Abstract
INTRODUCTION
Muscle changes after stroke cannot be explained solely on the basis of corticospinal bundle damage. Muscle-specific changes contribute to limited functional recovery but have been poorly characterized.
EVIDENCE ACQUISITION
We conducted a systematic review of muscular changes occurring at the histological, neuromuscular and functional levels during the first year after the onset of post-stroke hemiplegia. A literature search was performed on PubMed, Embase and CINHAL databases up to November 2022 using a keyword combination comprising cerebral stroke, hemiplegic, atrophy, muscle structure, paresis, skeletal muscle fiber type, motor unit, oxidative stress, strength, motor control.
EVIDENCE SYNTHESIS
Twenty-seven trial reports were included in the review, out of 12,798 articles screened. Structural modifications described on the paretic side include atrophy, transformation of type II fibers into type I fibers, decrease in fiber diameter and apparent myofilament disorganization from the first week post-stroke up to the fourth month. Reported biochemical changes comprise the abnormal presence of lipid droplets and glycogen granules in the subsarcolemmal region during the first month post-stroke. At the neurophysiological level, studies indicate an early decrease in the number and activity of motor units, correlated with the degree of motor impairment. All these modifications were present to a lesser degree on the non-paretic side. Although only sparse data concerning the subacute stage are available, these changes seem to appear during the first two weeks post-stroke and continue up to the third or fourth month.
CONCLUSIONS
Considering these early pathophysiological changes on both the paretic and non-paretic sides, it seems crucial to promptly stimulate central and also peripheral muscular activation after stroke through specific rehabilitation programs focused on the maintenance of muscle capacities associated with neurological recovery or plasticity.
Key words: Stroke, Hemiplegia, Muscles, Histology, Biochemistry, Recovery of function
Introduction
Stroke is the third cause of death and the first cause of disability in developed countries.1 The prevalence of ischemic stroke was 24.5 million in 2015, leading to 3 million years lived with disability (YLDs).2, 3 Stroke may lead to various disabilities, the most frequent presentation being hemiplegia.4 Rehabilitation conditions and provision of post-stroke care vary widely between countries.5 Recommendations have been proposed for post-stroke rehabilitation, the techniques employed depending on the accessibility of available rehabilitation platforms.6 They include active or passive central or peripheral re-activation approaches aiming to limit the effects of immobility and stimulate brain plasticity to restore autonomy.7
Loss of central activation is not the only factor explaining motor impairment. The preservation of skeletal striated muscle in hemiplegia remains insufficiently considered in rehabilitation.8 Better awareness of the time course of the muscular consequences of stroke (paresis, atrophy, retraction or fibrosis) appears essential to optimize potential rehabilitation benefits.8 Complementary protocols focused on the determinants of motor recovery and mutual plasticity between muscle and brain should also be developed.9 Previous reviews have reported a reduction in lower-limb muscle mass and strength on both the paretic side (PS) and the non-paretic side (NPS).9-11
Despite these structural changes, recovery on the PS is confirmed by an increase in muscle strength independent of the decrease in muscle thickness from the first months after stroke.12 In this latest review, confounding variables such as neural activation, spasticity, sarcopenia, immobilization, and their specific effects were not considered. Electrical activity and muscle structure data are still lacking to explain how muscle changes occur in the first year after stroke. Clear data on changes in muscle structure and physiology directly related to stroke, as well as those related to immobility or advancing age, are needed to tailor early rehabilitation management.
We propose a systematic review of skeletal muscle damage at the acute and subacute stages, extending up to one year after stroke. Our objective was to characterize muscle changes at the histological and neuromuscular levels, and describe the possible functional consequences.
Evidence acquisition
Study selection
We performed a systematic review of clinical trials published from 1950 to November 2022, using Medline, Embase, Cochrane and CINHAL databases, according to PRISMA guidelines. The main search strategy is presented in Supplementary Digital Material 1 (Supplementary Text File 1).
The search results were independently screened on the identified abstracts and full texts by two reviewers (O.A. and E.O.), sharing the retrieved citations. If necessary, a consensus was reached by consulting a third reviewer (P.C.).
Criteria for study inclusion comprised studies:
published in peer-reviewed journals;
published in English;
including adult patients having experienced ischemic or hemorrhagic stroke;
focusing on skeletal striated muscle (in the lower and/or upper limbs) and including data on muscle structure, motor control, or chemical or metabolic muscle properties;
describing muscular changes between stroke onset and 12 months post-stroke;
evaluating post-stroke muscular changes using the following techniques: dual-energy X-ray absorptiometry (DEXA), bioelectrical analysis (BIA), computed tomography scan (CT-scan), magnetic resonance imaging (MRI), electromyography (EMG), ultrasonography or biopsy.
Studies were excluded if:
the abstracts or full texts were not available;
the study design was a literature review;
the post-stroke interval considered was >1 year;
the study involved evaluation of a drug intervention or any other technique for muscle preservation;
the main purpose of the study was to evaluate specific stroke consequences due to spasticity or co-contractions;
Sources from citations in other studies, reviews, and meta-analyses were also identified.
Data extraction and quality assessment
For each clinical trial, details were extracted pertaining to study characteristics (authors’ names, year of publication, number of patients, biases, funding sources), patient characteristics (age, time since stroke, gender), treatment information, data on muscle structure or motor control, or chemical or metabolic muscle properties. Methodological quality was evaluated using the Check List developed by Downs and Black.9 For the observational studies included, questions 4, 8, 14, 15, 15, 19, 23, and 24 (concerning interventional studies) and for the cross-sectional studies, questions 9 and 26 were not applicable. The maximum total Check List score was therefore 18 for cross-sectional studies and 20 for longitudinal studies. Attainment of a minimum score, defined as 65% of the maximum possible score according to the type of study, was required to consider the study as being of good quality.13, 14 To be included, cross-sectional studies had to achieve a minimum score of 11.7 and longitudinal studies a minimum score of 13. This evaluation was conducted independently by two authors (O.A. and E.O.) and if necessary, by a third author (P.C.).
Evidence synthesis
We identified 13,298 potentially relevant articles. After discarding duplicates and selecting articles according to their titles and abstracts, 241 articles were fully analyzed, of which 27 were included and subjected to qualitative analysis (Figure 1).
Figure 1.
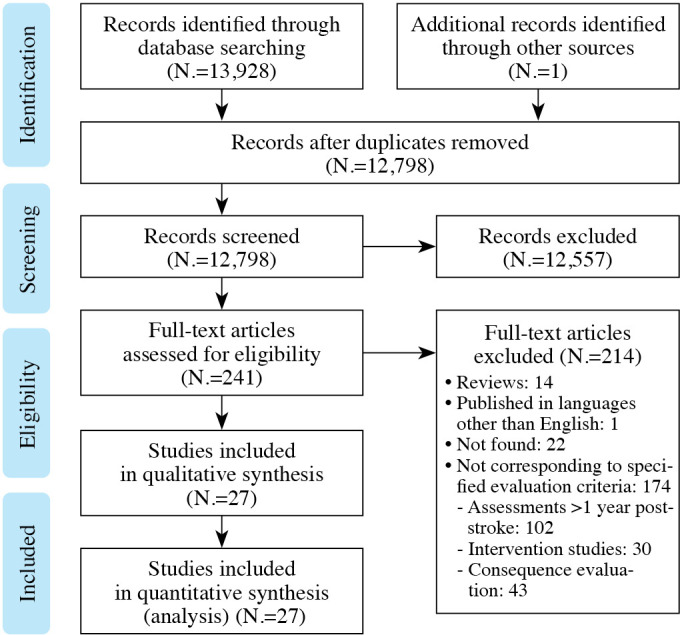
—Flow chart of study selection.
Eleven were longitudinal studies15-31 and 16 cross-sectional.32-41 The scores on the Downs and Black questionnaire were 17-18/20 for cross-sectional studies and 19-29/20 for longitudinal studies (Table I).15-41 All the studies compared the PS and the NPS. Eight cross-sectional studies had a control group,15-20, 22-24 but only one of the longitudinal studies.24
Table I. —Methodological quality analysis (Black and Downs Check List questionnaire).15-41.
| Study | Q1 | Q2 | Q3 | Q5 | Q6 | Q7 | Q9 | Q10 | Q11 | Q12 | Q13 | Q16 | Q17 | Q18 | Q20 | Q21 | Q22 | Q25 | Q26 | Scores |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Longitudinal studies | ||||||||||||||||||||
| Ramnemark et al.15 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 19 | |
| Harris et al.16 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 20 |
| Jorgensen and Jacobsen17 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 20 |
| Carin-levy et al.18 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 20 |
| Lazoura et al.19 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 20 |
| Nozoe et al.20 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 20 |
| Nozoe et al.21 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 20 |
| Ishimoto et al.22 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 20 |
| Nozoe et al.23 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 20 |
| Kostka et al.24 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 20 |
| Irisawa and Mizushima25 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 20 |
| Cross-sectional studies | ||||||||||||||||||||
| Chow et al.26 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 | ||
| Arasaki et al.27 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 | |||
| Chow and Stokic28 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 | ||
| Chow and Stokic29 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 | ||
| Maeda et al.30 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 | ||
| Bitencourt et al.31 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 | ||
| Scelsi et al.32 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 | ||
| Dietz et al.33 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 | ||
| Bohanon et al.34 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 | ||
| Andrews and Bohanon35 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 | ||
| Metoki et al.36 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 | ||
| Hara et al.37 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 | ||
| Horstman et al.38 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 | ||
| Horstman et al.39 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 | ||
| Horstman et al.40 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 | ||
| MacIntyre et al.41 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 |
Post-stroke muscular consequences at the structural, histological and biochemical levels
Ten studies focused on the analysis of muscle mass and structure.15-18, 20, 32, 33, 36, 41 Five studies used DEXA,15, 17, 19, 23 two CT-scans,37, 41 one ultrasound20 and two biopsies32, 33 (Table II).15, 17-20, 25, 32, 33, 36, 41
Table II. —Studies investigating post-stroke muscular consequences at the structural, histological and biochemical levels.15, 17-20, 25, 32, 33, 36, 41.
