Abstract
Background:
Living in neighborhoods with higher levels of walkability has been associated with a reduced risk of obesity and higher levels of physical activity. Obesity has been linked to increased risk of 13 cancers in women. However, long-term prospective studies of neighborhood walkability and risk for obesity-related cancer are scarce.
Objectives:
We evaluated the association between long-term average neighborhood walkability and obesity-related cancer risk in women.
Methods:
The New York University Women’s Health Study (NYUWHS) is a prospective cohort with 14,274 women recruited between 1985 and 1991 in New York City and followed over nearly three decades. We geocoded residential addresses for each participant throughout follow-up and calculated an average annual measure of neighborhood walkability across years of follow-up using data on population density and accessibility to destinations associated with geocoded residential addresses. We used ICD-9 codes to characterize first primary obesity-related cancers and employed Cox proportional hazards models to assess the association between average neighborhood walkability and risk of overall and site-specific obesity-related cancers.
Results:
Residing in neighborhoods with a higher walkability level was associated with a reduced risk of overall and site-specific obesity-related cancers. The hazards ratios associated with a 1-standard deviation increase in average annual neighborhood walkability were 0.88 (95% CI: 0.85, 0.93) for overall obesity-related cancer, 0.89 (95% CI: 0.84, 0.95) for postmenopausal breast cancer, 0.82 (95% CI: 0.68, 0.99) for ovarian cancer, 0.87 (95% CI: 0.76, 0.99) for endometrial cancer, and 0.68 (95% CI: 0.49, 0.94) for multiple myeloma, adjusting for potential confounders at both the individual and neighborhood level. The association between neighborhood walkability and risk of overall obesity-related cancer was stronger among women living in neighborhoods with higher levels of poverty compared with women living in areas with lower poverty levels ().
Discussion:
Our study highlights a potential protective role of neighborhood walkability in preventing obesity-related cancers in women. https://doi.org/10.1289/EHP11538
Introduction
The prevalence of obesity in the United States has been increasing steadily, reaching nearly 42% in adults by 2018.1 Obesity predisposes individuals to a higher risk of at least 13 cancers.2–5 Among women, obesity-related cancers constitute 55% of all cancers diagnosed, a higher percentage than the corresponding value among men (24%).6,7 Physical inactivity is a major risk factor for obesity-related cancers in women.8–10 Even low-intensity exercise in women has a protective effect against cancer risk.11,12 Yet, of Americans years of age do not achieve recommended levels of physical activity.13 Walking is an important component of total physical activity and can constitute moderate-intensity activity that contributes to meeting health recommendations.14,15 A major focus of active living research has been on how the built environment—human-made surroundings that provide the setting for human activities—can influence health and disease.16–21 There is growing evidence that built environment factors, such as neighborhood walkability, can foster outdoor walking.22,23 Neighborhood walkability refers to a combination of urban characteristics in the neighborhood that specifically support pedestrian activity and walking.24–26 For instance, in previous literature a linear positive relationship was found between residential density and walking for transport.27,28 Destination accessibility, which refers to density of accessible businesses and other local resources,29 has also been positively linked to walking behavior.30 However, evidence on the association between neighborhood walkability and cancer risk from prospective studies is limited. Studies that evaluate whether the effect of neighborhood walkability is modifiable by other risk factors at the individual or neighborhood level are also lacking.
A few studies assessing the impact of neighborhood walkability-related variables on cancer risk have yielded inconsistent findings. A prospective cohort study measuring street connectivity and population density with 2 y of follow-up showed no association with overall cancer risk.31 Findings from the prospective Multiethnic Cohort (MEC) showed that baseline neighborhood walkability was inversely related to risk of postmenopausal breast cancer.32 In subsequent analyses from the MEC, there was an inverse association between increasing recreational facilities and colorectal cancer risk; yet, a positive association was observed between increasing neighborhood population density and colorectal cancer risk.33 One ecologic study in New York City (NYC) reported an inverse association of neighborhood walkability on the incidence of multiple myeloma. These studies were either ecologic in design,34 had a limited follow-up time,31 or lacked data on important confounding factors at both the individual and neighborhood level.31,32,34,35 In addition, no study considered a time-weighted measure of neighborhood walkability based on residential history over time. Using mortality data, we previously reported an inverse association between neighborhood walkability at the time of recruitment and risk of death from obesity-related cancers in the New York University Women’s Health Study (NYUWHS).36 In the present study, we constructed an average annual residential walkability score for each woman using residential history throughout the cohort follow-up period (1985–2016), accounting for changes in residential environments and residential moves. We investigated the association between average annual neighborhood walkability during follow-up and risk of any incident obesity-related cancer and site-specific obesity-related cancers.
Methods
Study Population
The NYUWHS is a prospective cohort of 14,274 women between 34 and 65 years of age recruited at a mammography screening center in NYC between 1985 and 1991. Women were ineligible for enrollment if they had previously used hormonal medications or had been pregnant or lactating in the previous 6 months. Participants completed a baseline and up to six follow-up questionnaires mailed every 3–5 y capturing data on sociodemographic factors, lifestyle variables, and health conditions (Figure S1). Addresses were checked and updated every 1–2 y during the process of active follow-up, either through self-report, or by accessing the National Change of Address database. Lost participants were located using telephone directories and calls to participants or their next-of-kin. Women who withdrew from active follow-up completed a request, and we stopped contacting them. Participants were lost to follow-up when we no longer had a valid contact (addresses or phone number) available. As of 1 January 2017, participants in the cohort had an average follow-up of y. This study was approved by the New York University School of Medicine and the Columbia Presbyterian Medical Center Institutional Review Boards.
Outcome
Our primary outcome was overall incident obesity-related cancer, including each of the individual cancers that have been linked to obesity2–5: breast cancer diagnosed after menopause, colorectal (colon and rectal), pancreatic, endometrial (including uterine), ovarian, renal, thyroid, liver, gallbladder, and esophageal cancers, as well as meningioma and multiple myeloma (Table S1).3,4 Briefly, incident cancer cases were ascertained by record linkages to statewide tumor registries from New York, New Jersey, and Florida, where 86%–95% of the study population resided over the years during the follow-up. Self-reported data and medical records were used for participants who lived outside New York, New Jersey, or Florida or moved out of these states indefinitely. Using medical records obtained from reported cases, the diagnosis and cancer subtypes were confirmed by a medical doctor on our team and coded by trained personnel under supervision using the AJCC Cancer Staging Manual (Eighth Edition).37 A capture–recapture analysis showed the overall cancer ascertainment rate in our study to be 95%.38 For breast cancer, we also considered subtypes defined by hormone receptor (estrogen and progesterone) status. Estrogen (ER) and progesterone receptor (PR) receptor status was derived from medical records, tumor block immunohistochemistry assays, and/or tumor registry data. International Classification of Diseases, Ninth Revision (ICD-9)39 codes were assigned for tumor topography.
Exposure
A total of 401,239 addresses were geocoded throughout all follow-up years, of which 98.8% () were considered to be of an acceptable spatial quality and with no errors. A total of 33 women and their baseline addresses were excluded owing to missing addresses during all of their follow-up. We also excluded women without body mass index (BMI) information () and women with a history of cancer at baseline (). After these exclusions, 372,639 addresses and 13,240 participants remained. We constructed annual population density [American Community Survey (ACS) data 1990, 2000, and 2010] and destination accessibility [National Establishment Time Series (NETS) data containing the Dun and Bradstreet data of 1990–2014] derived from census tract-level data from date of enrollment until year of loss to follow-up, withdrawal from active follow-up, death, date of first incident obesity-related cancer, or 1 January 2017 [date of our last linkage to the National Death Index (NDI) and tumor registries], whichever came first. The NETS is an annual census of business establishments, nonprofits, or public-sector organizations that are compiled in the Dun and Bradstreet database for each year from 1990 onward.40 For population density, we used the 1990 ACS data for years prior to 1990, and we carried forward the value in 2010 for years after 2010. We interpolated population density measures between 1990 and 2000, as well as between 2000 and 2010, using the slope between decennial years.41–43 For instance, to obtain values for time points 1991–1999, we first ran a linear regression between years (1990–2000) and population density to obtain the slope estimating the difference in population density associated per year. Then, we estimated the year-specific population density by adding the product of the slope and the number of years since 1990 to the population density 1990 value. For destination accessibility, we used the 1990 destination accessibility value for years prior to 1990, and we carried forward the value in 2014 for years after 2014 until end of follow-up. An alternate exposure characterization for sensitivity analyses was conducted with extrapolated data: for population density, we ran a linear regression between years (2000–2010) and population density to estimate the data for years after 2010, whereas for destination accessibility, we similarly first ran a linear regression between years (1990–2014) and destination accessibility, and then estimated the data for years after 2014.
We calculated -scores for both annual population density and destination accessibility across data from all women and follow-up years. For both annual population density and destination accessibility, we calculated -scores by subtracting the ACS U.S. national census tract average in 2010 from the value associated with a given follow-up year for each participant and dividing it by the standard deviation (SD) from the ACS in 2010. The neighborhood walkability score was then calculated by summing the two -scores for each given follow-up year. Last, we averaged for each individual the annual neighborhood walkability score throughout all follow-up years, creating an average annual neighborhood walkability score for each participant (Figure S2). In our prior work, we constructed and validated the Built Environment and Health Neighborhood Walkability Index (BEH-NWI) using four items, including population density, destination accessibility, street connectivity, and rail transit density, described previously.29 Owing to data being unavailable for street connectivity and rail transit density for multiple follow-up years, we characterized annual neighborhood walkability using population density and destination accessibility throughout all follow-up years the study was active. The baseline two-item walkability scores were highly correlated with the original baseline four-item composite BEH-NWI score (Pearson correlation coefficient, ).
Additional Variables
Using self-reported data on amount (miles, blocks, or minutes) of outdoor walking per week collected at baseline, we calculated metabolic equivalent task (MET)-hours per week. MET-hours were assigned according published guidelines specified elsewhere assuming that walking at a () requires 3.3 METs of energy.44,45 Self-reported height and weight at baseline, as well as weight throughout each follow-up wave, were used to calculate BMI. Information on dietary intake was collected at baseline using a validated, semi-quantitative modified Block Food Frequency Questionnaire. We estimated a healthy dietary index—using Dietary Approaches to Stop Hypertension (DASH)—a diet that is rich in fruits, vegetables, whole grains, and low-fat dairy foods, developed from healthy dietary guidelines using the Fung et al. method,46 given that it has been associated with estimates of obesity and obesity-related cancers.47,48 Because the main focus in this study was obesity-related cancers, we deemed it appropriate to use the DASH diet score in our analyses.49,50 Neighborhood poverty rate (percentage of neighborhood population with a ratio of income to federal poverty level of ) from the 1990 census data was used to describe baseline neighborhood-level socioeconomic status (SES).
