Abstract
Eunicellane diterpenoids are a remarkable family of terpene natural products and have been of high interest for over five decades. Widely distributed in soft corals and rare in plants, eunicellanes were also recently identified in actinobacteria. These terpenoids have foundational 6/10-bicyclic frameworks that are frequently oxidized into structures containing transannular ether bridges. Interest in their unique structures and promising biological activities, such as the paclitaxel-like activities of eleutherobin and the sarcodictyins, has led to advancements in natural product isolation, total synthesis, medicinal chemistry, and drug lead development. Until recently, however, there was little known about the biosynthesis and enzymology of these natural products, but several recent studies in both bacteria and coral have opened up the field. This review summarizes recent advancements in the biosynthesis and enzymology of eunicellane diterpenoids and highlights future research prospects in the field.
One-Sentence Summary
A summary of recent advancements in the biosynthesis and enzymology of eunicellane diterpenoids, a structurally unique and biologically active family of natural products found in coral, plants, and bacteria.
Keywords: Eunicellanes, Diterpenoids, Biosynthesis, Terpene synthase, Genome mining
Graphical Abstract
Graphical Abstract.
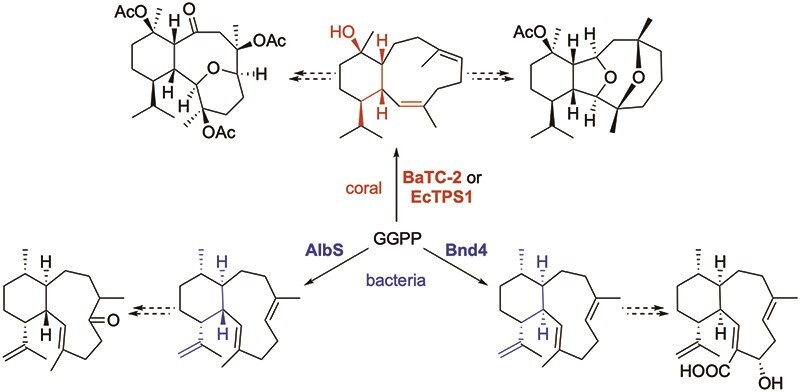
Biosynthesis of eunicellanes in bacteria and coral, highlighting the structural diversity of the terpene synthase-derived skeletons and the functionalized natural products.
Introduction
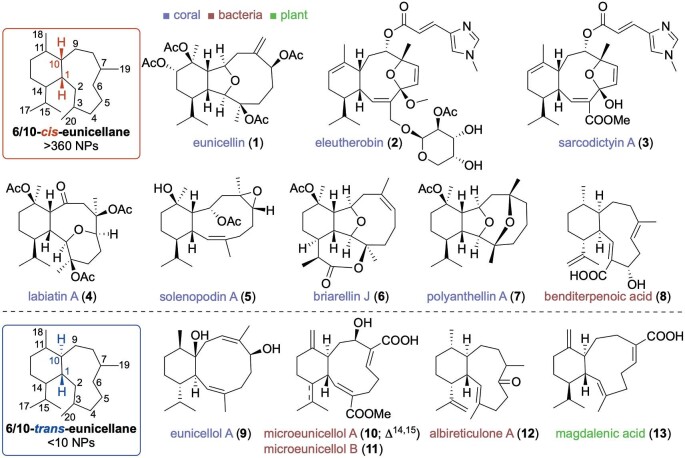
Terpenoids are the largest and most structurally diverse family of natural products, found in all living organisms and serving roles as both primary and secondary metabolites (Gross & König, 2006; Quin et al., 2014; Rudolf et al., 2021; Tholl, 2015). Terpenoids are of immense interest to chemical, biochemical, medicinal, and industrial communities due to their unique skeletons, synthetically challenging structures, genetic and enzymatic diversities, and promising commercial and therapeutic applications. Appropriately, the eunicellane diterpenoids have garnered a lot of interest since eunicellin (1; Fig. 1), the first member of this family, was identified in 1968 from the octocoral Eunicella stricta (currently accepted name Eunicella singularis) (Kennard & Watson, 1968). Since then, nearly 400 eunicellanes have been reported, mostly from soft corals, with biological activities including cytotoxic, anti-inflammatory, antiviral, antimalarial, and antibacterial properties (Fig. 1) (Dictionary of Natural Products, 2023; Li et al., 2020a; Welford & Collins, 2011). The most well-known members are eleutherobin (2) and the sarcodictyins (3), as they exhibit the paclitaxel-like mode of action of microtubule stabilization (Lindel et al., 1997; Long et al., 1998). However, these metabolites are found in extremely low quantities in coral (<0.2% dry mass of organism), and their repeated isolation is not practical, economical, or environmentally friendly (Krasnoslobodtseva et al., 2007); their chemical syntheses are lengthy and challenging (Chen et al., 1999; Nicolaou et al., 1998).
