ABSTRACT
Irritable bowel syndrome is a common functional gastrointestinal disorder, and it has been shown that the etiology of irritable bowel syndrome is a multifactorial complex of neurological, inflammatory, and immunological changes. There is growing evidence of low-grade chronic inflammation in irritable bowel patients. The peripheral action response of their intestinal immune factors is integrated into the central nervous system, while the microbiota interacts with the brain-gut axis contributing to the development of low-grade chronic inflammation. The objective of this review is to present a discussion about the impact of immune-brain-gut axis-inflammation interactions on irritable bowel syndrome, its clinical relevance in the course of irritable bowel syndrome disease, and possible therapeutic modalities.
KEYWORDS: Irritable bowel syndrome, inflammation, brain-gut axis, immunity, gut microbiome
Introduction
Irritable bowel syndrome (IBS) as a widespread functional gastrointestinal condition marked with prolonged periods of chronic abdominal pain or discomfort as well as bowel habit alterations.1 Currently, the basis for the designation of IBS is the Rome Diagnostic Criteria IV, pertaining to repeated episodes of abnormal abdominal pain averaging a minimum of about 3 days/week in the past month accompanied by one or both of the above: 1) associated to defecation; 2) related to changes of stool counts; and 3) linked to changes in fecal material presentation.2,3 IBS affects approximately 9–16% with a somewhat elevated prevalence among females and impacts substantially on healthcare affordability and overall life outcomes.3 Clinically, currently, four distinct IBS types are identified: constipated IBS (IBS-C), diarrheal IBS (IBS-D), mixed pattern constipated and diarrheal IBS (IBS-M), and unclassified IBS.4
Despite its relatively high incidence, the underlying pathogenesis of IBS remains incompletely clear.5 The etiology may be pleiotropic including identification with mucosal immunity, neurologic, endocrine, microbial, and intestinal permeability abnormalities.6 IBS is conventionally thought of as a component of the spectrum of what is known as “cerebral intestinal disorders”.7 Recent findings suggest that low-grade inflammation and gut microbiota dysbiosis are important factors in the normal function of the brain-gut axis.8
The aim for the present review is to discuss what role immune-center-brain-gut axis interactions play in the disease process of irritable bowel syndrome, as well as their impact and clinical relevance in the development of irritable bowel syndrome disease. An improved appreciation of the roles of all three in irritable bowel syndrome disease will assist in furthering current therapeutic approaches and the improvement of therapeutic clinical efficacy in this refractory disease.
IBS and intestinal immunity
Increased immunity in the lamina propria
Intestinal immune cells can sense multiple environmental changes and activate to make an immune response when the intestinal environment changes in IBS patients. The immune system of the lamina propria of the intestine includes a variety of immune cells, including MCs, dendritic cells (DC), etc. Some external or internal factors can cause these immune cells to produce an allergic reaction, which in turn leads to an inflammatory response.9 Increased cells in the lamina propria of IBS patients were found by colonoscopic biopsy.10 Further studies have shown a significant increase in intestinal chromophores11 and MCs12 in the colonic mucosa of IBS patients. Spiller et al.13 compared the results of lamina propria biopsies in IBS patients with an increased number of lymphocytes in the lamina propria and intraepithelial among the subject group versus the healthy group.
MC is known to be multifunctional cells capable of releasing and producing various inflammatory mediators upon activation and are usually present near neurons.14 MC density was increased in the lamina propria compared to healthy controls.10 Increased numbers of MC were found in the terminal ileum and rectosigmoid mucosa of IBS patients.15 mucosal biopsies from IBS patients released mediators, of which MC secretory products 5-HT, histamine and trypsin-like levels were higher than in controls.16 Whereas MC mediators excite injurious visceral sensory nerves in rats, MC infiltration may be associated with visceral hypersensitivity in IBS.17 MCs also promote the proliferation and polarization from B lymphocytes to IgA-producing Plasma cells.18
Intrinsic dendritic cells (LPDC) are important antigen-presenting agents found within the lamina propria of the intestines and are now considered essential for innate and acquired immunity.19 Studies of LPDCs in patients with IBS or inflammatory bowel disease (IBD) have shown that LPDCs significantly elevate TNF-α secretion, expression of microbial recognition receptors is upregulated, and DCs produce more pathologically relevant cytokines.20 Instead, DCs appear to become activated and participate in the immune response via generation of IL-10 producing CD4(+) T cell proliferation.21
Enterochromaffin cells (EC) are specialized cells in the gut that are capable of synthesizing and liberating 5-hydroxytryptamine (5-HT) and ATP.These substances can activate or modulate intestinal nerve reflexes, which convey signals related to visceral/pain sensations.22 5-HT is one of the most important neurotransmitters in the etiology of IBS because it affects peristalsis, nociception, visceral inflammation, immune response, and brain activity, all of which comprise the pathology of IBS.23 A recent study using an animal model of maternal isolation simulating early life stress showed that maternal isolation-induced intestinal stem cell expansion and its differentiation toward the secretory lineage resulted in the proliferation of ECs, enhanced 5-HT generation, and sensitization to visceral nociception. This indicates that early life stress-induced irritable bowel syndrome could be connected with enhanced ECs-5-HT modeling. In addition, ECs are closely associated with CRH, MCs, neuronal and neuronal growth factors, together with bile acids, which have been implicated in the pathogenesis of IBS.24Cecum biopsies taken from IBS patients revealed increased MC density25 and T cells activation,26 which is also consistent with the hypothesis of an underlying level of immunologic activation during the course of IBS illness.
Toll-like receptors (TLRs) are a family of pathogen recognition receptors of the innate immune system and a bridge between nonspecific and specific immunity.27 Visceral hypersensitivity and compromised intestinal barrier have a major role in the pathophysiology of IBS. TLR4 is a key molecule in pattern recognition of the innate immune system.28 In animal model studies, these changes are known to be mediated through the pro-adrenocorticotropic hormone-releasing factor-TLR-pro-inflammatory cytokine signaling pathway.29 Data indicate that peripheral cytokine levels and TLRs are elevated in IBS sufferers, demonstrating that some immune dysregulation exists in IBS patients.8 The IBS-M subgroup showed significant upregulation by TLR2 with TLR4 within the colonic mucosa.30 Brint and colleagues31 studied TLR expression from colonic biopsy samples acquired by 26 IBS sufferers in comparison to 19 health subjects, and the results supported the presence of immune activation in the colon of IBS patients. The study suggests that environmental alterations lead to immune cell activation in the intestine and cause inflammatory responses, but the direct factors that ultimately lead to damage to the intestinal immune system need to be further explored.