Statistical Analyses
We computed person-time for each participant from date of enrollment until either date of cancer diagnosis, death, or 1 January 2017 (date of our last linkage to NDI and tumor registries), whichever came first. We further censored women who stopped responding to active follow-up, were lost to follow-up, or withdrew from active follow-up and who last resided outside New York, New Jersey, or Florida at the year they moved out of the three states () or at their last follow-up questionnaire response (), whichever came last, given that we did not have data on their cancer status for later years. For women who last resided in New York, New Jersey, or Florida, if they did not respond to active follow-up, were lost to follow-up (), or withdrew from active follow-up (), no censoring was applied because we could ascertain their cancer status through state linkages, assuming that those who were lost to follow-up or who withdrew from active follow-up did not move out of the three states in later years. Average annual walkability was estimated using residential history until the end of the study for nonrespondents in the three states—given that we continued sending participant questionnaires and their addresses were confirmed through the U.S. Postal Service—or at last contact for those who were lost to follow-up or who withdrew from active follow-up. Therefore, the follow-up period for which we had cancer data could be longer than the period for which we had walkability exposure data for those who were lost to follow-up or who withdrew from active follow-up last residing in the three states (New York, New Jersey, and Florida). Participants who had a nonobesity-related cancer of any type prior to an obesity-related cancer were censored at the date of first cancer diagnosis. We used Cox proportional hazard (PH) models to compute hazard ratios (HRs) and 95% confidence intervals (CIs) assessing the association between average annual neighborhood walkability and risk of any first primary incident obesity-related cancer, and we reported estimates for site-specific cancers with cases. We also tested for linearity using survival models with cubic splines (for which we extracted both nonlinearity -values and effect -values) and found that associations between walkability and cancer risk did not deviate from linearity for all outcomes (), with the exception of renal cancer (; Table S2). Thus, we modeled average neighborhood walkability using quartiles, as a dichotomized variable with the median as the cut point, and as a continuous variable that was scaled using the and of the average neighborhood walkability measure. The data indicated that the PH assumption was not violated; the -values associated with the cross-product term between the scaled neighborhood walkability measure and the log function of survival time for overall and site-specific cancers ranged from to (Table S3).
We determined potential confounders based on their association with both neighborhood walkability and obesity-related cancer in our data as well as in the literature. In model 1, potential confounders included age at enrollment (in years), baseline educational attainment (high school or less, college or vocational school, and graduate school), race/ethnicity (non-Hispanic white, non-Hispanic Black, Hispanic, and an Other category collapsing Asians, Native Americans, or mixed races owing to smaller sample size), and smoking (ever vs. never smoker). All sociodemographic and lifestyle variables were self-reported at baseline. Women who ever moved from baseline address were younger, had a lower average neighborhood walkability, and were less likely to die during the follow-up,36 less likely to have cancer, and more likely to be lost to follow-up or withdraw from active follow-up (Table S4). Therefore, we controlled for ever moving residence (yes/no) as a covariate that affects exposure and follow-up completion.51 We additionally controlled for average daily alcohol intake dichotomized into above or below the Recommended Dietary Allowance (RDA: ) for women, menopausal status (yes/no), parity (yes/no), and neighborhood-level poverty (continuous variable) (model 2, main model). We employed in all models multiple imputation with 10 iterations52–54 for missing information on alcohol consumption (15.7%), smoking status (9.4%), education level (18.1%), and race/ethnicity (12.3%). Given that BMI, diabetes, and outdoor walking were considered potential mediators, we controlled for these factors in a separate model to assess whether the association between neighborhood walkability and cancer was attenuated (model 3). BMI was characterized as baseline BMI and BMI change (the difference between the second to last reported BMI before incident obesity-related cancer or end of follow-up, and BMI at enrollment).
In addition, to assess the robustness of the findings from the main model (model 2), we conducted several sensitivity and exploratory analyses, including a) models employing an average neighborhood walkability measure based on extrapolated population density for years after 2010 and extrapolated destination accessibility for years after 2014 (); b) analyses additionally censoring individuals at the year of loss to follow-up () or withdrawal () from active follow-up (); c) analyses using a 3-y lag between the exposure and outcome (); d) exploratory analyses assessing a potential window of susceptibility using average walkability exposure estimated at younger () and older ages (), with a cutoff of 60 years of age; e) analyses controlling additionally for continuous DASH diet (); f) analyses recoding as a category missing values in lieu of multiple imputation; g) analyses using a time-varying measure of the cumulative average annual walkability calculated from baseline to each follow-up year; and h) exploratory analyses for the main outcomes of interest (overall obesity-related cancers as well as postmenopausal breast cancer) using age as the timescale and stratifying by birth cohort in 10-y intervals in models, which adjusts for calendar effects. The latter analyses used time-on-study as the timescale with two slopes for age to allow different effects for younger and older ages (using a cutoff for the median age of 51 y, or 60 y, respectively).55,56
Last, we conducted exploratory analyses assessing neighborhood walkability in relation to risk of hormone receptor-defined postmenopausal breast cancer; we considered breast cancer subtypes based on ER and PR status employing Cox PH models accounting for competing risks.57 For cancers with sufficient sample size, we also conducted analyses stratified by potential effect modifiers including age (dichotomized at the median age at enrollment), BMI (dichotomized at the median BMI), DASH score (dichotomized at the median healthy diet adherence), smoking status, parity, race/ethnicity (White, Black, Hispanic, or Other), education level (high school or less, college/vocational school/other, or graduate school) and neighborhood-level poverty at baseline (dichotomized by median poverty). We tested for multiplicative interaction using the -value for the cross-product between scaled continuous walkability and each of the dichotomized effect modifiers. We additionally conducted stratified analyses by presence or absence of potential comorbid conditions (diabetes/no diabetes) or explanatory variables (above and below the median outdoor walking level). All analyses were implemented using SAS (version 9.4; SAS Institute Inc.).
Results
At baseline, the participants had an average age of 50.6 y, 78.5% of participants were White, 68.8% had completed an education level beyond high school, and 68.2% were parous (Table 1). Women with the highest average neighborhood walkability quartile had the lowest baseline BMI. Women were followed up for an average of 23.9 y, accumulating a total person-time of 316,783.5 y in the cohort. On average, women had annual walkability scores assigned for 93.2% of their total follow-up (Table S5). Nearly 48.6% moved from the baseline residence at some point during follow-up (Table 1). Those who moved from the baseline address had walkability data assigned for 94.3% of their follow-up time in the study (Table S5). Of the 13,240 women, 18.2% participants had a first obesity-related cancer () by the end of 2016 (Table S1). The most common cancer was postmenopausal breast cancer (), followed by colorectal cancer () and endometrial cancer ().
Table 1.
Baseline characteristics by average annual neighborhood walkability (NW) quartiles in the NYUWHS, years 1985–2016 ().
| Baseline characteristics | NYUWHS total ()a | NW Q1 [, 0.9 ()] | NW Q2 [0.9, 3.3 ()] | NW Q3 [3.3, 8.1 ()] | NW Q4 [8.1, 44.6 ()] |
|---|---|---|---|---|---|
| Age at enrollment [y ()] | |||||
| Baseline BMI [ ()] | |||||
| Education [ (%)] | |||||
| High school or less | 3,379 (31.2) | 912 (32.5) | 1,045 (39.1) | 938 (35.8) | 484 (17.7) |
| College/vocational/technical school/other | 4,402 (40.6) | 1,189 (42.4) | 1,023 (38.3) | 1,048 (40.0) | 1,142 (41.7) |
| Graduate school | 3,061 (28.2) | 706 (25.2) | 604 (22.6) | 636 (24.3) | 1,115 (40.7) |
| Race/ethnicity [ (%)] | |||||
| Non-Hispanic White | 9,115 (78.5) | 2,513 (84.4) | 2,185 (76.0) | 2,017 (71.0) | 2,400 (82.4) |
| Non-Hispanic Black | 1,370 (11.8) | 253 (8.5) | 405 (14.1) | 487 (17.2) | 225 (7.7) |
| Hispanic | 726 (6.3) | 131 (4.4) | 185 (6.4) | 220 (7.8) | 190 (6.5) |
| Other | 397 (3.4) | 81 (2.7) | 101 (3.5) | 116 (4.1) | 99 (3.4) |
| Menopausal status [ (%)] | |||||
| Premenopausal | 6,896 (52.1) | 1,632 (49.3) | 1,542 (46.6) | 1,722 (52.0) | 2,000 (60.4) |
| Postmenopausal | 6,344 (47.9) | 1,678 (50.7) | 1,768 (53.4) | 1,588 (48.0) | 1,310 (39.6) |
| Smoking status [ (%)] | |||||
| Never smoker | 5,692 (47.5) | 1,556 (50.3) | 1,433 (48.1) | 1,443 (49.5) | 1,260 (41.9) |
| Ever smoker | 6,304 (52.6) | 1,539 (49.7) | 1,545 (51.9) | 1,474 (50.5) | 1,746 (58.1) |
| Parity at enrollment [ (%)] | |||||
| No | 4,212 (31.8) | 627 (18.9) | 795 (24.0) | 1,078 (32.6) | 1,712 (51.7) |
| Yes | 9,028 (68.2) | 2,683 (81.1) | 2,515 (76.0) | 2,232 (67.4) | 1,598 (48.3) |
| Neighborhood poverty rate ()b | |||||
| Outdoor walking [MET-hours ()] | |||||
| Alcohol intake (g/d) [ (%)] | |||||
| 9,906 (88.8) | 2,667 (91.4) | 2,564 (92.3) | 2,383 (88.9) | 2,292 (82.3) | |
| 1,254 (11.2) | 251 (8.6) | 214 (7.7) | 297 (11.1) | 492 (17.7) | |
| Moving from baseline residence [ (%)]c | |||||
| No | 6,804 (51.4) | 1,295 (39.1) | 1,529 (46.2) | 1,779 (53.8) | 2,201 (66.5) |
| Yes | 6,436 (48.6) | 2,015 (60.9) | 1,781 (53.8) | 1,531 (46.3) | 1,109 (33.5) |
Note: BMI, body mass index; MET, metabolic equivalent of task; NYUWHS, New York University Women’s Health Study; Q, quartile; SD, standard deviation.