| Study, design/type | Variables (parameters and evaluation techniques used) |
N. (age, years) (follow-up) |
Results |
|---|---|---|---|
| Ramnemark et al.15 1999 Longitudinal |
Fat/lean mass (entire body, arms and legs, PS and NPS; DEXA) | 19 (12 men, mean age 73.3±4.3 years and 7 women mean age 76.3±10.4 y.) (From 2 to 4 weeks post-stroke and at 4, 7 and 12 months) |
Non-significant changes in lean mass and fat mass on the PS and the NPS between inclusion and study end. Substantial individual variations. Between inclusion and 1 year of follow-up: significant increase in fat mass (P<0.01), but no decrease in lean mass (P=NS) on the PS compared to the NPS. At 4 months of follow-up: lean mass: -1.5% on the PS versus -0.2% on the NPS (P=NS) fat mass: -0.1% on the PS versus -3.5% on the NPS (P=NS) At 7 months of follow-up: lean mass decrease: -2% on the PS versus -0.5% on NPS (P=NS) fat mass decrease: +1.5% on the PS versus -3% on the NPS (P=NS) At 12 months of follow-up: lean mass decrease: -0.5% on the PS versus -1% on the NPS (P=NS) fat mass increase: +8% on the PS versus +2.2% on the NPS (P<0.01) |
| Jørgensen and Jacobsen17 2001 Longitudinal |
Fat/lean mass (entire body, arms and legs, PS and NPS; DEXA) |
28 (18 men; mean age 75±7 years), comparison of two groups constituted according to patient ambulatory level (From 7 days after stroke to 12 months) |
At 2 months of follow-up: Decrease in lean mass: non-walking patients (N.=12): decrease -6% on the PS P<0.05 versus -5% on the NPS P<0.05 walking patients (N.=13): decrease -2% on the PS versus -1% on the PS, P>0.05 At 12 months of follow-up: recovery of lean mass on the NPS, not on the PS non-walking patients (N.=3): -5% on the PS versus -1% on the NPS P<0.05 (PS vs. NPS <0.001) walking patients (N.=18): -2% on the PS (P>0.05) versus +5% on the NPS (P<0.05) Increase in fat mass: non-walking patients (N.=3): +15% (P=0.04) on the PS versus +7% on the NPS (P=0.3) walking patients (N.=18): +3% on the PS versus +2% on the NPS, P=0.7 |
| Carin-Levy et al.18 2006 Longitudinal |
Muscle mass (anthropometric measures: girth and skin folds of arms, thighs and calves) Quality muscle: fat/lean mass (DEXA) |
17 stroke patients (mean age 66 years) (From 72 h post-stroke, at 1 to 4 weeks, at 3 months and at 6 months) (FIM: initial value: 107; at 3 months: 121; at 6 months: 121.5) |
Muscle mass (N.=11 patients): No significant changes over time between the PS and NPS in the upper and lower limbs Quality (N.=11 patients): At 3 weeks: Lean mass significantly reduced in the paretic leg compared to the non-paretic leg (7.51 vs. 7.83 kg, P=0.05) Significant increase in fat mass in the entire body (P=0.01) At 6 months: No longer significant difference in lean mass (7.63 vs. 8.08 kg) Significant increase in fat mass in the entire body (P=0.01) |
| Lazoura et al.19 2010 Longitudinal |
Fat/lean mass (DEXA) |
58 patients (36 men, mean age 65.75 years and 22 women, mean age 62.36 years) (At 3, 6 and 12 months post-stroke) |
Global fat mass: significant increase 6% from the 3rd to the 6th month post-stroke (P=0.004) and 8% between the 6th and the 12th month (P=0.16), Significant difference between men and women (P<0.0067) Global lean mass: 0.5% decrease between the 3rd and the 6th months (P=0.004) and 0.2% decrease between the 6th and the 12th month (P=0.16). Decrease constant in women, but only evident during the first 6 months in men. Limb lean mass decreased on the PS and the NPS with a significant difference between inclusion and the 3rd month (P<0.04) but with no difference between men and women. Significant increase in lean mass only on the NPS between the 3rd and the 6th months (P<0.001). Limb fat mass increased significantly on both the PS and NPS over time, but between the 3rd and the 6th month the difference was significant only on the NPS (P=0.001). |
| Nozoe et al.20 2016 Longitudinal |
Quadriceps muscle thickness (B-mode ultrasound imaging Rectus femoris and vastus intermedius) |
16 (11 men, mean age 72.1 y.) (Every week between the 1st and 3rd week post-stroke) |
Significant decrease in quadriceps muscle thickness in on the PS compared to the NPS (P<0.05). PS: muscle thickness decreased significantly every week, by 12.8% (95% CI 5.3-20.2%) between the 1st and the 2nd week and by 10.1% (95% CI 5.2-14.9%) between the 2nd and the 3rd week. NPS: muscle thickness decreased significantly by 9.3% (95% CI 2.5-16;1%) between the 1st and the 2nd week and by 5.3% between the 2nd and the 3rd week but the difference was not significant between the 2nd and the 3rd week (95% CI -1.6 to 12.1%). |
| Irisawa and Mizushima25 2022 Longitudinal without control group |
Muscle mass and muscle quality (bioelectrical impedance analysis) - muscle quality: PsA - mass: body muscle % |
179 (90 men/89 women, mean age 79.7±11.5y) at subacute stage (<1 month poststroke) Comparison between the 1st day and after 4 weeks. |
After 4 weeks in a rehabilitation center Muscle quality: increased in both men (4.2 vs. 4.5-degree, P<0.001) and women (3.3 vs. 3.5-degree, P<0.001). Muscle mass: no significant change in muscle mass in either men (40 vs. 39.6%, P=0.21) or women (35.8% vs. 35.4%, P=0.24) |
| Scelsi et al.32 1984 Cross-sectional |
Histological and structural fiber morphology: (fiber type, fiber distribution, fiber diameter, electron-microscopic characteristics) (Biopsy of anterior tibialis) |
16 (9 men, 7 women, mean age 62.1±2.8 years) 4 groups according to hemiplegia duration: G1: 1-2 months G2: 3-4 months G3: 5-7 months G4: 8-17 months |
From 1 to 2 months - on the PS: normal distribution of muscle fibers (type I: 66%; type II: 34%) preservation of muscle fiber structure, but abnormal presence of lipid droplets and glycogen granules in the subsarcolemmal region From 3 to 4 months - on the PS: muscle fiber distribution: type I 75.5%; type II 24.5% 40% to 70% with disorganized myofilaments additional presence of lipofuscin in the sarcolemma microvascular changes From 5 to 17 months: - no additional specific changes except a further decrease in muscle fiber diameter, predominantly with respect to type II fibers |
| Dietz et al.33 1986 Cross-sectional |
Histological and structural fiber morphology assessed by electron microscopy: fiber type distribution, fiber diameter) (Biopsy of left and right gastrocnemius medialis) |
Case study (n=4 patients: initials RR, MA, MR, HR): RR: 44 y., hematoma in left hemisphere MA: 56 y., benign glioma in left hemisphere MR: 63 y., tumor on the left PS HR: 53 y., two ischemic infarctions |
Patient RR at 7 weeks: equal proportions of type I and type II fibers on the NPS and type II predominance on the PS Six months later: No further modifications on the NPS. On the PS, atrophy of type II fibers leading to equal proportions of type I and type II fibers. Focal areas of myofibrillar destruction, loss of myofilaments and so-called ‘streaming Z-lines’ associated with loss of mitochondria. Patient MA at ten months later: strong predominance of type I fibers and slight selective type II atrophy in the biopsy taken from the PS. |
| Metoki et al.36 2003 Cross-sectional |
MVT (Cross-sectional CT-scan on both the PS and the NPS) |
50 (31 men, 19 women, mean age 62.1±2.8 years), more than 6 months post-stroke 2 groups (P=0.30) according to age (investigation of a possible senile effect): G1 <65 years: 74.6±30.3 years G2 >65 years: 83.2±22.9 years |
MVT Significantly lower on the PS than on the NPS (155.6±56.6 and 198.0±67.2 cm3, respectively, P<0.0001) Significantly diminished with age (172.7±60.3 versus 227.4±68.8 cm3, P=0.0045). Positive correlation with the Barthel Index and negatively with age. No significant relationship with Brunnstrom’s leg score or illness duration. |
| MacIntyre et al.41 2010 Cross-sectional with a control group |
Muscle mass and density (pQCT of calf and tibial muscles) | Subacute Stroke Group (<6 months post-stroke): 11 hemiparetic patients (6 men, mean age 69±9 years) Chronic Stroke group (>12 months post-stroke) 10 patients able to walk (6 men, mean age 72±12 years) Control group 13 healthy volunteers (6 men, mean age 71±13 years) |
No significant difference in muscle mass or density between the PS and the NPS. Muscle mass decreased in the subacute stroke group versus controls: 456.5±110.7 (NPS) and 435.9±111.1 mg/mm (PS) versus 492.9±58.1 (right side) and 484.0±55.2 (left side) mg/mm. Muscle mass also decreased in the chronic stroke group (460.5±83.4 (NPS) and 456.8±92.4 (PS) mg/mm |
BIA: bioelectrical impedance analysis; CI: confidence interval; CT: computed tomography; DEXA: dual-energy X-ray absorptiometry; FIM: functional independence measure; G: Group; GM: gastrocnemius medialis; MVT: muscular volume of the thigh; NPS: non-paretic side; NS: non-significant; P: significance; pQCT: peripheral quantitative computed tomography; PS: paretic side; NPS: non-paretic side; PsA: muscle quality.
