C, 2014. Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats. Psychopharmacology 231, 4309–4321. 10.1007/s00213-014-3571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix J, Nijs J, Ickmans K, Godderis L, Ghosh M, Polli A, 2020. The interplay between oxidative stress, exercise, and pain in health and disease: potential role of autonomic regulation and epigenetic mechanisms. Antioxidants 9, 1166. 10.3390/antiox9111166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeck DM, Fitek DJ, Svikis DS, Montoya ID, Marcus SC, West JC, 2005. Treatment compliance in patients with comorbid psychiatric and substance use disorders. Am. J. Addict 14 (3), 195–207. 10.1080/10550490590949488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Song C, Kuo L, Tennen H, Armeli S, Kranzler HR, 2006. Targeted versus daily naltrexone: secondary analysis of effects on average daily drinking. Alcohol Clin. Exp. Res 30, 860–865. 10.1111/j.1530-0277.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- Herz A, 1997. Endogenous opioid systems and alcohol addiction. Psychopharmacology 129, 99–111. 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Hickey JW, Neumann EK, Radtke AJ, Camarillo JM, Beuschel RT, Albanese A, McDonough E, Hatler J, Wiblin AE, Fisher J, Croteau J, Small EC, Sood A, Caprioli RM, Angelo RM, Nolan GP, Chung K, Hewitt SM, Germain RN, Spraggins JM, Lundberg E, Snyder MP, Kelleher NL, Saka SK, 2022. Spatial mapping of protein composition and tissue organization: a primer for multiplexed antibody-based imaging. Nat. Methods 19, 284–295. 10.1038/s41592-021-01316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, 2009. Opioid receptors in the gastrointestinal tract. Regul. Pept 155, 11–17. 10.1016/j.regpep.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtani T, Nishi M, Takeshima H, Nukada T, Sugimoto T, 1996. Structure and regional distribution of nociceptin/orphanin FQ precursor. Biochem Bioph Res Co 219, 714–719. 10.1006/bbrc.1996.0300. [DOI] [PubMed] [Google Scholar]
- Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR, 1975. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature 258, 577–579. 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- In Y, Minoura K, Tomoo K, Sasaki Y, Lazarus LH, Okada Y, Ishida T, 2005. Structural function of C-terminal amidation of endomorphin. FEBS J. 272, 5079–5097. 10.1111/j.1742-4658.2005.04919.x. [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, O’Donohue TL, 1978. alpha-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc. Natl. Acad. Sci. USA 75, 6300–6304. 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James IF, Chavkin C, Goldstein A, 1982. Selectivity of dynorphin for κ opioid receptors. Life Sci. 31, 1331–1334. 10.1016/0024-3205(82)90374-5. [DOI] [PubMed] [Google Scholar]
- Jarjour S, Bai L, Gianoulakis C, 2009. Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alcohol Clin. Exp. Res 33, 1033–1043. 10.1111/j.1530-0277.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- Jarvis BP, Holtyn AF, Subramaniam S, Tompkins DA, Oga EA, Bigelow GE, Silverman K, 2018. Extended-release injectable naltrexone for opioid use disorder: a systematic review. Addiction 113, 1188–1209. 10.1111/add.14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop DS, Major GN, Coventry TL, Kaye SJ, Fulford AJ, Harbuz MS, Bree FMD, 2000. Novel opioid peptides endomorphin-1 and endomorphin-2 are present in mammalian immune tissues. J. Neuroimmunol 106, 53–59. 10.1016/s0165-5728(99)00216-7. [DOI] [PubMed] [Google Scholar]
- Johanning K, Juliano MA, Juliano L, Lazure C, Lamango NS, Steiner DF, Lindberg I, 1998. Specificity of prohormone convertase 2 on proenkephalin and proenkephalin-related substrates. J. Biol. Chem 273, 22672–22680. 10.1074/jbc.273.35.22672. [DOI] [PubMed] [Google Scholar]
- Johnson BA, 2007. Naltrexone long-acting formulation in the treatment of alcohol dependence. Therapeut. Clin. Risk Manag 3, 741–749. [PMC free article] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC, 2014. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 311, 1889–1900. 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- Jones BN, Stern AS, Lewis RV, Kimura S, Stein S, Udenfriend S, Shively JE, 1980. Structure of two adrenal polypeptides containing multiple enkephalin sequences. Arch. Biochem. Biophys 204, 392–395. 10.1016/0003-9861(80)90048-x. [DOI] [PubMed] [Google Scholar]
- Judd AK, Kaushanskaya A, Tuttle DJ, Sanchez A, Khroyan T, Polgar W, Toll L, 2003. N-terminal modifications leading to peptide ORL1 partial agonists and antagonists. J. Pept. Res 62, 191–198. 10.1034/j.1399-3011.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- Julius D, Renault P (Eds.), 1976. Narcotic Antagonists: Naltrexone Progress Report 9, NIDA Research Monograph Series. US Dept of Health, Education, and Welfare. [PubMed] [Google Scholar]
- Jung JH, Szule JA, Stouder K, Marshall RM, McMahan UJ, 2018. Active zone material-directed orientation, docking, and fusion of dense core vesicles alongside synaptic vesicles at neuromuscular junctions. Front. Neuroanat 12, 72. 10.3389/fnana.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczyńska K, Wojciechowski P, 2021. Non-opioid peptides targeting opioid effects. Int. J. Mol. Sci 22, 13619 10.3390/ijms222413619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Südhof TC, 2011. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 144, 282–295. 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy R, Hillhouse TM, Livingston KE, Kochan KE, Meurice C, Eans SO, Li M-H, White AD, Roques BP, McLaughlin JP, Ingram SL, Burford NT, Alt A, Traynor JR, 2021. Positive allosteric modulation of the mu-opioid receptor produces analgesia with reduced side effects. Proc. Natl. Acad. Sci. USA 118, e2000017118. 10.1073/pnas.2000017118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanvah S, Joseph J, Schuster GB, Barnett RN, Cleveland CL, Landman U, 2010. Oxidation of DNA: damage to nucleobases. Accounts Chem. Res 43, 280–287. 10.1021/ar900175a. [DOI] [PubMed] [Google Scholar]
- Kasai H, Takahashi N, Tokumaru H, 2012. Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol. Rev 92, 1915–1964. 10.1152/physrev.00007.2012. [DOI] [PubMed] [Google Scholar]
- Kayser V, Fournie-Zaluski MC, Guilbaud G, Roques BP, 1989. Potent antinociceptive effects of Kelatorphan (a highly efficient inhibitor of multiple enkephalin-degrading enzymes) systemically administered in normal and arthritic rats. Brain Res. 497, 94–101. 10.1016/0006-8993(89)90974-8. [DOI] [PubMed] [Google Scholar]
- Kemppainen H, Raivio N, Suo-Yrjo V, Kiianmaa K, 2012. Opioidergic modulation of ethanol self-administration in the ventral pallidum. Alcohol Clin. Exp. Res 36, 286–293. 10.1111/j.1530-0277.2011.01611.x. [DOI] [PubMed] [Google Scholar]
- Khan MIH, Sawyer BJ, Akins NS, Le HV, 2022. A systematic review on the kappa opioid receptor and its ligands: new directions for the treatment of pain, anxiety, depression, and drug abuse. Eur. J. Med. Chem 243, 114785 10.1016/j.ejmech.2022.114785. [DOI] [PubMed] [Google Scholar]