Cohort total after restricting data set to women with available geocoded addresses in the continental United States, available BMI, and no baseline cancer. Daily alcohol intake was missing in 2,080; race/ethnicity was missing in 1,632; education level was missing in 2,398; outdoor walking was missing in 2,679; and smoking status was missing in 1,244 women.
Percentage of people in residential neighborhood living in baseline year with a ratio of income to federal poverty level (FPL) below 1. Census block groups aggregated to radial buffers.
Moving from baseline residence at any time during the follow-up.
Neighborhood walkability was inversely associated with risk of obesity-related cancer in the NYUWHS (Table 2). Effect estimates for site-specific cancers were reported for cancers with cases. For overall obesity-related cancer and postmenopausal breast cancer, there was a significant pattern of decreasing risk with increasing quartile of neighborhood walkability. In models adjusted for individual-level and neighborhood-level covariates (model 2), women with average neighborhood walkability in the top quartile had a 26% lower risk of obesity-related cancer [ (95% CI: 0.65, 0.85)] and a 27% lower risk of postmenopausal breast cancer [ (95% CI: 0.61, 0.87)], compared with women with average neighborhood walkability in the bottom quartile. Similarly, when we considered average neighborhood walkability as a continuous variable, for every 1-SD increase in average neighborhood walkability, there was a 12% lower risk of overall obesity-related cancer [ (95% CI: 0.85, 0.93)] and an 11% lower risk of postmenopausal breast cancer [ (95% CI: 0.84, 0.95)].
Table 2.
Obesity-related cancer HRs and 95% CIs based on neighborhood walkability (NW) in the NYUWHS, years 1985–2016 ().
| Cancer subtype | NW Q1 [, 0.9 (Ref)] | NW Q2 [0.9, 3.3 HR (95% CI)] | NW Q3 [3.3, 8.1 HR (95% CI)] | NW Q4 [8.1, 44.6 HR (95% CI)] | Per SD (continuous NW) HR (95% CI)a |
|---|---|---|---|---|---|
| Overall (any first incident obesity-related cancer) | |||||
| Cases () | 614 | 603 | 619 | 575 | 2,411 |
| Model 1b | Ref | 0.94 (0.84, 1.05) | 0.93 (0.83, 1.04) | 0.76 (0.68, 0.86) | 0.90 (0.86, 0.94) |
| Model 2c | Ref | 0.94 (0.84, 1.05) | 0.92 (0.81, 1.03) | 0.74 (0.65, 0.85) | 0.89 (0.85, 0.93) |
| Model 3d | Ref | 0.94 (0.84, 1.05) | 0.92 (0.82, 1.04) | 0.77 (0.68, 0.88) | 0.90 (0.86, 0.94) |
| Postmenopausal breast cancere | |||||
| Cases () | 336 | 311 | 311 | 311 | 1,269 |
| Model 1b | Ref | 0.88 (0.76, 1.03) | 0.84 (0.72, 0.99) | 0.73 (0.62, 0.86) | 0.89 (0.84, 0.95) |
| Model 2c | Ref | 0.89 (0.76, 1.05) | 0.86 (0.72, 1.01) | 0.73 (0.61, 0.87) | 0.89 (0.84, 0.95) |
| Model 3d | Ref | 0.89 (0.76, 1.05) | 0.86 (0.73, 1.02) | 0.75 (0.63, 0.89) | 0.90 (0.85, 0.96) |
| Colorectal cancer | |||||
| Cases () | 88 | 83 | 90 | 82 | 343 |
| Model 1b | Ref | 0.88 (0.65, 1.19) | 0.93 (0.69, 1.25) | 0.82 (0.60, 1.13) | 0.92 (0.82, 1.04) |
| Model 2c | Ref | 0.87 (0.64, 1.18) | 0.91 (0.66, 1.25) | 0.79 (0.56, 1.11) | 0.91 (0.80, 1.03) |
| Model 3d | Ref | 0.87 (0.64, 1.19) | 0.92 (0.67, 1.27) | 0.83 (0.57, 1.17) | 0.92 (0.82, 1.05) |
| Colon cancer | |||||
| Cases () | 69 | 63 | 79 | 64 | 275 |
| Model 1b | Ref | 0.85 (0.60, 1.19) | 1.04 (0.74, 1.44) | 0.82 (0.58, 1.17) | 0.94 (0.83, 1.07) |
| Model 2c | Ref | 0.84 (0.59, 1.19) | 1.01 (0.71, 1.43) | 0.80 (0.54, 1.17) | 0.92 (0.80, 1.06) |
| Model 3d | Ref | 0.84 (0.59, 1.19) | 1.03 (0.72, 1.46) | 0.83 (0.57, 1.23) | 0.94 (0.82, 1.08) |
| Rectal cancer | |||||
| Cases () | 19 | 20 | 11 | 18 | 68 |
| Model 1b | Ref | 0.98 (0.52, 1.86) | 0.52 (0.25, 1.11) | 0.82 (0.42, 1.60) | 0.87 (0.67, 1.14) |
| Model 2c | Ref | 1.00 (0.53, 1.90) | 0.52 (0.23, 1.15) | 0.77 (0.36, 1.61) | 0.86 (0.65, 1.14) |
| Model 3d | Ref | 1.00 (0.53, 1.91) | 0.53 (0.24, 1.17) | 0.80 (0.38, 1.68) | 0.86 (0.65, 1.15) |
| Cancer of the uterus and endometrium | |||||
| Cases () | 71 | 68 | 75 | 68 | 282 |
| Model 1b | Ref | 0.96 (0.69, 1.34) | 1.00 (0.72, 1.39) | 0.80 (0.56, 1.13) | 0.91 (0.80, 1.03) |
| Model 2c | Ref | 0.93 (0.66, 1.31) | 0.93 (0.66, 1.33) | 0.71 (0.49, 1.04) | 0.87 (0.76, 0.99) |
| Model 3d | Ref | 0.93 (0.66, 1.30) | 0.94 (0.66, 1.34) | 0.77 (0.52, 1.12) | 0.90 (0.79, 1.03) |
| Ovarian cancer | |||||
| Cases () | 29 | 34 | 43 | 32 | 138 |
| Model 1b | Ref | 1.19 (0.72, 1.95) | 1.43 (0.89, 2.31) | 0.85 (0.51, 1.43) | 0.85 (0.71, 1.02) |
| Model 2c | Ref | 1.20 (0.72, 1.98) | 1.48 (0.89, 2.46) | 0.84 (0.48, 1.47) | 0.82 (0.68, 0.99) |
| Model 3d | Ref | 1.21 (0.73, 2.00) | 1.50 (0.90, 2.49) | 0.85 (0.48, 1.51) | 0.83 (0.68, 1.00) |
| Pancreatic cancer | |||||
| Cases () | 28 | 29 | 27 | 24 | 108 |
| Model 1b | Ref | 1.02 (0.60, 1.72) | 0.95 (0.56, 1.64) | 0.85 (0.48, 1.50) | 0.92 (0.75, 1.13) |
| Model 2c | Ref | 0.97 (0.57, 1.64) | 0.83 (0.47, 1.48) | 0.70 (0.38, 1.30) | 0.86 (0.69, 1.08) |
| Model 3d | Ref | 0.97 (0.57, 1.65) | 0.86 (0.48, 1.52) | 0.75 (0.40, 1.40) | 0.88 (0.70, 1.11) |
| Multiple myeloma and malignant plasma cell neoplasms | |||||
| Cases () | 17 | 26 | 20 | 10 | 73 |
| Model 1b | Ref | 1.41 (0.76, 2.62) | 1.06 (0.55, 2.06) | 0.55 (0.25, 1.24) | 0.77 (0.58, 1.03) |
| Model 2c | Ref | 1.25 (0.67, 2.33) | 0.77 (0.38, 1.55) | 0.38 (0.16, 0.91) | 0.68 (0.49, 0.94) |
| Model 3d | Ref | 1.26 (0.67, 2.34) | 0.76 (0.38, 1.54) | 0.40 (0.17, 0.94) | 0.69 (0.50, 0.96) |
| Renal cancer | |||||
| Cases () | 18 | 26 | 16 | 13 | 73 |
| Model 1b | Ref | 1.29 (0.70, 2.38) | 0.73 (0.37, 1.46) | 0.57 (0.27, 1.19) | 0.80 (0.61, 1.05) |
| Model 2c | Ref | 1.28 (0.69, 2.37) | 0.74 (0.36, 1.54) | 0.61 (0.27, 1.36) | 0.83 (0.62, 1.11) |
| Model 3d | Ref | 1.28 (0.69, 2.36) | 0.74 (0.35, 1.53) | 0.63 (0.28, 1.40) | 0.85 (0.63, 1.14) |
| Thyroid cancer | |||||
| Cases () | 16 | 15 | 17 | 22 | 70 |
| Model 1b | Ref | 0.95 (0.47, 1.93) | 0.99 (0.50, 1.98) | 0.98 (0.50, 1.92) | 0.93 (0.73, 1.17) |
| Model 2c | Ref | 1.07 (0.52, 2.19) | 1.30 (0.62, 2.71) | 1.43 (0.69, 2.97) | 1.03 (0.80, 1.31) |
| Model 3d | Ref | 1.07 (0.52, 2.18) | 1.26 (0.60, 2.64) | 1.35 (0.64, 2.81) | 1.00 (0.78, 1.29) |
Note: Cox proportional hazard (PH) models were implemented for neighborhood walkability as predictor of first incident obesity-related cancer adjusted for covariates. Missing observations for covariates alcohol (15.7%), smoking status (9.4%), education level (18.1%), and race/ethnicity (12.3%) were included in the models using multiple imputation with 10 iterations. Only cancer subtypes with a number of first obesity-related malignant cancer cases are shown in the table. A total of 316,783.5 person-years at risk were accrued throughout the study. BMI, body mass index; CI, confidence interval; HR, hazard ratio; NYUWHS, New York University Women’s Health Study; Q, quartile; Ref, reference; SD, standard deviation.
Continuous neighborhood walkability variable scaled to the SD ().