Increased intestinal mucosal permeability
The intestinal epithelial barrier is one of the largest interfaces between the environment and the body’s internal environment. There is growing evidence that ecological dysregulation, immune activation and intestinal epithelial barrier dysfunction are manifested in a variety of diseases. Disruption of the epithelial barrier may also be involved in the production of persistent abdominal pain and discomfort.32 Impaired intestinal epithelial barrier function is a marker for a variety of pathological conditions including inflammatory enteropathy and IBS.33
Intestinal permeability can be increased by physiological effects on luminal nutrients or pathologically by a combination of immune cells and cytokines in the mucosa, the enteric nervous system, and disease-causing factors.34 MC dysfunction can lead to altered intestinal function and soreness because it also disrupts epithelial barrier function, thereby altering mucosal permeability. Manufacturers of MCs can be involved in hypersensitivity reactions and permeability defects in IBS-D.35 Ewa and colleagues36 Studies have shown low levels of E-calmodulin, which is a closely linked component of the protein involved in the regulation of paracellular permeability, in the colonic mucosa of patients with IBS, mainly in the form of diarrhea or alternating symptoms. These findings correspond to the symptoms of IBS-D and allow us to add to the improved understanding of epithelial barrier damage related to IBS. Increasing evidence suggests that at least a proportion of patients with IBS have an impaired epithelial barrier related to low-level immune activation and intestinal malfunction.37 Zhou et al.38 evaluated intestinal membrane permeability in 54 IBS-D patients and 22 healthy controls. About 39% have elevated permeability of the intestinal membrane in IBS-D sufferers. In rectal biopsy experiments using horseradish peroxidase to assess 16 IBS-D patients and 7 normal subjects, the number of mucosal MCs and permeability were significantly increased in the IBS-D group compared to the control group. It was shown that the number of mucosal MCs was positively correlated with intestinal permeability and that mucosal MCs play an important role in the increased intestinal permeability in IBS-D patients.39 Mujagic et al.40 experiment included 37 patients with distinct characteristics of various types of IBS and different parts of the gastrointestinal tract in terms of intestinal permeability in otherwise healthy subjects. Small bowel permeability was increased in IBS-D patients compared to healthy subjects in controls, independent of confounding factors.
Most patients with IBS have a visceral hypersensitivity reaction that could lead to pain in the abdomen. MC malfunction may as well destroy the epithelial barrier functionality, thus altering the mucociliary permeability and possibly causing functional and painful changes in the intestine.35 Clinical evaluation and jejunal biopsies were performed in IBS-D patients and healthy subjects, and jejunal mucosa in IBS-D patients showed MC activation and disruption of the integrity of the apical junction complex associated with clinical manifestations, findings that provide evidence of intestinal epithelial barrier damage in IBS-D.41 IBS-D is distinguished with the abdominal distress or diarrhea and the change in defecation habits, associated with excessive intestinal permeability.42 MicroRNA (miRNA) is implicated and involved in the modulation for intestinal permeability of IBS-D. Eight upregulated and 18 downregulated miRNAs were identified in a rat model of IBS-D. Among them, miR-144 was significantly upregulated, and upregulation of miR-144 promotes intestinal hyperpermeability and impairs the protective effect of the epithelial barrier, which may be a new target for the treatment of IBS-D.43 Xu et al.44 had 28 patients with IBS-D and 12 healthy controls. Patients exhibited increased psychiatric symptoms, greater visceral hypersensitivity and intestinal barrier dysfunction. The data showed significant elevation of nerve growth factor gene expressed, MC count as well as sensory nerve fibers in the patients. Elevated mucosal nerve growth factor might interplay between MCs and sensory nerve fibers, leading to visceral hypersensitivity responses in IBS-D patients with compromised intestinal barrier function. Interestingly, increased intestinal permeability contributes to inflammation, and increased inflammation further enhances intestinal permeability.
Dysbiosis of microbiome ecology
The gut microbiome represents a diverse ecosystem that influences proper physiological function and predisposition to disease by its metabolic activity and collective effects on the host.45 The gut microbiome has a significant impact on antimicrobial, immune and metabolic activities as an essential component of the human body.46 The intactness observed for the intestinal barrier is primarily due to the existence of a well-established interaction among the gut microbiome, mucosal lining, host cells, the immunological defense system as well as the intestinal vasal barrier, which involves a complex network of two-way interactions and the control of inflammatory processes. It is essential for the maintenance of intestinal homeostasis.47Systemic chronic inflammation induced by the intestinal microbiota mainly refers to excessive intestinal inflammation caused by immune dysregulation of the intestinal mucosa, exacerbated by the creation or disruption of the intestinal barrier interface, which triggers various common chronic diseases along its diffusion pathway.48
Disturbances to the homeostasis of the enteric microbiome compromise the intestinal mucosal barrier from which immunity is reduced, and perturbations to the structure of complex commensal communities can lead to inadequate education of the host immune system and subsequent immune-mediated disease.49 The overgrowth of small intestinal bacteria features unusually high numbers of bacteria in the small intestine, which is considered to be an important positive sign in patients with IBS.50 The fecal microbiota of patients meeting the Rome criteria were measured against control recipients who were age and sex marked. Quantitative alterations of the gastrointestinal microbiota in IBS patients were found by assay comparison.51 Bacterial commensals in the intestine, including the cell surface lipopolysaccharides of the cell wall components of Gram bacteria, can serve as potential ligands for TLRs, and the TLR5 receptor is able to detect flagellin.,52 suggesting that changes in the small intestinal microbiome of IBS patients can affect immune activation. Altered immune activation in response to a dysregulated microbiota may promote intestinal inflammation in a subset of IBS patients.53 During the development process in the central nervous system, the production of neurons is influenced from various environmental factors. Intestinal microbiome has an essential part to regulate and guide the neural development of the central nervous system.54
These changes could explain some of the symptoms of IBS, including visceral hypersensitivity, effects of gut microbes on the host immune system and intestinal barrier function, and the brain-gut axis (Figure 1).
Figure 1.
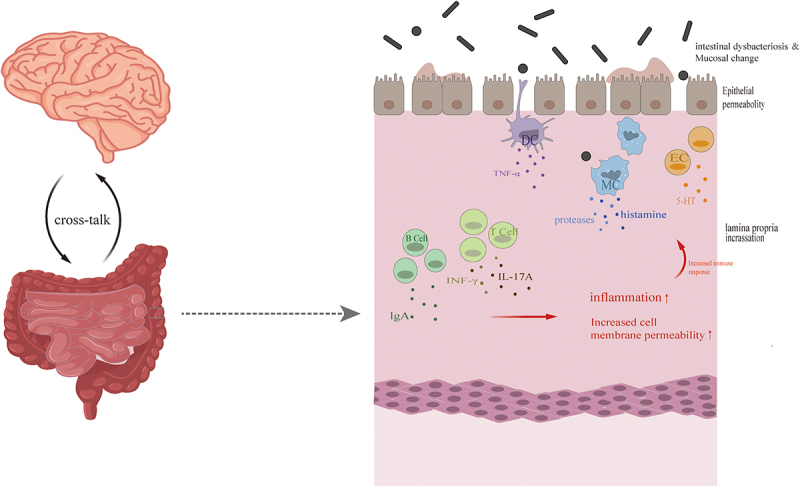
The presence of intestinal immune activation in irritable bowel syndrome.
In the intestinal tissues of the Irritable Bowel Syndrome, impaired intestinal mucosal barrier due to dysbiosis may activate a pro-inflammatory dendritic cell phenotype that stimulates an immune response by T-cell B cells and increases cell permeability. DC, dendritic cell; EC, enterophilic cell; IL-17A, interleukin 17A; INF-γ, tumor necrosis factor-γ; IgA, immunoglobulin A; MC, mast cell; TNF-α, tumor necrosis factor-α; 5-HT, 5-hydroxytryptamine.
IBS and low-grade inflammation
Clinical observation
Recently, the issue of low-grade inflammation or immune activation and the ecological dysregulation that may trigger or exacerbate IBS has been raised.27 The importance of low-grade inflammation for the progression of IBS is now supported and confirmed by observations.
