Abstract
Studies with animals and in vitro studies have demonstrated that flucytosine plus amphotericin B or fluconazole has significantly improved mycologic activity against meningitis caused by Cryptococcus neoformans compared to the activity of amphotericin B or fluconazole used alone. However, few doses have been tested in combination. This study evaluated the antifungal efficacy of amphotericin B colloidal dispersion (ABCD) combined with flucytosine with and without fluconazole in a murine model of cryptococcal meningitis. The following dosages were tested: ABCD at 0 to 12.5 mg/kg of body weight given intravenously 3 days/week, flucytosine at 0 to 110 mg/kg/day, and fluconazole at 0 to 50 mg/kg/day. Meningitis was established in male BALB/c mice by intracerebral injection of C. neoformans. Treatment with flucytosine with or without fluconazole dissolved in the sole source of drinking water was started on day 2; animals were sacrificed at 16 days, and the numbers of fungal colonies in the brain were quantified. A survival rate of 100% was achieved with ABCD plus flucytosine without fluconazole; however, the addition of fluconazole was required to prevent weight loss (P < 0.00001) and to achieve the maximum antifungal effect (P < 0.00001). The only region of dose combinations for which the 99% confidence intervals were less than 100 CFU/g of brain was defined by ABCD at 5.0 to 7.5 mg/kg combined with flucytosine at 20 to 60 mg/kg/day and fluconazole at 30 to 40 mg/kg/day. The triple combination of ABCD plus flucytosine and fluconazole was necessary to achieve the greatest antifungal activity.
The use of combinations of antifungal agents has been necessary to achieve the greatest level of clinical success when treating meningitis caused by Cryptococcus neoformans. In those clinical studies that have pitted a single antifungal agent against combination therapy, the combination regimen has resulted in high rates of success, usually in the range of 55 to 65% for patients with and without human immunodeficiency virus coinfection compared to rates of success of 35 to 40% when fluconazole or amphotericin B are used alone (3, 13–15). However, even these modest improvements in the rates of success are associated with significant drug toxicity (15, 17, 19). Nevertheless, treatment with combinations of antifungal agents appears to be necessary and offers the most immediate opportunity for significant improvement in outcome. Higher doses of fluconazole alone and in combination with flucytosine have been evaluated in clinical studies (4, 17) and in experimental models of cryptococcal meningitis (1, 8, 12). In vitro studies have evaluated amphotericin B plus flucytosine (20). However, to date, no studies have evaluated the potential for improved antifungal activity by combining these three most commonly used agents, amphotericin B, fluconazole, and flucytosine, for the treatment of cryptococcal meningitis. The present study was designed to evaluate the fungicidal activity of amphotericin B (herein given by the preparation amphotericin B colloidal dispersion [ABCD]) alone and in combination with flucytosine with or without fluconazole over a wide dose range in a murine model of cryptococcal meningitis.
MATERIALS AND METHODS
Animal protocol.
Pathogen-free BALB/c male mice (age, approximately 6 weeks; weight, 21 to 26 g) were used in all experiments. The animals were weighed individually, housed in isolation cages at four or five mice per cage, and given free access to food and water. The mice were briefly anesthetized (CO2 narcosis) and were challenged intracerebrally with approximately 500 CFU of C. neoformans 1597. The inoculum was delivered in a volume of 0.06 ml through a 27-gauge needle by direct puncture through the cranial vault approximately 6 mm posterior to the orbit. The animal protocol was approved by the University of Southern California Institutional Animal Care and Use Committee.
Chemotherapy.