Adjusting for baseline age, race/ethnicity, education level, smoking status, and ever moving residence at any time during the study follow-up.
Adjusting for covariates in model 1 and additionally for alcohol intake, menopausal status, parity, and percentage below the poverty level living in neighborhood.
Adjusting for model 2 covariates and additionally for potential mediators outdoor walking, baseline BMI, and BMI change (the difference between the second to last reported BMI before incident obesity-related cancer or end of follow-up and BMI at enrollment). Missing observations for outdoor walking (20.2%) were included in the models using multiple imputation with 10 iterations.
Breast cancer diagnosed after menopause. Twenty-one women had unknown menopausal status at breast cancer diagnosis that were treated as a postmenopausal diagnosis if age at diagnosis was and treated as premenopausal if age at diagnosis was years of age.
Similar results were observed for endometrial cancer, although the association was apparent only in the analysis with walkability modeled as a continuous variable, likely owing to the smaller number of cases; for every 1-SD increase in average neighborhood walkability, there was a 13% lower risk of endometrial cancer [ (95% CI: 0.76, 0.99)]. For multiple myeloma, the risk reduction was limited to the fourth quartile, such that women with average neighborhood walkability in the top quartile had a 62% lower risk of multiple myeloma [ (95% CI: 0.16, 0.91)], compared with women in the bottom quartile, after adjusting for potential confounders at both individual and neighborhood level (model 2). When we considered the exposure as a continuous variable, we observed that for every 1-SD increase in average neighborhood walkability, there was a 32% lower risk of multiple myeloma [ (95% CI: 0.49, 0.94)]. For ovarian cancer, although there was no consistent evidence of a trend across quartiles, for every 1-SD increase in average neighborhood walkability, there was an 18% lower risk of ovarian cancer [ (95% CI: 0.68, 0.99)]. Except for thyroid cancer, the HR estimates suggested a lower risk of other cancers comparing the highest to the bottom quartile of average neighborhood walkability, although the CIs were wide. HR estimates from analyses using a dichotomized measure of average neighborhood walkability were also consistent for the rest of the cancer subtypes. When we adjusted for change in BMI and outdoor walking, the associations remained, although attenuated (Table 2, model 3). Similarly, findings from analyses with a time-varying measure of neighborhood walkability were also consistent with an inverse association for risk of obesity-related cancer (Table 3).
Table 3.
Obesity-related cancer HRs and 95% CIs based on time-varying neighborhood walkability (NW) sensitivity analyses ().
| Cases () | NW HR (95% CI) | |
|---|---|---|
| Overall (any first incident obesity-related cancer) | 2,411 | 0.90 (0.86, 0.94) |
| Postmenopausal breast cancera | 1,269 | 0.90 (0.85, 0.96) |
| Colorectal cancer | 343 | 0.93 (0.92, 1.05) |
| Colon cancer | 275 | 0.95 (0.82, 1.09) |
| Rectal cancer | 68 | 0.85 (0.64, 1.14) |
| Cancer of the uterus (including endometrium) | 282 | 0.88 (0.77, 1.00) |
| Ovarian cancer | 138 | 0.84 (0.69, 1.02) |
| Pancreatic cancer | 108 | 0.88 (0.70, 1.10) |
| Multiple myeloma and malignant plasma cell neoplasms | 73 | 0.68 (0.49, 0.95) |
| Renal cancer | 73 | 0.85 (0.63, 1.13) |
| Thyroid cancer | 70 | 1.06 (0.82, 1.36) |
Note: Time-varying Cox proportional hazard (PH) models were conducted to estimate the obesity-related cancer risk or HR associated with a per unit difference in continuous NW (SD-scaled), where . Annual walkability was considered for data points within the 31 y of follow-up. Models adjusted for covariates age, race/ethnicity, education level, smoking status, alcohol intake, menopausal status, parity, percentage below the poverty level living in neighborhood at baseline, and ever moving from baseline residence at any time during follow-up. Covariates alcohol, smoking status, education level, and race/ethnicity had missing observations and were included in the model using multiple imputation with 10 iterations for missing covariates. A total of 316,783.5 person-years at risk were accrued throughout the study. CI, confidence interval; HR, hazard ratio; SD, standard deviation.
Breast cancer diagnosed in menopause. Twenty-one women had unknown menopausal status at breast cancer diagnosis that were treated as a postmenopausal diagnosis if age at diagnosis was and treated as premenopausal if age at diagnosis was years of age.
Analyses using an extrapolated average measure of neighborhood walkability (Table S6, model 1) indicated very similar inverse associations to those found using a carried forward measure of the exposure. Analyses censoring women at withdrawal or at loss to follow-up also yielded almost identical results (Table S6, model 2). When we used a 3-y exposure lag, the inverse associations for overall obesity-related cancer, including postmenopausal breast cancer and multiple myeloma, attenuated but remained apparent (Table S6, model 3). In addition, higher levels of average neighborhood walkability exposures measured at younger and older age were both associated with lower risk of obesity-related cancer and postmenopausal breast cancer (Table S6, model 5). Analyses controlling for DASH diet (Table S6, model 6), without imputation (Table S6, model 7), analyses using different time scales (Table S7), and analyses scaling by IQR in lieu of SD were consistent (Table S8). Last, in sensitivity analyses assessing linearity using cubic splines, we found similar linear associations between walkability with overall obesity-related cancer risk () and postmenopausal breast cancer () (Table S2).
The inverse association between neighborhood walkability and risk of obesity-related cancer and risk of postmenopausal breast cancer was apparent in every subgroup (Figures 1 and 2). The association was stronger (Figure 1, ) in those who lived at baseline in a neighborhood with a poverty level (percentage of neighborhood population with a ratio of income to federal poverty level of ) above the sample median value [ (95% CI: 0.75, 0.87)] compared with the association in those who lived at baseline in a neighborhood with a poverty level lower than the sample median [ (95% CI: 0.88, 1.00)]. A similar pattern was also observed for postmenopausal breast cancer (Figure 2, ) where the association was more apparent in those who resided at baseline in areas with a higher poverty rate [ (95% CI: 0.73, 0.90)] compared with that of those who lived in an area with a lower poverty rate [ (95% CI: 0.86, 1.01)]. For other obesity-related cancers, no apparent interactions by sociodemographic lifestyle were observed on cancer risk (Tables S9–S12). A similar pattern of associations was also found for diabetic and nondiabetic women, as well as for women with higher and lower levels of outdoor walking (Table S13). Last, we found that the association between higher neighborhood walkability and lower risk of postmenopausal breast cancer was more apparent than the association for other subtypes of postmenopausal breast cancer, although the CI overlapped with CIs for other subtypes. Women in the highest quartile of neighborhood walkability had a 33% [ (95% CI: 0.51, 0.88)] lower risk of postmenopausal breast cancer compared with those in the lowest quartile (Table S14).
Figure 1.
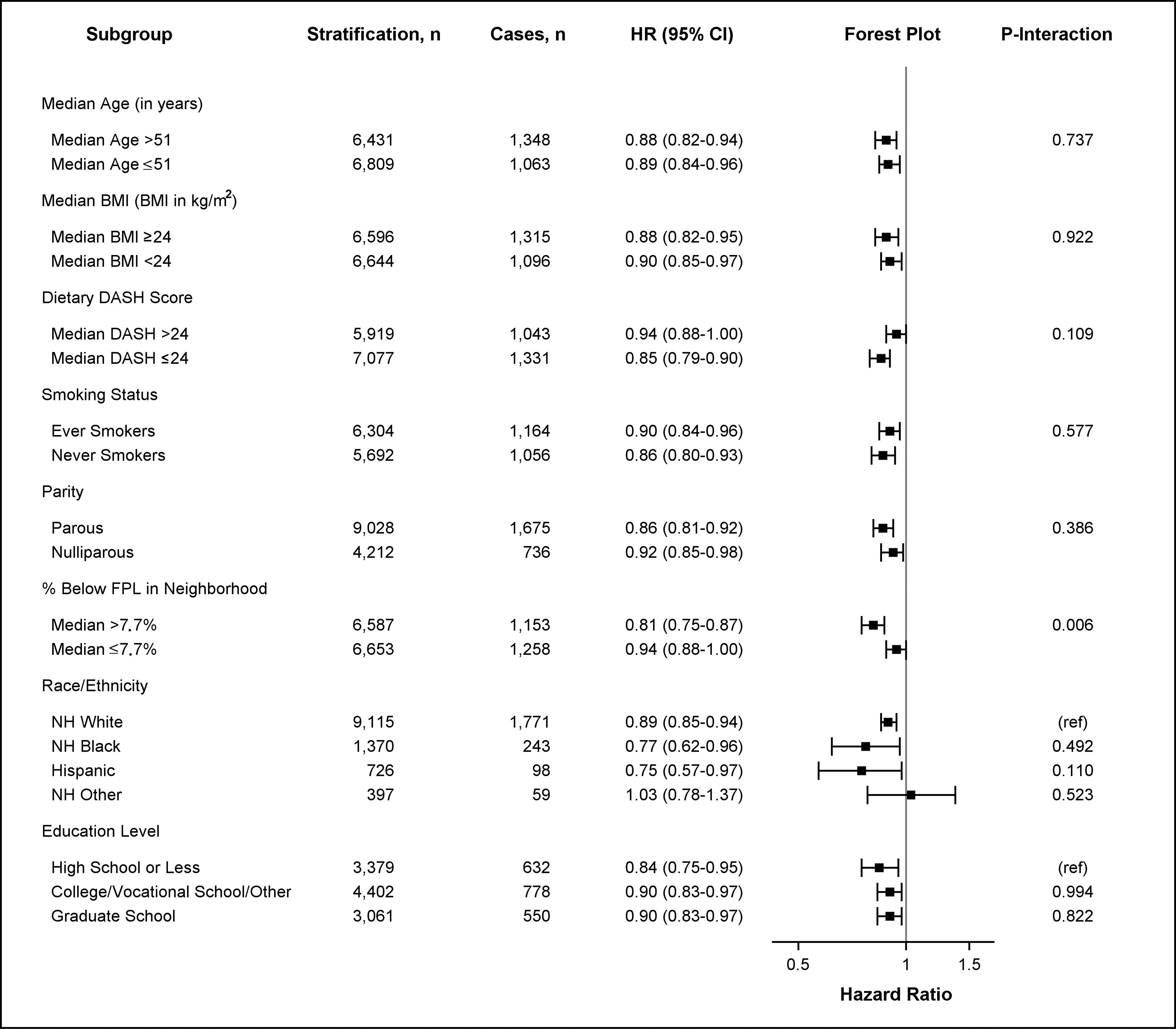
HRs for obesity-related cancer risk based on neighborhood walkability by age, BMI, DASH score, smoking status, parity, poverty level, education level, and race/ethnicity. HRs and 95% CIs are shown for stratified survival models assessing the association between continuous neighborhood walkability (SD-scaled, ) and obesity-related cancer risk by potential effect modifiers. Models were adjusted for all covariates (age, race/ethnicity, education level, smoking status, alcohol intake, menopausal status, parity, percentage below the poverty level living in neighborhood at baseline, and ever moving from baseline residence at any time during follow-up) except for the stratifying variable. Age was adjusted as a continuous variable in all models (including models stratified by median age). The -axis in the forest plot shows the untransformed HRs on the log-scale. HR estimates are represented by the squares, bars represent the 95% CIs of the estimates, and the whiskers represent the lower and upper confidence limits. Obesity-related cancers include breast cancer diagnosed after menopause, colorectal (colon and rectal), pancreatic, endometrial (including uterine), ovarian, renal, thyroid, liver, gallbladder, and esophageal cancers, as well as meningioma, and multiple myeloma. represents the -value of the coefficient for the cross-product of continuous neighborhood walkability and the effect modifier. Interaction models were computed treating effect modifiers as dichotomized variables. All interactions had 13,240 observations except for analyses including dietary DASH score (), smoking status (), education level (), and race/ethnicity (), which were restricted to nonmissing values. Note: %, percentage; BMI, body mass index; CI, confidence interval; DASH, Dietary Approaches to Stop Hypertension; FPL, federal poverty level; HR, hazard ratio; NH, non-Hispanic.