In a subgroup with IBS, elevated innate immune activation was observed both in the intestinal mucosa and the blood. The hallmark of the disease is intestinal leakage, the pathology where the intestinal integrity of the intestinal blood barrier is impaired, allowing intestinal fluids (such as the immune cells and microbiota) into the circulation, resulting in low-grade generalized systemic inflammation.55 Flagellin, a major structural component of the bacterial flagellum, was shown to activate innate and adaptive immunity in patients with inflammatory bowel disease, with significantly higher flagellin antibodies in the IBS group compared to the normal healthy group in 112 IBS sufferers and 43 healthy control experiments.56 Guven et al.57 compared 107 IBS patients with 107 controls, the IBS group had higher platelet and neutrophil counts were higher and lymphocyte counts were lower in the IBS group. The results suggest that patients with IBS have a higher index of systemic immune inflammation.
The generation of pathophysiological mechanisms of IBS may be associated with abnormal activation and a disturbed state of intestinal immune function.58 The results of the study showed an association between patients with typical IBS symptoms and increased immune cells in the lamina propria of the colonic mucosa compared to healthy controls.10 Interestingly, the persistence of mucosal inflammation, increased recruitment of enteroendocrine cells, increased mast cell(MC) density, and intestinal activity in IBS patients highlighted in the experimental results may be associated with symptoms of visceral hypersensitivity reactions. In recent studies, there is increasing evidence of T cell-mediated hypo-inflammation in the intestinal mucosa of post-infectious irritable bowel syndrome (PI-IBS).59 As demonstrated by the disease progression in patients with PI-IBS, the infection may lead to generalized inflammation and alter the diversity of the microbiome, instead potentially perpetuating a cycle of permanent, low-grade, and subclinical infections.60 Also, the activation of immunity could account for the dominance of women and fluctuating immunological activation, leading to changes in symptoms over time.61
By analyzing the retrieved studies, patients with IBS were observed to have a higher frequency of activation of immunity compared to healthy subjects, with increased numbers of MCs in IBS patients and lymphocytes, altered levels of cytokines, and increased permeability of the intestine.62 An increase in intestinal mucosal and blood-innate immune activity was also observed in the IBS subgroup of subjects.55 Cermon et al.63 compared 48 IBS patients, 12 patients with colitis, 20 patients with ulcerative colitis, and 24 healthy controls for intestinal immune cells, and IBS patients had significantly increased mucosal immune cells. Further analysis showed that increased immune cells were present in 50% of IBS patients. Immune cells such as T cells and MCs were increased in IBS patients compared to controls. Shulman et al.64 measured fecal β-defensin-72 in children with IBS and healthy controls, and children with IBS had higher β-defensin-2 concentrations than the healthy group. Elevated fecal β-defensin-2 values of children with IBS suggest innate immune system activation with increased intestinal permeability in some patients, and these changes appear to be associated with symptoms of IBS associated with abdominal distress. Together, they suggest the presence of intestinal immune system activation in IBS patients.
Inflammatory signals transmitted through the brain-intestine axis
Clinical evidence suggests that mental disorders are important factors in the development and progression of irritable bowel syndrome, a disease with multiple causative factors.13 Early life stress(ELS) is one of the most common factors in the deterioration of IBS patients.24 This stress may come from family, work or other social environments, or it may be caused by individual personality factors. The psychological and physical development of children and adolescents exposed to ELS can have serious consequences. The literature shows that important brain structures are formed in early childhood and adolescence and that the negative effects of traumatic events are long-lasting and can persist throughout a child’s life.65 A study of infants with social and family problems found that their cortisol awakening response was higher than that of infants without social and family problems.66 A study of the effects of early childhood trauma on HPA axis reactivity in patients with IBS found that patients who had an early adverse life event had significantly higher levels of cortisol in their saliva. This suggests that early childhood trauma can have an impact on IBS patients, which also provides important information for studying the relationship between early childhood trauma and IBS, the researchers said.67 High levels of cortisol can cause damage to the hippocampus, as demonstrated in those with a history of emotional neglect at an early age. Compared to patients without a history of emotional neglect, white matter in the left hippocampus is reduced.68 The hippocampus possesses more glucocorticoid receptors than any other organ and is now recognized as an important site for the regulation of glucocorticoid synthesis. Stress in childhood has been shown to cause irreversible changes in hypothalamic axons that lead to anxiety in adulthood; dysfunction of the HPA axis may be due to an imbalance between glucocorticoid and mineralocorticoid receptors. Studies on the HPA axis in patients with IBS have shown decreased plasma cortisol concentrations, decreased cortisol responses to adrenocorticotropic hormone, and increased vagal responses to rectal distention.69
The cell bodies of vagal afferent fibers are found in the nodal ganglia and project to solitary bundle nuclei in the brainstem, where they receive messages before sending them to higher-order cerebral areas. The hypothalamic-pituitary-adrenal (HPA) axis is activated and neuronal circuits involved in abnormal behavior are activated when vagal afferent fibers are stimulated by inflammation.70 Through the vagal descending pathway of the dorsal motor nucleus of the vagus nerve, intestinal inflammation is centrally regulated. Enteric neurons are stimulated by cholinergic vagal efferent fibers, which also prevent macrophages from releasing inflammatory cytokines.71 Local activation of neurons due to inflammation leads to the release of neuropeptides, which also play an important immunomodulatory role by stimulating immune cells.72 Despite the fact that mucosal macrophages are close to vagal nerve terminals, it is not apparent if vagal stimulation directly modifies intestinal immune cells or whether messages must first go indirectly through enteric neurons, glial cells, or intestinal epithelial cells.
Inflammation leads to the local activation of neurons resulting in the release of neuropeptides, which also play an important immunomodulatory role by stimulating immune cells. The intestinal epithelial enteroendocrine cells located throughout the intestinal epithelium are key regulators of coordinated communicating agents operating alongside the brain-gut-microbiome axis. Intestinal endocrine cells sense changes in luminal microorganisms through microbial inclusions; at the same time, the cells correspond to the host system through neuroendocrine molecules.73 Neurons and glial cells of the intestinal nervous system are involved with immunity in the gut, and besides providing support to intestinal neurons, the glial protects the intestinal barrier through secretion of RET receptor for ligands, which provoke type 3 natural lymphocyte-dependent production of interleukin 2.74 There is growing evidence that eosinophils are key neuroimmune players in the regulation of gastrointestinal function and that eosinophil-neuron interactions are facilitated by chemotactic and adhesion molecules.75 The presence of mucosal eosinophilia and eosinophil activation has been identified in IBS.76 When activated, eosinophils release a variety of cytotoxic substances and immunomodulatory cytokines, leading to local inflammation and tissue damage.77
Gut immune cells themselves directly regulate the regulation of neuroimmunity and the brain’s response to inflammation, in addition to the endocrine signaling of immune factors via the gut-brain axis. To control the exchange of gut microbes, gut antigens stimulate the differentiation of B cells into immunoglobulin A (IgA)-secreting plasma cells. In autoimmune neurological diseases, massive migration of gut IgA+ macrophages into the brain and medulla attenuates neuroinflammation in an interleukin-10 (IL-10)-dependent manner.78 These findings may open new avenues for the treatment of neuroimmune diseases by using intestinal antigens to promote IgA+ B cell production and facilitate neuroimmune suppression.79
The central nervous system can also regulate the composition and homeostasis of the gut microbial community (mainly Gram-negative bacteria) through the stress system (autonomic nervous system locus, HPA axis).80 Differences in the total abundance of specific bacterial taxa were observed in patients with PTSD compared to trauma-exposed controls.81 Evidence for the interactions between the microbiota and the brain-gut axis in clinical practice comes from the correlation between ecological dysregulation and functional gastrointestinal illnesses and central neurological disorders, such as autism and anxiety-depressive behaviors.82 The composition of the microbiota of the human body changes dynamically during the life cycle, forming a close relationship with organisms from the earliest stages of life. Thus, the development of the gut microbiota occurs in parallel with the central nervous system, with rapid and profound developmental changes during infancy, childhood and adolescence. Disturbances in the gut microbiota early in life can affect neurodevelopment and may lead to unfavorable morbidity in adulthood.83 For example, neonatal adversity increases the likelihood of functional gastrointestinal disorders in adulthood.84
In recent years, brain-gut-brain interactions have been recognized as a theoretical model for the pathogenesis of functional gastrointestinal disorders such as IBS and have become an important part of clinical research. Generally, the relationship between the center and the small intestine is bidirectional, especially when the balance in the body is disrupted. Currently, national and international studies agree that there are two main pathogenic mechanisms of IBS: central (top-down) and peripheral (bottom-up). Notably, much of the literature suggests that the brain-gut axis may be affected by several stressors at the same time, i.e., the brain-gut axis may be affected by both central (external stress) and intestinal (internal stress), which leads to the onset and exacerbation of IBS. However, the question of which mechanism comes first is as unanswered as the question of “which came first, the chicken or the egg”, and needs to be explored in the future.