Only two studies described precisely the histological changes occurring at an early-stage early stage post-stroke. The prospective trial conducted by Scelsi et al.32 reported the changes in fiber composition of the anterior tibial muscle. On the PS, a normal fiber type distribution was observed from 1 to 2 months post-stroke. Some fibers started to show displacement of the nucleus to a central location, as well as fatty infiltration. From 3 to 4 months, a change in the proportions of type I and type II fibers was seen, as well as a decrease in the diameter of both types of fiber (approximately -20%). Beyond 5 months, the relative proportions of type I and type II fibers showed no further change, but the diameter of type I fibers continued to decrease. From the first 3 months onward, biopsies revealed disorganization of 40-70% of the myofilaments with rupture of the Z-line. As regards microvascularization, the basement membrane and media of the capillaries were thickened and perivascular fibrosis of the small arteries continued up to 5 months post-stroke. On the NPS, moderate ultrastructural and histological changes were observed up to 5 months. Biochemical changes were reported only on the PS, between 1 month and 2 months post-stroke, specifically the abnormal presence of lipid droplets and glycogen granules in the subsarcolemmal area. These changes persisted beyond 3 months. After 5 months, lipofuscin storage was evident in the sarcolemma and swollen mitochondria were noticeable in areas of myofibrillar disorganization. This study constitutes the most robustly documented investigation of the structural and histological consequences of stroke in hemiplegic patients.
Dietz et al. also reported in a case-series of 4 patients with hemiparesis a greater decrease in the number of muscle fibers, particularly type II fibers, on the PS than on the NPS from 5 weeks post-stroke.33 After 5 months, a predominance of type I fibers was evident. The changes on the NPS remained moderate. Biochemical changes included myofibrillar lesions, and loss of myofilaments and mitochondria on the PS. The oxidative capacity of the muscles decreased owing to reduced NADH-TR enzyme activity, particularly in type I fibers.
Concerning muscle mass measured by CT scan, Metoki et al.36 observed a significantly greater reduction in thigh muscle volume at one week post-stroke on the PS than on the NPS. MacIntyre et al.41 reported no changes in the mass or density of the calf muscles in both either subacute or chronic stroke patients. Nozoe et al.20 reported a significant decrease in muscle thickness analyzed by ultrasound on the PS during the first week post-stroke, associated with a smaller decrease on the NPS. Separate evaluation of lean and fat mass by DEXA or BIA monitoring showed a decrease in lean mass on the PS, whereas fat mass increased to an extent varying according to locomotion recovery. Recently, Irisawa and Mizushima reported that muscle quality analyzed by-BIA was correlated with functional recovery at the end of 4 weeks of rehabilitation.25 In patients at the acute stage post-stroke, Ramnemark et al.15 showed in the body no significant change in either lean or-fat mass in the body as a whole, whereas a significant loss of fat mass at 4 months, greater at 7 months, was observed in the lower limbs. Subsequently, lean mass started to recover progressively. Jørgensen and Jacobsen,17 showed a significant loss of lean body mass in the non-ambulatory group at 2 months, but during the following 10 months, significant recovery was evident on the NPS, particularly in patients who had recovered the ability to walk. Carin-Levy et al.,18 reported a significantly lower limb lean mass on the PS than on the NPS at 3 weeks post-stroke, but no significant change in limb lean tissue cross-sectional area or muscle mass at 6 months. No further significant change occurred after 6 months. A significant overall increase in fat mass was noted. Lazoura et al.19 reported a decrease in lean mass and an increase in total body fat mass at 12 months post-stroke. Total lean mass loss remained minor during the entire year post-stroke, fat mass even increasing during the second semester.
Post-stroke effects on muscular function at the neuromuscular level
Changes in neuromuscular function during stroke were studied by electromyography (EMG) in eight studies,21, 26-29, 31, 33, 37, 38 four of which included a control group of healthy subjects26-29, 38 (Table III).21, 26-28, 31, 33, 37, 38
Table III. —Studies investigating post-stroke effects on muscular function at the neuromuscular level.21, 26-28, 31, 33, 37, 38.
| Study, design/type | Variables (localizations and techniques) |
N. (age, years) (following) |
Results |
|---|---|---|---|
| Nozoe et al.21 2020 Longitudinal |
Motor Nerve Conduction (CMAP and MCV after electrical stimulation of peroneal nerve) Lower limb muscle wasting (QMT with ultrasound) Lower paretic leg muscle strength (MI) |
18 patients evaluated (4 women, 14 men) Median age: 68±14 years 10 ischemic stroke and 8 hemorrhagic stroke Two evaluations at 2±2 days post-stroke and 2 weeks later |
Between 1st and 2nd evaluation: CMAP amplitude or MCV did not change significantly. MI increased significantly (P=0.001) and QMT decreased significantly on both sides (PS P=0.014; NPS P=0.003). QMT was less in the PS than on the NPS. The percentage differences in CMAP amplitude were significantly correlated with the percentage differences in QMT only on the PS (R=0.604, P=0.008). The percentage differences in MCV were not significantly correlated with the percentage differences in QMT. There was no significant correlation between the percentage differences in the CMAP amplitude, and the percentage differences in MCV and the lower limb MI variation. |
| Chow and Stokic26 2011 Cross-sectional |
Isometric quadriceps strength on the NPS and PS in stroke patients and on the dominant leg in the control group. Peak force measured at 10%, 20%, 30%, 50% and 100% of MVC. The CV was used to quantify force variability. Different power spectra were tested for evaluation of the force signal: 0-3, 4-6, and 8-12 Hz bands. |
34 patients (18 men, mean age 65±15 years and 16 women; 61±11 years) Time post-stroke: 17±4 days Control group: 20 (15 men; mean age 64±12 years, 5 women; mean age 64±12 years) |
Mean quadriceps strength was higher in the control group than on the PS and NPS in the stroke patients (179±58 versus 133±55 versus 93±44 Nm, respectively). The relative power increased in the 0–3 Hz band and decreased in both the 4-6 and 8-12 Hz bands in the paretic leg only (P<0.001). Progressively stronger contractions resulted in a significant decrease in relative power in the 0-3 Hz band and an increase in the 8-12 Hz band in the control subjects but not in the stroke patients. |
| Arasaki et al.27 2009 Cross-sectional with a control group |
Estimated number of MUs (MUNE) F-wave MUNE method and needle EMG (Hypothenar muscle group) |
Stroke patients: 3 groups Group 1: 6 patients with cerebral infarction but no motor hand weakness during the first 24 hours after stroke onset. Group 2: 8 patients with acute motor hand weakness during the first 24 hours after stroke onset. Group 3: 16 patients with motor hand weakness due to subacute or chronic unilateral cerebral infarction. Control group: 13 healthy volunteers |
The patients in group 1, with no hand weakness, manifested no reduction in the MUNE either on the PS or on the NPS. The MUNE decreased on the PS in the group with cerebral infarction (24 patients) with no reduction on the NPS. A significant decrease in the MUNE was seen on the PS in the 8 patients tested within 4-30 h after the onset of cerebral infarction. |
| Chow and Stokic28 2014 Cross-sectional and retrospective |
Isometric quadriceps strength on the NPS and PS in stroke patients and on the dominant leg in the control group. Peak force was measured at 10%, 20%, 30%, 50% and 100% of MVC. The CV was used to quantify force variability. Different power spectra were tested for evaluation of the force signal: 0-3, 4-6, and 8-12 Hz bands. |
23 chronic stroke patients (mean age 65±14 years), from 6-12 months post-stroke (no clinical hypertonia) of whom 10 (64±15 years) underwent two evaluations: at 11-22 days (subacute stage) and at 6-8 months post-stroke. Control group: 15 healthy subjects (mean age 65±8 years) tested on their dominant leg |
The MVC torques were significantly lower in the paretic leg of the stroke patients (99±45 Nm) compared to either the non-paretic leg (138±51 Nm) or the dominant leg in the controls (P<0.001) Significantly smaller MVC torques were persistently observed after normalization on the body mass (164±57 Nm). From the subacute to chronic stage, the CV decreased significantly only in the paretic leg (P<0.001) with a similar trend in the non-paretic leg (P=0.028). No significant changes in spectral frequency and entropy parameters were seen. In the paretic leg, the MVC difference was not correlated with the CV, or with frequency or entropy measures (P=0.093). |
| Chow and Stokic29 2013 Cross-sectional |
Evaluation of the force signal NPS, NPS and dominant leg in the control group with different power spectra: 0-3, 4-6, and 8-12 Hz bands |