- Kim K, Na M, Oh K, Cho E, Han SS, Chang S, 2022. Optimized single-step optical clearing solution for 3D volume imaging of biological structures. Commun Biology 5, 431. 10.1038/s42003-022-03388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CM, Hentges ST, 2011. Relative number and distribution of murine hypothalamic proopiomelanocortin neurons innervating distinct target sites. PLoS One 6, e25864. 10.1371/journal.pone.0025864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL, Walker BM, 2016. Dissociating motivational from physiological withdrawal in alcohol dependence: role of central amygdala κ-opioid receptors. Neuropsychopharmacology 41, 560–567. 10.1038/npp.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi RM, Palkovits M, Miller RJ, Chang K-J, Cuatrecasas P, 1978. Brain enkephalin distribution is unaltered by hypophysectomy. Life Sci. 22, 527–530. 10.1016/0024-3205(78)90434-4. [DOI] [PubMed] [Google Scholar]
- Komiya E, Tominaga M, Hatano R, Kamikubo Y, Toyama S, Sakairi H, Honda K, Itoh T, Kamata Y, Tsurumachi M, Kishi R, Ohnuma K, Sakurai T, Morimoto C, Takamori K, 2022. Peripheral endomorphins drive mechanical alloknesis under the enzymatic control of CD26/DPPIV. J. Allergy Clin. Immunol 149, 1085–1096. 10.1016/j.jaci.2021.08.003. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Linden A, Hytönen HR, Paasikoski N, Vashchinkina E, Dudek M, Herr DR, Hyytiä P, 2017. Continuous delivery of naltrexone and nalmefene leads to tolerance in reducing alcohol drinking and to supersensitivity of brain opioid receptors. Addiction Biol. 22, 1022–1035. 10.1111/adb.12393. [DOI] [PubMed] [Google Scholar]
- Kosterlitz HW, 1987. Endogenous opioids and their receptors. Pol. J. Pharmacol. Pharm 39, 571–576. [PubMed] [Google Scholar]
- Kranzler HR, Soyka M, 2018. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 320, 815–824. 10.1001/jama.2018.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Pizzagalli DA, Smoski M, Mathew SJ, Nurnberger J, Lisanby SH, Iosifescu D, Murrough JW, Yang H, Weiner RD, Calabrese JR, Sanacora G, Hermes G, Keefe RSE, Song A, Goodman W, Szabo ST, Whitton AE, Gao K, Potter WZ, 2020. A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat. Med 26, 760–768. 10.1038/s41591-020-0806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunselman JM, Gupta A, gomes I, Devi LA, Puthenveedu MA, 2021. Compartment-specific opioid receptor signaling is selectively modulated by different Dynorphin peptides. Elife 10, e60270. 10.7554/elife.60270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW, 2015. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat. Neurosci 18, 1230–1232. 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch V, Bornschein G, Loreth D, Bank J, Jordan J, Baur D, Watanabe M, Kulik A, Heckmann M, Eilers J, Schmidt H, 2018. Munc13–3 is required for the developmental localization of Ca2+ channels to active zones and the nanopositioning of Cav2.1 near release sensors. Cell Rep. 22, 1965–1973. 10.1016/j.celrep.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Kuspiel S, Wiemuth D, Gründer S, 2021. The neuropeptide nocistatin is not a direct agonist of acid-sensing ion channel 1a (ASIC1a). Biomol 11, 571. 10.3390/biom11040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Bazov I, Sheedy D, Garrick T, Harper C, Bakalkin G, 2009. Expression of pronociceptin and its receptor is downregulated in the brain of human alcoholics. Brain Res. 1305, S80–S85. 10.1016/j.brainres.2009.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Madjid N, Terenius L, Ogren SO, Bakalkin G, 2006. Big dynorphin, a prodynorphin-derived peptide produces NMDA receptor-mediated effects on memory, anxiolytic-like and locomotor behavior in mice. Neuropsychopharmacology 31, 1928–1937. 10.1038/sj.npp.1300959. [DOI] [PubMed] [Google Scholar]
- Lam MP, Nurmi H, Rouvinen N, Kiianmaa K, Gianoulakis C, 2009. Effects of acute ethanol on β-endorphin release in the nucleus accumbens of selectively bred lines of alcohol-preferring AA and alcohol-avoiding ANA rats. Psychopharmacology 208, 121. 10.1007/s00213-009-1733-y. [DOI] [PubMed] [Google Scholar]
- Lapalu S, Moisand C, Butour J-L, Mollereau C, Meunier J-C, 1998. Different domains of the ORL1 and κ-opioid receptors are involved in recognition of nociceptin and dynorphin A. Febs Lett 427, 296–300. 10.1016/s0014-5793(98)00452-9. [DOI] [PubMed] [Google Scholar]
- Larhammar D, Bergqvist C, Sundström G, 2015. Chapter three ancestral vertebrate complexity of the opioid system. Vitam. Horm 97, 95–122. 10.1016/bs.vh.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Larrinaga G, Perez I, Sanz B, Irazusta A, Zarrazquin I, Sanchez Clara Elena, Sanchez Carmen Elena, Rey ASD, Zabala A, Santaolalla F, 2011. Activity of soluble aminopeptidase A and dipeptidyl peptidase IV and membrane-bound aminopeptidase B and pyroglutamyl peptidase I in adenoid hyperplasia, tonsillar hyperplasia and chronic tonsillitis. Int. J. Pediatr. Otorhinolaryngol 75, 1399–1403. 10.1016/j.ijporl.2011.07.037. [DOI] [PubMed] [Google Scholar]
- Lasher RS, Lutz EM, Mulholland F, Sanderson R, Stewart JM, Bublitz C, 1990. Immunocytochemical localization of endopeptidase-24.11 in the nucleus tractus solitarius of the rat brain. Neurosci. Lett 117, 43–49. 10.1016/0304-3940(90)90117-r. [DOI] [PubMed] [Google Scholar]
- Lee HS, Basbaum AI, 1984. Immunoreactive pro-enkephalin and pro-dynorphin products are differentially distributed within the nucleus of the solitary tract of the rat. J. Comp. Neurol 230, 614–619. 10.1002/cne.902300409. [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Novo P, Bachrach K, Bailey GL, Bhatt S, Farkas S, Fishman M, Gauthier P, Hodgkins CC, King J, Lindblad R, Liu D, Matthews AG, May J, Peavy KM, Ross S, Salazar D, Schkolnik P, Shmueli-Blumberg D, Stablein D, Subramaniam G, Rotrosen J, 2018. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet 391, 309–318. 10.1016/s0140-6736(17)32812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger L, Zheng Z, Bonnet C, Cespuglio R, 1997. Ultrastructural relationships of the pro-opiomelanocortin axons with the serotoninergic neurons in the dorsal raphe nucleus of the rat. Neurosci. Lett 222, 155–158. 10.1016/s0304-3940(97)13363-8. [DOI] [PubMed] [Google Scholar]
- Leighty AE, Ansara ED, 2019. Treatment outcomes of long-acting injectable naltrexone versus oral naltrexone in alcohol use disorder in veterans. Ment Heal Clin 9, 392–396. 10.9740/mhc.2019.11.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy F, Brann DH, Meira T, Siegelbaum SA, 2017. Input-timing-dependent plasticity in the hippocampal CA2 region and its potential role in social memory. Neuron 95, 1089–1102. 10.1016/j.neuron.2017.07.036 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy F, de Solis CA, Boyle LM, Bock T, Lofaro OM, Buss EW, Asok A, Kandel ER, Siegelbaum SA, 2021. Enkephalin release from VIP interneurons in the hippocampal CA2/3a region mediates heterosynaptic plasticity and social memory. Mol. Psychiatr 1–22 10.1038/s41380-021-01124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Keith DE, Evans CJ, 1996. Multiple opioid receptor-like genes are identified in diverse vertebrate phyla. Febs Lett 397, 25–29. 10.1016/s0014-5793(96)01126-x. [DOI] [PubMed] [Google Scholar]