The mice were randomly assigned to treatment groups 2 days after intracerebral challenge, and treatment was initiated with the assigned concentrations of flucytosine or fluconazole, or both, dissolved in the sole source of drinking water. ABCD was administered intravenously via the lateral tail vein starting on day 3 and then thrice weekly for 2 weeks. ABCD was reconstituted in sterile water and was diluted with sterile 5% dextrose on the day of administration of the concentration calculated to deliver the assigned dose in a constant volume of 10 ml/kg of body weight. The water intake by the mice in each cage was recorded daily. Water containing the treatment was replaced every 3 to 4 days; the concentrations of fluconazole and flucytosine were recalculated on the basis of the weights of the animals in each cage, measured water intake during the preceding days, and assigned drug doses. Treatment was continued for 14 days. ABCD was tested at a dosage of 0 to 7.5 mg/kg of body weight 3 days/week in combination with flucytosine at 0 to 110 mg/kg/day without fluconazole. When fluconazole (10 to 50 mg/kg/day) was added, flucytosine was tested at dosages of 20 to 110 mg/kg/day. In addition, ABCD was tested alone at 2.5 to 12.5 mg/kg. Four to five animals in one cage were treated with each dosage combination tested.
Mycologic procedures.
The C. neoformans isolate (isolate 1597) was obtained from a patient with AIDS-associated cryptococcal meningitis who responded promptly to treatment with fluconazole and flucytosine. Two days prior to use, the isolate was plated onto Sabouraud dextrose agar. Twenty-four hours prior to infection of the mice, 1 CFU was placed in brain heart infusion broth and the broth was incubated at 35°C overnight. The organisms were washed twice with normal pyrogen-free saline before suspension in saline. The concentration of the organisms injected into the mice was confirmed by making serial 10-fold dilutions of the initial suspension and by counting the numbers of CFU on plates prepared from 0.06 ml of the suspension ejected from the inoculation syringe just before and just after inoculation of the mice in each cage.
For measurement of the brain fungal burden, the animals were killed and the brains were removed, weighed, and homogenized in 1.0 ml of normal saline. Serial dilutions of the whole-brain homogenate were prepared for quantitative counts of CFU. A 0.01-ml aliquot from each dilution was plated onto Sabouraud dextrose agar, the agar plate was incubated at 35°C for 72 to 96 h, and the numbers of CFU were recorded. In addition, the remaining original whole-brain homogenate was plated on a large agar plate to assess the sample for low colony counts or sterility.
Measurements of efficacy.
Treatment activity was determined by measuring or evaluating the following endpoints: measuring the survival rate and weight change relative to the initial weight and evaluating the brain tissue mycologically. Survival was considered in two forms: first, as the duration of the survival time and, second, as the proportion of animals in each cage which were alive at the end of the 16-day experiment. The weight change for each animal was based on the weight at the time of killing relative to the initial weight recorded at the time of infection. Groups of four to five control mice were killed on day 2 and at additional time points following infection to estimate the growth curve for the numbers of CFU of C. neoformans. Animals exhibiting signs of distress were killed, and the day following the date of killing was considered the date of death. Survival times for animals alive on the scheduled day of killing were censored at that day. Any animal that died prior to the scheduled day of killing was considered a death, with survival time equal to the day of death.
Statistical analysis.
The primary objective of this experiment was to evaluate the dependence of observed measures of efficacy (survival, weight loss, and fungicidal activity) on the doses of ABCD alone and in combination with flucytosine or fluconazole, or both. Loess regression was used to estimate the dose-response surface for each measure of response (2, 6, 7). Unlike classical least-squares regression methods, loess regression estimates the response to each dose combination by using locally weighted linear or quadratic regression on the observed responses to nearby dose combinations. Thus, the patterns of association are not forced to be the same over the entire range of doses. Loess regression is nonparametric, in that it does not require the specification of an explicit equation for the association between response and doses. The loess method also does not require the assumption of normally distributed errors. Robust resistant iterative methods are used to minimize the distortion of the estimated surface by unusually large or small observations (5, 10).