Inflammatory susceptibility and HPA axis reactive damage
IBS pathogenesis is often associated with negative emotions, and stress is thought to be an underlying process. Although the pathophysiological basis is not fully understood, data consistently show that inflammatory mediators and HPA axis activation play a key role in stress-induced disease. An enhanced stress response is considered an underlying cause in terms of the IBS pathophysiology, mainly in terms of changes in the HPA axis and the function of the sympathetic nervous system. These two systems can regulate mucosal immunity.85 Stress triggers activation of the hypothalamic-pituitary axis and the autonomic nervous system with increased cortisol levels and pro-inflammatory cytokines, while stress leads to alterations in the HPA axis and systemic release of gut-derived inflammatory factors can alter the integrity of the blood-brain barrier and lead to developmental defects in the brain, in addition, inflammation-induced HPA axis activity can trigger the systemic release of glucocorticoids, which can alter gut function.86
The HPA axis activity represents by far the main emanative body fluid axis recorded for the gut-brain axis, where peripheral responses to environmental stressors or gut Inflammation become consolidated within the central nervous system as well as provoking the HPA axis, that mediates adrenal glands to deliver glucocorticoids. This powerful stress-inducing hormone can restore homeostasis or promote gastrointestinal dysfunction in vivo by modulating intestinal immune cell activity, gut function and microbial composition.87 Stress-induced dysbiosis can in turn trigger intestinal inflammation through helper T cell 17-dependent release of IL-17A, which contributes to feedforward activation of the stress response.88 The gut microbiome is also involved in the regulation of the HPA axis during homeostasis, as microbiota deficiency exacerbates the HPA axis in response to moderate stress.87
Recently, experimental data have shown a critical role of stress in damaging the blood-brain barrier, thereby activating the HPA axis of peripheral immune stimulation and inducing pro-inflammatory cytokine gene expression in the hypothalamus.89 Also, stress increases intestinal permeability through pro-adrenocorticotropic hormone-releasing hormone (CRH)-mediated MC activation.90 CRH administration has been shown to exacerbate visceral pain hypersensitivity in IBS patients.91 CRH receptor-1 antagonists significantly prevented the increase in intestinal sensitivity in rats.92 De-inhibition of the hypothalamic paraventricular nucleus projecting GABAergic neurons in the ventral anterior region of the bed nucleus contributes to the excitation of CRH neurons, which mediates visceral hypersensitivity responses.93,94 Autonomic nervous system function and neuroimmune axis show specific features among IBS sufferers.90 However, additional studies are required to verify a variable relationship between HPA axis reactive damage and IBS inflammation.
Modern treatment progress
Currently there is a lack of effective treatment for IBS, and the clinical approach is based on symptom reduction.95 An international study found that patients gave up 25% of their remaining life expectancy (an average of 15 years) in order to receive treatment to relieve their symptoms, and 14% had a 1 in 1,000 chance of dying.96 Current treatments for irritable bowel syndrome include lifestyle changes, dietary modifications, probiotics, and medications to improve sensation and homeostasis in people with irritable bowel syndrome. This article summarizes current treatments for irritable bowel syndrome, focusing on medications, dietary modifications, probiotics, and other alternative therapies. (Table 1) Despite recent advances, current treatments still do not respond well to all the changes caused by irritable bowel syndrome. There is a need to develop new therapies that can alleviate the suffering of people with irritable bowel syndrome without causing deleterious central effects or other adverse effects.
Table 1.
Modern treatments for IBS.
| Type of treatment | Concrete method | Effect | References |
|---|---|---|---|
| Medicines | rifaximin | 2 weeks of treatment provides significant relief from IBS symptoms, bloating, abdominal pain and loose or watery stools | 97 |
| loperamide | For first-line treatment of IBS-D diarrhea | 98 | |
| Bile acid sequestrants | Improves stool consistency and reduces bowel movement frequency | 99 | |
| antispasmodic | It is very effective for abdominal pain in patients with IBS, but can lead to more adverse effects such as dry mouth, vertigo, and constipation | 100 | |
| peppermint oil | Superior to placebo in the treatment of IBS, but adverse events are more frequent and the quality of evidence is very low | 101 | |
| antidepressant | Antidepressant medications provide better relief for IBS. However, there are limitations in the information, so estimates of efficacy may be overestimated | 102 | |
| pregabalin | In patients with allergic IBS, it can significantly increase their rectal sensory threshold to a dilated state | 103 | |
| Diet | FODMAP diet | Effective for many patients, but not for all due to complexity of operation | 104–106 |
| Increased Dietary Fiber | The clinical symptoms of IBS were only marginally improved and the beneficial effects were limited to psyllium seeds, while bran had no significant effect on them | 107 | |
| Gluten-free Diet (GFD) | GFD was associated with overall symptom improvement compared with controls, but there was insufficient evidence to confirm that GFD improved IBS symptoms | 108 | |
| Gut microbiota | probiotics | Reduces pain and symptom severity scores | 109,110 |
| Synergistic combination of prebiotics and probiotics (called synbiotics) | Beneficial for overall IBS symptoms and abdominal pain, but unable to draw definitive conclusions about its efficacy | 111,112 | |
| Fecal microbiota transplantation | Recommended for the treatment of recurrent C. difficile infections accompanying IBD, but very time-consuming and labor-intensive | 113–116 | |
| complementary alternative therapy | hypnotherapy | Applications are limited by considerable cost and long duration as well as adverse patient and clinician restrictions | 117,118 |
| acupuncture | It may be possible to improve intestinal motility and visceral sensitivity to IBS treatment by modulating brain gut peptide levels in the central nervous system, intestines, and blood. However, the reasons for the effectiveness of acupuncture treatment still need to be further explored | 119–123 | |
| Psychotherapeutic Approaches | cognitive behavioral therapy (CBT) | Patients with IBS develop positive clinical symptoms after treatment, which may be related to their brain network function, altered structural connectivity, and altered gut microbiome. However, there is a lack of reference for evaluating microbiome persistence and neuroanatomical alterations in CBT-responsive populations | 124 |
Conclusion
In the last few years, it has been demonstrated that altered gastrointestinal environment, persistent low-grade inflammation, and abnormal neuro-immune interactions have a significant part to perform in the IBS pathophysiology. The research focus has also gradually shifted toward immune activation and gut ecological dysregulation. The triad of altered immune cell activation in the intestinal environment, intestinal flora, and neuroimmune interactions contribute to the development of low-grade chronic inflammation (Figure 2). Although there is growing evidence of low-grade chronic inflammation in patients with irritable bowel, it remains uncertain which triggers ultimately disrupt the intestinal immune system. Many other mechanisms can also lead to immune activation. The effects of stress on the HPA axis, as well as alterations in intestinal permeability or interactions with the immune system in response to food intolerance, require further investigation. The current clinical is the difficulty in identifying and describing subgroups of patients with the same pathophysiological mechanisms, leading to difficulties in the design and improvement of therapeutic regimens.