Figure 2.
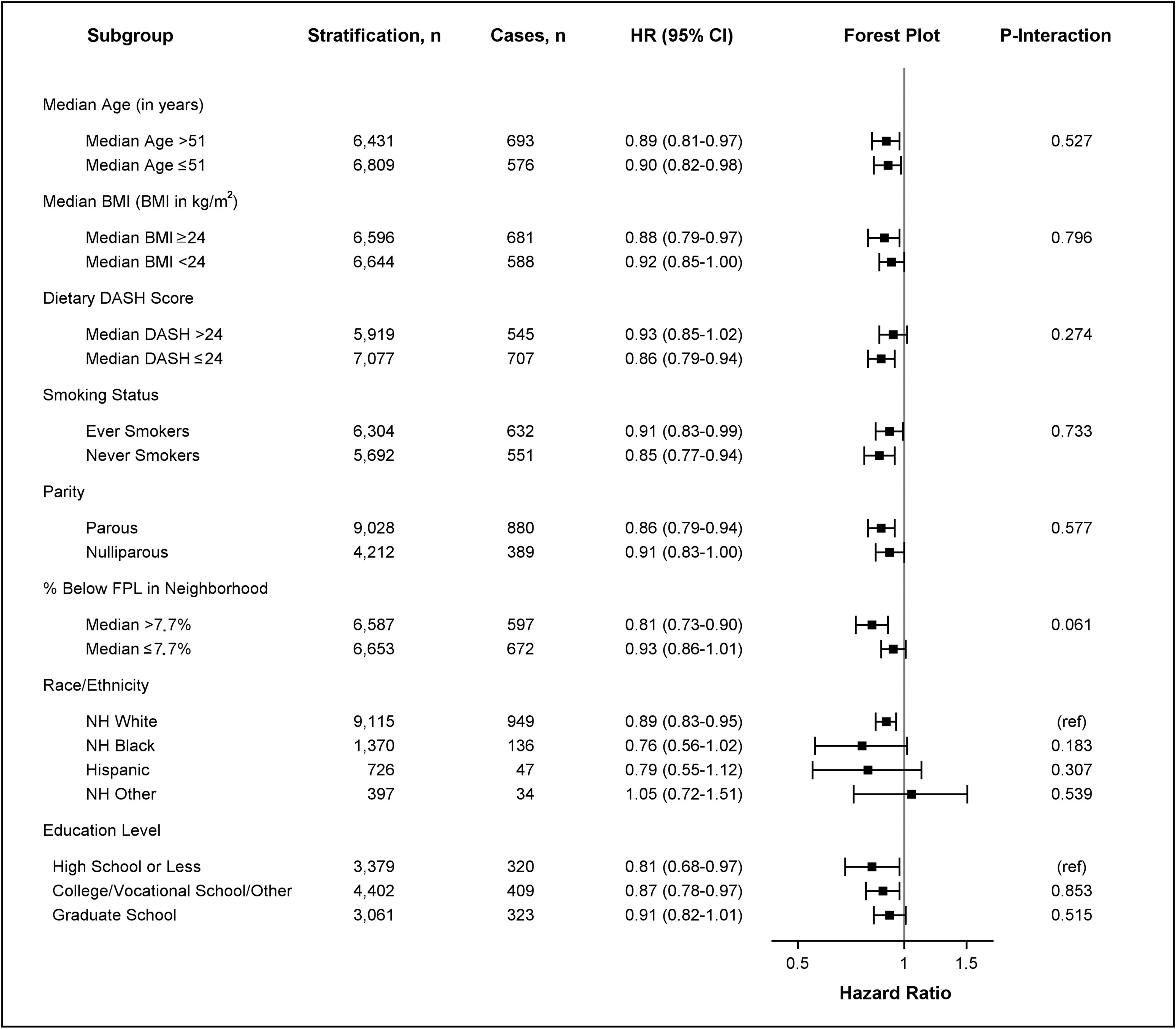
HRs for postmenopausal breast cancer risk based on neighborhood walkability by age, BMI, DASH score, smoking status, parity, poverty level, education level, and race/ethnicity. HRs and 95% CIs are shown for stratified survival models assessing the association between continuous neighborhood walkability (SD-scaled, ) and postmenopausal breast cancer risk by potential effect modifiers. Models were adjusted for all covariates (age, race/ethnicity, education level, smoking status, alcohol intake, menopausal status, parity, percentage below the poverty level living in neighborhood at baseline, and ever moving from baseline residence at any time during follow-up) except for the stratifying variable. Age was adjusted as a continuous variable in all models (including models stratified by median age). The -axis in the forest plot shows the untransformed HRs on the log-scale. HR estimates are represented by the squares, bars represent the 95% CIs of the estimates, and the whiskers represent the lower and upper confidence limits. represents the -value of the coefficient for the cross-product of continuous neighborhood walkability and the effect modifier. Interaction models were computed treating effect modifiers as dichotomized variables. All interactions had 13,240 observations except for analyses including dietary DASH score (), smoking status (), education level (), and race/ethnicity (), which were restricted to nonmissing values. Note: %, percentage; BMI, body mass index; CI, confidence interval; DASH, Dietary Approaches to Stop Hypertension; FPL, federal poverty level; HR, hazard ratio; NH, non-Hispanic.
Discussion
In this prospective cohort, we found that women who resided in neighborhoods with higher walkability levels, as measured by average destination accessibility and population density over y of follow-up, had lower risk of obesity-related cancers, particularly postmenopausal breast cancer. Moderate associations were also found for endometrial cancer, ovarian cancer, and multiple myeloma. We also observed that the inverse association between average neighborhood walkability and overall obesity-related cancer risk was modified by neighborhood poverty level.
Previous epidemiologic studies assessing the effects of neighborhood walkability on risk of cancer had major methodological limitations. An ecologic study showed inverse correlations between neighborhood walkability (measured as a 5-item scale including land use, population density, intersection density, transit distance, and ratio of retail building floor to retail land area) and incidence of multiple myeloma in a NYC population.34 However, the unit of analysis was at the ZIP code level, rendering the study susceptible to ecological fallacy. Another study in an older adult population from the University of Michigan Health and Retirement Study showed no association with overall cancer incidence using residential density and street connectivity as proxies for walkability.31 However, that study had only 2 y of follow-up and did not differentiate obesity-related cancers from other cancers. In the MEC study with long-term follow-up, higher levels of baseline mixed land development and urban environments were protective against postmenopausal breast cancers in Hispanics and Asians.32 In the same cohort, neighborhood obesogenic environment score, constructed using baseline street connectivity and population density, was not associated with risk of colorectal cancer after controlling for individual-level factors.35 In analyses taking into consideration residential changes over time, increasing population density was associated with increased risk of colorectal cancer, but increasing recreational and business facilities were related to a lower risk.33 However, that study investigated only the effects of changes in neighborhood with a limited temporal resolution using three data points or 1–3 y measurement windows. Importantly, previous studies either used a one-time measurement of neighborhood variables31,32,34,35 or used a limited quantity of data points that were not time weighted,33 potentially leading to substantial measurement errors of exposure. Despite these differences in study design and exposure assessment, our findings were consistent with those reported in the MEC regarding the inverse association between neighborhood walkability and breast cancer, and with the ecologic study in NYC regarding multiple myeloma. To our knowledge, the present study is the first prospective study on neighborhood walkability and risk of obesity-related cancer with a time-weighted average walkability construct based on a trajectory of residential history. The availability of longitudinal data on neighborhood walkability reduced measurement error and enhanced the validity of our findings.
The results of our study contribute to the growing evidence of how urban design affects the health and well-being of aging populations. Obesity and physical inactivity have each been associated with increased risk of premature death and cancer in women,58,59 but individual-level interventions to increase physical activity and reduce obesity are costly and have short-term effects.60–63 Neighborhood walkable spaces can promote walking behaviors, physical activity, and reduce car dependency,64 which could lead to subsequent improvements in preventing diseases attributed to obesity.65–67 Mounting data suggests that neighborhood walkability is linked to lower BMI22,68,69 and physical activity.70–72 Our findings are consistent with previous studies linking walking to lower risk of postmenopausal breast,73 endometrial,74 and renal75 cancers. After adjusting for BMI and outdoor walking in our study, the associations were attenuated, as expected, but the inverse association remained. There may be other effects of neighborhood walkability on physical activity, such as strenuous outdoor physical activity via biking or running. In addition, potential residual confounding associated with measurement error from potential mediators is possible. Possible mechanisms that underlie the association of neighborhood walkability with the risk of cancer include the potential effects of obesity and walking on hormone levels, chronic inflammation, or metabolic changes, such as insulin resistance.76–80 Future studies may also consider mediators measured at the molecular level.