- Lindsay NM, Chen C, Gilam G, Mackey S, Scherrer G, 2021. Brain circuits for pain and its treatment. Sci. Transl. Med 13, eabj7360. 10.1126/scitranslmed.abj7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisi TL, Sluka KA, 2006. A new electrochemical HPLC method for analysis of enkephalins and endomorphins. J. Neurosci. Methods 150, 74–79. 10.1016/j.jneumeth.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Liu S. (Steve), Pickens S, Burma NE, Ibarra-Lecue I, Yang H, Xue L, Cook C, Hakimian JK, Severino AL, Lueptow L, Komarek K, Taylor AMW, Olmstead MC, Carroll FI, Bass CE, Andrews AM, Walwyn W, Trang T, Evans CJ, Leslie FM, Cahill CM, 2019. Kappa opioid receptors drive a tonic aversive component of chronic pain. J. Neurosci 39, 4162–4178. 10.1523/jneurosci.0274-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhao L, Wang Y, Zhou J, Wang D, Zhang Y, Zhang X, Wang Z, Yang D, Mou L, Wang R, 2017. MEL-N16: a series of novel endomorphin analogs with good analgesic activity and a favorable side effect profile. ACS Chem. Neurosci 8, 2180–2193. 10.1021/acschemneuro.7b00097. [DOI] [PubMed] [Google Scholar]
- Livett BG, Dean DM, Whelan LG, Udenfriend S, Rossier J, 1981. Co-release of enkephalin and catecholamines from cultured adrenal chromaffin cells. Nature 289, 317–319. 10.1038/289317a0. [DOI] [PubMed] [Google Scholar]
- Lord JAH, Waterfield AA, Hughes J, Kosterlitz HW, 1977. Endogenous opioid peptides: multiple agonists and receptors. Nature 267, 495–499. 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Luo F, Li M, Florence C, 2021. State-level economic costs of opioid use disorder and fatal opioid overdose — United States, 2017. Morbidity Mortal Wkly Rep 70, 541–546. 10.15585/mmwr.mm7015a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrouk OS, Li Q, Song P, Kennedy RT, 2011. Microdialysis and mass spectrometric monitoring of dopamine and enkephalins in the globus pallidus reveal reciprocal interactions that regulate movement. J. Neurochem 118, 24–33. 10.1111/j.1471-4159.2011.07293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malovan G, Hierzberger B, Suraci S, Schaefer M, Santos K, Jha S, Macheroux P, 2022. The emerging role of dipeptidyl peptidase 3 in pathophysiology. FEBS J. 10.1111/febs.16429. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Peindl K, Masand PS, Patkar AA, 2014. Long-acting injectable naltrexone for the treatment of alcohol dependence. Expert Rev. Neurother 7, 1265–1277. 10.1586/14737175.7.10.1265. [DOI] [PubMed] [Google Scholar]
- Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H, 1995. The cloned μ, δ and κ receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 700, 89–98. 10.1016/0006-8993(95)00928-j. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA, 1998. Chronic administration of cannabinoids regulates proenkephalin mRNA levels in selected regions of the rat brain. Mol. Brain Res 55, 126–132. 10.1016/s0169-328x(97)00371-9. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Densmore VS, Osborne PB, 2007. Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J. Comp. Neurol 504, 702–715. 10.1002/cne.21464. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM, 2008. δ-Opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J. Neurosci 28, 12672–12681. 10.1523/jneurosci.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL, 2003. κ-Opioid agonists directly inhibit midbrain dopaminergic neurons. J. Neurosci 23, 9981–9986. 10.1523/jneurosci.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Karkhanis AN, 2019. Dopaminergic cellular and circuit contributions to kappa opioid receptor mediated aversion. Neurochem. Int 129, 104504 10.1016/j.neuint.2019.104504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie BS, 2019. Assessing patients’ risk for opioid use disorder. AACN Adv. Crit. Care 30, 343–352. 10.4037/aacnacc2019931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Bai L, Quirion R, Gianoulakis C, 2005. A microdialysis profile of met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcohol Clin. Exp. Res 29, 1821–1828. 10.1097/01.alc.0000183008.62955.2e. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C, 2006. A microdialysis profile of dynorphin A1–8 release in the rat nucleus accumbens following alcohol administration. Alcohol Clin. Exp. Res 30, 982–990. 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C, 2003. A microdialysis profile of β-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology 169, 60–67. 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE, 1976. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Therapeut 197, 517–532. [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE, 1999. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J. Comp. Neurol 405, 450–471. [PubMed] [Google Scholar]
- Martin-Schild S, Zadina JE, Gerall AA, Vigh S, Kastin AJ, 1997. Localization of endomorphin-2-like immunoreactivity in the rat medulla and spinal cord. Peptides 18, 1641–1649. 10.1016/s0196-9781(97)00320-3. [DOI] [PubMed] [Google Scholar]
- Matsushima A, Sese J, Koyanagi KO, 2019. Biosynthetic short neuropeptides: a rational theory based on experimental results for the missing pain-relief opioid endomorphin precursor gene. Chembiochem 20, 2054–2058. 10.1002/cbic.201900317. [DOI] [PubMed] [Google Scholar]
- Matzeu A, Terenius L, Martin-Fardon R, 2018. Exploring sex differences in the attenuation of ethanol drinking by naltrexone in dependent rats during early and protracted abstinence. Alcohol Clin. Exp. Res 42, 2466–2478. 10.1111/acer.13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConalogue K, Grady EF, Minnis J, Balestra B, Tonini M, Brecha NC, Bunnett NW, Sternini C, 1999. Activation and internalization of the μ-opioid receptor by the newly discovered endogenous agonists, endomorphin-1 and endomorphin-2††These authors contributed equally to this work. Neuroscience 90, 1051–1059. 10.1016/s0306-4522(98)00514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentlein R, 1999. Dipeptidyl-peptidase IV (CD26)-role in the inactivation of regulatory peptides. Regul. Pept 85, 9–24. 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- Merg F, Filliol D, Usynin I, Bazov I, Bark N, Hurd YL, Yakovleva T, Kieffer BL, Bakalkin G, 2006. Big dynorphin as a putative endogenous ligand for the κ-opioid receptor. J. Neurochem 97, 292–301. 10.1111/j.1471-4159.2006.03732.x. [DOI] [PubMed] [Google Scholar]
- Merrer JL, Becker JAJ, Befort K, Kieffer BL, 2009. Reward processing by the opioid system in the brain. Physiol. Rev 89, 1379–1412. 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz MJ, Daimon CM, King CM, Rau AR, Hentges ST, 2021. Individual arcuate nucleus proopiomelanocortin neurons project to select target sites. Am J Physiology-regulatory Integr Comp Physiology 321, R982–R989. 10.1152/ajpregu.00169.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz MJ, Pennock RL, Krapf D, Hentges ST, 2019. Temporal dependence of shifts in mu opioid receptor mobility at the cell surface after agonist binding observed by single-particle tracking. Sci Rep-uk 9, 7297. 10.1038/s41598-019-43657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J-C, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour J-L, Guillemot J-C, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J, 1995. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377, 532–535. 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Millington GW, 2007. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr. Metabol 4, 18. 10.1186/1743-7075-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami K, Sudo Y, Shiraishi S, Seo M, Uezono Y, 2010. Analysis of the effects of anesthetics and ethanol on μ-opioid receptor. J. Pharmacol. Sci 112, 424–431. 10.1254/jphs.10003fp. [DOI] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A, 2011. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Db Syst Rev CD001333. 