Fig. 1.
Structures and sources of selected eunicellane diterpenoids. The 6/10-bicyclic skeleton is conserved throughout the family, with main structural differences being the configuration of their ring fusion at C1 and C10, the absence or presence of transannular ether bridges, and the prevalence and location of oxidized sites.
Eunicellane diterpenoids are a structurally unique subset of terpenoids. The eunicellanes exhibit a 6/10-bicyclic framework and possess three main structural differences: the configuration of their ring fusion at C1 and C10, the absence or presence of transannular ether bridges, and the prevalence and location of oxidized sites (Fig. 1). First, the vast majority of known eunicellanes (>98%) are found as cis-fused bicycles (Dictionary of Natural Products, 2023). This may be reflective of their prominence in corals and their conserved biosynthetic routes in these organisms, as almost all coral eunicellanes are cis-fused. In bacteria and plants, however, trans-eunicellanes appear to be much more common, albeit with a significantly smaller number (<10) of natural products to evaluate. Second, the transannular ether bridges are a distinctive feature of coral eunicellanes and are most commonly seen between C2–C9 (eunicellins; e.g., 1, 6, and 7), C4–C7 (eleuthesides; e.g., 2 and 3), and C2–C6 (labiatins; e.g., 4); coral eunicellanes without a transannular ether bridge are within the solenopodin (e.g., 5) class (Li et al., 2020a). Third, of the 20 carbons of the eunicellane skeleton, only 2, C1 and C5, are not yet known to be functionalized with oxygens (Dictionary of Natural Products, 2023; Li et al., 2020a). One additional structural element to consider is that many eunicellanes also retain one or two double bonds, which can be found in either E or Z configurations, within their 10-membered ring, giving rise to different conformations and reactivities (Mancini et al., 2000). As the biosynthesis of these molecules has long been expected to originate from E,E,E,-geranylgeranyl diphosphate (GGPP), many of these alkene transformations are expected to be enzymatic in nature.
Biosynthetically, not much was known about how these molecules are formed in nature until recently. Based on their overall structures and their similarities to the cembrenoids, another common family of diterpenoids found in corals and other marine organisms, biosynthetic routes were proposed (Welford & Collins, 2011; Li et al., 2020a). In fact, eunicellanes are frequently referred to as 2,11-cyclized cembranoids (Welford & Collins, 2011; Li et al., 2020a), although that implies that the biosynthesis of all eunicellanes proceeds via a cembrane intermediate. While that may be true for the coral natural products (Scesa et al., 2022), recent evidence supports that at least some bacterial eunicellanes do not go through a cembrenyl cationic intermediate (Li et al., 2023; Zhu et al., 2021).
In the last three years, there have been significant achievements in eunicellane discoveries, the identification of biosynthetic genes in both bacteria and coral, and mechanistic enzymology. In this mini-review, we summarize these recent advancements in the biosynthesis and enzymology of eunicellane diterpenoids and discuss how these studies will stimulate the field and accelerate the study and use of these structurally fascinating and biologically important natural products.
Bacterial Eunicellanes
The first bacterial eunicellane diterpenoids were only recently discovered, with several additional natural products following in quick succession. Microeunicellols A (10) and B (11; Fig. 1), isolated from the coral-associated Streptomyces albogriseolus SY67903, were the first eunicellane-type diterpenoids reported from bacteria (Ma et al., 2020). The microeunicellols are trans-fused eunicellanes with an incipient Z-configured C2–C3 alkene. Microeunicellol A (10) exhibited cytotoxicity against two breast cancer cell lines at low μM concentrations; 11 was inactive (Ma et al., 2020). Soon after the microeunicellols were reported, a third bacterial eunicellane diterpenoid was discovered. Benditerpenoic acid (8, BND; Fig. 1), a cis-fused eunicellane with an E,E-configured cyclodecadiene, was identified in a bioactivity-guided screen from the soil-dwelling Streptomyces sp. (CL12-4) (Zhu et al., 2021). BND (8) had moderate antibacterial activity against Bacillus subtilis and Staphylococcus aureus.
