ABSTRACT
Autophagy requires a tightly controlled and adjustable subcellular distribution of Atg9. Although the machinery that regulates the transport of Atg9-positive vesicles has attracted much interest, the mechanism controlling Atg9 trafficking beyond standard laboratory conditions remains poorly understood. Recently, we demonstrated that the lipid flippase Drs2 participates in the transport of Atg9 vesicles to the phagophore assembly site, and this Drs2 function becomes necessary as temperature drops. Drs2, through an I(S/R)TTK motif nested in a conserved N-terminal cavity, binds and stabilizes the transport protein particle III (TRAPPIII) on Atg9-positive membranes. This is a new function for this lipid flippase and illustrates the necessity to investigate the mechanism of selective autophagy beyond standard laboratory conditions.
Abbreviations
Autophagy-related 9 (Atg9); cytoplasm-to-vacuole targeting (Cvt); Golgi-associated retrograde protein (GARP); multisubunit tethering complexes (MTCs); phagophore assembly site (PAS); phosphatidylserine (PS); Protein interactions from Imaging Complexes after Translocation (PICT); transport protein particle III (TRAPPIII); type IV P-type ATPases (P4-ATPases)
KEYWORDS: Atg9, cytoplasm-to-vacuole targeting, Drs2, multisubunit tethering complex, P4-ATPases, TRAPPIII
Text
Atg9 (autophagy-related 9) is a transmembrane lipid scramblase that plays an essential role in autophagy. In growing Saccharomyces cerevisiae cells, Atg9 cycles between endocytic Atg9 reservoirs and the Golgi, a transport mediated by the transport protein particle III (TRAPPIII) complex. TRAPPIII is crucial for delivering Atg9 to the phagophore assembly site (PAS), a step that is strictly required for the cytoplasm-to-vacuole targeting (Cvt) pathway, a biosynthetic type of selective autophagy. After Atg9 has reached the PAS, Cvt vesicles mainly containing the precursor aminopeptidase Ape1 are generated and eventually fuse with the vacuole, releasing it in the vacuolar lumen where it is processed to its active form. Remarkably, yeast adjusts the machinery controlling Atg9 transport in response to environmental stimuli. For instance, the Golgi-associated retrograde protein (GARP) complex bypasses the function of TRAPPIII upon nutrient deprivation. Most of the studies on Atg9 transport have been limited to standardized laboratory conditions that cannot capture the ample molecular adaptations that take place in nature. We investigated the transport of Atg9 vesicles in the context of the Cvt pathway and we discovered a new mechanism that regulates TRAPPIII as a response to environmental thermal shifts [1].
We first explored protein interactions relevant to the function of multisubunit tethering complexes (MTCs), a group of protein complexes essential for vesicle transport that include TRAPPIII and GARP. We conducted a genome-wide search based on reported genetic interactions and the PICT (Protein interactions from Imaging Complexes after Translocation) assay, a live-cell imaging technique that detects protein-protein interactions in situ. The interactions found uncovered a possible interplay between MTCs and type IV P-type ATPases (P4-ATPases), a family of lipid flippases. Our results suggested that P4-ATPases Drs2, Dnf1, and Dnf2 interact with the GARP, TRAPII, and TRAPIII complexes. To better understand the relevance of this association, we focused on the functional relationship between TRAPPIII and Drs2. Drs2, which traffics between early endosomes and the trans-Golgi network, is a flippase that maintains phosphatidylserine (PS) asymmetry in the cytosolic leaflet of membranes. Interestingly, Drs2 was recently detected in Atg9-positive vesicles, but the relevance of this flippase in Atg9 transport was unknown.
Live-cell imaging and correlative light-electron microscopy showed that cells lacking TRAPPIII or Drs2 present similar defects in Ape1 processing and the formation of Cvt bodies, supporting a direct functional relationship between them. However, though TRAPPIII is required for the normal transport of Ape1 regardless of the growth temperature, we found that Drs2 contribution in the Cvt pathway is important in cells incubated at colder temperatures. Thus, while Ape1 was normally delivered into the vacuole in the drs2∆ strain grown at 37°C, these same cells minimally processed Ape1 at 16°C. The PICT assay enables the study of protein interactions under controlled cellular conditions, which allowed monitoring the impact of the temperature on the TRAPPIII-Drs2 interaction. We discovered that cells regulate the interplay between Drs2 and TRAPPIII in response to temperature shifts by enhancing their interaction at lower temperatures (Figure 1). Why Drs2 is a dispensable regulatory partner of TRAPPIII at higher temperatures still needs to be addressed. A possible explanation could be a compensation by Dnf1 and Dnf2, two flippases partially redundant with Drs2.
Figure 1.