Our findings suggest that the relationship between neighborhood walkability and obesity-related cancer could be primarily driven by postmenopausal breast cancer. In our data, there was a positive association between prevalence of some postmenopausal breast cancer risk factors at the individual level (high educational attainment, low parity) and walkability. On the other hand, poverty rate was higher in the top walkability quartile in our study. This is not uncommon in NYC given that an area with high walkability can have a wide range of income levels and include a high poverty rate. We found a stronger inverse association between average neighborhood walkability and risk of obesity-related cancer in women who resided in neighborhoods with a higher poverty rate. Consistently, the effect of walkability in women with lower educational attainment was stronger than that in women with higher educational attainment, although the effect modification was not apparent. Individuals who are relatively wealthier may be more likely to own cars or engage in exercise regardless of neighborhood walkability, reducing the effect of walkable neighborhoods. Unfortunately, we did not have data on income at the individual level. Taken together, the data suggest that effect of walkability on cancer risk in women may be modifiable by neighborhood SES variables, but future research is needed to confirm this observation.
Interestingly, we found a more apparent inverse association between walkability and risk of breast cancers. Although sample size was limited for receptor-defined breast cancer subtypes, our findings are consistent with several prospective studies81,82 that reported stronger associations between physical activity and breast cancers in postmenopausal women, compared with the associations with other subtypes. Hormone receptor-positive breast cancers tend to occur at older ages83 and are more strongly associated with adult weight gain and obesity in postmenopausal women84,85 and circulating steroid hormones.84–86 Increased physical activity can reduce inflammation and estrogen production by limiting androgen aromatization in older women.87,88 In an NYUWHS study of control participants from previous nested case–control studies of breast, endometrial, and ovarian cancer, we observed that higher levels of neighborhood walkability were associated with lower levels of androgens and higher levels of sex hormone binding globulin at baseline, suggesting a role of neighborhood walkability in hormone levels.89 However, future studies are warranted to confirm these findings and evaluate other mechanisms by which neighborhood walkability is involved in the risk of obesity-related cancer.
In addition to postmenopausal breast cancer, we observed inverse associations between neighborhood walkability and risk of ovarian and endometrial cancer. Epidemiologic studies have consistently shown that obesity and physical activity influence the risk of postmenopausal breast cancer.90,91 Nonstrenuous activity has also been linked to reduced breast cancer risk in postmenopausal women.73,92 Endometrial and ovarian cancers are also heavily influenced by adiposity. Physical activity, including walking, also was inversely related to endometrial cancer74,93,94 and moderately related to ovarian cancer.95–97 In terms of potential windows of susceptibility, the effect of walkability was observed at both younger and older ages in our study. Given that most of the cases were diagnosed at older age, the data suggests that concurrent neighborhood walkability at older ages may have an immediate beneficial effect. Our findings are in agreement with the notion that walkable neighborhoods are thought to promote aging in place.98–100
There are a few limitations in our study. First, we used an adapted version of a 4-item neighborhood walkability measure29 without annual data on transit and street connectivity, which may lead to nondifferential measurement errors. These data were unavailable owing to the cost of collecting these data for each year of follow-up and the inaccuracies in the national street network data before the early 2000s. The reduced version of our exposure measure may not fully capture neighborhood walkability. Nonetheless, the 2-item and 4-item walkability scores at baseline maintained a high correlation (). We did not interpolate transit stops because no data supports that the number of transit stops increases or decreases in a given neighborhood linearly across years. We acknowledge that there is measurement error from using two items to construct average annual walkability instead of using the four items. However, the two measures at baseline were highly correlated, suggesting that the walkability measure based on the two items is a good proxy for the more comprehensive 4-item walkability measurement. Although we were unable to adjust for access to cancer screening services in our models, walkability is positively correlated with poverty in our cohort and, in turn, potentially also related to lower levels of cancer screenings, suggesting a lack of adjustment for negative confounding and a potential bias toward the null. We also acknowledge that women who withdrew from active follow-up could have resided last in a state without a tumor registry linkage, leading to incomplete cancer ascertainment among these women at later years of follow-up. However, average walkability was constructed taking into account a censoring for nonrespondent women who moved last to states for which we do not have a linkage. Given that the recruitment was based on a visit at one mammography center, the relatively homogenous NYUWHS sample may not be representative of the U.S. female population; however, the observed effect did not differ by race and ethnic background or educational attainment. We acknowledge as a limitation that data on neighborhood poverty were available only at baseline, but evidence suggests that people tend to move to neighborhoods with similar SES characteristics.42,101,102 Future studies are needed to investigate the interrelationship between neighborhood walkability and neighborhood on the risk of health outcomes. Furthermore, although a large proportion of the cohort participants were recruited in a highly walkable setting (i.e., NYC), there was a wide range of walkability level across participants, ranging from to 44.6 average walkability scores. Last, our sample sizes for specific cancers were small, limiting our ability to detect smaller effects or describe a dose–response relationship.
This study has also several strengths. We constructed a time-weighted measure of neighborhood walkability that considered changes in the neighborhood environment and residential changes over a follow-up period of 23 y on average. Our measure of neighborhood walkability was grounded in urban design theory and based on data collected uniformly across the United States since 1985. Moreover, detailed data was collected in our study, which allowed us to control for potential confounders at both the individual and neighborhood level. Last, we included both overall obesity-related cancer, as well as site-specific cancers, as outcomes. Our study is unique in that the long-term follow-up allowed us to study effects of walkability with potential long latency periods of cancer. Overall, these findings may help substantiate policies targeting the built environment as a preventive or palliative measure to reduce obesity-related cancer risk in older women.
Conclusion
This study supports the hypothesis that residing in a more walkable neighborhood protects against the risk of overall obesity-related cancers in women, specifically postmenopausal breast cancer, as well as ovarian cancer, endometrial cancer, and multiple myeloma. Further research is needed in more diverse populations to replicate our findings.
Supplementary Material
Acknowledgments
The contributions of the authors are as follows. Y.C., A.G.R., and A.Z.J.: conceptualization; S.I.A., Y.A., J.W.Q.: data curation, formal analysis; Y.C. and A.Z.J.: funding acquisition; S.I.A.: investigation, software, visualization; S.I.A., Y.C. and M.L.: methodology; S.I.A.: roles/writing—original draft; A.G.R., A.Z.J., T.V.C., K.L.K., K.M.N., L.E.T., and Y.C.: writing—review and editing.
The study was supported by the U.S. National Institutes of Health (grants UM1CA182934-01A1, UM1CA182934-05-S1, P30CA016087, and P30ES000260, all to Y.C.). The data used in this research was also supported by the National Institute of Aging (grants 1R01AG049970 and 3R01AG049970-04S1, to primary investigator Gina Lovasi).
References
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. 2020. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 360:1–8, PMID: , https://www.cdc.gov/nchs/data/databriefs/db360-h.pdf. [PubMed] [Google Scholar]
- 2.Haslam DW, James WPT. 2005. Obesity. Lancet 366(9492):1197–1209, PMID: , 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Basen-Engquist K, Chang M. 2011. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep 13(1):71–76, PMID: , 10.1007/s11912-010-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. 2016. Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med 375(8):794–798, PMID: , 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.OECD (Organisation for Economic Co-operation and Development). 2017. Obesity Update 2017. https://www.oecd.org/health/health-systems/Obesity-Update-2017.pdf [accessed 21 September 2023].
- 6.Massetti GM, Dietz WH, Richardson LC. 2017. Excessive weight gain, obesity, and cancer: opportunities for clinical intervention. JAMA 318(20):1975–1976, PMID: , 10.1001/jama.2017.15519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, et al. 2017. Vital signs: trends in incidence of cancers associated with overweight and obesity—United States, 2005–2014. MMWR Morb Mortal Wkly Rep 66(39):1052–1058, PMID: , 10.15585/mmwr.mm6639e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godinho-Mota JCM, Gonçalves LV, Soares LR, Mota JF, Martins KA, Freitas-Junior I, et al. 2018. Abdominal adiposity and physical inactivity are positively associated with breast cancer: a case-control study. Biomed Res Int 2018:4783710, PMID: , 10.1155/2018/4783710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannioto R, Etter JL, Guterman LB, Joseph JM, Gulati NR, Schmitt KL, et al. 2017. The association of lifetime physical inactivity with bladder and renal cancer risk: a hospital-based case-control analysis. Cancer Epidemiol 49:24–29, PMID: , 10.1016/j.canep.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannioto R, LaMonte MJ, Risch HA, Hong CC, Sucheston-Campbell LE, Eng KH, et al. 2016. Chronic recreational physical inactivity and epithelial ovarian cancer risk: evidence from the Ovarian Cancer Association Consortium. Cancer Epidemiol Biomarkers Prev 25(7):1114–1124, PMID: , 10.1158/1055-9965.EPI-15-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, et al. 2003. Recreational physical activity and the risk of breast cancer in postmenopausal: the Women’s Health Initiative Cohort Study. JAMA 290(10):1331–1336, PMID: , 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein L, Patel AV, Ursin G, Sullivan-Halley J, Press MF, Deapen D, et al. 2005. Lifetime recreational exercise activity and breast cancer risk among black women and white women. J Natl Cancer Inst 97(22):1671–1679, PMID: , 10.1093/jnci/dji374. [DOI] [PubMed] [Google Scholar]
- 13.Clarke T, Norris T, Schiller JS. 2017. Early release of selected estimates based on data from the 2016 National Health Interview Survey. Data tables for figures 7.1, 7.4. https://www.cdc.gov/nchs/data/nhis/earlyrelease/earlyrelease201705.pdf [accessed 21 September 2023].