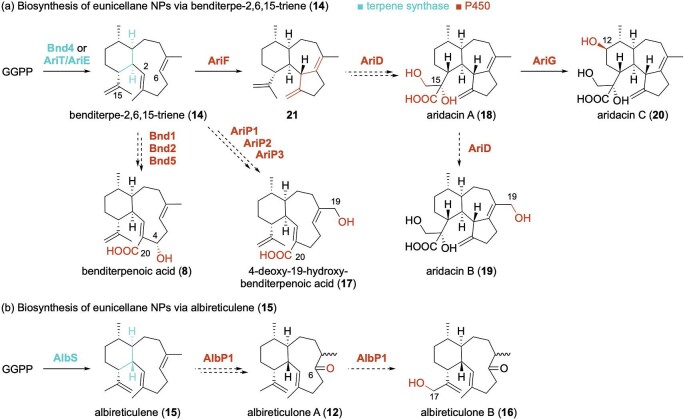
Biosynthetically, the discovery of 8 led to the identification of its biosynthetic gene cluster (BGC) and the first terpene synthase (TS) responsible for producing the 6/10-bicyclic eunicellane skeleton from GGPP (Zhu et al., 2021). Genome sequencing of Streptomyces sp. (CL12-4) and subsequent bioinformatic analysis of all terpene-related genes found in its genome revealed a promising BGC consisting of a putative GGPP synthase, type I TS, and several cytochromes P450 (P450s). Characterization of the TS, Bnd4, confirmed the formation of a single diterpene product, the cis-fused eunicellane benditerpe-2,6,15-triene (14; Fig. 2a). After using Bnd4 to search for related BGCs in bacteria, it was apparent that several actinobacteria have the biosynthetic machinery to produce the same terpene skeleton but decorate the core differently to yield distinct natural products (Zhu et al., 2021).
Fig. 2.
Proposed biosynthetic routes of the bacterial eunicellanes. Dotted arrows signify that the proposed enzymatic step(s) are experimentally supported but not confirmed. AriT/AriE, AriP1/AriD, AriP2/AriF, and AriP3/AriG are the same enzymes from the ari BGC but were named independently and expressed in different heterologous hosts.
Genome mining for novel TSs led to the discovery of the second bacterial eunicellane synthase (Li et al., 2023). A putative terpene BGC (alb) from Streptomyces albireticuli was selected based on the diversity of its type I TS and its colocalization with a putative GGPP synthase, two P450s, and an unknown protein directly downstream of the TS gene. The TS, named AlbS, produced only one terpene when incubated with GGPP. The structure of albireticulene (15) was quickly identified as an eunicellane, but it took extensive spectroscopic characterization and chemical derivatization to confirm it as the trans-fused C1 diastereomer of 14 (Fig. 2b). Thus, AlbS was the first trans-eunicellane synthase identified in nature (Li et al., 2023). AlbS only shares 24% sequence identity over 75% coverage with Bnd4, supporting that sequence diverse TSs can form the same planar 6/10-bicyclic eunicellane skeleton.
Recently, Streptomyces albus J1074M, a chassis strain encoding additional farnesyl diphosphate (FPP) and GGPP synthase genes, was used to heterologously express 13 bacterial terpene BGCs, two of which produced new eunicellane diterpenoids (Hu et al., 2023). Expression of the alb BGC described above resulted in two unnamed trans-eunicellane ketones, named here as albireticulones A (12) and B (16; Fig. 2b). Expression of the ari cluster, a BGC related to bnd with a TS known to produce 14 (Zhu et al., 2021), yielded 4-deoxy-19-hydroxy-BND (17; Fig. 2a) (Hu et al., 2023). Interestingly, in the hands of another group, expression of the same ari cluster (including the same four-gene construct as well as larger genomic regions) in S. albus J1074 produced highly oxidized 6/7/5-tricyclic terpenoids, which were named the aridacins (18–20; Fig. 2a) (Wang et al., 2023). The tricyclic nature and oxidation pattern of the aridacins are quite distinct from those of the BNDs. Through a combination of in vivo and in vitro experiments, the P450 AriF was determined to catalyze the cyclization of 14 into the nascent 6/7/5 skeleton (21), a fascinating use of a P450 enzyme to further diversify the terpene skeleton prior to functionalization. Oxidation at C12, C14–C17, and C19 by the clustered P450s completes the biosynthesis of 18–20 (Wang et al., 2023).