10.1002/14651858.cd001333.pub4. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Bergren LJ, Chen KS, Rowbotham MC, Fields HL, 2009. Naltrexone aversion and treatment efficacy are greatest in humans and rats that actively consume high levels of alcohol. Neurobiol. Dis 33, 72–80. 10.1016/j.nbd.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL, 2005. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology 182, 384–392. 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL, 2012. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci. Transl. Med 4, 116ra6. 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Tavares VC, Fields HL, D’Esposito M, Boettiger CA, 2007. Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls. Neuropsychopharmacology 32, 439–449. 10.1038/sj.npp.1301226. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour J-L, Moisand C, Chalon P, Caput D, Vassart G, Meunier J-C, 1994. ORL1, a novel member of the opioid receptor family. Febs Lett 341, 33–38. 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Morales M, Margolis EB, 2017. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci 18, 73–85. 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
- Moss M, Glazer E, Basbaum A, 1983. The peptidergic organization of the cat periaqueductal gray. I. The distribution of immunoreactive enkephalin-containing neurons and terminals. J. Neurosci 3, 603–616. 10.1523/jneurosci.03-03-00603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss MS, Basbaum AI, 1983. The fine structure of the caudal periaqueductal gray of the cat: morphology and synaptic organization of normal and immunoreactive enkephalin-labeled profiles. Brain Res. 289, 27–43. 10.1016/0006-8993(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Mumford RA, Pierzchala PA, Strauss AW, Zimmerman M, 1981. Purification of a membrane-bound metalloendopeptidase from porcine kidney that degrades peptide hormones. Proc. Natl. Acad. Sci. USA 78, 6623–6627. 10.1073/pnas.78.11.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakawa H, Iwata K, Takeshita T, Ozawa H, 2016. Immunoelectron microscopic observation of the subcellular localization of kisspeptin, neurokinin B and dynorphin A in KNDy neurons in the arcuate nucleus of the female rat. Neurosci. Lett 612, 161–166. 10.1016/j.neulet.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Murray E, Cho JH, Goodwin D, Ku T, Swaney J, Kim S-Y, Choi H, Park Y-G, Park J-Y, Hubbert A, McCue M, Vassallo S, Bakh N, Frosch MP, Wedeen VJ, Seung HS, Chung K, 2015. Simple, scalable proteomic imaging for high-dimensional profiling of intact systems. Cell 163, 1500–1514. 10.1016/j.cell.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA, 2014. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc. Natl. Acad. Sci. USA 111, E1648–E1655. 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T, 2006. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 31, 739–750. 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Narita M, Mizoguchi H, Oji GS, Tseng EL, Suganuma C, Nagase H, Tseng LF, 1998. Characterization of endomorphin-1 and −2 on [35S]GTPγS binding in the mouse spinal cord. Eur. J. Pharmacol 351, 383–387. 10.1016/s0014-2999(98)00395-1. [DOI] [PubMed] [Google Scholar]
- Neal CR, Newman SW, 1989. Prodynorphin peptide distribution in the forebrain of the syrian hamster and rat: a comparative study with antisera against dynorphin A, dynorphin B, and the C-terminus of the prodynorphin precursor molecule. J. Comp. Neurol 288, 353–386. 10.1002/cne.902880302. [DOI] [PubMed] [Google Scholar]
- NRC, 1978. Clinical evaluation of naltrexone treatment of opiate-dependent individuals: report of the national research council committee on clinical evaluation of narcotic antagonists. Arch. Gen. Psychiatr 35, 335–340. 10.1001/archpsyc.1978.01770270085008. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Volpicelli LA, Volpicelli JR, 1996. Naltrexone in the treatment of alcoholism: a clinical review. Alcohol 13, 35–39. 10.1016/0741-8329(95)02038-1. [DOI] [PubMed] [Google Scholar]
- Okuda-Ashitaka E, Minami T, Tachibana S, Yoshihara Y, Nishiuchi Y, Kimura T, Ito S, 1998. Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature 392, 286–289. 10.1038/32660. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Huang Y, Goodwin S, Ciobanu DC, Lu L, Sutter TR, Winder DG, 2008. Microarray analysis reveals distinctive signaling between the bed nucleus of the stria terminalis, nucleus accumbens, and dorsal striatum. Physiol. Genom 32, 283–298. 10.1152/physiolgenomics.00224.2006. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR, 2007. Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. J. Clin. Psychopharmacol 27, 507–512. 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Rode S, Schottenfeld R, Meyer RE, Rounsaville B, 1996. Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Arch. Gen. Psychiatr 53, 217–224. 10.1001/archpsyc.1996.01830030039007. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B, 1992. Naltrexone and coping skills therapy for alcohol dependence: a controlled study. Arch. Gen. Psychiatr 49, 881–887. 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, O’Connor PG, 2000. Naltrexone-induced nausea in patients treated for alcohol dependence: clinical predictors and evidence for opioid-mediated effects. J. Clin. Psychopharmacol 20, 69–76. 10.1097/00004714-200002000-00012. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Lynch KG, Pettinati HM, Kampman KM, Gariti P, Gelfand L, Have TT, Wortman S, Dundon W, Dackis C, Volpicelli JR, O’Brien CP, 2008. A placebo-controlled randomized clinical trial of naltrexone in the context of different levels of psychosocial intervention. Alcohol Clin. Exp. Res 32, 1299–1308. 10.1111/j.1530-0277.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmakov DI, Koshelev SG, Ivanov IA, Andreev YA, Kozlov SA, 2019. Endogenous neuropeptide nocistatin is a direct agonist of acid-sensing ion channels (ASIC1, ASIC2 and ASIC3). Biomol 9, 401. 10.3390/biom9090401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CB, Meyrath M, Canals M, Kostenis E, Chevigné A, Szpakowska M, 2021. Atypical opioid receptors: unconventional biology and therapeutic opportunities. Pharmacol. Therapeut 233, 108014 10.1016/j.pharmthera.2021.108014. [DOI] [PubMed] [Google Scholar]
- Palmer RHC, Brick LA, Chou Y-L, Agrawal A, McGeary JE, Heath AC, Bierut L, Keller MC, Johnson E, Hartz SM, Schuckit MA, Knopik VS, 2019. The etiology of DSM-5 alcohol use disorder: evidence of shared and non-shared additive genetic effects. Drug Alcohol Depend. 201, 147–154. 10.1016/j.drugalcdep.2018.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KE, Pedersen CE, Gomez AM, Spangler SM, Walicki MC, Feng SY, Stewart SL, Otis JM, Al-Hasani R, McCall JG, Sakers K, Bhatti DL, Copits BA, Gereau RW, Jhou T, Kash TJ, Dougherty JD, Stuber GD, Bruchas MR, 2019. A paranigral VTA nociceptin circuit that constrains motivation for reward. Cell 178, 653–671. 10.1016/j.cell.2019.06.034 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KE, Sugiarto E, Taylor AM, Pradhan A, Al-Hasani R, 2020. Pain, motivation, migraine and the microbiome: new frontiers for opioid systems and disease. Mol. Pharmacol 98 10.1124/mol.120.119438 mol.120.119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak GW, Pan Y-X, 2013. Mu opioids and their receptors: evolution of a concept. Pharmacol. Rev 65, 1257–1317. 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, McKee SA, 2019. Sex differences in stress-related alcohol use. Neurobiology Stress 10, 100149. 10.1016/j.ynstr.2019.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocchi JA, de Almeida DL, Paiva-Lima P, Queiroz-Junior C, Caliari MV, Duarte IDG, Romero TRL, 2019. Peripheral antinociception induced by ketamine is mediated by the endogenous opioid system. Eur. J. Pharmacol 865, 172808 10.1016/j.ejphar.2019.172808. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Silverman BL, Battisti JJ, Forman R, Schweizer E, Gastfriend DR, 2011. Efficacy of extended-release naltrexone in patients with relatively higher severity of alcohol dependence. Alcohol Clin. Exp. Res 35, 1804–1811. 10.1111/j.1530-0277.2011.01524.x. [DOI] [PubMed] [Google Scholar]