The relative association of potentially explanatory variables with response was assessed by robust analysis of variance (7, 10). Pointwise 99% confidence intervals (CIs) for the estimated response were used to identify regions of dose combinations with similar levels of response for each measure of efficacy (2). For survival and weight change, dose combinations for which the lower limits of the 99% CIs were greater than selected values were identified. For fungicidal activity, dose combinations for which the upper limits of the 99% CIs were less than selected values of the numbers of CFU per gram of brain tissue were identified.
The duration of survival for untreated controls was estimated by the method of Kaplan and Meier (11). CIs for the proportion of mice surviving up to a time point were estimated by Greenwood’s (9) formula by the method described by Link (16). Analysis of survival for treated animals was based on the proportion of animals alive at the end of the 14-day treatment period, determined separately for the animals in each cage. Analysis of fungicidal activity for treated animals was based on the numbers of CFU per gram of brain tissue. Descriptive statistics were based on medians and robust 99% CIs (10). All statistical analyses were performed with S-plus statistical software (21, 22). Because of the exploratory nature of these analyses, only P values less than 0.001 were considered significant.
Since no animals were treated with combinations of ABCD plus fluconazole without flucytosine, these data cannot be used to test whether the addition of flucytosine affected the response but can be used to test whether the response was associated with the dosage of flucytosine in the range evaluated (20 to 110 mg/kg/day) in the three-drug combination.
RESULTS
Survival and growth curve of C. neoformans for control mice.
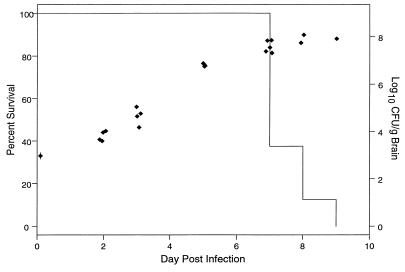
Figure 1 shows the Kaplan-Meier survival curve for control animals. Median survival was 7 days. The median inoculum for controls was 933 CFU/g of brain tissue (99% CI; 645 to 1,260 CFU/g). By day 8, the fungal burden had reached 108 CFU/g of brain tissue. ice tolerated up to 7 days of untreated infection, resulting in 108 CFU/g of brain tissue, before exhibiting signs of distress or death.
FIG. 1.
Kaplan-Meier survival (—) and numbers of CFU of C. neoformans per gram of brain tissue (⧫) by day postinfection for untreated control animals.
Survival for treated mice.
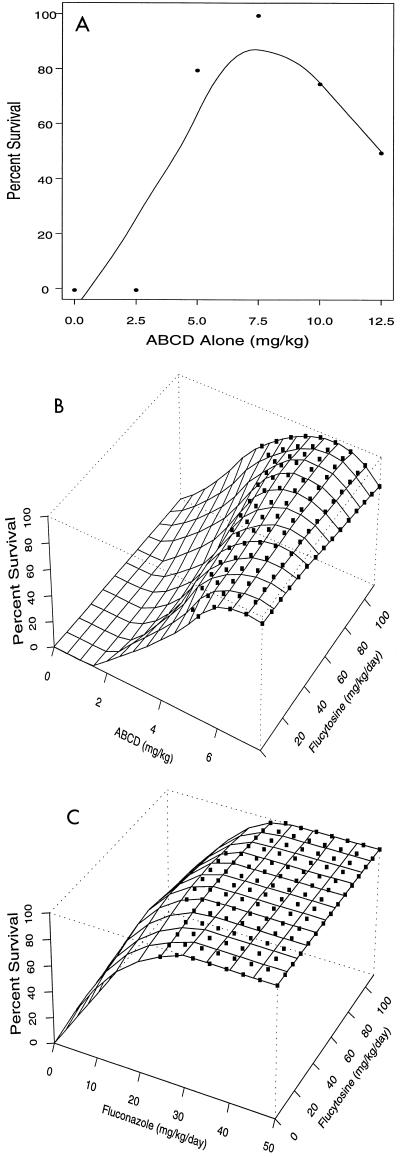
The proportion of animals treated with ABCD alone surviving to the end of treatment increased from 0% for mice receiving 2.5 mg/kg to 100% for mice receiving 7.5 mg/kg and then decreased to 50% for mice receiving 12.5 mg/kg (Fig. 2A). Two of the four animals treated with 12.5 mg of ABCD per kg alone died during injection (days 8 and 13, respectively), with >105 and 103 CFU/g being recovered from the brains, respectively.