Coral Eunicellanes
Less than a year after the reports of the bnd BGC and Bnd4, the first TSs from octocorals were discovered and characterized (Burkhardt et al., 2022; Scesa et al., 2022). Natural products from sessile marine animals, such as octocorals, were known (Piel et al., 2004; Wakimoto et al., 2014) or often considered to be produced by microbial symbionts (Piel, 2008). After eunicellane diterpenoids were found in bacteria (Ma et al., 2020; Zhu et al., 2021), it seemed feasible that symbiotic organisms may also provide the biosynthetic machinery in corals for eunicellane production. However, the Schmidt and Moore groups independently identified coral TSs responsible for the production of the skeletons of several well-known families of terpenoids, including the eunicellanes (Burkhardt et al., 2022; Scesa et al., 2022).
Using hidden Markov models (HMMs) generated from a diverse set of microbial-type TSs, hits were found in coral genomic and transcriptomic data (Burkhardt et al., 2022; Scesa et al., 2022). Despite having very low sequence identities with both microbial and plant TSs (e.g., <25% identity over <30% coverage), these sequences possessed the canonical DDxxD and NSE/DTE metal-binding motifs and RY motifs that are highly conserved in bacterial di-TSs. Additionally, a crystal structure of one of these TSs confirmed the overall fold is conserved in octocoral and microbial type I TSs (Burkhardt et al., 2022). BaTC-2, which was found in transcriptomic data from Briareum asbestinum, produced klysimplexin R (22; Fig. 3) (Burkhardt et al., 2022); B. asbestinum is known to produce briarellins and polyanthellins (Fig. 1; e.g., 6 and 7) (Gutiérrez et al., 2020), eunicellanes that presumably originate from 22. EcTPS1, from the eleutherobin producer Erythropodium caribaeorum, also produced 22 (Fig. 3) (Scesa et al., 2022); BaTC-2 and EcTPS1 share only 30.4% identity over 92% coverage. Along with the discovery of the coral TSs, one of the most exciting realizations in these studies was the fact that these genes were found colocalized in a single chromosomal region with genes commonly seen in microbial terpene BGCs (e.g., isoprenyl diphosphate synthases, P450s, and acyltransferases) (Burkhardt et al., 2022; Scesa et al., 2022).
Fig. 3.
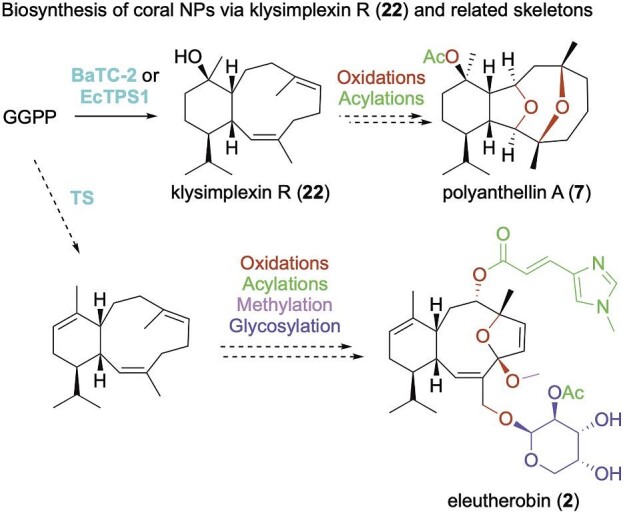
Proposed biosynthetic routes of the coral eunicellanes. Dotted arrows signify that the proposed enzymatic step(s) are supported by genetic evidence.