Stroke patients: 34 patients (18 men, mean age 65±15 years and 16 women; 61±11 years) Time post-stroke: 17±4 days Control group: 20 (15 men; mean age 64±12 years, 5 women; mean age 64±12 years) |
The relative power increased in the 0-3 Hz band and decreased in both the 4-6 and 8-12 Hz bands in the paretic leg only (P<0.001). Progressively stronger contractions resulted in a significant decrease in relative power in the 0-3 Hz band and an increase in the 8-12 Hz band in the control subjects but not in the stroke patients. |
| Bitencourt31 2022 Cross-sectional |
EMG patterns with motor and sensory conduction at rest, slight effort, and maximum effort |
20 patients (10 male, mean age 65±14 years) Evaluation <72 h post-stroke in the PS |
Abnormal EMG patterns for 40% of patients on the PS: At rest: 50% of PSW, 62.5% of fibrillation, and 25% of fasciculations mainly in the distal arm and hand muscles. During contraction: no activity 35%, normal activity 50%, increased activity 50%. Positive correlation between stroke severity (NIHSS score) and the presence of an abnormal EMG. |
| Dietz et al.33 1986 Cross-sectional |
MU activation (EMG on GM and TA) |
2 cases studied: patients RR and MA (patient initials) (cf. Table I) | RR: From stroke onset to 7 weeks: Discharge patterns of 12 different MUs recorded on the NPS and those of 11 MUs on the PS: 5.55±0.9 versus 5.35±0.5 imp/s. MA: From stroke onset to 9 months: Both patterns showed clear differences in the mode of leg muscle activation between the NPS and the PS. GM activation was reduced on the PS. |
| Hara et al.37 2004 Cross-sectional |
Estimated number of MUs (MUNE) (F-wave MUNE method and needle EMG on the median innervated thenar muscle and APB) |
First EMG at a mean of 19 days after stroke in 14 stroke hemiplegia subjects (9 men, mean age 65.3 years) 2nd and 3rd EMG in 9 of the patients at 3 months and 1 year after stroke onset (5 men, 4 women, mean age 59.1±8 years) |
During the first month post-stroke: The maximum M-potential negative amplitude on the PS (mean±SD: 9.4±3.0 mV) was significantly smaller than that on the NPS (mean±SD: 11.5±2.8 mV) (P<0.01). Negative amplitudes of S-MUAP were the same on the PS (37.3±9.8 mV) and NPS (37.6±12.9 mV). The mean±SD number of MUs on the PS (237±50) was significantly smaller than that on the NPS (316±43) (P<0.01). MU loss began at 9 days post-stroke on the PS and was greater in patients with severe hemiparesis. At 3 months and 1 year post-stroke: All the stroke patients showed spontaneous activity in the hemiparetic APB muscles 3-4 months after stroke, but only one patient 1 year later. No statistically significant difference in MU number between the PS and the NPS was seen at 3 months and 1 year post-stroke No significant difference was found between the PS and NPS for median motor nerve conduction velocity (52.7 vs. 57.8 m/s) or minimum F-wave latency (mean: 27.7 vs. 26.7 ms). |
| Horstman et al.38 2008 Cross-sectional with a control group |
Isometric muscle strength MVC and electrically evoked forces of KF/KE with LEXS Voluntary activation and coactivation of KE MVC with electrical stimulation (Digitimer DSH7 stimulator) on both knee extensor muscles for the stroke patients and on the right side only for the control group EMG (Biotel 99) |
14 stroke patients, mean age 55.9±10.4 years (10 men, 4 women) 12 able-bodied controls, mean age 58.1±12.2 years (7 men, 5 women) (4 evaluation sessions with at least 1 day of rest in between). |
Isometric muscle strength: significant decreases in force KE: control vs. PS vs. NPS: 223±48 vs. 152±55 vs. 62±48 newtons, P<0.05 for control and NPS compared to PS KF: control vs. PS vs. NPS: 90±29 vs. 56±27 vs. 10±16 N, P<0.05 KE/KF: 0.4±0.07 vs. 0.36±0.09 vs. 0.14±0.1, P<0.05 Voluntary activation (%): significant decrease Control vs. NPS vs. PS: 93.6±4.1 vs. 75.1±7.3 vs. 57.8±24.6, P<0.05 for control and NPS compared to PS. Coactivation The rsEMG/rsEMGmax ratio used as a measure of coactivation of the KE during flexion did not differ significantly across groups but was higher for the biceps femoris and medial gastrocnemius during knee extension on the PS vs. the NPS and vs. controls |
APB: abductor pollicis brevis; CMAP: compound muscle action potential amplitude; CV: coefficient of variation; EMG: electromyogram; GM: gastrocnemius medialis; KE: knee extensor; KF: knee flexion; LEXS: lower extremity system; MCV: motor conduction velocity; MVC: maximal voluntary contraction; MI: Motricity Index; MU: motor unit; MUNE: motor unit number estimate; NIHSS: National Institute of Health Stroke Scale; NPS: non-paretic side; PS: paretic side; PSW: positive sharp waves; QMT: Quadriceps Muscle Thickness; S-MUAP: surface motor unit action potentials; TA: tibialis anterior.
Two case reports showed a decrease in motor unit (MU) activity in the lower limb after chronic post-cerebral lesions with concomitant reduced spontaneous activity.33 These changes were related to the severity of the paretic deficiency and were significantly greater on the PS than on the NPS. Arasaki et al.-reported a rapid decrease in the number of MUs reflected both by a reduced number of electrically excitable motor axons and a decreased excitability of spinal α-motor neurons and their axons.27
Decreased MU activity on the PS is also explained by the difficulty in recruiting motor units. Horstman et al.38 reported a significant decrease in EMG-assessed activity of the knee extensors (KE) and flexors (KF) during maximal KE contraction on the PS compared to both the NPS and the right leg of control subjects. The coactivation was significantly higher on the PS as regards the KF during maximum voluntary extensor contraction. For the authors, this reflected activation failure of the descending motor tracts on the PS and to a lesser extent on the NPS, contributing to inability to exploit contractile velocity for extensor strength. According to Chow and Stokic this could be explained by adverse changes in the force-power spectrum developed on both the PS and the NPS with increasing contraction intensity.28 Chow and Stokic considered that this change in modulation with increasing contraction could be explained by a lesser capacity to execute motor tasks and recruit additional MUs.29 These same authors, in 2014,28 reported that these changes were accompanied by a decrease in the maximum voluntary isometric muscle strength on the PS which disappeared in patients with good motor recovery. Nozoe et al. evaluated in acute stroke patients motor nerve conduction by electrical stimulation from the peroneal nerve, lower limb muscle wasting (expressed by quadriceps muscle thickness [QMT]) by ultrasound, and muscle strength.21 They reported a significant correlation between differences in compound motor action potential amplitude (CMAP) and differences in PS QMT on the PS. In contrast, they found no significant relationship between the differences in motor nerve conduction velocity (MCV), QMT and lower-limb muscle strength. For these authors, differences in the decrease in CMAP amplitude on the PS were due to the loss of MU. Interestingly, neuromuscular changes can begin as early as the first few hours after stroke.
Abnormal activity is detected in 40% of paralyzed upper extremity muscles, with variability in responses correlated with stroke severity and muscle strength deficit.31
Consequences of functional changes
Fourteen studies investigating functional changes were identified,16, 18, 22-26, 30, 34, 35, 38-41 of which eight did not include a control group (Table IV).22, 23, 30, 38-41 Many of the studies reported muscle weakness and a decrease in muscle strength, on both the PS and the NPS. As regards the upper limbs, Bohannon et al. noted a significant deficit of upper limb strength between 10 and 36 days post-stroke, as well as a significant difference between the PS and NPS.34, 35 Irisawa and Mizushima confirmed an increase in shoulder abduction strength after 4 weeks of stroke rehabilitation.25 A similar progression in deficit was seen on both sides for grip strength and KE strength from 72 hours post-stroke to the end of the 6-month follow-up, significantly greater on the PS than on the NPS.18 Horstman et al.38 also found a decrease in KE and KF strength evident on both sides but greater on the PS than on the NPS. These authors additionally reported a correlation between loss of Maximal Voluntary Contraction (MVC) and functional performance. In a further published study concerning the same cohort and using the same methodology, they reported a correlation between muscle weakness and short muscle length.39 A subsequent comparison of the rates of muscle contraction and muscle fatigue in the KE of hemiplegic patients and control subjects revealed a slowing of muscle contractibility and relaxation in the hemiplegic patients. Both contractibility and relaxation were slower and weaker on the PS than on the NPS.40 Chow and Stokic29 assessed the KE muscle isometric strength of hemiplegic patients. The patients have a pronounced decline in maximum strength of the PS quadriceps compared to the NPS, and on both sides compared to the control group. In stroke survivors with subacute hemiparesis, MacIntyre et al.41 found a decrease in KE and ankle plantar flexor (APF) muscle isometric strength, greater on the PS than on the NPS. A correlation between muscle strength and muscle density was found only for the APF muscle.