FIG. 2.
Response surfaces showing the loess fit of the association between the proportion of animals alive at the end of treatment and dose combinations of ABCD (A), ABCD plus flucytosine (B), and fluconazole plus flucytosine (C). (A) ABCD alone. (B) ABCD plus flucytosine without fluconazole. The loess fit used a neighborhood of 75% with a local regression quadratic for ABCD and linear for flucytosine. The association between ABCD and survival was highly significant (P < 0.00001); the additional contribution of flucytosine was not significant (P = 0.42). (C) Flucytosine plus fluconazole without ABCD. The loess fit used a neighborhood of 70% with a local regression quadratic for fluconazole and linear for flucytosine. The association between fluconazole and survival was highly significant (P < 0.00001); flucytosine had no additional contribution (P = 0.6). ▪, 99% CIs contain 100% survival.
For animals treated with ABCD plus flucytosine without fluconazole, the association between survival and dose of ABCD was highly significant (P < 0.00001); the additional contribution of flucytosine was not significant (P = 0.4; Fig. 2B).
For animals treated with flucytosine plus fluconazole without ABCD, 100% survival was observed when mice received fluconazole at ≥20 mg/kg/day (P < 0.00001; Fig. 2C), regardless of the dose of flucytosine (P = 0.6). For animals treated with the three-drug combination, 100% survived to the end of treatment with all dose combinations (data not shown).
Weight change.
Untreated control animals lost 20% of their initial weight; the median weight change for treated animals ranged from −20 to +10%. ABCD alone did not protect animals from weight loss, regardless of the dose (median weight loss, 20% for mice receiving 2.5 mg/kg and 10 to 15% for mice receiving 12.5 mg/kg; Fig. 3A). The combination of ABCD plus flucytosine without fluconazole did not protect animals from significant weight loss (Fig. 3B). Animals treated with flucytosine plus fluconazole at 10 to 45 mg/kg/day without ABCD maintained their weight, regardless of the flucytosine dose (Fig. 3C). However, weight loss was seen for mice receiving fluconazole at dosages of 50 alone and >40 mg/kg/day in combination with flucytosine at >60 mg/kg/day.
FIG. 3.
Loess fit of the association between percent weight change and combinations of doses of ABCD (A), ABCD plus flucytosine (B), fluconazole plus flucytosine (C), and the three-drug combination (D). (A) ABCD alone. (B) ABCD plus flucytosine without fluconazole. The loess fit used a neighborhood of 75% with a local regression quadratic for ABCD (P = 0.002) and linear for flucytosine (P = 0.01). (C) Flucytosine plus fluconazole without ABCD. The loess fit used a neighborhood of 75% with a local regression quadratic for fluconazole (P < 0.00001) and linear for flucytosine (P = 0.38). (D) Three-drug combination with 20 to 110 mg of flucytosine per kg per day. The loess fit used a neighborhood of 50% with a local regression quadratic for fluconazole (P < 0.00001) and linear for ABCD (P < 0.00001). There was no additional association with the flucytosine dose over the range tested (P = 0.9). Lower limits of 99% CIs for weight change: >0% (○) and −2.5 to 0% (⧫).