Mechanisms of Eunicellane Synthases
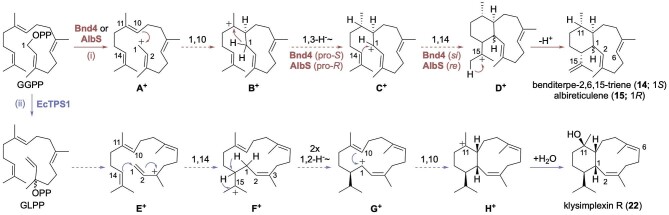
To form the 6/10-bicyclic eunicellane skeleton, two general mechanistic proposals are possible (Fig. 4). The first pathway (i) initially forms a 10-membered ring through a 1,10-ring closure, followed by either a single or series of hydride shifts to relocate the carbocation back to C1, which is then attacked by C14 to form the six-membered ring. The second pathway (ii) switches the order of ring formation with the formation of an initial 14-membered ring (presumably via a cembrenyl cation), hydride shift(s), and 1,10-ring closure. The second pathway was previously hypothesized as the likely mechanism for the coral skeletons, based on the location of alkenes and hydroxyl groups commonly seen on the six-membered ring in these natural products (Li et al., 2020a). In addition, many of the C2–C3 olefins are Z-configured, implicating the isomerization of GGPP into geranyllinalyl diphosphate (GLPP) prior to cyclization. However, the structure of 14 with its terminal alkene at C15–C16 made the first pathway the simpler option for Bnd4.
Fig. 4.
Proposed mechanisms for terpene cyclization into the eunicellanes. Deuterium labeling studies, mutagenesis, and quantum chemical calculations support the mechanisms.
Incubation of Bnd4 with 1,1-2H2-GGPP yielded 14 with deuteriums on C1 and C11 (Xu et al., 2021). A follow-up study concluded that the pro-R hydrogen on C1 of GGPP stays on C1 and the pro-S hydrogen moves to C11, based on incubation with 1R-2H-GGPP (Li et al., 2023). Quantum chemical calculations of the relative free energies of intermediates and transition state structures, along with the cis configuration of the methyl and isopropylene substituents on the cyclohexane ring, supported pathway (i) as the more viable mechanism (Xu et al., 2021). Thus, the Bnd4 mechanism was proposed to proceed via 1,10-cyclization (B+), a 1,3-hydride shift from C1 to C11 (C+), 1,14-cyclization (D+), and deprotonation at C15 to yield the cis-fused 14 (Fig. 4).
Similar experiments conducted with the coral EcTPS1 differentiated the bacterial and animal mechanisms. Incubation of EcTPS1 with 1,1-2H2-GGPP produced 22 with deuteriums on C1 and C14 (Scesa et al., 2022). That difference, together with the location of the hydroxy group at C11 and the Z-configured alkene, suggested pathway (ii) as the likely mechanism. Klysimplexin R (22) is made through the initial formation of a cembrenyl cation F+ via 1,14-cyclization and E to Z isomerization of the C2–C3 alkene perhaps via GLPP, followed by a series of 1,2-hydride shifts from C14 to C15 and C1 to C14 (G+), 1,10-cyclization (H+), and final water quench at C11 (Fig. 4). Density functional theory calculations supported this feasible mechanism (Scesa et al., 2022).
For trans-eunicellane formation catalyzed by AlbS, mechanistic investigation revealed a similar pathway to that of Bnd4 with two significant differences (Fig. 4). First, the pro-R hydrogen on C1 of GGPP is transferred to C11, the opposite of that seen in Bnd4, implicating differing conformers of the monocyclic C11 cationic intermediates B+ and C+ (Li et al., 2023). Second, a conformational change of the monocylic allylic cationic intermediate C+ is proposed to allow stereoselective 1,14-ring closure.
The importance of active site residues in the bacterial eunicellane synthases was probed by site-directed mutagenesis. In Bnd4, the catalytically essential Asp-rich motif was mapped to D94NxxxD, with only Asp94 being absolutely essential (Xu et al., 2021). Two other positions, Glu169 and Arg173, also likely have roles in binding Mg2+ ions or the diphosphate moiety, respectively, as mutation at those positions completely abolished activity. Mutation of some aromatic residues in the active site of Bnd4, particularly W316, altered product formation to include cembrenes (Xu et al., 2022). While it is not unusual to see cembrene formation in TS variants (Driller et al., 2019) or by simply changing reaction conditions (Rinkel et al., 2018), this result was intriguing considering the mechanism of eunicellane formation in coral TSs runs through the cembrenyl cation F+. If a simple change to Bnd4 adjusts initial cyclization from 10-membered to 14-membering ring formation, continued engineering may be able to divert activity to a coral-like mechanism. Similarly, engineering of cembrene synthases may yield novel eunicellane synthases, although initial attempts with DtCycA were unsuccessful (Xu et al., 2022).