Figure 2.
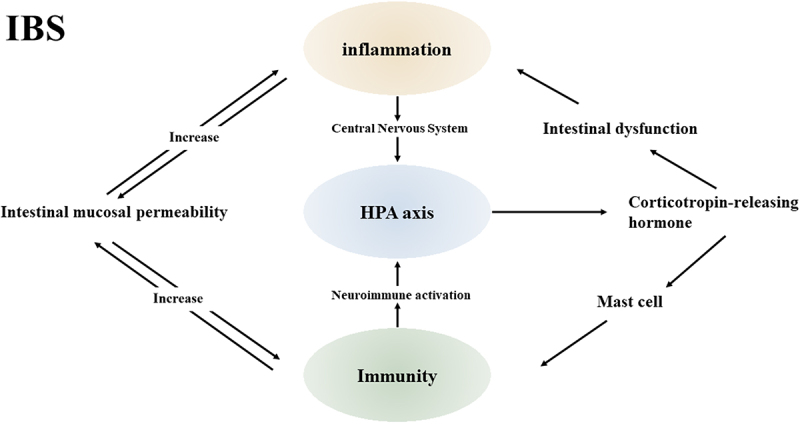
Inflammation, immunity and HPA axis in IBS.
The inflammatory, immune and HAP axes interact with each other in IBS disease. Peripheral responses to environmental stressors or intestinal inflammation are integrated in the central nervous system, triggering the HPA axis. The HPA axis coordinates the release of glucocorticoids from the adrenal glands to promote gastrointestinal dysfunction. Activation of intestinal immune cells may disrupt epithelial barrier function, thereby altering mucosal permeability and potentially leading to intestinal inflammation. Intestinal inflammation in turn enhances intestinal mucosal permeability. Increased intestinal immune response through CRH-mediated mast cell activation. Intestinal immunity drives neuroimmune activation.
In summary, IBS is a multifactorial complex of immunological, microbiota, and gut-brain axis signaling changes, and the results of various experiments provide compelling evidence that this is indeed the case. This article provides a framework for advancing the concept of IBS as an immune-brain-gut axis-microbial disease to further our understanding of this disorder. It is believed that the shortcomings of current clinical treatment options will be addressed in the future.
Funding Statement
The work was supported by the National Natural Science Foundation of China [81973936]; Anhui Province Scientific Research Planning Project [2022AH050438].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150(6):1262–1279.e2. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Akehurst RL, Brazier JE, Mathers N, O’keefe C, Kaltenthaler E, Morgan A, Platts M, Walters SJ. Health-related quality of life and cost impact of irritable bowel syndrome in a UK primary care setting. Pharmacoeconomics. 2002;20(7):455–15. doi: 10.2165/00019053-200220070-00003. [DOI] [PubMed] [Google Scholar]
- 3.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(7):712–21 e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Bonetto S, Fagoonee S, Battaglia E, Grassini M, Saracco GM, Pellicano R. Recent advances in the treatment of irritable bowel syndrome. Pol Arch Intern Med. 2021;131(7–8):709–715. doi: 10.20452/pamw.16067. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Lin E, Pimentel M. Biomarkers of irritable bowel syndrome. J Neurogastroenterol Motil. 2017;23(1):20–26. doi: 10.5056/jnm16135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbara G, Cremon C, De Giorgio R, Dothel G, Zecchi L, Bellacosa L, Carini G, Stanghellini V, Corinaldesi R. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. 2011;13(4):308–315. doi: 10.1007/s11894-011-0195-7. [DOI] [PubMed] [Google Scholar]
- 7.Fagoonee S, Pellicano R. Does the microbiota play a pivotal role in the pathogenesis of irritable bowel syndrome? J Clin Med. 2019;8(11):1808. doi: 10.3390/jcm8111808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mckernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther. 2011;33(9):1045–1052. doi: 10.1111/j.1365-2036.2011.04624.x. [DOI] [PubMed] [Google Scholar]
- 9.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell. Front Immunol. 2015;6:620. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salzmann JL, Peltier-Koch F, Bloch F, Petite JP, Camilleri JP. Morphometric study of colonic biopsies: a new method of estimating inflammatory diseases. Lab Invest. 1989;60:847–851. [PubMed] [Google Scholar]
- 11.Arevalo F, Aragon V, Montes P, Guzmán E, Monge E. Increase of intraepithelial lymphocytes in patients with irritable bowel syndrome. Rev Gastroenterol Peru. 2011;31:315–318. [PubMed] [Google Scholar]
- 12.Guilarte M, Santos J, De Torres I, Alonso C, Vicario M, Ramos L, Martinez C, Casellas F, Saperas E, Malagelada JR. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56(2):203–209. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47(6):804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiological Reviews. 1997;77(4):1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 15.Wang LH. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53(8):1096–1101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137(4):1425–1434. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132(1):26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 18.Merluzzi S, Frossi B, Gri G, Parusso S, Tripodo C, Pucillo C. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA-secreting plasma cells. Blood. 2010;115(14):2810–2817. doi: 10.1182/blood-2009-10-250126. [DOI] [PubMed] [Google Scholar]
- 19.Chirdo FG, Millington OR, Beacock-Sharp H, Mowat A. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol. 2005;35(6):1831–1840. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- 20.Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, Kamm MA, Stagg AJ. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129(1):50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Psarras A, Antanaviciute A, Alase A, Carr I, Wittmann M, Emery P, Tsokos GC, Vital EM. TNF-α regulates human plasmacytoid dendritic cells by suppressing IFN-α production and enhancing T cell activation. J Immunol. 2021;206(4):785–796. doi: 10.4049/jimmunol.1901358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linan-Rico A, Ochoa-Cortes F, Zuleta-Alarcon A, Alhaj M, Tili E, Enneking J, Harzman A, Grants I, Bergese S, Christofi FL. UTP – gated signaling pathways of 5-HT release from BON cells as a model of human enterochromaffin cells. Front Pharmacol. 2017;8:429. doi: 10.3389/fphar.2017.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishima Y, Ishihara S. Enteric microbiota-mediated serotonergic signaling in pathogenesis of irritable bowel syndrome. Int J Mol Sci. 2021;22(19):10235. doi: 10.3390/ijms221910235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao E, Zhu Z, Hu C, Long G, Chen B, Guo R, Fang M, Jiang M. Potential roles of enterochromaffin cells in early life stress-induced irritable bowel syndrome. Front Cell Neurosci. 2022;16:837166. doi: 10.3389/fncel.2022.837166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Sullivan M, Clayton N, Breslin NP. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12(5):449–457. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 26.Ohman L, Isaksson S, Lindmark AC, Posserud I, Stotzer PO, Strid H, Sjövall H, Simrén M. T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104(5):1205–1212. doi: 10.1038/ajg.2009.116. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Fandino O, Hernandez-Ruiz J, Schmulson M. From cytokines to toll-like receptors and beyond - current knowledge and future research needs in irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16(4):363–373. doi: 10.5056/jnm.2010.16.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen ZY, Zhang XW, Yu L, Hua R, Zhao XP, Qin X, Zhang YM. Spinal toll-like receptor 4-mediated signalling pathway contributes to visceral hypersensitivity induced by neonatal colonic irritation in rats. Eur J Pain. 2015;19(2):176–186. doi: 10.1002/ejp.534. [DOI] [PubMed] [Google Scholar]