For animals treated with the three-drug combination, weight change was strongly associated with the dosages of both ABCD and fluconazole (P < 0.00001); there was no additional association with flucytosine in the range of dosages tested (P = 0.9; Fig. 3D). The region of dosage combinations at which weight was maintained included fluconazole at ≥20 mg/kg/day combined with flucytosine without ABCD and fluconazole at ≥10 mg/kg/day combined with flucytosine plus ABCD at 5.0 or 7.5 mg/kg. Again, weight loss was seen in mice receiving the highest dosages of the three-drug combination, fluconazole at ≥40 mg/kg/day and ABCD at 7.5 mg/kg, regardless of the dose of flucytosine.
Fungicidal activity.
ABCD alone at 10 to 12.5 mg/kg reduced the numbers of CFU per gram of brain tissue from 108 for untreated controls to 105 for treated animals (Fig. 4A). The combination of ABCD at 7.5 mg/kg plus flucytosine at 110 mg/kg/day without fluconazole reduced the numbers of CFU per gram of brain from 108 to 104 (Fig. 4B; for ABCD, P < 0.00001; for flucytosine, P < 0.0001). For animals treated with fluconazole plus flucytosine without ABCD, the numbers of CFU per gram of brain tissue recovered had a strong association with the fluconazole dosage (P < 0.00001) and a moderate association with the dosage of flucytosine (P < 0.01; Fig. 4C).
FIG. 4.
Loess fit of the association between the numbers of CFU per gram of brain tissue at end of treatment and combinations of doses of ABCD (A), ABCD plus flucytosine (B), and fluconazole plus flucytosine (C). (A) ABCD alone. (B) ABCD plus flucytosine without fluconazole. The loess fit used a neighborhood of 75% with a local regression quadratic for ABCD (P < 0.00001) and linear for flucytosine (P < 0.0001). (C) Flucytosine plus fluconazole without ABCD. The loess fit used a neighborhood of 70% with a local regression quadratic for fluconazole (P < 0.00001) and linear for flucytosine (P = 0.006). Upper limit of 99% CI (CFU per gram of brain tissue): <101 (○), 101 to 102 (◊), and 102 to 103 (•).
The most potent fungicidal effect was seen in the group of animals receiving the three-drug combination. The numbers of CFU recovered per gram of brain tissue had a strong association with the dosages of both ABCD and fluconazole (P < 0.00001) and a moderate association with the dosage of flucytosine (P < 0.01). The loess fit of the association between the numbers of CFU per gram of brain tissue and the dosages of the three-drug combinations are displayed as slices from the four-dimensional surface, which are dose-response curves for one drug at selected dosages of the other two drugs (Fig. 5). The effect of ABCD at each dosage of fluconazole was to decrease the overall level of the dose-response curve for flucytosine as the dose of ABCD increased. However, this reduction in the numbers of CFU per gram of brain tissue appeared primarily in the range of 0 to 5 mg of ABCD per kg, with little additional reduction for ABCD at 7.5 mg/kg (Fig. 5, two right panels in each row). As the fluconazole dosage increased, the level of the flucytosine dose-response curve decreased and the slope of the curve changed from negative to positive, so that at fluconazole dosages of ≥20 mg/kg/day, the numbers of CFU per gram of brain tissue increased slightly with increasing dosages of flucytosine. This interaction between fluconazole and flucytosine was seen at all doses of ABCD, but it was somewhat more pronounced at ABCD doses of ≥5.0 mg/kg (Fig. 5, two right panels in each row). The upper limits of the 99% CIs were not below 100 CFU/g until both ABCD was used at ≥5.0 mg/kg and fluconazole was used at ≥30 mg/kg/day (Figure 5; two right panels in two top rows).
FIG. 5.
Flucytosine dose-response curves for selected levels of ABCD (increasing from 0 mg/kg on the left to 7.5 mg/kg on the right) and fluconazole (increasing from 0 mg/kg/day in the bottom row to 40 mg/kg/day in the top row). The solid black curves represent the loess estimate of the dose-response curve; the dots show the upper and lower limits of the pointwise 99% CIs for the estimated response. The gray horizontal reference lines are 102 CFU/g.