While mutation studies of Bnd4 yielded limited mechanistic insights, one mutation in AlbS, Y214F, provided additional support for the timing of cyclization (Li et al., 2023). Located in the presumed active site, the hydroxy group of Y214 is proposed to play a role in stabilizing the monocyclic cationic intermediate B+, as Y214F only produced prenylgermacrene A, the deprotonation product of B+. This mutation supported that 1,10-cyclization indeed occurs first in AlbS catalysis (Li et al., 2023). The corresponding change in Bnd4, Y197F, had no effect on product formation (Xu et al., 2022).
Conclusion
The advancement of eunicellanes as drug leads has been hampered by a lack of supply, arduous total syntheses, and a general lack of knowledge about their biosynthetic pathways. Genome mining in both bacteria and coral, which resulted in the identification of multiple distinct BGCs, will revolutionize this field. In the first three BGCs discovered, three different eunicellane synthases, including both cis- and trans-synthases, were characterized. These enzymes have already been utilized to produce the eunicellane hydrocarbon skeletons, providing potential starting points for semi-synthesis or other synthetic biology applications. In fact, a semi-synthetic strategy to access coral natural products from klysimplexin R (22) was reported during the revision of this review (Scesa & Schmidt, 2023). Non-functionalized hydrocarbon skeletons may not always be ideal starting points. However, clustered with these TSs are oxidative enzymes, specifically P450s. When these P450s are characterized and can be turned into biocatalysts, the oxidation of these skeletons may yield better starting points for synthetic schemes.
As natural products are successfully paired with their biosynthetic genes, genome mining efforts begin to pervade the field, and rightly so. Nature has created a vast array of combinatorial biosynthetic pathways that diversify conserved skeletons. Genome mining-based natural product discovery has already led to new eunicellanes in bacteria, as evidenced by the recent report on the aridacins (Hu et al., 2023; Wang et al., 2023). The same can be expected for the coral eunicellanes, perhaps even to a greater extent. Not only were TSs identified to be encoded by the coral genomes and not those of symbiotic organisms, but the realization that putative biosynthetic genes were clustered with the TSs will surely invigorate the field to follow the highly successful template that microbial natural product biosynthetic chemists have developed over the last 20 years. Plants should also not be forgotten. With at least six eunicellane diterpenoids found in plants and the biosynthetic genes still unknown, it is possible that a completely different set of genes may be responsible for their biosyntheses. Alternatively, it is also possible that symbiotes may also be biosynthetically contributing.
Mechanistically, it is truly fascinating to consider that three different enzymes, sharing little to no sequence identities, can produce the hydrocarbon foundation for all known eunicellane natural products. Only initial mechanistic investigations have been conducted, and it will be of interest to fully understand how these enzymes control cis vs. trans ring fusion, shepherd GGPP into GLPP to yield a Z-alkene, and direct deprotonation or water quench. In hindsight, the eunicellane skeleton is present in the cyclization cascades of other bacterial TSs: 6/10-eunicellane cations have been proposed as intermediates in the formation of the 5/5/6/7-tetracyclic venezuelaene (catalyzed by VenA) and the 6/7/5-tricyclic catenul-14-en-6-ol (CaCS) (Li et al., 2020b, 2021); a cis-eunicellane neutral intermediate, prehydropyrene, is on pathway to the 6/6/6/6-tetracyclic hydropyrene (HpS) (Rinkel et al., 2017). It would appear there may be initial mechanistic parallels between each of these TSs, eventually diverging in how they control the bicyclic cations or neutral eunicellanes. Each of these enzymes, along with the eunicellane synthases, is an intriguing launching point for future TS engineering or directed evolution studies.
Contributor Information
Zining Li, Department of Chemistry, University of Florida, Gainesville, FL 32611-7011, USA.
Jeffrey D Rudolf, Department of Chemistry, University of Florida, Gainesville, FL 32611-7011, USA.
Funding
This work is supported in part by the National Institutes of Health (grant number R35 GM142574) and the University of Florida.
Conflict of Interest
The authors declare no conflict of interest.