Table IV. —Studies investigating on the functional consequences of muscle changes.16, 18, 22-26, 30, 34, 38-41.
| Study, design/type | Variables (localizations and techniques) |
N. (age, years) (following) |
Results |
|---|---|---|---|
| Harris et al.16 2001 Longitudinal with a control group |
Isometric quadriceps strength (Magnetic femoral nerve stimulation (Tw Q) and MVC - Test on the NPS of stroke patients and on the right side of control subjects) TCT |
Stroke group: 10 patients (6 men, 4 women) with supratentorial cerebral infarction (5 cortical and 5 lacunar), mean age: 73.6±11 years Control group: 10 healthy subjects, mean age: 71.9±6.8 years (Two evaluations separated by 7 days, within 48 h after stroke onset for the first evaluation of the stroke group) |
Isometric quadriceps strength First evaluation Stroke group: median (95% CI) Tw Q and MVC values were respectively 7.6 kg (4.4-9.9 kg) and 12.15 kg (7.9-30.8 kg). Control group: median (95% CI) Tw Q and MVC values were respectively 9.4 kg (6.1-12.5 kg) and 37.2 kg (23.8-54.6 kg) Second evaluation Stroke patients: median (95% CI) Tw Q and MVC values were respectively -16.2% (-6% to -25.9%) and -30.45% (0% to -78.6%) (P<0.01) Control group: median (95% CI) Tw Q and MVC values were respectively +1.75% (-9.8% to 8%) and +5.45% (-15.1% to 22.7%) (NS) TCT: A significant correlation was observed between the percentage fall in Tw Q and both change in TCT score (rs=0.83, P<0.01) and percentage change in body weight (rs=0.83, P<0.01) |
| Carin-Levy et al.18 2006 Longitudinal |
Isometric muscle force (Hand grip strength, MVC of KE) |
17 stroke patients (mean age 66 years) (From 72 h post-stroke, at 1 to 4 weeks, and at 3 and 6 months) FIM initial, 107; 3 months, 121; 6 months, 121.5) |
Hand grip strength (N): PS vs. NPS: 78.5 (IQR 0-188.7) vs. 171.6 (IQR 103-318.7) P<0.004 at week 1, and 98.1 (IQR 0-220.6) vs. 245.2 (IQR 107.9-313.8) at week 24, P>0.05 for repeated measures over time on both sides. MVC KE (N): PS vs. NPS: 258.4 (IQR 156-360.9) vs. 270.3 (IQR 135.9-404.7); P=0.66 at week 1, and 228 (IQR 166.4-356.2) vs. 324 (IQR 200.8-414); P=0.018 at week 24, P>0.05 for repeated measures over time on both sides |
| Ishimoto et al.22 2020 Longitudinal |
QMT - fat and muscle mass (B mode ultrasound imaging of RF and VI) FM FIM |
11 patients (8 women, 3 men). 4 hemorrhagic stroke and 7 ischemic stroke. Mean age: 76.1±5.1 y. Time post-stroke at 1st evaluation: 28.8±10.6 days. Time between 1st and 2nd evaluation: 81.9 days FIM gait score: at 1st evaluation: 3.0 (1.5-4.0) at 2nd evaluation: 6.0 (5.0-6.0) |
PS: quadriceps echo intensity was significantly lower than at admission (88.6±23.8 versus 68.6±13.2; P=0.01) NPS: QMT was higher than that at admission (18.3±4.5 versus 20.8±4.0; P=0.02). A significant negative correlation was noted between the quadriceps echo intensity changes and the FIM gait score. A significant positive correlation was noted between the QMT changes and the FIM gait score gain on the NPS. |
| Nozoe et al.23 2020 Longitudinal |
QMT (B mode ultrasound imaging of RF and VI on PS and NPS 1 week post-stroke and 2 weeks after 1st examination % QMT differences was evaluated FAC |
55 acute post-stroke patients (14 women, 41 men) with inability to walk without assistance within one after admission. Mean age: 65±10 years 28 hemorrhages et 27 infarcts Two evaluations: 3±3 days post stroke and 2 weeks later 28 in dependent group FAC 0-3 and 27 in independent group FAC 4-5) at 3 months post-stroke |
PS: The % QMT differences were not significant between the ambulatory independent and dependent groups. NPS: the % QMT differences were significant between the ambulatory independent and dependent groups (5.5% [8.7%] vs. 16.8% [13.6%], respectively; P<0.001). % QMT difference remained significantly associated with dependent ambulation, odds ratio of 0.86 (95% CI, 0.75-0.98; P=0.02). |
| Kostka et al.24 2019 Longitudinal with a control group |
Muscle function deficit Pmax υopt Functional capacities (TUG, BI, RMI) |
67 patients (22 women, 45 men) 67 controls, matched for age and sex Time post-stroke between 2 weeks and 3 months |
Significant correlation between Pmax/kg, υopt and TUG for post-stroke patients Post-stroke patients: Pmax/kg and υopt related to the BI (ρ=0.48; P<0.001 for Pmax/kg; ρ=0.42; P<0.001 for υopt) and to the RMI total results (ρ=0.58; P<0.001 for Pmax/kg; ρ=0.46; P<0.001 for υopt) |
| Irisawa and Mizushima25 2022 Longitudinal without control group |
Muscle strength (Dynamometer) Shoulder abduction on the NPS (kg) FIM score |
179 (90 men/89 women, mean age 79.7±11.5 years) at subacute stage (<1 month poststroke) Comparison between the 1st day and after 4 weeks. |
After 4 weeks in a rehabilitation center - Muscle strength increased in men (18.6 vs. 22.9 kg, P<0.001) and in women (182.4 vs. 16.8 kg, P<0.001). FIM score increased in men (39.5 vs. 55.7, P<0.001) and in women (38.6 vs. 52.8, P<0.001). |
| Chow and Stokic26 2011 Cross-sectional with a control group |
Isometric muscle strength measured on quadriceps MVC and submaximal force control 10%, 20%, 30%, or 50% of the MVC. CV and RMSE to quantify force variability and error. Functional performance Lower extremity motor section of the FM and RMI to calculate the correlation between functional tests and force measures Tests on the less-affected and more-affected legs in stroke patients and on the dominant leg in control subjects |
33 patients (13 women, 20 men; mean age: 62±13 years) with sufficiently high motor abilities to walk. Time post-stroke: 16±2 days Control group: 20 healthy subjects (6 women, 14 men; mean age: 62±10 years) |
The MVC was significantly smaller in the more-affected leg than in the less-affected leg in stroke patients (97±43 vs. 140±56 N/m; P<0.001) and significantly lower than in the dominant leg of control subjects, after normalization of performances to body mass. The CV was significantly smaller in controls compared with either the more-affected leg (P<0.006) or the less-affected leg (P<0.023) of stroke patients across all force levels. The RMSE was significantly different between groups only at higher force levels, specifically at 50% force when all groups differed significantly from one another. No significant correlation between force variability and motor scores was seen. |
| Chow and Stokic29 2013 Cross-sectional |
Isometric quadriceps strength on the NPS and PS and on the dominant leg in the control group. Peak force measured at 10%, 20%, 30%, 50% and 100% of MVC. CV was used to quantify force variability. |
Stroke patients: 34 patients (18 men, mean age 65±15 years and 16 women; 61±11 years) Time post-stroke: 17±4 days Control group: 20 (15 men; mean age 64± 12 y, 5 women; mean age 64±12 years) |
Mean quadriceps strength was higher in the control group than on the PS and NPS in the stroke patients (179±58 versus 133±55 versus 93±44 Nm, respectively). |
| Maeda et al.30 2020 Cross-sectional |
Relationships of gait independence with MT and EI in quadriceps femoris | 43 post-stroke patients (21 mild, 22 severe; 27 men, 16 women) Mean age: 66.3±13.1 years Time since stroke onset: 84.6±39.0; 73.2±36.3; 95.5±40.0 (mean days) Rehabilitation hospital stay 52.4±34.5; 43.7±32.6; 60.6±35.0 (mean days) |
- MT PS (19.8±5.5 mm) < NPS (25.0±6.6) P<0.05 for all patients (20.5±6.0 for mild and 19.0±5.1 for severe cases vs. 22.9±6.2 and 27.0±6.5). - EI PS (87.0±12.3 mm) > NPS (79.8±12.4) for all patients (87.2±13.2 for mild cases and 86.9±11.6 for severe cases vs. 82.3±12.6 and 77.4±11.9 - FIM gait scores In the mild hemiparetic group, correlation with PS MT (rho = 0.60, P<0.01) and PS EI (rho = -0.57, P<0.01). In the severe hemiparetic group, correlation with PS MT (rho = 0.67, P<0.01), PS EI (rho = -0.43, P<0.05), NPS MT (rho = 0.86, P<0.01), and NPS EI (rho = -0.56, P<0.01) |
| Bohannon et al.34 1991 Cross-sectional |
Elbow flexion strength (Dynamometer) |
24 stroke patients (mean age: 70±10.3 years) Time post-stroke: 36.4±33.5 days |
Elbow flexion force on the PS was lower than on the NPS: • Shoulder adducted position Trial 1: 5.79±4.38 versus 16.47±5.69 kg Trial 2: 6.06±4.60 versus 16.57±5.66 kg • Shoulder abducted position Trial 1: 5.66±4.38 versus 17.23±5.81 kg Trial 2: 6.06±4.60 versus 16.69±5.99 kg |
| Andrews and Bohannon35 2000 Cross-sectional |
Strength (Hand-held dynamometer) |
31 stroke patients (mean age: 70±10.3 years) (Follow-up: 2 evaluations at 10.0±6.3 and 27.7±14.6 days post-stroke) |
Strength was impaired with respect to all muscle actions on both the PS and the NPS (P<0.001) with a significant difference between the PS and the NPS (P<0.001) Initial assessment: PS: strength decrease ranged from 19.8% to 33.9% of normal NPS: strength decrease ranged from 60.1% to 89.5% of normal Final assessment: PS: strength decrease ranged from 29.3 to 44.5% of normal NPS: strength decrease ranged from 65.4 to 88.8% of normal |
| Horstman et al.38 2008 Cross-sectional with a control group |
Functional performance assessed by TUG, 10-meter walk test, BBS, MI, FA, Fugl-Meyer test (FM) for lower limb and RMI |
14 stroke patients, mean age 55.9±10.4 years (10 men, 4 women) 12 able-bodied controls, mean age 58.1±12.2 years (7 men, 5 women) (Four evaluation sessions with at least 1 day of rest in between.) |