- Phillips RA, Tuscher JJ, Black SL, Andraka E, Fitzgerald ND, Ianov L, Day JJ, 2022. An atlas of transcriptionally defined cell populations in the rat ventral tegmental area. Cell Rep. 39, 110616 10.1016/j.celrep.2022.110616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Sesack SR, 1993. Cellular substrates for interactions between dynorphin terminals and dopamine dendrites in rat ventral tegmental area and substantia nigra. Brain Res. 602, 275–289. 10.1016/0006-8993(93)90693-h. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Veznedaroglu E, Milner TA, 1995. Neuropeptide Y and dynorphin-immunoreactive large dense-core vesicles are strategically localized for presynaptic modulation in the hippocampal formation and substantia nigra. Synapse 19, 160–169. 10.1002/syn.890190303. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Sumal KK, Beckley SC, Miller RJ, Reis DJ, 1980. Immunocytochemical localization of enkephalin in the neostriatum of rat brain: a light and electron microscopic study. J. Comp. Neurol 189, 721–740. 10.1002/cne.901890408. [DOI] [PubMed] [Google Scholar]
- Piskorowski RA, Chevaleyre V, 2013. Delta-opioid receptors mediate unique plasticity onto parvalbumin-expressing interneurons in area CA2 of the Hippocampus. J. Neurosci 33, 14567–14578. 10.1523/jneurosci.0649-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Smoski M, Ang Y-S, Whitton AE, Sanacora G, Mathew SJ, Nurnberger J, Lisanby SH, Iosifescu DV, Murrough JW, Yang H, Weiner RD, Calabrese JR, Goodman W, Potter WZ, Krystal AD, 2020. Selective kappa-opioid antagonism ameliorates anhedonic behavior: evidence from the fast-fail trial in mood and anxiety spectrum disorders (FAST-MAS). Neuropsychopharmacology 45, 1656–1663. 10.1038/s41386-020-0738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova D, Desai N, Blendy JA, Pang ZP, 2019. Synaptic regulation by OPRM1 variants in reward neurocircuitry. J. Neurosci 39, 5685–5696. 10.1523/jneurosci.2317-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese PS, 1989. Bivalent ligands and the message-address concept in the design of selective opioid receptor antagonists. Trends Pharmacol. Sci 10, 230–235. 10.1016/0165-6147(89)90267-8. [DOI] [PubMed] [Google Scholar]
- Poulin J, Castonguay-Lebel Z, Laforest S, Drolet G, 2008. Enkephalin co-expression with classic neurotransmitters in the amygdaloid complex of the rat. J. Comp. Neurol 506, 943–959. 10.1002/cne.21587. [DOI] [PubMed] [Google Scholar]
- Poulin J-F, Arbour D, Laforest S, Drolet G, 2009. Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacology Biological Psychiatry 33, 1356–1365. 10.1016/j.pnpbp.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Poulin J-F, Caronia G, Hofer C, Cui Q, Helm B, Ramakrishnan C, Chan SC, Dombeck DA, Deisseroth K, Awatramani R, 2018. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci 21, 1260–1271. 10.1038/s41593-018-0203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gavériaux-Ruff C, Kieffer BL, 2011. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol. Sci 32, 581–590. 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psifogeorgou K, Psigfogeorgou K, Terzi D, Papachatzaki MM, Varidaki A, Ferguson D, Gold SJ, Zachariou V, 2011. A unique role of RGS9–2 in the striatum as a positive or negative regulator of opiate analgesia. J. Neurosci 31, 5617–5624. 10.1523/jneurosci.4146-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillan JM, Sadée W, 1997. Dynorphin peptides: antagonists of melanocortin receptors. Pharmaceut. Res 14, 713–719. 10.1023/a:1012185919153. [DOI] [PubMed] [Google Scholar]
- Ravanat J-L, Douki T, Duez P, Gremaud E, Herbert K, Hofer T, Lasserre L, Saint-Pierre C, Favier A, Cadet J, 2002. Cellular background level of 8-oxo-7,8-dihydro-2′-deoxyguanosine: an isotope based method to evaluate artefactual oxidation of DNA during its extraction and subsequent work-up. Carcinogenesis 23, 1911–1918. 10.1093/carcin/23.11.1911. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Tennant FS, 1984. Five-year follow-up of opiate addicts with naltrexone and behavior therapy. NIDA Res. Monogr 49, 289–295. [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, 2007. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch. Gen. Psychiatr 64, 1069–1077. 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ray LA, Meredith LR, Kiluk BD, Walthers J, Carroll KM, Magill M, 2020. Combined pharmacotherapy and cognitive behavioral therapy for adults with alcohol or substance use disorders. JAMA Netw. Open 3, e208279. 10.1001/jamanetworkopen.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, 1986. The co-occurrence of substance P-like immunoreactivity and dynorphin-like immunoreactivity in striatopallidal and striatonigral projection neurons in birds and reptiles. Brain Res. 371, 155–161. 10.1016/0006-8993(86)90821-8. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Ardati A, Monsma FJ, Civelli O, 1996. Structure-activity relationship studies on the novel neuropeptide orphanin FQ. J. Biol. Chem 271, 14163–14168. 10.1074/jbc.271.24.14163. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker H-P, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, M FJ Jr., Civelli O, 1995. Orphanin fq: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270, 792–794. 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Reyes BAS, Glaser JD, Magtoto R, Bockstaele EJV, 2006. Pro-opiomelanocortin colocalizes with corticotropin- releasing factor in axon terminals of the noradrenergic nucleus locus coeruleus. Eur. J. Neurosci 23, 2067–2077. 10.1111/j.1460-9568.2006.04744.x. [DOI] [PubMed] [Google Scholar]
- Riesenberg R, Nitsch C, 1990. Two different types of dynorphin-A-immunoreactive terminals in rat substantia nigra. Cell Tissue Res. 261, 107–113. 10.1007/bf00329443. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Vardy E, DiBerto JF, Chefer VI, White KL, Fish EW, Chen M, Gigante E, Krouse MC, Sun H, Thorsell A, Roth BL, Heilig M, Malanga CJ, 2015. Receptor reserve moderates mesolimbic responses to opioids in a humanized mouse model of the OPRM1 A118G polymorphism. Neuropsychopharmacology 40, 2614–2622. 10.1038/npp.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Ung RL, Nomura H, Otis JM, Basiri ML, Namboodiri VMK, Zhu X, Robinson JE, van den Munkhof HE, McHenry JA, Eckman LEH, Kosyk O, Jhou TC, Kash TL, Bruchas MR, Stuber GD, 2020. Prepronociceptin-expressing neurons in the extended amygdala encode and promote rapid arousal responses to motivationally salient stimuli. Cell Rep. 33, 108362 10.1016/j.celrep.2020.108362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, 2004. What place does naltrexone have in the treatment of alcoholism? CNS Drugs 18, 547–560. 10.2165/00023210-200418090-00001. [DOI] [PubMed] [Google Scholar]