References
- Burkhardt I., de Rond T., Chen P. Y.-T., Moore B. S. (2022). Ancient plant-like terpene biosynthesis in corals. Nature Chemical Biology, 18, 664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-T., Bhattacharya S. K., Zhou B., Gutteridge C. E., Pettus T. R. R., Danishefsky S. J. (1999). The total synthesis of eleutherobin. Journal of the American Chemical Society, 121, 6563–6579. [Google Scholar]
- Dictionary of Natural Products . (2023, March 31). http://dnp.chemnetbase.com
- Driller R., Garbe D., Mehlmer N., Fuchs M., Raz K., Major D. T., Brück T., Loll B. (2019). Current understanding and biotechnological application of the bacterial diterpene synthase CotB2. Beilstein Journal of Organic Chemistry, 15, 2355–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H., König G. M. (2006). Terpenoids from marine organisms: Unique structures and their pharmacological potential. Phytochemistry Reviews, 5, 115–141. [Google Scholar]
- Gutiérrez M., Santamaría R., Gómez-Reyes J. F., Guzmán H. M., Ávila-Román J., Motilva V., Talero E. (2020). New eunicellin-type diterpenes from the Panamanian octocoral Briareum asbestinum. Marine Drugs, 18, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. L., Zhang Q., Liu S. H., Sun J. L., Yin F. Z., Wang Z. R., Shi J., Jiao R. H., Ge H. M. (2023). Building Streptomyces albus as a chassis for synthesis of bacterial terpenoids. Chemical Science, 14, 3661–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard O., Watson D. G. (1968). Chemical studies of marine invertebrates. IV. Terpenoids LXII. Eunicellin, a diterpenoid of the gorgonian Eunicella stricta. X-ray diffraction analysis of eunicellin dibromide. Tetrahedron Letters, 24, 2879–2884. [Google Scholar]
- Krasnoslobodtseva O. Y., Salikhov S. M., Sharipov B. T., Valeev F. A., Tolstikov G. A. (2007). Diterpenoids of eunicellane series. Chemistry for Sustainable Development, 15, 265–285. [Google Scholar]
- Li G., Dickschat J. S., Guo Y.-W. (2020a). Diving into the world of marine 2,11-cyclized cembranoids: A summary of new compounds and their biological activities. Journal of Natural Products, 37, 1367–1383. [DOI] [PubMed] [Google Scholar]
- Li G., Guo Y.-W., Dickschat J. S. (2021). Diterpene biosynthesis in Catenulispora acidiphila: On the mechanism of catenul-14-en-6-ol synthase. Angewandte Chemie International Edition, 60, 1488–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Jiang Y., Zhang X., Chang Y., Li S., Zhang X., Zheng S., Geng C., Men P., Ma L.i, Yang Y., Gao Z., Tang Y.-J., Li S. (2020b). Fragrant venezuelaenes A and B with a 5-5-6-7-tetracyclic skeleton: Discovery, biosynthesis, and mechanisms of central catalysts. ACS Catalysis, 10, 5846–5851. [Google Scholar]
- Li Z., Xu B., Kojasoy V., Ortega T., Adpressa D. A., Ning W., Wei X., Liu J., Tantillo D. J., Loesgen S., Rudolf J. D. (2023). First trans-eunicellane terpene synthase in bacteria. Chemistry, 9, 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindel T., Jensen P. R., Fenical W., Long B. H., Casazza A. M., Carboni J., Fairchild C. R. (1997). Eleutherobin, a new cytotoxin that mimics paclitaxel (Taxol) by stabilizing microtubules. Journal of the American Chemical Society, 119, 8744–8745. [Google Scholar]
- Long B. H., Carboni J., Wasserman A. J., Cornell L. A., Casazza A. M., Jensen P. R., Lindel T., Fenical W., Fairchild C. R. (1998). Eleutherobin, a new cytotoxic agent that induces tubulin polymerization, is similar to paclitaxel (Taxol). Cancer Research, 58, 1111–1115. [PubMed] [Google Scholar]
- Ma L.-F., Chen M.-J., Liang D.-E., Shi L.-M., Ying Y.-M., Shan W.-G., Li G.-Q., Zhan Z.-J. (2020). Streptomyces albogriseolus SY67903 produces eunicellin diterpenoids structurally similar to terpenes of the gorgonian Muricella sibogae, the bacterial source. Journal of Natural Products, 83, 1641–1645. [DOI] [PubMed] [Google Scholar]