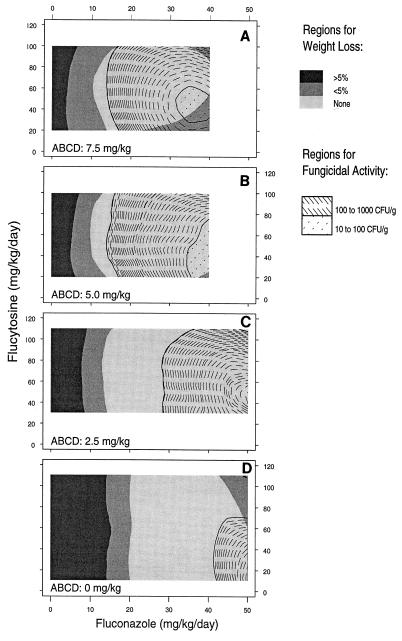
The maximum fungicidal effect of the three-drug combination was seen at dosages of fluconazole of ≥30 mg/kg/day, flucytosine in the dosage range of 20 to 60 mg/kg/day, and ABCD at doses of 5.0 and 7.5 mg/kg, with the exception that the numbers of CFU per gram of brain tissue increased slightly at the highest dosages of the three drugs. Figure 6 shows the regions of dose combinations with similar fungicidal activities superimposed on the regions of the dose combinations resulting in similar weight loss in the animals treated with the three-drug combination. The region of dose combinations of fluconazole and flucytosine with the greatest fungicidal activity and no weight loss were similar when they were used in combination with ABCD at both 5.0 and 7.5 mg/kg (Fig. 6A and B, respectively). The three-drug combination with ABCD at 2.5 mg/kg and the drug combination without ABCD (Fig. 6C and D, respectively) had similar regions where weight was maintained; however, the level of fungicidal activity achieved at the two higher doses of ABCD was not achieved with ABCD at 2.5 mg/kg or in its absence.
FIG. 6.
Regions with the most effective dose combinations. For each dose of ABCD tested, the regions of combinations of fluconazole and flucytosine dosages with similar fungicidal activities are superimposed on the regions where similar weight loss was found. The size and locations of the regions for weight loss (indicated by gray shading) were similar for all doses of ABCD tested in the three-drug combination, with the exception of the slight increase in weight loss for ABCD at 7.5 mg/kg with higher doses of fluconazole in combination with low dosages of flucytosine (A). In contrast, the regions with fungicidal activity in the range 100 to 1,000 CFU per gram of brain tissue showed a strong shift toward the inclusion of lower doses of fluconazole as the dose of ABCD in the three-drug combination increased, from 0 mg/kg (D) to 7.5 mg/kg (A). However, the maximum fungicidal effect (less than 100 CFU/g of brain tissue) was achieved only for the three-drug combination with ABCD 5.0 to 7.5 mg/kg (as indicated by the presence of regions with dotted lines in panels A and B and their absence in panels C and D).
DISCUSSION
We studied the fungicidal activity of ABCD alone and in combination with flucytosine or fluconazole, or both, in a murine model of cryptococcal meningitis. A 100% survival rate was achieved with ABCD plus flucytosine without fluconazole; however, in the absence of fluconazole, neither ABCD nor flucytosine, alone or in combination, prevented weight loss. ABCD alone at 10 to 12.5 mg/kg decreased the numbers of CFU per gram of brain tissue from 108 to 105 but proved to have lethal toxicity for some animals. When ABCD was tested in combination with flucytosine without fluconazole, the numbers of CFU recovered per gram of brain tissue were 104 for the highest dose combination (ABCD at 7.5 mg/kg plus flucytosine at 110 mg/kg/day). In contrast, when fluconazole was added, the numbers of CFU recovered per gram of brain tissue were between 10 and 100 for animals treated with ABCD at 5 or 7.5 mg/kg combined with fluconazole at 30 to 40 mg/kg/day and flucytosine at 20 to 60 mg/kg/day. For one animal treated with ABCD at 7.5 mg/kg, fluconazole at 40 mg/kg/day, and flucytosine at 40 mg/kg/day, cultures of whole-brain samples were sterile.