- 29.Nozu T, Miyagishi S, Ishioh M, Takakusaki K, Okumura T. Peripheral apelin mediates visceral hypersensitivity and impaired gut barrier in a rat irritable bowel syndrome model. Neuropeptides. 2022;94:102248. doi: 10.1016/j.npep.2022.102248. [DOI] [PubMed] [Google Scholar]
- 30.Belmonte L, Beutheu Youmba S, Bertiaux-Vandaele N, Antonietti M, Lecleire S, Zalar A, Gourcerol G, Leroi AM, Déchelotte P, Coëffier M, et al. Role of toll like receptors in irritable bowel syndrome: differential mucosal immune activation according to the disease subtype. PloS One. 2012;7(8):e42777. doi: 10.1371/journal.pone.0042777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brint EK, Macsharry J, Fanning A, Shanahan F, Quigley EM. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am J Gastroenterol. 2011;106(2):329–336. doi: 10.1038/ajg.2010.438. [DOI] [PubMed] [Google Scholar]
- 32.Barbara G, Barbaro MR, Fuschi D, Palombo M, Falangone F, Cremon C, Marasco G, Stanghellini V. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front Nutr. 2021;8:718356. doi: 10.3389/fnut.2021.718356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M, Oshima T, Ito C, Yamada M, Tomita T, Fukui H, Miwa H. Glutamine blocks interleukin-13-induced intestinal epithelial barrier dysfunction. Digestion. 2021;102(2):170–179. doi: 10.1159/000502953. [DOI] [PubMed] [Google Scholar]
- 34.Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013;11(9):1075–1083. doi: 10.1016/j.cgh.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasler WL, Grabauskas G, Singh P, Owyang C. Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterol Motil. 2022;34(7):e14339. doi: 10.1111/nmo.14339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilcz-VIllega E, Mcclean S, O’Sullivan M. Reduced E-cadherin expression is associated with abdominal pain and symptom duration in a study of alternating and diarrhea predominant IBS. Neurogastroenterol Motil. 2014;26(3):316–325. doi: 10.1111/nmo.12262. [DOI] [PubMed] [Google Scholar]
- 37.Piche T. Tight junctions and IBS–the link between epithelial permeability, low-grade inflammation, and symptom generation? Neurogastroenterol Motil. 2014;26(3):296–302. doi: 10.1111/nmo.12315. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146(1–2):41–46. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee H, Park JH, Park DI, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Chae SW. Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J Neurogastroenterol Motil. 2013;19(2):244–250. doi: 10.5056/jnm.2013.19.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mujagic Z, Ludidi S, Keszthelyi D, Hesselink MAM, Kruimel JW, Lenaerts K, Hanssen NMJ, Conchillo JM, Jonkers DMAE, Masclee AAM, et al. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther. 2014;40(3):288–297. doi: 10.1111/apt.12829. [DOI] [PubMed] [Google Scholar]
- 41.Martinez C, Lobo B, Pigrau M, Ramos L, González-Castro AM, Alonso C, Guilarte M, Guilá M, de Torres I, Azpiroz F, et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62(8):1160–1168. doi: 10.1136/gutjnl-2012-302093. [DOI] [PubMed] [Google Scholar]
- 42.Altomare A, Di Rosa C, Imperia E, Emerenziani S, Cicala M, Guarino MPL. Diarrhea predominant-irritable bowel syndrome (IBS-D): effects of different nutritional patterns on intestinal dysbiosis and symptoms. Nutrients. 2021;13(5):1506. doi: 10.3390/nu13051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou Q, Huang Y, Zhu S, Li P, Chen X, Hou Z, Liu F. MiR-144 increases intestinal permeability in IBS-D rats by targeting OCLN and ZO1. Cell Physiol Biochem. 2017;44(6):2256–2268. doi: 10.1159/000486059. [DOI] [PubMed] [Google Scholar]
- 44.Xu XJ, Zhang YL, Liu L, Pan L, Yao SK. Increased expression of nerve growth factor correlates with visceral hypersensitivity and impaired gut barrier function in diarrhoea-predominant irritable bowel syndrome: a preliminary explorative study. Aliment Pharmacol Ther. 2017;45(1):100–114. doi: 10.1111/apt.13848. [DOI] [PubMed] [Google Scholar]
- 45.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kogut MH, Lee A, Santin E. Microbiome and pathogen interaction with the immune system. Poult Sci. 2020;99(4):1906–1913. doi: 10.1016/j.psj.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicoletti A, Ponziani FR, Biolato M, Valenza V, Marrone G, Sganga G, Gasbarrini A, Miele L, Grieco A. Intestinal permeability in the pathogenesis of liver damage: from non-alcoholic fatty liver disease to liver transplantation. WJG. 2019;25(33):4814–4834. doi: 10.3748/wjg.v25.i33.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mou Y, Du Y, Zhou L, Yue J, Hu X, Liu Y, Chen S, Lin X, Zhang G, Xiao H, et al. Gut microbiota interact with the brain through systemic chronic inflammation: implications on neuroinflammation, neurodegeneration, and aging. Front Immunol. 2022;13:796288. doi: 10.3389/fimmu.2022.796288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Black CJ, Ford AC. Chronic idiopathic constipation in adults: epidemiology, pathophysiology, diagnosis and clinical management. Med J Aust. 2018;209(2):86–91. doi: 10.5694/mja18.00241. [DOI] [PubMed] [Google Scholar]
- 50.Ford AC, Spiegel BM, Talley NJ, Moayyedi P. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1279–1286. [DOI] [PubMed] [Google Scholar]
- 51.Malinen E, Rinttila T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100(2):373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 52.Bhattarai Y, Muniz Pedrogo DA, Kashyap PC. Irritable bowel syndrome: a gut microbiota-related disorder? Am J Physiol Gastrointest Liver Physiol. 2017;312(1):G52–G62. doi: 10.1152/ajpgi.00338.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shukla R, Ghoshal U, Ranjan P, Ghoshal UC. Expression of toll-like receptors, pro-, and anti-inflammatory cytokines in relation to gut microbiota in irritable bowel syndrome: the evidence for its micro-organic basis. J Neurogastroenterol Motil. 2018;24(4):628–642. doi: 10.5056/jnm18130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Q, Xing C, Long W, Wang HY, Liu Q, Wang RF. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflammation. 2019;16(1):53. doi: 10.1186/s12974-019-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7(3):163–173. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 56.Schoepfer AM, Schaffer T, Seibold-Schmid B, Müller S, Seibold F. Antibodies to flagellin indicate reactivity to bacterial antigens in IBS patients. Neurogastroenterol Motil. 2008;20(10):1110–1118. doi: 10.1111/j.1365-2982.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 57.Guven IE, Baspinar B, Atalay R. Relationship between systemic immune-inflammation index and irritable bowel syndrome. Turk J Gastroenterol. 2022;33(1):30–34. doi: 10.5152/tjg.2021.21321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ringel Y, Maharshak N. Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2013;305(8):G529–41. doi: 10.1152/ajpgi.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong LW, Ma ZC, Fu J, Huang BL, Liu FJ, Sun D, Lan C. Upregulated adenosine 2A receptor accelerates post-infectious irritable bowel syndrome by promoting CD4+ T cells’ T helper 17 polarization. World J Gastroenterol. 2022;28(25):2955–2967. doi: 10.3748/wjg.v28.i25.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ng QX, Soh AYS, Loke W, Lim DY, Yeo WS. The role of inflammation in irritable bowel syndrome (IBS). JIR. 2018;11:345–349. doi: 10.2147/JIR.S174982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Talley NJ. What causes functional gastrointestinal disorders? A proposed disease model. Am J Gastroenterol. 2020;115(1):41–48. doi: 10.14309/ajg.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 62.Matricon J, Meleine M, Gelot A, Piche T, Dapoigny M, Muller E, Ardid D. Review article: associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36(11–12):1009–1031. doi: 10.1111/apt.12080. [DOI] [PubMed] [Google Scholar]