• Functional performance Significant correlations and trends were found between voluntary muscle contraction and both FM and MI. A significant correlation was also seen between activation on both sides and RMI, 10 m walk test results, and FAC, BBS, TUG, FM and MI scores. |
| Horstman et al.39 2009 Cross-sectional with a control group |
Isometric muscle strength MVC measured LEXS on KF/KE of both legs at 30°, 60° and 90° knee angles Electromyographic activity EMG (Biotel 99) on extensor and flexor muscles of both knees |
14 stroke patients, mean age 55.9±10.4 years (10 men, 4 women) 12 able-bodied control subjects, mean age 58.1±12.2 years (7 men, 5 women) |
Extensors and flexors of both knees showed significant group and angle effects (P<0.01). As regards the paretic knee, extensors showed lower normalized maximal torques (73%) and lower normalized activation (71%) at 30°. Flexors at 60° and 90° were also significantly weaker 64% and 45% respectively than control. Lower muscle torque at shorter muscle lengths was associated with a length-dependent lower voluntary activation. Co-activation did not differ significantly between the PS and NPS in stroke patients or between the stroke patient and control groups. |
| Horstman et al.40 2010 Cross-sectional with a control group |
Isometric muscle strength and fatigue and fast voluntary isometric contractions HRTs and MRTDs |
Patients with subacute stroke (N.=14) Able-bodied age-matched control subjects (N.=12) |
In stroke patients: MRTDs was significantly lower during voluntary contractions on both the PS (53% of control, P=0.022) and the NPS (71% of control, P=0.001), but there were no differences between groups during electrically evoked contractions (P=0.117) HRTs were significantly higher on both the PS (134% of control values, P=0.001) and the NPS (123% of control values, P=0.032) indicating slowing of muscle function. On the PS, muscle fatigue was greater and occurred more rapidly than in control subjects (P=0.011) and on both the PS and the NPS, recovery was slower than in control subjects (P=0.001). |
| MacIntyre et al.41 2010 Cross-sectional |
Isometric strength (APF and KE) |
Subacute Stroke Group (<6 months): N.=11 (6 men; mean age 69±9 years) Chronic Stroke group (>12 months): N.=10 (6 men; mean age 72±12 years) Control group: N.=13 (6 men; mean age 71±13 years) |
Side-to-side differences were observed in acute stroke patients with regard to mean KE strength (PS: 0.69±0.34 Nm/kg; NPS: 1.04±0.40 Nm/kg) and its decrease compared to control group values (right side: 1.15±0.26; left side: 1.12±0.33 Nm/kg). Side-to-side differences in stroke patients were also observed with respect to mean APF strength (PS: 0.21±0.21 Nm/kg NPS: 0.47±0.18 Nm/kg) and its decrease compared to control values (right side: 0.71±0.29; left side: 0.70±0.26 Nm/kg). |
APF: ankle plantar flexor; BBS: Berg Balance Scale; BI: Barthel Index; CG: control group; CI: Confidence Interval; CV: coefficient of variation; EI: echo intensity; EMG: electromyogram; FAC: functional ambulation category scores; FIM: Functional Independence Measure; FM: Fugl-Meyer test; HRTs: Half-relaxation times; IQR: Interquartile; MRTs: maximal rates of torque development; MI: motricity index; KE: knee extensor; KF: knee flexion; BBS: Berg Balance scale; MVC: maximal voluntary contraction; MRTD: maximal rates of torque development; HRTs: half-relaxation times; KE: knee extensor; MT: muscle thickness; NPS: non-paretic side; NS: not significant; Pmax: Maximal muscle power; PS: paretic side; RF: rectus femoris; RMI: Rivermead mobility index; RMSE: root-mean square error; QMT: quadriceps muscle thickness; TCT: Trunk Control Test; TUG: Timed Up-and-Go Test; Tw Q: magnetic femoral nerve stimulation; VI: vastus intermedius; υopt: Optimal shortening velocity; %QMT: differences in QMT on both sides.
Kostka et al.24 assessed maximal muscle power (Pmax) and optimal shortening velocity (υopt, the velocity at which power reaches a maximum value) of the KE muscles using a specially equipped cycle ergometer. The results obtained revealed significant deficits in Pmax/kg, υop, Timed Up and Go test variables and correlations in the post-stroke group. The muscle power generated by post-stroke patients was only 49.6% of that of the control group, muscle contraction velocity being only 65.5% of the control value.
With regard to muscle thickness, Nozoe et al.23 evaluated quadriceps muscle (QMT) changes in 55 hemiplegic patients at 1 week and 3 weeks post-stroke. A significant reduction in percentage QMT differences according to functional ambulation ability at 3 months (expressed as the Functional Ambulation Category ([FAC]) was observed only in the non-paretic limb. Ishimoto et al.22 evaluated intramuscular fat and muscle mass by ultrasound in stroke patients between their admission to and discharge from a rehabilitation unit. At discharge, the quadriceps echo intensity on the PS was significantly lower than at admission, the decrease being correlated with gait independence. QMT was significantly greater on the NPS. Maeda et al.30 found that QMT was associated with the degree of gait independence attained. Muscle atrophy may therefore be a limiting factor for achieving gait independence.
Harris et al.16 evaluated quadriceps strength within the first 48 hours post-stroke and at one week. Their methodology combined assessment of maximal voluntary contraction and the contraction induced by magnetic stimulation of the femoral nerve. The results showed a decrease in both contractions during the first week post-stroke compared to the control group.
Summary of the evidence
This literature review, focusing on the earliest post-stroke muscular changes, indicates:
a loss of lean mass starting from the first week post-stroke, in both upper- and lower-limb muscle tissue18, 20 and continuing up to 3 months predominantly on the PS.17, 20 This loss seemed to be more significant in the lower limbs at 4 months, reaching a maximum at around 7 months.15, 19 After 6 months, the loss of muscle mass was correlated with functional recovery,36 but with individual variations,15 the loss of lean mass being associated with an increase in body fat.15, 18, 19 This decrease in muscle mass has already been reported at the chronic stage on both the PS and the NPS, correlated with the degree of muscle underutilization;10, 42
a profound alteration of muscle structure at the subacute stage was observed, with changes in the relative proportions of type II fibers (decreased) and type I fibers (increased), as well as in fiber size and microvascularization. Hafer-Macko et al.43 and von Walden et al.44 highlighted the fiber phenotype variations at the chronic stage, correlated with the severity of the patients’ neurological deficit. Structural modifications were predominantly seen on the PS32 and have already been reported in the upper limbs;11
a lack of data regarding histological changes in early-stage post-stroke patients. Only Sclesi et al.32 and Dietz et al.33 described early structural changes in fiber distribution, as well as a decrease in fiber size and changes in myofibrils and the vascular network. These authors also reported a link between an inflammatory state and the implication of TNF-α, insulin resistance and myostatin in the histological changes. The existence of a pro-inflammatory state, as well as nutritional status, should therefore be taken into consideration, especially in frail patients and those displaying multiple comorbidities;
in terms of neuromuscular activity, an early decrease in MU number and activity possibly explained by a lower recruitment of motor neurons and a decrease in their excitability.27, 33, 37 The decrease in the number of MUs is associated with structural reorganization of neuromuscular anatomy, with degeneration of the motor neurons responsible for axonal sprouting and collateral re-innervation.45 Trans-synaptic degeneration of α-motor neurons has been described, secondary to deprivation of the inputs normally received through descending motor pathways.46 Based on the limited data available, these changes seem to commence during the first two weeks after stroke and continue up to the end of the 3rd or 4th month. Moreover, impairment of the central control of MU activation could induce a further exacerbation of residual muscle contraction as a result of potentially inefficient and disorganized peripheral MU control;47
functionally, a decrease in maximum voluntary force at a very early stage, on both the PS and the NPS.16, 26, 33, 35, 41 The data reported by Harris et al.16 and Horstmann et al.38 show a decrease in the force induced by electrical stimulation, independent of neuronal activation deficiency. This reflects lower intrinsic muscle activation in addition to the decrease in central neuronal activation on the PS. For Horstman et al.,39 these factors are related to post-stroke effects associating muscular atrophy, more or less persistent neuromotor deficiency and disturbances in excitability. These mechanisms hamper the rapid development of maximum force and lead to longer relaxation times and lower fatigue resistance. Further deficiencies due to advanced age or non-use of muscles may also be observed.