- 14.Besser LM, Dannenberg AL. 2005. Walking to public transit: steps to help meet physical activity recommendations. Am J Prev Med 29(4):273–280, PMID: , 10.1016/j.amepre.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Shephard RJ. 2008. Is active commuting the answer to population health? Sports Med 38(9):751–758, PMID: , 10.2165/00007256-200838090-00004. [DOI] [PubMed] [Google Scholar]
- 16.Yang S, Chen X, Wang L, Wu T, Fei T, Xiao Q, et al. 2021. Walkability indices and childhood obesity: a review of epidemiologic evidence. Obes Rev 22(suppl 1):e13096, PMID: , 10.1111/obr.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou Y, Ma Y, Wu Z, Liu Y, Xu M, Qiu G, et al. 2021. Neighbourhood residential density and childhood obesity. Obes Rev 22(suppl 1):e13037, PMID: , 10.1111/obr.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lachapelle U, Frank L, Saelens BE, Sallis JF, Conway TL. 2011. Commuting by public transit and physical activity: where you live, where you work, and how you get there. J Phys Act Health 8(suppl 1):S72–S82, PMID: , 10.1123/jpah.8.s1.s72. [DOI] [PubMed] [Google Scholar]
- 19.Saelens BE, Sallis JF, Frank LD. 2003. Environmental correlates of walking and cycling: findings from the transportation, urban design, and planning literatures. Ann Behav Med 25(2):80–91, PMID: , 10.1207/S15324796ABM2502_03. [DOI] [PubMed] [Google Scholar]
- 20.Handy SL, Boarnet MG, Ewing R, Killingsworth RE. 2002. How the built environment affects physical activity: views from urban planning. Am J Prev Med 23(suppl 2):64–73, PMID: , 10.1016/s0749-3797(02)00475-0. [DOI] [PubMed] [Google Scholar]
- 21.Pikora T, Giles-Corti B, Bull F, Jamrozik K, Donovan R. 2003. Developing a framework for assessment of the environmental determinants of walking and cycling. Soc Sci Med 56(8):1693–1703, PMID: , 10.1016/s0277-9536(02)00163-6. [DOI] [PubMed] [Google Scholar]
- 22.Creatore MI, Glazier RH, Moineddin R, Fazli GS, Johns A, Gozdyra P, et al. 2016. Association of neighborhood walkability with change in overweight, obesity, and diabetes. JAMA 315(20):2211–2220, PMID: , 10.1001/jama.2016.5898. [DOI] [PubMed] [Google Scholar]
- 23.Frank LD, Andresen MA, Schmid TL. 2004. Obesity relationships with community design, physical activity, and time spent in cars. Am J Prev Med 27(2):87–96, PMID: , 10.1016/j.amepre.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Frank LD, Engelke PO. 2001. The built environment and human activity patterns: exploring the impacts of urban form on public health. J Plan Lit 16(2):202–218, 10.1177/08854120122093339. [DOI] [Google Scholar]
- 25.Freeman L, Neckerman K, Schwartz-Soicher O, Quinn J, Richards C, Bader MDM, et al. 2013. Neighborhood walkability and active travel (walking and cycling) in New York City. J Urban Health 90(4):575–585, PMID: , 10.1007/s11524-012-9758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christiansen LB, Cerin E, Badland H, Kerr J, Davey R, Troelsen J, et al. 2016. International comparisons of the associations between objective measures of the built environment and transport-related walking and cycling: IPEN Adult Study. J Transp Health 3(4):467–478, PMID: , 10.1016/j.jth.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsyth A, Krizek KJ. 2010. Promoting walking and bicycling: assessing the evidence to assist planners. Built Environ 36(4):429–446, 10.2148/benv.36.4.429. [DOI] [Google Scholar]
- 28.Kerr J, Emond JA, Badland H, Reis R, Sarmiento O, Carlson J, et al. 2016. Perceived neighborhood environmental attributes associated with walking and cycling for transport among adult residents of 17 cities in 12 countries: the IPEN study. Environ Health Perspect 124(3):290–298, PMID: , 10.1289/ehp.1409466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rundle AG, Chen Y, Quinn JW, Rahai N, Bartley K, Mooney SJ, et al. 2019. Development of a neighborhood walkability index for studying neighborhood physical activity contexts in communities across the U.S. over the past three decades. J Urban Health 96(4):583–590, PMID: , 10.1007/s11524-019-00370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathan A, Pereira G, Foster S, Hooper P, Saarloos D, Giles-Corti B. 2012. Access to commercial destinations within the neighbourhood and walking among Australian older adults. Int J Behav Nutr Phys Act 9:133, PMID: , 10.1186/1479-5868-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman VA, Grafova IB, Rogowski J. 2011. Neighborhoods and chronic disease onset in later life. Am J Public Health 101(1):79–86, PMID: , 10.2105/AJPH.2009.178640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conroy SM, Clarke CA, Yang J, Shariff-Marco S, Shvetsov YB, Park SY, et al. 2017. Contextual impact of neighborhood obesogenic factors on postmenopausal breast cancer: the Multiethnic Cohort. Cancer Epidemiol Biomarkers Prev 26(4):480–489, PMID: , 10.1158/1055-9965.EPI-16-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shvetsov YB, Shariff-Marco S, Yang J, Conroy SM, Canchola AJ, Albright CL, et al. 2020. Association of change in the neighborhood obesogenic environment with colorectal cancer risk: the Multiethnic Cohort Study. SSM Popul Health 10:100532, PMID: , 10.1016/j.ssmph.2019.100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamath GR, Renteria AS, Jagannath S, Gallagher EJ, Parekh S, Bickell NA. 2020. Where you live can impact your cancer risk: a look at multiple myeloma in New York City. Ann Epidemiol 48:43–50.e44, PMID: , 10.1016/j.annepidem.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Canchola AJ, Shariff-Marco S, Yang J, Albright C, Hertz A, Park SY, et al. 2017. Association between the neighborhood obesogenic environment and colorectal cancer risk in the Multiethnic Cohort. Cancer Epidemiol 50(pt A):99–106, PMID: , 10.1016/j.canep.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.India-Aldana S, Rundle AG, Zeleniuch-Jacquotte A, Quinn JW, Kim B, Afanasyeva Y, et al. 2021. Neighborhood walkability and mortality in a prospective cohort of women. Epidemiology 32(6):763–772, PMID: , 10.1097/EDE.0000000000001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. , eds. 2017. AJCC Cancer Staging Manual, Eighth Edition. Cham, Switzerland: Springer Cham. [Google Scholar]
- 38.Kato I, Toniolo P, Koenig KL, Kahn A, Schymura M, Zeleniuch-Jacquotte A. 1999. Comparison of active and cancer registry-based follow-up for breast cancer in a prospective cohort study. Am J Epidemiol 149(4):372–378, PMID: , 10.1093/oxfordjournals.aje.a009823. [DOI] [PubMed] [Google Scholar]
- 39.WHO (World Health Organization). 1978. International Classification of Diseases, Ninth Revision, Basic Tabulation List with Alphabetic Index. https://apps.who.int/iris/handle/10665/39473 [accessed 21 September 2023].
- 40.Kaufman TK, Sheehan DM, Rundle A, Neckerman KM, Bader MDM, Jack D, et al. 2015. Measuring health-relevant businesses over 21 years: refining the National Establishment Time-Series (NETS), a dynamic longitudinal data set. BMC Res Notes 8:507, PMID: , 10.1186/s13104-015-1482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.South SJ, Huang Y, Spring A, Crowder K. 2016. Neighborhood attainment over the adult life course. Am Sociol Rev 81(6):1276–1304, PMID: , 10.1177/0003122416673029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowder K, South SJ. 2008. Spatial dynamics of White flight: the effects of local and extralocal racial conditions on neighborhood out-migration. Am Sociol Rev 73(5):792–812, PMID: , 10.1177/000312240807300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirsch JA, Moore KA, Barrientos-Gutierrez T, Brines SJ, Zagorski MA, Rodriguez DA, et al. 2014. Built environment change and change in BMI and waist circumference: Multi-Ethnic Study of Atherosclerosis. Obesity (Silver Spring) 22(11):2450–2457, PMID: , 10.1002/oby.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, et al. 2011. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 43(8):1575–1581, PMID: , 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 45.ODPHP (Office of Disease Prevention and Health Promotion). 2021. 2008 Physical Activity Guidelines for Americans. https://health.gov/our-work/nutrition-physical-activity/physical-activity-guidelines/previous-guidelines/2008-physical-activity-guidelines [accessed 21 September 2023].
- 46.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. 2008. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 168(7):713–720, PMID: , 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 47.Jafari Nasab S, Ghanavati M, Rafiee P, Bahrami A, Majidi N, Clark CCT, et al. 2021. A case-control study of Dietary Approaches to Stop Hypertension (DASH) diets, colorectal cancer and adenomas among Iranian population. BMC Cancer 21(1):1050, PMID: , 10.1186/s12885-021-08786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toorang F, Sasanfar B, Hadji M, Esmaillzadeh A, Zendehdel K. 2020. Adherence to “Dietary Approaches to Stop Hypertension” eating plan in relation to gastric cancer. Nutr J 19(1):40, PMID: , 10.1186/s12937-020-00560-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. 2019. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol 15(6):367–385, PMID: , 10.1038/s41581-019-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Temu TM, Macharia P, Mtui J, Mwangi M, Ngungi PW, Wanjalla C, et al. 2021. Obesity and risk for hypertension and diabetes among Kenyan adults: results from a national survey. Medicine (Baltimore) 100(40):e27484, PMID: , 10.1097/MD.0000000000027484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nohr EA, Liew Z. 2018. How to investigate and adjust for selection bias in cohort studies. Acta Obstet Gynecol Scand 97(4):407–416, PMID: , 10.1111/aogs.13319. [DOI] [PubMed] [Google Scholar]
- 52.Rubin DB. 1987. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons Inc. [Google Scholar]
- 53.van Buuren S. 2007. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 16(3):219–242, PMID: , 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 54.Rubin DB. 1976. Inference and missing data. Biometrika 63(3):581–592, 10.1093/biomet/63.3.581. [DOI] [Google Scholar]
- 55.Canchola AJ, Stewart SL, Bernstein L, West DW, Ross RK, Deapen D, et al. 2003. Cox regression using different time-scales. https://www.lexjansen.com/wuss/2003/DataAnalysis/i-cox_time_scales.pdf [accessed 21 September 2023].