- 63.Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104(2):392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 64.Shulman RJ, Devaraj S, Heitkemper M. Activation of the innate immune system in children with irritable bowel syndrome evidenced by increased fecal human beta-defensin-2. Clin Gastroenterol Hepatol. 2021;19(10):2121–2127. doi: 10.1016/j.cgh.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teicher MH. Scars that won’t heal: the neurobiology of child abuse. Sci Am. 2002;286(3):68–75. doi: 10.1038/scientificamerican0302-68. [DOI] [PubMed] [Google Scholar]
- 66.Saridjan NS, Huizink AC, Koetsier JA, Jaddoe VW, Mackenbach JP, Hofman A, Kirschbaum C, Verhulst FC, Tiemeier H. Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? The generation R study. Horm Behav. 2010;57(2):247–254. doi: 10.1016/j.yhbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Videlock EJ, Adeyemo M, Licudine A, Hirano M, Ohning G, Mayer M, Mayer EA, Chang L. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137(6):1954–1962. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res. 2010;44(13):799–807. doi: 10.1016/j.jpsychires.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie–Doyle S, Smith E, Drew P, Talley NJ, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132(3):913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 70.Bluthe RM, Michaud B, Kelley KW, Dantzer R. Vagotomy blocks behavioural effects of interleukin-1 injected via the intraperitoneal route but not via other systemic routes. Neuroreport. 1996;7(15):2823–2827. doi: 10.1097/00001756-199611040-00083. [DOI] [PubMed] [Google Scholar]
- 71.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rijnierse A, Nijkamp FP, Kraneveld AD. Mast cells and nerves tickle in the tummy: implications for inflammatory bowel disease and irritable bowel syndrome. Pharmacol Ther. 2007;116(2):207–235. doi: 10.1016/j.pharmthera.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 73.Yu L, Li Y. Involvement of intestinal enteroendocrine cells in neurological and psychiatric disorders. Biomedicines. 2022;10(10):2577. doi: 10.3390/biomedicines10102577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huh JR, Veiga-Fernandes H. Neuroimmune circuits in inter-organ communication. Nat Rev Immunol. 2020;20(4):217–228. doi: 10.1038/s41577-019-0247-z. [DOI] [PubMed] [Google Scholar]
- 75.Jung Y, Rothenberg ME. Roles and regulation of gastrointestinal eosinophils in immunity and disease. The J Immunol. 2014;193(3):999–1005. doi: 10.4049/jimmunol.1400413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salvo-Romero E, Rodino-Janeiro BK, Albert-Bayo M, Lobo B, Santos J, Farré R, Martinez C, Vicario M. Eosinophils in the gastrointestinal tract: key contributors to neuro-immune crosstalk and potential implications in disorders of brain-gut interaction. Cells. 2022;11(10):1644. doi: 10.3390/cells11101644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan BM, Shaffer EA. Primary eosinophilic disorders of the gastrointestinal tract. Gut. 2009;58(5):721–732. doi: 10.1136/gut.2008.165894. [DOI] [PubMed] [Google Scholar]
- 78.Rojas OL, Probstel AK, Porfilio EA, Wang AA, Charabati M, Sun T, Lee DSW, Galicia G, Ramaglia V, Ward LA, et al. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell. 2019;177(2):492–493. doi: 10.1016/j.cell.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 79.Fitzpatrick Z, Frazer G, Ferro A, Clare S, Bouladoux N, Ferdinand J, Tuong ZK, Negro-Demontel ML, Kumar N, Suchanek O, et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature. 2020;587(7834):472–476. doi: 10.1038/s41586-020-2886-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galley JD, Bailey MT. Impact of stressor exposure on the interplay between commensal microbiota and host inflammation. Gut Microbes. 2014;5(3):390–396. doi: 10.4161/gmic.28683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hemmings SMJ, Malan-Muller S, Van Den Heuvel LL, Demmitt BA, Stanislawski MA, Smith DG, Bohr AD, Stamper CE, Hyde ER, Morton JT, et al. The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med. 2017;79(8):936–946. doi: 10.1097/PSY.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 83.Borre YE, O’keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20(9):509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 84.Leserman J, Drossman DA. Relationship of abuse history to functional gastrointestinal disorders and symptoms: some possible mediating mechanisms. Trauma Violence Abuse. 2007;8(3):331–343. doi: 10.1177/1524838007303240. [DOI] [PubMed] [Google Scholar]
- 85.Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21(2):149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Sci. 2021;374(6571):1087–1092. doi: 10.1126/science.abi6087. [DOI] [PubMed] [Google Scholar]
- 87.Cryan JF, O’riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TF, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 88.Xu C, Lee SK, Zhang D, Frenette PS. The gut microbiome regulates psychological-stress-induced inflammation. Immunity. 2020;53(2):417–428.e4. doi: 10.1016/j.immuni.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Welcome MO, Mastorakis NE. Stress-induced blood brain barrier disruption: molecular mechanisms and signaling pathways. Pharmacol Res. 2020;157:104769. doi: 10.1016/j.phrs.2020.104769. [DOI] [PubMed] [Google Scholar]
- 90.Vanuytsel T, Van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Salim Rasoel S, Tόth J, Holvoet L, Farré R, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63(8):1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 91.Fukudo S. Stress and visceral pain: focusing on irritable bowel syndrome. Pain. 2013;154(Suppl 1):S63–S70. doi: 10.1016/j.pain.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 92.Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26(Suppl 3):119–121. doi: 10.1111/j.1440-1746.2011.06640.x. [DOI] [PubMed] [Google Scholar]
- 93.Huang ST, Song ZJ, Liu Y, Luo WC, Yin Q, Zhang YM. BNST(AV) (GABA)-PVN(CRF) circuit regulates visceral hypersensitivity induced by maternal separation in Vgat-Cre mice. Front Pharmacol. 2021;12:615202. doi: 10.3389/fphar.2021.615202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song Y, Meng QX, Wu K, Hua R, Song ZJ, Song Y, Qin X, Cao JL, Zhang YM. Disinhibition of PVN-projecting GABAergic neurons in AV region in BNST participates in visceral hypersensitivity in rats. Psychoneuroendocrinology. 2020;117:104690. doi: 10.1016/j.psyneuen.2020.104690. [DOI] [PubMed] [Google Scholar]