This review confirms the objective consequences of stroke on both the PS and the NPS. With regard to recuperation, even though imaging and neurophysiological markers are currently available for analyzing the potential for recovery through resumption of cortico-spinal bundle activity, the availability of a muscle-specific biomarkers to monitor muscular damage and recovery would be very useful at the acute post-stroke stage.45
Our review reinforces the need to set up rehabilitation programs focused designed to-maintain muscle capacities as soon as possible after post-stroke paresis,38 recovery occurring predominantly within 3 to 6 months after stroke.48-50
Limitations of the study
This review has several limitations:
the limited number of studies: only a few studies were selected on the basis of the search criteria, but these studies were of good methodological quality according to the Black and Down Checklist questionnaire evaluation. This low yield is consistent with the observations and references lists of previous reviews;11, 42
the small and heterogeneous population: combining all the trials selected, few patients were studied (in total 635 with small individual sample sizes ranging from 4 to 67). Furthermore, the populations included were heterogeneous in terms of age, lesion type, location and size, motor and cognitive repercussions, and early functional consequences;
the limited and heterogeneous evaluations of muscle changes: various techniques were used to evaluate post-stroke muscle, status, according to the study objectives, the exploration possibilities, the precocity of the investigations, the muscular groups studied, and the type of study design. In addition, certain limitations have been reported concerning the sensitivity of muscle mass measurement by ultrasound,28 DEXA or BIA with regard to assessing changes;
the question of muscle tone damage: our analysis did not take into account certain variables potentially affecting muscle status after stroke, such as the existence of spasticity and/or muscular co-contraction phenomena.38, 41
Conclusions
The investigation of post-stroke muscular adaptations as early as possible, particularly the various aspects of neuromuscular physiology and the pathophysiological changes-is crucial for the conception of appropriate early therapeutic rehabilitation measures. This review reveals that there are still gaps in our knowledge of post-stroke muscular changes. It indicates the possibility of a kinetic approach to the loss of muscle mass and to muscular adaptations, with progression from the acute to the chronic stage. It also highlights the potential reversibility of these impairments up to the stage of muscle atrophy, reinforcing the value of early preventive therapeutic interventions.
An approach based on motor control recovery (brain plasticity) and the preservation of muscle capacities (passive or active peripheral solicitations) could contribute to optimizing post-stroke rehabilitation.
Supplementary Digital Material 1
Supplementary Text File 1
Search strategies in PubMed
Footnotes
Conflicts of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.World Health Organization. World Health Statistics 2018: monitoring health for the SDGs, sustainable development goals; 2018 [Internet]. Available from: https://apps.who.int/iris/handle/10665/272596 [cited 2023 Jul 18].
- 2.Gimigliano F, Negrini S. The World Health Organization “Rehabilitation 2030: a call for action”. Eur J Phys Rehabil Med 2017;53:155–68. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28382807&dopt=Abstract 10.23736/S1973-9087.17.04746-3 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organisation. Rehabilitation 2030: A call for action; 2023 [Internet]. Available from: https://www.who.int/publications/m/item/rehabilitation-2030-a-call-for-action [cited 2023 Jul 18].
- 4.GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017;16:877–97. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28931491&dopt=Abstract 10.1016/S1474-4422(17)30299-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kossi O, Batcho CS, Adoukonou T, Thonnard JL. Functional recovery after stroke in Benin: A six-month follow-up study. J Rehabil Med 2016;48:671–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27563697&dopt=Abstract 10.2340/16501977-2128 [DOI] [PubMed] [Google Scholar]
- 6.Pak S, Patten C. Strengthening to promote functional recovery poststroke: an evidence-based review. Top Stroke Rehabil 2008;15:177–99. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18647724&dopt=Abstract 10.1310/tsr1503-177 [DOI] [PubMed] [Google Scholar]
- 7.Hofstad H, Naess H, Gjelsvik BE, Eide GE, Skouen JS. Subjective health complaints predict functional outcome six months after stroke. Acta Neurol Scand 2017;135:161–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27345529&dopt=Abstract 10.1111/ane.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haute Autorité de la Santé. Accident vasculaire cérébral : méthodes de rééducation de la fonction motrice chez l’adulte; 2023 [Internet]. Available from: https://www.has-sante.fr/jcms/c_1334330/fr/accident-vasculaire-cerebral-methodes-de-reeducation-de-la-fonction-motrice-chez-l-adulte [cited 2023, Jul 18].
- 9.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9764259&dopt=Abstract 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunnicutt JL, Gregory CM. Skeletal muscle changes following stroke: a systematic review and comparison to healthy individuals. Top Stroke Rehabil 2017;24:463–71. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28251861&dopt=Abstract 10.1080/10749357.2017.1292720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faturi FM, Lopes Santos G, Ocamoto GN, Russo TL. Structural muscular adaptations in upper limb after stroke: a systematic review. Top Stroke Rehabil 2019;26:73–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30222075&dopt=Abstract 10.1080/10749357.2018.1517511 [DOI] [PubMed] [Google Scholar]
- 12.Beckwée D, Cuypers L, Lefeber N, De Keersmaecker E, Scheys E, Van Hees W, et al. Skeletal muscle changes in the first three months of stroke recovery: A systematic review. J Rehabil Med 2022;54:jrm00308. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35848335&dopt=Abstract 10.2340/jrm.v54.573 [DOI] [PMC free article] [PubMed]
- 13.Weierink L, Vermeulen RJ, Boyd RN. Brain structure and executive functions in children with cerebral palsy: a systematic review. Res Dev Disabil 2013;34:1678–88. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23500162&dopt=Abstract 10.1016/j.ridd.2013.01.035 [DOI] [PubMed] [Google Scholar]
- 14.Meyer S, Karttunen AH, Thijs V, Feys H, Verheyden G. How do somatosensory deficits in the arm and hand relate to upper limb impairment, activity, and participation problems after stroke? A systematic review. Phys Ther 2014;94:1220–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24764072&dopt=Abstract 10.2522/ptj.20130271 [DOI] [PubMed] [Google Scholar]
- 15.Ramnemark A, Nyberg L, Lorentzon R, Olsson T, Gustafson Y. Hemiosteoporosis after severe stroke, independent of changes in body composition and weight. Stroke 1999;30:755–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10187874&dopt=Abstract 10.1161/01.STR.30.4.755 [DOI] [PubMed] [Google Scholar]
- 16.Harris ML, Polkey MI, Bath PM, Moxham J. Quadriceps muscle weakness following acute hemiplegic stroke. Clin Rehabil 2001;15:274–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11386397&dopt=Abstract 10.1191/026921501669958740 [DOI] [PubMed] [Google Scholar]
- 17.Jørgensen L, Jacobsen BK. Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: a 1 year prospective study. Bone 2001;28:655–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11425655&dopt=Abstract 10.1016/S8756-3282(01)00434-3 [DOI] [PubMed] [Google Scholar]
- 18.Carin-Levy G, Greig C, Young A, Lewis S, Hannan J, Mead G. Longitudinal changes in muscle strength and mass after acute stroke. Cerebrovasc Dis 2006;21:201–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16401884&dopt=Abstract 10.1159/000090792 [DOI] [PubMed] [Google Scholar]
- 19.Lazoura O, Papadaki PJ, Antoniadou E, Groumas N, Papadimitriou A, Thriskos P, et al. Skeletal and body composition changes in hemiplegic patients. J Clin Densitom 2010;13:175–80. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20347365&dopt=Abstract 10.1016/j.jocd.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 20.Nozoe M, Kanai M, Kubo H, Kitamura Y, Shimada S, Mase K. Changes in quadriceps muscle thickness in acute non-ambulatory stroke survivors. Top Stroke Rehabil 2016;23:8–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26094880&dopt=Abstract 10.1179/1945511915Y.0000000002 [DOI] [PubMed] [Google Scholar]
- 21.Nozoe M, Kubo H, Kanai M, Yamamoto M, Shimada S, Mase K. Peripheral motor nerve conduction abnormality, muscle strength, and muscle wasting in patients with acute stroke: A pilot study. J Clin Neurosci 2020;75:80–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32178990&dopt=Abstract 10.1016/j.jocn.2020.03.021 [DOI] [PubMed] [Google Scholar]