In our laboratory, murine models of cryptococcal meningitis consistently demonstrate that flucytosine has increased antifungal activity when it is combined with fluconazole (8, 12). This dramatic effect of fluconazole on the fungicidal activity of flucytosine, which has also been demonstrated in in vitro studies (18), has now been demonstrated for fluconazole in combination with amphotericin B. In these models, we took advantage of the availability of new statistical methods for estimating and visualizing the dose-response surfaces for survival, weight loss, and fungicidal activity (2, 6, 7, 21, 22). By evaluating these easily determined endpoints over a wide range of dose combinations, we were able to identify a range of dosages of fluconazole and flucytosine that, in combination with ABCD, define a region of doses which has promising fungicidal activity, which has a 100% survival rate, and where weight is maintained. The reproducibility of the results obtained by these methods is demonstrated by comparing the regions of fluconazole and flucytosine dosages with the greatest fungicidal activity obtained in this experiment with the regions obtained in previous experiments (8).
The identification of a region of dose combinations with the most promising therapeutic effect provides more useful information than statistical summaries based on statistically significant differences between specific dose combinations and controls or estimation of specific dose combinations, e.g., the 50% lethal dose, for guiding investigators in designing subsequent experiments and clinical trials (2). Furthermore, these regions can be used to evaluate the effect of systematic changes in experimental conditions such as severity of meningitis, host immune status, and the possible need to alter therapies based on known clinical conditions that affect the rates of clinical success.
It is widely recognized that animal models are not likely to provide the precise doses which will produce the optimum therapeutic effect in the clinic. However, information which is essential for the design of clinical trials can be determined from the location and size of the region of dose combinations which provide the best response in the animal model. For example, in the present model, the region of dose combinations with the most promising therapeutic effect is defined by higher doses of fluconazole combined with low to moderate doses of flucytosine and ABCD rather than the combination of all three drugs at their respective maximum tolerated doses.
ACKNOWLEDGMENTS
This work was supported in part by SEQUUS Pharmaceuticals, Inc., grants from the National Institute of Allergy and Infectious Diseases (N01-AI-15082) and the State of California (California Collaborative Treatment Group), contributions from Robert B. Henry, Jr., and Edwin G. Snyder, and an anonymous grant in honor of the family of Richard Pitts.
We thank Hoi Pham for expertise in database management and Lucia Noll for expert technical editing.
REFERENCES
- 1.Allendoerfer R, Marquis A J, Rinaldi M G, Graybill J R. Combined therapy with fluconazole and flucytosine in murine cryptococcal meningitis. Antimicrob Agents Chemother. 1991;35:726–729. doi: 10.1128/aac.35.4.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer M, Thomas A M. Loess regression in evaluation of dose response surfaces. Biom Bull. 1996;13(4):10. [Google Scholar]
- 3.Bennett J E, Dismukes W E, Duma R J, Medoff G, Sande M A, Gallis H, Leonard J L, Fields B T, Bradshaw M, Haywood H, McGee Z A, Cate T R, Cobbs C G, Warner J F, Alling D W. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N Engl J Med. 1979;301:126–131. doi: 10.1056/NEJM197907193010303. [DOI] [PubMed] [Google Scholar]