- Roldán-Sastre A, Aguado C, Martín-Belmonte A, Alfaro-Ruiz R, Moreno-Martínez AE, Luján R, 2021. Cellular diversity and differential subcellular localization of the G-protein Gαo subunit in the mouse cerebellum. Front. Neuroanat 15, 686279 10.3389/fnana.2021.686279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rónai AZ, Székely JI, Berzétei I, Miglécz E, Bajusz S, 1979. Tetrapeptide-amide analogues of enkephalin: the role of C-terminus in determining the character of opioid activity. Biochem Bioph Res Co 91, 1239–1249. 10.1016/0006-291x(79)91200-2. [DOI] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Ciccocioppo R, Wong CJ, Witkin JM, Martinez-Grau MA, Stopponi S, Adams BL, Katner JS, Perry KW, Toledo MA, Diaz N, Lafuente C, Jiménez A, Benito A, Pedregal C, Weiss F, Statnick MA, 2016. A novel, orally bioavailable nociceptin receptor antagonist, LY2940094, reduces ethanol self-administration and ethanol seeking in animal models. Alcohol Clin. Exp. Res 40, 945–954. 10.1111/acer.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, McCool BA, Jones SR, 2016. Supersensitive kappa opioid receptors promotes ethanol withdrawal-related behaviors and reduce dopamine signaling in the nucleus accumbens. Int. J. Neuropsychopharmacol 19, pyv127. 10.1093/ijnp/pyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi GC, Pellegrino M, Shane R, Abbadie CA, Dustman J, Jimenez C, Bodnar RJ, Pasternak GW, Allen RG, 2002. Characterization of rat prepro-orphanin FQ/Nociceptin(154–181): nociceptive processing in supraspinal sites. J. Pharmacol. Exp. Therapeut 300, 257–264. 10.1124/jpet.300.1.257. [DOI] [PubMed] [Google Scholar]
- Sakurada C, Sakurada S, Hayashi T, Katsuyama S, Tan-No K, Sakurada T, 2003. Degradation of endomorphin-2 at the supraspinal level in mice is initiated by dipeptidyl peptidase IV: an in vitro and in vivo study. Biochem. Pharmacol 66, 653–661. 10.1016/s0006-2952(03)00391-5. [DOI] [PubMed] [Google Scholar]
- Sandin J, Tan-No K, Kasakov L, Nylander I, Winter A, Silberring J, Terenius L, 1997. Differential metabolism of dynorphins in substantia nigra, striatum, and Hippocampus. Peptides 18, 949–956. 10.1016/s0196-9781(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Sapio MR, Iadarola MJ, Loydpierson AJ, Kim JJ, Thierry-Mieg D, Thierry-Mieg J, Maric D, Mannes AJ, 2020. Dynorphin and enkephalin opioid peptides and transcripts in spinal cord and dorsal root ganglion during peripheral inflammatory hyperalgesia and allodynia. J. Pain 21, 988–1004. 10.1016/j.jpain.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Kawano S, Kohara H, Watanabe H, Ambo A, 2006. ORL1 and opioid receptor preferences of nociceptin and dynorphin A analogues with Dmp substituted for N-terminal aromatic residues. Bioorg. Med. Chem 14, 2433–2437. 10.1016/j.bmc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Gähwiler BH, Charpak S, 1998. Target cell-specific modulation of transmitter release at terminals from a single axon. Proc. Natl. Acad. Sci. USA 95, 12004–12009. 10.1073/pnas.95.20.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick MR, Spillane NS, Hostetler KL, 2020. A call to action: a systematic review examining the failure to include females and members of minoritized racial/ethnic groups in clinical trials of pharmacological treatments for alcohol use disorder. Alcohol Clin. Exp. Res 44, 1933–1951. 10.1111/acer.14440. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Cassin J, Zhang Y, Levran O, Ho A, Kreek MJ, 2011. Regional mRNA expression of the endogenous opioid and dopaminergic systems in brains of C57BL/6J and 129P3/J mice: strain and heroin effects. Pharmacol., Biochem. Behav 100, 8–16. 10.1016/j.pbb.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Han Y, Kochubey O, 2012. Ca2+ channels and transmitter release at the active zone. Cell Calcium 52, 199–207. 10.1016/j.ceca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, 2004. Findings across subgroups regarding the level of response to alcohol as a risk factor for alcohol use disorders: a college population of women and latinos. Alcohol Clin. Exp. Res 28, 1499–1508. 10.1097/01.alc.0000141814.80716.32. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Danko GP, 2005. A cross-generational comparison of alcohol challenges at about age 20 in 40 father-offspring pairs. Alcohol Clin. Exp. Res 29, 1921–1927. 10.1097/01.alc.0000187154.94681.65. [DOI] [PubMed] [Google Scholar]
- Schwyzer R, 1977. ACTH: a short introductory review. Ann Ny Acad Sci 297, 3–26. 10.1111/j.1749-6632.1977.tb41843.x. [DOI] [PubMed] [Google Scholar]
- Sesack S, Pickel V, 1992. Dual ultrastructural localization of enkephalin and tyrosine hydroxylase immunoreactivity in the rat ventral tegmental area: multiple substrates for opiate-dopamine interactions. J. Neurosci 12, 1335–1350. 10.1523/jneurosci.12-04-01335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM, 1995. Ultrastructural relationships between terminals immunoreactive for enkephalin, GABA, or both transmitters in the rat ventral tegmental area. Brain Res. 672, 261–275. 10.1016/0006-8993(94)01391-T. [DOI] [PubMed] [Google Scholar]
- Shane R, Wilk S, Bodnar RJ, 1999. Modulation of endomorphin-2-induced analgesia by dipeptidyl peptidase IV. Brain Res. 815, 278–286. 10.1016/s0006-8993(98)01121-4. [DOI] [PubMed] [Google Scholar]
- Sherwood TW, Askwith CC, 2009. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J. Neurosci 29, 14371–14380. 10.1523/jneurosci.2186-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silm K, Yang J, Marcott PF, Asensio CS, Eriksen J, Guthrie DA, Newman AH, Ford CP, Edwards RH, 2019. Synaptic vesicle recycling pathway determines neurotransmitter content and release properties. Neuron 102, 786–800. 10.1016/j.neuron.2019.03.031 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons ML, Terman GW, Gibbs SM, Chavkin C, 1995. L-type calcium channels mediate dynorphin neuropeptide release from dendrites but not axons of hippocampal granule cells. Neuron 14, 1265–1272. 10.1016/0896-6273(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Smith HS, 2009. Opioid metabolism. Mayo Clin. Proc 84, 613–624. 10.1016/s0025-6196(11)60750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, 1979. Immunoreactive β-endorphin and acth in the same neurons of the hypothalamic arcuate nucleus in the rat. Am. J. Anat 154, 283–289. 10.1002/aja.1001540212. [DOI] [PubMed] [Google Scholar]
- Solati J, 2011. Dorsal hippocampal N-methyl-d-aspartate glutamatergic and δ-opioidergic systems modulate anxiety behaviors in rats in a noninteractive manner. Kaohsiung J. Med. Sci 27, 485–493. 10.1016/j.kjms.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Solati J, Zarrindast M, Salari A, 2010. Dorsal hippocampal opioidergic system modulates anxiety-like behaviors in adult male Wistar rats. Psychiatr. Clin. Neurosci 64, 634–641. 10.1111/j.1440-1819.2010.02143.x. [DOI] [PubMed] [Google Scholar]