- 22.Ishimoto T, Taniguchi Y, Akazawa N. Longitudinal relationship between intramuscular fat in the quadriceps and gait independence in convalescent stroke patients. J Stroke Cerebrovasc Dis 2020;29:105287. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33066923&dopt=Abstract 10.1016/j.jstrokecerebrovasdis.2020.105287 [DOI] [PubMed] [Google Scholar]
- 23.Nozoe M, Kanai M, Kubo H, Yamamoto M, Shimada S, Mase K. Non-paretic lower limb muscle wasting during acute phase is associated with dependent ambulation in patients with stroke. J Clin Neurosci 2020;74:141–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32081597&dopt=Abstract 10.1016/j.jocn.2020.02.020 [DOI] [PubMed] [Google Scholar]
- 24.Kostka J, Niwald M, Guligowska A, Kostka T, Miller E. Muscle power, contraction velocity and functional performance after stroke. Brain Behav 2019;9:e01243. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30821102&dopt=Abstract 10.1002/brb3.1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irisawa H, Mizushima T. Assessment of changes in muscle mass, strength, and quality and activities of daily living in elderly stroke patients. Int J Rehabil Res 2022;45:161–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35170496&dopt=Abstract 10.1097/MRR.0000000000000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow JW, Stokic DS. Force control of quadriceps muscle is bilaterally impaired in subacute stroke. J Appl Physiol 2011;111:1290–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21885803&dopt=Abstract 10.1152/japplphysiol.00462.2011 [DOI] [PubMed] [Google Scholar]
- 27.Arasaki K, Igarashi O, Ichikawa Y, Machida T, Shirozu I, Hyodo A, et al. Reduction in the motor unit number estimate (MUNE) after cerebral infarction. J Neurol Sci 2006;250:27–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16904126&dopt=Abstract 10.1016/j.jns.2006.06.024 [DOI] [PubMed] [Google Scholar]
- 28.Chow JW, Stokic DS. Variability, frequency composition, and complexity of submaximal isometric knee extension force from subacute to chronic stroke. Neuroscience 2014;273:189–98. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24840274&dopt=Abstract 10.1016/j.neuroscience.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 29.Chow JW, Stokic DS. Impaired force steadiness is associated with changes in force frequency composition in subacute stroke. Neuroscience 2013;242:69–77. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23548515&dopt=Abstract 10.1016/j.neuroscience.2013.03.040 [DOI] [PubMed] [Google Scholar]
- 30.Maeda H, Imada K, Ishida K, Akima H. Quadriceps thickness and echo intensity predict gait independence in individuals with severe and mild hemiparetic stroke. Eur Neurol 2020;83:167–73. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32450559&dopt=Abstract 10.1159/000507548 [DOI] [PubMed] [Google Scholar]
- 31.Silva Bitencourt AC, Fernandes TD, Bazan R, Luvizutto GJ. Needle electromyography in the acute phase of stroke: correlation with severity and muscle strength. Neurol India 2022;70:1170–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35864658&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 32.Scelsi R, Lotta S, Lommi G, Poggi P, Marchetti C. Hemiplegic atrophy. Morphological findings in the anterior tibial muscle of patients with cerebral vascular accidents. Acta Neuropathol 1984;62:324–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6730908&dopt=Abstract 10.1007/BF00687615 [DOI] [PubMed] [Google Scholar]
- 33.Dietz V, Ketelsen UP, Berger W, Quintern J. Motor unit involvement in spastic paresis. Relationship between leg muscle activation and histochemistry. J Neurol Sci 1986;75:89–103. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3746341&dopt=Abstract 10.1016/0022-510X(86)90052-3 [DOI] [PubMed] [Google Scholar]
- 34.Bohannon RW, Warren M, Cogman K. Influence of Shoulder Position on Maximum Voluntary Elbow Flexion Forc; 2023 [Internet]. Available from: https://cufind.campbell.edu/physical_therapy/357/ [cited 2023, Jul 18].
- 35.Andrews AW, Bohannon RW. Distribution of muscle strength impairments following stroke. Clin Rehabil 2000;14:79–87. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10688348&dopt=Abstract 10.1191/026921500673950113 [DOI] [PubMed] [Google Scholar]
- 36.Metoki N, Sato Y, Satoh K, Okumura K, Iwamoto J. Muscular atrophy in the hemiplegic thigh in patients after stroke. Am J Phys Med Rehabil 2003;82:862–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14566154&dopt=Abstract 10.1097/01.PHM.0000091988.20916.EF [DOI] [PubMed] [Google Scholar]
- 37.Hara Y, Masakado Y, Chino N. The physiological functional loss of single thenar motor units in the stroke patients: when does it occur? Does it progress? Clin Neurophysiol 2004;115:97–103. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14706475&dopt=Abstract https://doi.org/ 10.1016/j.clinph.2003.08.002 [DOI] [PubMed] [Google Scholar]
- 38.Horstman AM, Beltman MJ, Gerrits KH, Koppe P, Janssen TW, Elich P, et al. Intrinsic muscle strength and voluntary activation of both lower limbs and functional performance after stroke. Clin Physiol Funct Imaging 2008;28:251–61. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18355344&dopt=Abstract 10.1111/j.1475-097X.2008.00802.x [DOI] [PubMed] [Google Scholar]
- 39.Horstman A, Gerrits K, Beltman M, Janssen T, Konijnenbelt M, de Haan A. Muscle function of knee extensors and flexors after stroke is selectively impaired at shorter muscle lengths. J Rehabil Med 2009;41:317–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19363562&dopt=Abstract 10.2340/16501977-0331 [DOI] [PubMed] [Google Scholar]
- 40.Horstman AM, Gerrits KH, Beltman MJ, Koppe PA, Janssen TW, de Haan A. Intrinsic properties of the knee extensor muscles after subacute stroke. Arch Phys Med Rehabil 2010;91:123–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20103406&dopt=Abstract https://doi.org/ 10.1016/j.apmr.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 41.MacIntyre NJ, Rombough R, Brouwer B. Relationships between calf muscle density and muscle strength, mobility and bone status in the stroke survivors with subacute and chronic lower limb hemiparesis. J Musculoskelet Neuronal Interact 2010;10:249–55. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21116061&dopt=Abstract [PubMed] [Google Scholar]
- 42.English C, McLennan H, Thoirs K, Coates A, Bernhardt J. Loss of skeletal muscle mass after stroke: a systematic review. Int J Stroke 2010;5:395–402. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20854624&dopt=Abstract 10.1111/j.1747-4949.2010.00467.x [DOI] [PubMed] [Google Scholar]
- 43.Hafer-Macko CE, Ryan AS, Ivey FM, Macko RF. Skeletal muscle changes after hemiparetic stroke and potential beneficial effects of exercise intervention strategies. J Rehabil Res Dev 2008;45:261–72. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18566944&dopt=Abstract 10.1682/JRRD.2007.02.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Walden F, Jakobsson F, Edström L, Nader GA. Altered autophagy gene expression and persistent atrophy suggest impaired remodeling in chronic hemiplegic human skeletal muscle. Muscle Nerve 2012;46:785–92. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22996233&dopt=Abstract 10.1002/mus.23387 [DOI] [PubMed] [Google Scholar]
- 45.Li X, Fisher M, Rymer WZ, Zhou P. Application of the F-Response for Estimating Motor Unit Number and Amplitude Distribution in Hand Muscles of Stroke Survivors. IEEE Trans Neural Syst Rehabil Eng 2016;24:674–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26168437&dopt=Abstract 10.1109/TNSRE.2015.2453274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev 2000;80:767–852. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10747207&dopt=Abstract 10.1152/physrev.2000.80.2.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu X, Suresh AK, Rymer WZ, Suresh NL. Altered motor unit discharge patterns in paretic muscles of stroke survivors assessed using surface electromyography. J Neural Eng 2016;13:046025. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27432656&dopt=Abstract 10.1088/1741-2560/13/4/046025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stinear CM. Prediction of motor recovery after stroke: advances in biomarkers. Lancet Neurol 2017;16:826–36. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28920888&dopt=Abstract 10.1016/S1474-4422(17)30283-1 [DOI] [PubMed] [Google Scholar]
- 49.Chow JW, Stokic DS. Gait impairments in patients without lower limb hypertonia early poststroke are related to weakness of paretic knee flexors. Arch Phys Med Rehabil 2019;100:1091–101. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30447195&dopt=Abstract 10.1016/j.apmr.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 50.Coleman ER, Moudgal R, Lang K, Hyacinth HI, Awosika OO, Kissela BM, et al. Early rehabilitation after stroke: a narrative review. Curr Atheroscler Rep 2017;19:59. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29116473&dopt=Abstract 10.1007/s11883-017-0686-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text File 1
Search strategies in PubMed