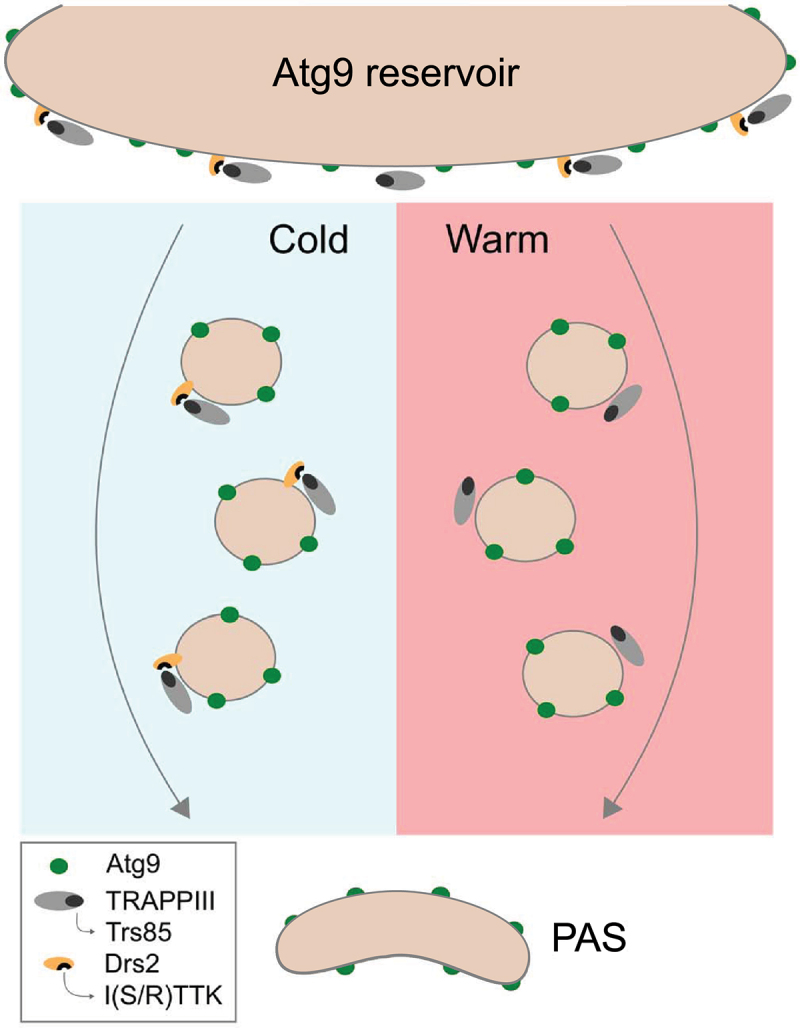
Temperature-dependent transport of Atg9 vesicles. the lipid flippase Drs2 is required to deliver Atg9-positive vesicles to the PAS at low temperatures. Drs2 binds and stabilizes the multisubunit tethering complex TRAPPIII through the interaction between Trs85, the specific subunit of TRAPPIII, and the I(S/R)TTK motif present in the N-terminus of Drs2. At higher temperatures, Drs2 is dispensable for Atg9 transport.
Next, we investigated the molecular mechanism mediating the interplay between Drs2 and TRAPPIII. Given its function as a PS flippase, our initial hypothesis was that Drs2 could accumulate negatively charged PS on the cytosolic leaflet of Atg9-positive compartments, stabilizing TRAPPIII through interactions with the positively charged amphipathic helix of its Trs85 subunit. However, cells expressing a mutant form of Drs2 that cannot flip lipids showed that electrostatic interactions between Drs2-flipped PS and Trs85 are not necessary for the Cvt pathway. Thus, we explored alternative mechanisms. Drs2 is known to have multiple functions in various vesicle transport pathways in which it mediates protein-protein interactions mostly through its C-terminal tail. Mutagenesis experiments, however, showed that the known functional motifs in the Drs2 C-terminal tail were not required for its role in the Cvt pathway. Overall, monitoring Ape1 processing in genetic backgrounds where the vesicle trafficking reported to be controlled by Drs2 had been abolished revealed that the Drs2 canonical functions are not required for the Cvt pathway.
Multiple amino acid sequence alignments identified a conserved stretch of 15 amino acids in the cytosolic N-terminal tail of P4-ATPases. Homology modeling of this stretch revealed a cavity that is present in flippases from yeast to humans, but whose function had not been investigated yet. Interestingly, the presence of the I(S/R)TTK motif within the cavity correlates with the ability of some P4-ATPases to bind MTCs (i.e. GARP, TRAPPII and TRAPPIII). We used mutagenesis and cross-linking mass spectrometry to show that Drs2 binds TRAPPIII through the Trs85 subunit via a direct or close proximity interaction that requires the I(S/R)TTK motif. In cells expressing a mutant form of Drs2 lacking this motif, TRAPPIII cannot bind Atg9-positive membranes, Atg9 vesicles are not properly transported to the PAS and the Cvt pathway is impaired. Furthermore, PICT assays revealed that, generally, binding to MTCs relies on the I(S/R)TTK motif in the N-terminal cavity of P4-ATPases. This finding suggests that the P4-ATPases share a common feature that is responsible for their interaction with MTCs. This opens up avenues for understanding the functional relevance of the interplay between MTCs and P4-ATPases. For instance, the relevance of the interplay between GARP and Drs2 in the transport of Atg9 during starvation remains unexplored. Binding to P4-ATPases seems to be a general feature of MTCs mode of action whose implications might extend to additional cellular processes.
Funding Statement
The work was supported by the Agencia Estatal de Investigación [PID2021-127773NB-I00, MDM-2014-0370]; Human Frontier Science Program [RGP0017/2020].
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- [1].Pazos I, Puig-Tintó M, Betancur L, et al. The P4-ATPase Drs2 interacts with and stabilizes the multisubunit tethering complex TRAPPIII in yeast. EMBO Rep. 2023;24(5):e56134. doi: 10.15252/embr.202256134 [DOI] [PMC free article] [PubMed] [Google Scholar]