- 95.El-Salhy M. Recent advances in the diagnosis of irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2015;9(9):1161–1174. doi: 10.1586/17474124.2015.1067138. [DOI] [PubMed] [Google Scholar]
- 96.Drossman DA, Morris CB, Schneck S, Hu YJ, Norton NJ, Norton WF, Weinland S, Dalton C, Leserman J, Bangdiwala SI. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol. 2009;43(6):541–550. doi: 10.1097/MCG.0b013e318189a7f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364(1):22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 98.Rawla P, Sunkara T, Raj JP. Updated review of current pharmacological and non-pharmacological management of irritable bowel syndrome. Life Sci. 2018;212:176–181. doi: 10.1016/j.lfs.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 99.Nee J, Zakari M, Lembo AJ. Novel therapies in IBS-D treatment. Curr Treat Options Gastroenterol. 2015;13(4):432–440. doi: 10.1007/s11938-015-0068-5. [DOI] [PubMed] [Google Scholar]
- 100.Ruepert L, Quartero AO, De Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Of Systematic Reviews. 2011;2011(3). CD003460. doi: 10.1002/14651858.CD003460.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ingrosso MR, Ianiro G, Nee J, Lembo AJ, Moayyedi P, Black CJ, Ford AC. Systematic review and meta-analysis: efficacy of peppermint oil in irritable bowel syndrome. Aliment Pharmacol Ther. 2022;56(6):932–941. doi: 10.1111/apt.17179. [DOI] [PubMed] [Google Scholar]
- 102.Ford AC, Lacy BE, Harris LA, Quigley EMM, Moayyedi P. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. 2019;114(1):21–39. doi: 10.1038/s41395-018-0222-5. [DOI] [PubMed] [Google Scholar]
- 103.Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD. Effect of a second-generation 2 ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut. 2007;56(9):1218–1225. doi: 10.1136/gut.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Molina-Infante J, Serra J, Fernandez-Banares F, Mearin F. The low-FODMAP diet for irritable bowel syndrome: lights and shadows. Gastroenterol Hepatol. 2016;39(2):55–65. doi: 10.1016/j.gastrohep.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 105.Mearin F, Pena E, Balboa A. Importance of diet in irritable bowel syndrome. Gastroenterol Hepatol. 2014;37(5):302–310. doi: 10.1016/j.gastrohep.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 106.Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol. 2010;25(2):252–258. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 107.Moayyedi P, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Ford AC. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109(9):1367–1374. doi: 10.1038/ajg.2014.195. [DOI] [PubMed] [Google Scholar]
- 108.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O’Neill J, Carlson P, Lamsam J, Janzow D, Eckert D, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144(5):903–911.e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: updated systematic review with meta-analysis. WJG. 2015;21(10):3072–3084. doi: 10.3748/wjg.v21.i10.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: new therapeutic strategies. World J Gastroenterol. 2016;22(7):2219–2241. doi: 10.3748/wjg.v22.i7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ford AC, Harris LA, Lacy BE, Quigley EM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044–1060. doi: 10.1111/apt.15001. [DOI] [PubMed] [Google Scholar]
- 112.Simon E, Calinoiu LF, Mitrea L, Vodnar DC. Probiotics, prebiotics, and synbiotics: implications and beneficial effects against irritable bowel syndrome. Nutrients. 2021;13(6):2112. doi: 10.3390/nu13062112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kump P, Hogenauer C. Any future for fecal microbiota transplantation as treatment strategy for inflammatory bowel diseases? Dig Dis. 2016;34(Suppl 1):74–81. doi: 10.1159/000447379. [DOI] [PubMed] [Google Scholar]
- 114.Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ. Clinical application and potential of fecal microbiota transplantation. Annu Rev Med. 2019;70(1):335–351. doi: 10.1146/annurev-med-111717-122956. [DOI] [PubMed] [Google Scholar]
- 115.Tkach S, Dorofeyev A, Kuzenko I, Sulaieva O, Falalyeyeva T, Kobyliak N. Fecal microbiota transplantation in patients with post-infectious irritable bowel syndrome: a randomized, clinical trial. Front Med. 2022;9:994911. doi: 10.3389/fmed.2022.994911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schmulson M, Bashashati M. Fecal microbiota transfer for bowel disorders: efficacy or hype? Curr Opin Pharmacol. 2018;43:72–80. doi: 10.1016/j.coph.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 117.Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20(22):6759–6773. doi: 10.3748/wjg.v20.i22.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Adriani A, Ribaldone DG, Astegiano M, Durazzo M, Saracco GM, Pellicano R. Irritable bowel syndrome: the clinical approach. Panminerva Med. 2018;60(4):213–222. doi: 10.23736/S0031-0808.18.03541-3. [DOI] [PubMed] [Google Scholar]
- 119.Wu HG, Jiang B, Zhou EH, Shi Z, Shi DR, Cui YH, Kou ST, Liu HR. Regulatory mechanism of electroacupuncture in irritable bowel syndrome: preventing MC activation and decreasing SP VIP secretion. Dig Dis Sci. 2008;53(6):1644–1651. doi: 10.1007/s10620-007-0062-4. [DOI] [PubMed] [Google Scholar]
- 120.Wu HG, Liu HR, Zhang ZA, Zhou EH, Wang XM, Jiang B, Shi Z, Zhou CL, Qi L, Ma XP. Electro-acupuncture relieves visceral sensitivity and decreases hypothalamic corticotropin-releasing hormone levels in a rat model of irritable bowel syndrome. Neurosci Lett. 2009;465(3):235–237. doi: 10.1016/j.neulet.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 121.Hirata T, Funatsu T, Keto Y, Nakata M, Sasamata M. Pharmacological profile of ramosetron, a novel therapeutic agent for IBS. Inflammopharmacology. 2007;15:5–9. [DOI] [PubMed] [Google Scholar]
- 122.Chu WC, Wu JC, Yew DT, Zhang L, Shi L, Yeung DK, Wang D, Tong RK, Chan Y, Lao L, et al. Does acupuncture therapy alter activation of neural pathway for pain perception in irritable bowel syndrome?: a comparative study of true and sham acupuncture using functional magnetic resonance imaging. J Neurogastroenterol Motil. 2012;18(3):305–316. doi: 10.5056/jnm.2012.18.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guo J, Yang L, He J, Yang Z. Comparison of therapeutic effects of different acupuncture and moxibustion therapies on irritable bowel syndrome: a protocol for systematic review and network meta-analysis. Medicine (Baltimore). 2021;100:e26920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jacobs JP, Gupta A, Bhatt RR, Brawer J, Gao K, Tillisch K, Lagishetty V, Firth R, Gudleski GD, Ellingson BM, et al. Cognitive behavioral therapy for irritable bowel syndrome induces bidirectional alterations in the brain-gut-microbiome axis associated with gastrointestinal symptom improvement. Microbiome. 2021;9(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]