- 4.Berry A J, Rinaldi M G, Graybill J R. Use of high-dose fluconazole as salvage therapy for cryptococcal meningitis in patients with AIDS. Antimicrob Agents Chemother. 1992;36:690–692. doi: 10.1128/aac.36.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Box G E P, Draper N R. Empirical model-building and response surfaces. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 6.Cleveland W S. Visualizing data. Summit, N.J: Hobart; 1993. [Google Scholar]
- 7.Cleveland W S, Grosse E, Shyu W M. Local regression models. In: Chambers J M, Hastie T, editors. Statistical models in S. New York, N.Y: Chapman & Hall; 1991. pp. 309–376. [Google Scholar]
- 8.Ding J C, Bauer M, Diamond D M, Leal M E, Johnson D, Thomas A M, Navjar L, Graybill J R, Larsen R A. Effects of delayed treatment on the fungicidal activity of flucytosine combined with fluconazole in murine cryptococcal meningitis. Antimicrob Agents Chemother. 1997;41:1589–1593. doi: 10.1128/aac.41.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwood M. Reports of public health and medical subjects. Vol. 33. London, United Kingdom: Her Majesty’s Stationery Office; 1926. The natural duration of cancer. [Google Scholar]
- 10.Hoaglin D C, Mosteller F, Tukey J W. Understanding robust and exploratory data analysis. New York, N.Y: John Wiley & Sons, Inc.; 1983. [Google Scholar]
- 11.Kaplan G, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 12.Larsen R A, Bauer M, Weiner J M, Diamond D M, Leal M E, Ding J C, Rinaldi M G, Graybill J R. Effect of fluconazole on fungicidal activity of flucytosine in murine cryptococcal meningitis. Antimicrob Agents Chemother. 1996;40:2178–2182. doi: 10.1128/aac.40.9.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen R A, Bozzette S A, Jones B E, Haghighat D, Leal M A, Forthal D, Bauer M, Tilles J G, McCutchan J A, Leedom J M. Fluconazole combined with flucytosine for the treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1994;19:741–745. doi: 10.1093/clinids/19.4.741. [DOI] [PubMed] [Google Scholar]
- 14.Larsen R A, Leal M E. Flucytosine use in patients with AIDS. Ann Intern Med. 1990;113:992. doi: 10.7326/0003-4819-113-3-183. [DOI] [PubMed] [Google Scholar]
- 15.Larsen R A, Leal M E, Chan L S. Fluconazole compared with amphotericin B plus flucytosine for the treatment of cryptococcal meningitis: a prospective randomized trial in patients with AIDS. Ann Intern Med. 1990;113:183–187. doi: 10.7326/0003-4819-113-3-183. [DOI] [PubMed] [Google Scholar]
- 16.Link C L. Confidence intervals for the survival function using Cox’s proportional hazards model with covariates. Biometrics. 1984;40:601–610. [PubMed] [Google Scholar]
- 17.Milefchik E, Leal M, Haubrich R, Haghighat D, Bozzette S, Larsen R. Program and abstracts of the 9th International Conference on AIDS. 1993. High dose fluconazole with and without flucytosine for cryptococcal meningitis in persons with AIDS. [Google Scholar]
- 18.Nguyen M H, Barchiesi F, McGough D A, Yu V L, Rinaldi M G. In vitro evaluation of combination of fluconazole and flucytosine against Cryptococcus neoformans var. neoformans. Antimicrob Agents Chemother. 1995;39:1691–1695. doi: 10.1128/aac.39.8.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saag M S, Powderly W G, Cloud G A, Robinson P, Grieco M H, Sharkey P K. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. N Engl J Med. 1992;326:83–89. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- 20.Shadomy S, Wagner G, Espinel-Ingroff A, Davis B. In vitro studies with combinations of 5-fluorocytosine and amphotericin B. Antimicrob Agents Chemother. 1975;28:117–121. doi: 10.1128/aac.8.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Statistical Sciences, Inc. S-plus guide to statistical and mathematical analysis, version 3.3 for Windows. Seattle, Wash: MathSoft, Inc.; 1995. [Google Scholar]
- 22.Venables W N, Ripley B D. Modern applied statistics with S-plus. 2nd ed. New York, N.Y: Springer-Verlag, Inc.; 1997. [Google Scholar]