- 56.Korn EL, Graubard BI, Midthune D. 1997. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 145(1):72–80, PMID: , 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 57.Fine JP, Gray RJ. 1999. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94(446):496–509, 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 58.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. 2008. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation 117(13):1658–1667, PMID: , 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 59.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, et al. 2000. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med 160(14):2117–2128, PMID: , 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 60.Groessl EJ, Kaplan RM, Blair SN, Rejeski WJ, Katula JA, King AC, et al. 2009. A cost analysis of a physical activity intervention for older adults. J Phys Act Health 6(6):767–774, PMID: , 10.1123/jpah.6.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sevick MA, Dunn AL, Morrow MS, Marcus BH, Chen GJ, Blair SN. 2000. Cost-effectiveness of lifestyle and structured exercise interventions in sedentary adults: results of project ACTIVE. Am J Prev Med 19(1):1–8, PMID: , 10.1016/s0749-3797(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 62.Vanroy J, Seghers J, Bogaerts A, Devloo K, De Cock S, Boen F. 2017. Short- and long-term effects of a need-supportive physical activity intervention among patients with type 2 diabetes mellitus: a randomized controlled pilot trial. PLoS One 12(4):e0174805, PMID: , 10.1371/journal.pone.0174805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sjöwall D, Hertz M, Klingberg T. 2017. No long-term effect of physical activity intervention on working memory or arithmetic in preadolescents. Front Psychol 8:1342, PMID: , 10.3389/fpsyg.2017.01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carlson JA, Saelens BE, Kerr J, Schipperijn J, Conway TL, Frank LD, et al. 2015. Association between neighborhood walkability and GPS-measured walking, bicycling and vehicle time in adolescents. Health Place 32:1–7, PMID: , 10.1016/j.healthplace.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richardson CR, Newton TL, Abraham JJ, Sen A, Jimbo M, Swartz AM. 2008. A meta-analysis of pedometer-based walking interventions and weight loss. Ann Fam Med 6(1):69–77, PMID: , 10.1370/afm.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy MH, Nevill AM, Murtagh EM, Holder RL. 2007. The effect of walking on fitness, fatness and resting blood pressure: a meta-analysis of randomised, controlled trials. Prev Med 44(5):377–385, PMID: , 10.1016/j.ypmed.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 67.Thorp AA, Owen N, Neuhaus M, Dunstan DW. 2011. Sedentary behaviors and subsequent health outcomes in adults: a systematic review of longitudinal studies, 1996–2011. Am J Prev Med 41(2):207–215, PMID: , 10.1016/j.amepre.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Nichani V, Turley L, Vena JE, McCormack GR. 2020. Associations between the neighbourhood characteristics and body mass index, waist circumference, and waist-to-hip ratio: findings from Alberta’s Tomorrow Project. Health Place 64:102357, PMID: , 10.1016/j.healthplace.2020.102357. [DOI] [PubMed] [Google Scholar]
- 69.Tarlov E, Silva A, Wing C, Slater S, Matthews SA, Jones KK, et al. 2020. Neighborhood walkability and BMI change: a national study of veterans in large urban areas. Obesity (Silver Spring) 28(1):46–54, PMID: , 10.1002/oby.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stevens RB, Brown BB. 2011. Walkable new urban LEED_Neighborhood-Development (LEED-ND) community design and children’s physical activity: selection, environmental, or catalyst effects? Int J Behav Nutr Phys Act 8:139, PMID: , 10.1186/1479-5868-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zuniga-Teran AA, Orr BJ, Gimblett RH, Chalfoun NV, Marsh SE, Guertin DP, et al. 2017. Designing healthy communities: testing the walkability model. Front Archit Res 6(1):63–73, 10.1016/j.foar.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orstad SL, McDonough MH, James P, Klenosky DB, Laden F, Mattson M, et al. 2018. Neighborhood walkability and physical activity among older women: tests of mediation by environmental perceptions and moderation by depressive symptoms. Prev Med 116:60–67, PMID: , 10.1016/j.ypmed.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC. 2010. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med 170(19):1758–1764, PMID: , 10.1001/archinternmed.2010.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Du M, Kraft P, Eliassen AH, Giovannucci E, Hankinson SE, De Vivo I. 2014. Physical activity and risk of endometrial adenocarcinoma in the Nurses’ Health Study. Int J Cancer 134(11):2707–2716, PMID: , 10.1002/ijc.28599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams PT. 2014. Reduced risk of incident kidney cancer from walking and running. Med Sci Sports Exerc 46(2):312–317, PMID: , 10.1249/MSS.0b013e3182a4e89c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Gaal LF, Mertens IL, De Block CE. 2006. Mechanisms linking obesity with cardiovascular disease. Nature 444(7121):875–880, PMID: , 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 77.Hopkins BD, Goncalves MD, Cantley LC. 2016. Obesity and cancer mechanisms: cancer metabolism. J Clin Oncol 34(35):4277–4283, PMID: , 10.1200/JCO.2016.67.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wertheim BC, Martínez ME, Ashbeck EL, Roe DJ, Jacobs ET, Alberts DS, et al. 2009. Physical activity as a determinant of fecal bile acid levels. Cancer Epidemiol Biomarkers Prev 18(5):1591–1598, PMID: , 10.1158/1055-9965.EPI-08-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winzer BM, Whiteman DC, Reeves MM, Paratz JD. 2011. Physical activity and cancer prevention: a systematic review of clinical trials. Cancer Causes Control 22(6):811–826, PMID: , 10.1007/s10552-011-9761-4. [DOI] [PubMed] [Google Scholar]
- 80.NCI (National Cancer Institute). 2020. Physical Activity and Cancer. https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/physical-activity-fact-sheet [accessed 21 September 2023].
- 81.Suzuki R, Iwasaki M, Yamamoto S, Inoue M, Sasazuki S, Sawada N, et al. 2011. Leisure-time physical activity and breast cancer risk defined by estrogen and progesterone receptor status—the Japan Public Health Center-based Prospective Study. Prev Med 52(3–4):227–233, PMID: , 10.1016/j.ypmed.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt ME, Steindorf K, Mutschelknauss E, Slanger T, Kropp S, Obi N, et al. 2008. Physical activity and postmenopausal breast cancer: effect modification by breast cancer subtypes and effective periods in life. Cancer Epidemiol Biomarkers Prev 17(12):3402–3410, PMID: , 10.1158/1055-9965.EPI-08-0479. [DOI] [PubMed] [Google Scholar]
- 83.Diab SG, Elledge RM, Clark GM. 2000. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 92(7):550–556, PMID: , 10.1093/jnci/92.7.550. [DOI] [PubMed] [Google Scholar]
- 84.Vrieling A, Buck K, Kaaks R, Chang-Claude J. 2010. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat 123(3):641–649, PMID: , 10.1007/s10549-010-1116-4. [DOI] [PubMed] [Google Scholar]
- 85.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. 2017. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin 67(5):378–397, PMID: , 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. 2004. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst 96(24):1856–1865, PMID: , 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 87.Lynch BM. 2010. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev 19(11):2691–2709, PMID: , 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 88.Neilson HK, Friedenreich CM, Brockton NT, Millikan RC. 2009. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev 18(1):11–27, PMID: , 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- 89.India-Aldana S, Rundle AG, Clendenen TV, Quinn JW, Arslan AA, Afanasyeva Y, et al. 2022. Neighborhood walkability and sex hormone levels in women. Environ Res 215(pt 2):114285, PMID: , 10.1016/j.envres.2022.114285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fournier A, Dos Santos G, Guillas G, Bertsch J, Duclos M, Boutron-Ruault MC, et al. 2014. Recent recreational physical activity and breast cancer risk in postmenopausal women in the E3N cohort. Cancer Epidemiol Biomarkers Prev 23(9):1893–1902, PMID: , 10.1158/1055-9965.EPI-14-0150. [DOI] [PubMed] [Google Scholar]
- 91.Wu Y, Zhang D, Kang S. 2013. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat 137(3):869–882, PMID: , 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 92.Hildebrand JS, Gapstur SM, Campbell PT, Gaudet MM, Patel AV. 2013. Recreational physical activity and leisure-time sitting in relation to postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 22(10):1906–1912, PMID: , 10.1158/1055-9965.EPI-13-0407. [DOI] [PubMed] [Google Scholar]
- 93.Borch KB, Weiderpass E, Braaten T, Jareid M, Gavrilyuk OA, Licaj I. 2017. Physical activity and risk of endometrial cancer in the Norwegian Women and Cancer (NOWAC) study. Int J Cancer 140(8):1809–1818, PMID: , 10.1002/ijc.30610. [DOI] [PubMed] [Google Scholar]
- 94.Schmid D, Behrens G, Keimling M, Jochem C, Ricci C, Leitzmann M. 2015. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol 30(5):397–412, PMID: , 10.1007/s10654-015-0017-6. [DOI] [PubMed] [Google Scholar]
- 95.Moorman PG, Jones LW, Akushevich L, Schildkraut JM. 2011. Recreational physical activity and ovarian cancer risk and survival. Ann Epidemiol 21(3):178–187, PMID: , 10.1016/j.annepidem.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee AH, Su D, Pasalich M, Wong YL, Binns CW. 2013. Habitual physical activity reduces risk of ovarian cancer: a case-control study in southern China. Prev Med 57(suppl):S31–S33, PMID: , 10.1016/j.ypmed.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 97.Olsen CM, Bain CJ, Jordan SJ, Nagle CM, Green AC, Whiteman DC, et al. 2007. Recreational physical activity and epithelial ovarian cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev 16(11):2321–2330, PMID: , 10.1158/1055-9965.EPI-07-0566. [DOI] [PubMed] [Google Scholar]
- 98.King AC, Sallis JF, Frank LD, Saelens BE, Cain K, Conway TL, et al. 2011. Aging in neighborhoods differing in walkability and income: associations with physical activity and obesity in older adults. Soc Sci Med 73(10):1525–1533, PMID: , 10.1016/j.socscimed.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weiss RL, Maantay JA, Fahs M. 2010. Promoting active urban aging: a measurement approach to neighborhood walkability for older adults. Cities Environ 3(1):12, PMID: , 10.15365/cate.31122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beard JR, Blaney S, Cerda M, Frye V, Lovasi GS, Ompad D, et al. 2009. Neighborhood characteristics and disability in older adults. J Gerontol B Psychol Sci Soc Sci 64(2):252–257, PMID: , 10.1093/geronb/gbn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Margerison-Zilko C, Cubbin C, Jun J, Marchi K, Braveman P. 2016. Post-partum residential mobility among a statewide representative sample of California women, 2003–2007. Matern Child Health J 20(1):139–148, PMID: , 10.1007/s10995-015-1812-0. [DOI] [PubMed] [Google Scholar]
- 102.Wang Q, Phillips NE, Small ML, Sampson RJ. 2018. Urban mobility and neighborhood isolation in America’s 50 largest cities. Proc Natl Acad Sci USA 115(30):7735–7740, PMID: , 10.1073/pnas.1802537115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