- Solecki W, Ziolkowska B, Krowka T, Gieryk A, Filip M, Przewlocki R, 2009. Alterations of prodynorphin gene expression in the rat mesocorticolimbic system during heroin self-administration. Brain Res. 1255, 113–121. 10.1016/j.brainres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP, 2010. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanolconditioned place preference and self-administration. Psychopharmacology 210, 199–209. 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Stevens CW, 2009. The evolution of vertebrate opioid receptors. Front. Biosci. ume, 1247. 10.2741/3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg MF, Volpicelli JR, O’Brien CP, 1998. Effects of naltrexone administered repeatedly across 30 or 60 Days on ethanol consumption using a limited access procedure in the rat. Alcohol Clin. Exp. Res 22, 2186–2191. 10.1111/j.1530-0277.1998.tb05932.x. [DOI] [PubMed] [Google Scholar]
- Sukhov RR, Walker LC, Rance NE, Price DL, Young WS, 1995. Opioid precursor gene expression in the human hypothalamus. J. Comp. Neurol 353, 604–622. 10.1002/cne.903530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Ritchie K, Kajikawa E, Fujiwara T, Kusumi A, 2005. Rapid hop diffusion of a G-protein-coupled receptor in the plasma membrane as revealed by single-molecule techniques. Biophys. J 88, 3659–3680. 10.1529/biophysj.104.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson V, Natarajan KN, Ly L-H, Miragaia RJ, Labalette C, Macaulay IC, Cvejic A, Teichmann SA, 2017. Power analysis of single-cell RNA-sequencing experiments. Nat. Methods 14, 381–387. 10.1038/nmeth.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori AE, Portoghese PS, 1984. Comparative antagonism by naltrexone and naloxone of mu, kappa, and delta agonists. Eur. J. Pharmacol 104 (1–2), 101–104. 10.1016/0014-2999(84)90374-1. [DOI] [PubMed] [Google Scholar]
- Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, Lao K, Surani MA, 2009. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382. 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- Tan-No K, Esashi A, Nakagawasai O, Niijima F, Tadano T, Sakurada C, Sakurada T, Bakalkin G, Terenius L, Kisara K, 2002. Intrathecally administered big dynorphin, a prodynorphin-derived peptide, produces nociceptive behavior through an N-methyl-d-aspartate receptor mechanism. Brain Res. 952, 7–14. 10.1016/s0006-8993(02)03180-3. [DOI] [PubMed] [Google Scholar]
- Terskiy A, Wannemacher KM, Yadav PN, Tsai M, Tian B, Howells RD, 2007. Search of the human proteome for endomorphin-1 and endomorphin-2 precursor proteins. Life Sci. 81, 1593–1601. 10.1016/j.lfs.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GL, Lane JR, Coudrat T, Sexton PM, Christopoulos A, Canals M, 2016. Systematic analysis of factors influencing observations of biased agonism at the mu-opioid receptor. Biochem. Pharmacol 113, 70–87. 10.1016/j.bcp.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Thompson GL, Lane JR, Coudrat T, Sexton PM, Christopoulos A, Canals M, 2015. Biased agonism of endogenous opioid peptides at the μ-opioid receptor. Mol. Pharmacol 88, 335–346. 10.1124/mol.115.098848. [DOI] [PubMed] [Google Scholar]
- Thurner P, Gsandtner I, Kudlacek O, Choquet D, Nanoff C, Freissmuth M, Zezula J, 2014. A two-state model for the diffusion of the A2A adenosine receptor in hippocampal neurons AGONIST-INDUCED SWITCH TO SLOW MOBILITY IS MODIFIED BY SYNAPSE-ASSOCIATED PROTEIN 102 (SAP102)*. J. Biol. Chem 289, 9263–9274. 10.1074/jbc.m113.505685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Bruchas MR, Calo’ G, Cox BM, Zaveri NT, 2016. Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol. Rev 68, 419–457. 10.1124/pr.114.009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treatment, C. for S.A. (Ed.), 2009. Incorporating Alcohol Pharmacotherapies into Medical Practice. [PubMed]
- Trieu BH, Remmers BC, Toddes C, Brandner DD, Lefevre EM, Kocharian A, Retzlaff CL, Dick RM, Mashal MA, Gauthier EA, Xie W, Zhang Y, More SS, Rothwell PE, 2022. Angiotensin-converting enzyme gates brain circuit–specific plasticity via an endogenous opioid. Science 375, 1177–1182. 10.1126/science.abl5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, Kendler KS, 2015. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol. Med 45, 1061–1072. 10.1017/s0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viden A, Ch’ng SS, Walker LC, Shesham A, Hamilton SM, Smith CM, Lawrence AJ, 2022. Organisation of enkephalin inputs and outputs of the central nucleus of the amygdala in mice. J. Chem. Neuroanat 125, 102167 10.1016/j.jchemneu.2022.102167. [DOI] [PubMed] [Google Scholar]
- Vincent S, Hökfelt T, Christensson I, Terenius L, 1982. Immunohistochemical evidence for a dynorphin immunoreactive striato-nigral pathway. Eur. J. Pharmacol 85, 251–252. 10.1016/0014-2999(82)90477-0. [DOI] [PubMed] [Google Scholar]
- Viveros OH, Diliberto EJ, Hazum E, Chang KJ, 1979. Opiate-like materials in the adrenal medulla: evidence for storage and secretion with catecholamines. Mol. Pharmacol 16, 1101–1108. [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP, 1992. Naltrexone in the treatment of alcohol dependence. Arch. Gen. Psychiatr 49, 876–880. 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP, 1997. Naltrexone and alcohol dependence: role of subject compliance. Arch. Gen. Psychiatr 54, 737–742. 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- de Waele J-P, Gianoulakis C, 1993. Effects of single and repeated exposures to ethanol on hypothalamic β-endorphin and CRH release by the C57bl/6 and DBA/2 strains of mice. Neuroendocrinology 57, 700–709. 10.1159/000126428. [DOI] [PubMed] [Google Scholar]
- Waksman G, Bouboutou R, Devin J, Bourgoin S, Cesselin F, Hamon M, Fournie-Zaluski M-C, Roques BP, 1985. In vitro and in vivo effects of kelatorphan on enkephalin metabolism in rodent brain. Eur. J. Pharmacol 117, 233–243. 10.1016/0014-2999(85)90608-9. [DOI] [PubMed] [Google Scholar]
- Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, Aliev F, Bacanu S-A, Batzler A, Bertelsen S, Biernacka JM, Bigdeli TB, Chen L-S, Clarke T-K, Chou Y-L, Degenhardt F, Docherty AR, Edwards AC, Fontanillas P, Foo JC, Fox L, Frank J, Giegling I, Gordon S, Hack LM, Hartmann AM, Hartz SM, Heilmann-Heimbach S, Herms S, Hodgkinson C, Hoffmann P, Hottenga JJ, Kennedy MA, Alanne-Kinnunen M, Konte B, Lahti J, Lahti-Pulkkinen M, Lai D, Ligthart L, Loukola A, Maher BS, Mbarek H, McIntosh AM, McQueen MB, Meyers JL, Milaneschi Y, Palviainen T, Pearson JF, Peterson RE, Ripatti S, Ryu E, Saccone NL, Salvatore JE, Sanchez-Roige S, Schwandt M, Sherva R, Streit F, Strohmaier J, Thomas N, Wang J-C, Webb BT, Wedow R, Wetherill L, Wills AG, Agee M, Alipanahi B, Auton A, Bell RK, Bryc K, Elson SL, Fontanillas P, Furlotte NA, Hinds DA, Huber KE, Kleinman A, Litterman NK, McCreight JC, McIntyre MH, Mountain JL, Noblin ES, Northover CAM, Pitts SJ, Sathirapongsasuti JF, Sazonova OV, Shelton JF, Shringarpure S, Tian C, Tung JY, Vacic V, Wilson CH, Boardman JD, Chen D, Choi D-S, Copeland WE, Culverhouse RC, Dahmen N, Degenhardt L, Domingue BW, Elson SL, Frye MA, Gäbel W, Hayward C, Ising M, Keyes M, Kiefer F, Kramer J, Kuperman S, Lucae S, Lynskey MT, Maier W, Mann K,Männistö S, Müller-Myhsok B, Murray AD, Nurnberger JI, Palotie A, Preuss U, Räikkönen K, Reynolds MD, Ridinger M, Scherbaum N, Schuckit MA, Soyka M, Treutlein J, Witt S, Wodarz N, Zill P, Adkins DE, Boden JM, Boomsma DI, Bierut LJ, Brown SA, Bucholz KK, Cichon S, Costello EJ, de Wit H, Diazgranados N, Dick DM, Eriksson JG, Farrer LA, Foroud TM, Gillespie NA, Goate AM, Goldman D, Grucza RA, Hancock DB, Harris KM, Heath AC, Hesselbrock V, Hewitt JK, Hopfer CJ, Horwood J, Iacono W, Johnson EO, Kaprio JA, Karpyak VM, Kendler KS, Kranzler HR, Krauter K, Lichtenstein P, Lind PA, McGue M, MacKillop J, Madden PAF, Maes HH, Magnusson P, Martin NG, Medland SE, Montgomery GW, Nelson EC, Nöthen MM, Palmer AA, Pedersen NL, Penninx BWJH, Porjesz B, Rice JP, Rietschel M, Riley BP, Rose R, Rujescu D, Shen P-H, Silberg J, Stallings MC, Tarter RE, Vanyukov MM, Vrieze S, Wall TL, Whitfield JB, Zhao H, Neale BM, Gelernter J, Edenberg HJ, Agrawal A, 2018. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat. Neurosci 21, 1656–1669. 10.1038/s41593-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-Y, Augustine GJ, 2015. Presynaptic nanodomains: a tale of two synapses. Front. Cell. Neurosci 8, 455. 10.3389/fncel.2014.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Guan J, Shioda S, 2001. Immunoelectron microscopic study of β-endorphinergic synaptic innervation of GABAergic neurons in the dorsal raphe nucleus. Synapse 42, 234–241. 10.1002/syn.10008. [DOI] [PubMed] [Google Scholar]