- Mancini I., Guella G., Zibrowius H., Pietra F. (2000). Configuration, conformation, and reactivity of highly functionalized eunicellane diterpenes isolated from the gorgonians Eunicella cavolinii and Eunicella singularis from Marseille. Helvetica Chimica Acta, 83, 1561–1575. [Google Scholar]
- Nicolaou K. C., Xu J. Y., Kim S., Pfefferkorn J., Ohshima T., Vourloumis D., Hosokawa S. (1998). Total synthesis of sarcodictyins A and B. Journal of the American Chemical Society, 120, 8661–8673. [Google Scholar]
- Piel J. (2008). Metabolites from symbiotic bacteria. Natural Product Reports, 26, 338–362. [DOI] [PubMed] [Google Scholar]
- Piel J., Hui D., Wen G., Butzke D., Platzer M., Fusetani N., Matsunaga S. (2004). Antitumor polyketide biosynthesis by a bacterial symbiont of the marine sponge Theonella swinhoei. Proceedings of the National Academy of Sciences, 101, 16222–16227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quin M. B., Flynn C. M., Schmidt-Dannert C. (2014). Traversing the fungal terpenome. Natural Product Reports, 31, 1449–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkel J., Lauterbach L., Rabe P., Dickschat J. S. (2018). Two diterpene synthases for spiroalbatene and cembrene A from Allokutzneria albata. Angewandte Chemie International Edition, 57, 3238–3241. [DOI] [PubMed] [Google Scholar]
- Rinkel J., Rabe P., Chen X., Köllner T. G., Chen F., Dickschat J. S. (2017). Mechanisms of the diterpene cyclases β-pinacene synthase from Dictyostelium discoideum and hydropyrene synthase from Streptomyces clavuligerus. Chemistry: A European Journal, 23, 10501–10505. [DOI] [PubMed] [Google Scholar]
- Rudolf J. D., Alsup T. A., Xu B., Li Z. (2021). Bacterial terpenome. Natural Product Reports, 38, 249–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scesa P. D., Lin Z., Schmidt E. W. (2022). Ancient defensive terpene biosynthetic gene clusters in the soft corals. Nature Chemical Biology, 18, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scesa P. D., Schmidt E. W. (2023). Biomimetic approach to diverse coral diterpenes from a biosynthetic scaffold. Angewandte Chemie International Edition, 62, e202311406. https://onlinelibrary.wiley.com/doi/10.1002/anie.202311406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholl D. (2015). Biosynthesis and biological functions of terpenoids in plants. In J. Schrader & J. Bohlmann (Eds.), Biotechnology of Isoprenoids. Advances in Biochemical Engineering/Biotechnology (148, pp. 63–106). Springer. [DOI] [PubMed] [Google Scholar]
- Wakimoto T., Egami Y., Nakashima Y., Wakimoto Y., Mori T., Awakawa T., Ito T., Kenmoku H., Asakawa Y., Piel J., Ikuro A. (2014). Calyculin biogenesis from a pyrophosphate protoxin produced by a sponge symbiont. Nature Chemical Biology, 10, 648–655. [DOI] [PubMed] [Google Scholar]
- Wang Z., Yang Q., He J., Li H., Pan X., Li Z., Xu H.-M., Rudolf J. D., Tantillo D. J., Dong L.-B. (2023). Cytochrome P450-mediated cyclization in eunicellane-derived diterpenoid biosynthesis. ChemRxiv. 10.26434/chemrxiv-2023-wc46q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford A. J., Collins I. (2011). The 2,11-cyclized cembranoids: Cladiellins, asbestinins, and briarellins (period 1998–2010). Journal of Natural Products, 74, 2318–2328. [DOI] [PubMed] [Google Scholar]
- Xu B., Ning W., Wei X., Rudolf J. D. (2022). Mutation of the eunicellane synthase Bnd4 alters its product profile and expands its prenylation ability. Organic & Biomolecular Chemistry, 20, 8833–8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Tantillo D. J., Rudolf J. D. (2021). Mechanistic insights into the formation of the 6,10-bicyclic eunicellane skeleton by the bacteria diterpene synthase Bnd4. Angewandte Chemie International Edition, 60, 23159–23163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Xu B., Adpressa D. A., Rudolf J. D., Loesgen S. (2021). Discovery and biosynthesis of a structurally dynamic antibacterial diterpenoids. Angewandte Chemie International Edition, 60, 14163–14170. [DOI] [PMC free article] [PubMed] [Google Scholar]