- Wang Q-P, Zadina JE, Guan J-L, Kastin AJ, Shioda S, 2003. Electron microscopic examination of the endomorphin 2-like immunoreactive neurons in the rat hypothalamus. Brain Res. 969, 126–134. 10.1016/s0006-8993(03)02290-x. [DOI] [PubMed] [Google Scholar]
- Wang XM, Zhang KM, Mokha SS, 1996. Nociceptin (orphanin FQ), an endogenous ligand for the QRL1 (opioid-receptor-like1) receptor; modulates responses of trigeminal neurons evoked by excitatory amino acids and somatosensory stimuli. J. Neurophysiol 76, 3568–3572. 10.1152/jn.1996.76.5.3568. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xing Y, Liu X, Ji H, Kai M, Chen Z, Yu J, Zhao D, Ren H, Wang R, 2012. A new class of highly potent and selective endomorphin-1 analogues containing α-Methylene-β-aminopropanoic acids (map). J. Med. Chem 55, 6224–6236. 10.1021/jm300664y. [DOI] [PubMed] [Google Scholar]
- Watson SJ, Akil H, Fischli W, Goldstein A, Zimmerman E, Nilaver G, van Griedanus TBW, 1982. Dynorphin and vasopressin: common localization in magnocellular neurons. Science 216, 85–87. 10.1126/science.6121376. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Benjannet S, Rovère C, Barbero P, Seidah NG, Epelbaum J, Dournaud P, 2000. Regional and cellular localization of the neuroendocrine prohormone convertases PC1 and PC2 in the rat central nervous system. J. Comp. Neurol 424, 439–460. . [DOI] [PubMed] [Google Scholar]
- Winters BL, Gregoriou GC, Kissiwaa SA, Wells OA, Medagoda DI, Hermes SM, Burford NT, Alt A, Aicher SA, Bagley EE, 2017. Endogenous opioids regulate moment-to-moment neuronal communication and excitability. Nat. Commun 8, 14611 10.1038/ncomms14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, Tucker RC, Ciccocioppo R, 2014. The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol. Therapeut 141, 283–299. 10.1016/j.pharmthera.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Wallace TL, Martin WJ, 2019. The nociceptin/orphanin FQ peptide receptor. Handb. Exp. Pharmacol 254, 399–415. 10.1007/164_2018_186. [DOI] [PubMed] [Google Scholar]
- Xie H, Woods JH, Traynor JR, Ko M-C, 2008. The spinal antinociceptive effects of endomorphins in rats: behavioral and G protein functional studies. Anesth. Analg 106, 1873–1881. 10.1213/ane.0b013e31817300be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovleva T, Bazov I, Cebers G, Marinova Z, Hara Y, Ahmed A, Vlaskovska M, Johansson B, Hochgeschwender U, Singh IN, Bruce-Keller AJ, Hurd YL, Kaneko T, Terenius L, Ekström TJ, Hauser KF, Pickel VM, Bakalkin G, Yakovleva T, Bazov I, Cebers G, Marinova Z, Hara Y, Ahmed A, Vlaskovska M, Johansson B, Hochgeschwender U, Singh IN, Bruce-Keller AJ, Hurd YL, Kaneko T, Terenius L, Ekström TJ, Hauser KF, Pickel VM, Bakalkin G, 2006. Prodynorphin storage and processing in axon terminals and dendrites. Faseb. J 20, 2124–2126. 10.1096/fj.06-6174fje. [DOI] [PubMed] [Google Scholar]
- Yanagawa M, Hiroshima M, Togashi Y, Abe M, Yamashita T, Shichida Y, Murata M, Ueda M, Sako Y, 2018. Single-molecule diffusion-based estimation of ligand effects on G protein–coupled receptors. Sci. Signal 11 10.1126/scisignal.aao1917. [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Sierra V, Lutfy K, 1989. Chronic opioid antagonist treatment: assessment of receptor upregulation. Eur. J. Pharmacol 170, 193–200. 10.1016/0014-2999(89)90539-6. [DOI] [PubMed] [Google Scholar]
- Yoon G, Kim SW, Petrakis IL, Westermeyer J, 2016. High-dose naltrexone treatment and gender in alcohol dependence. Clin. Neuropharmacol 39, 165–168. 10.1097/wnf.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Williams C, Sabol SL, 1984. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J. Biol. Chem 259, 14301–14308. [PubMed] [Google Scholar]
- Yu Y, Shao X, Cui Yun, Liu H, Wang C, Fan Y, Liu J, Dong S, Cui Yu-xing, Wang R, 2007. Structure–activity study on the spatial arrangement of the third aromatic ring of endomorphins 1 and 2 using an atypical constrained C terminus. ChemMedChem 2, 309–317. 10.1002/cmdc.200600274. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge L-J, Kastin AJ, 1997. A potent and selective endogenous agonist for the μ-opiate receptor. Nature 386, 499–502. 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Nilges MR, Morgenweck J, Zhang X, Hackler L, Fasold MB, 2016. Endomorphin analog analgesics with reduced abuse liability, respiratory depression, motor impairment, tolerance, and glial activation relative to morphine. Neuropharmacology 105, 215–227. 10.1016/j.neuropharm.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Zalewska-Kaszubska J, Czarnecka E, 2005. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides 26, 701–705. 10.1016/j.peptides.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Zhang G, Murray TF, Grandy DK, 1997. Orphanin FQ has an inhibitory effect on the Guinea pig ileum and the mouse vas deferens. Brain Res. 772, 102–106. 10.1016/s0006-8993(97)00858-5. [DOI] [PubMed] [Google Scholar]
- Zhang S, Qi J, Li X, Wang H-L, Britt JP, Hoffman AF, Bonci A, Lupica CR, Morales M, 2015. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat. Neurosci 18, 386–392. 10.1038/nn.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cui F, Chen H, Zhang T, Yang K, Wang Y, Jiang Z, Rice KC, Watkins LR, Hutchinson MR, Li Y, Peng Y, Wang X, 2018. Dissecting the innate immune recognition of opioid inactive isomer (+)-Naltrexone derived toll-like receptor 4 (TLR4) antagonists. J. Chem. Inf. Model 58, 816–825. 10.1021/acs.jcim.7b00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadée W, 2005. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J. Biol. Chem 280, 32618–32624. 10.1074/jbc.m504942200. [DOI] [PubMed] [Google Scholar]
- Zheng F, Grandy DK, Johnson SW, 2002. Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br. J. Pharmacol 136, 1065–1071. 10.1038/sj.bjp.0704806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PC, Thureson-Klein Å, Klein RL, 1986. Exocytosis from large dense cored vesicles outside the active synaptic zones of terminals within the trigeminal subnucleus caudalis: a possible mechanism for neuropeptide release. Neuroscience 19, 43–54. 10.1016/0306-4522(86)90004-7. [DOI] [PubMed] [Google Scholar]
- Zukin RS, Sugarman JR, Fitz-Syage ML, Gardner EL, Zukin SR, Gintzler AR, 1982. Naltrexone-induced opiate receptor supersensitivity. Brain Res. 245, 285–292. 10.1016/0006-8993(82)90811-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


