Abstract
The judicious use of perioperative antibiotic prophylaxis reduces the infectious complications of surgery. However, increased bacterial resistance within hospitals may make antibiotic prophylaxis less effective in the future and alternative strategies are needed. New immunomodulatory agents might prevent wound infections by stimulation of the host immune system. To test this hypothesis, we administered poly-[1-6]-β-d-glucopyranosyl-[1-3]-β-d-glucopyranose glucan (PGG glucan), which enhances neutrophil microbicidal activity, intravenously to guinea pigs in doses ranging from 0.015 to 4 mg/kg of body weight on the day before, on the day of, and on the day after intermuscular inoculation with methicillin-resistant strains of Staphylococcus aureus and Staphylococcus epidermidis. Abscesses were identified at 72 h, and median infective doses (ID50) and statistical significance were determined by logistic regression. Guinea pigs receiving PGG glucan and inoculated with methicillin-resistant S. aureus and S. epidermidis exhibited ID50 of as much as 2.5- and 60-fold higher, respectively, than those of control guinea pigs not receiving PGG glucan. Maximal protection was observed with a dose of 1 mg of PGG glucan per kg, and efficacy was reduced at higher as well as at lower PGG glucan doses. Furthermore, a single dose of PGG glucan given 24 h following bacterial inoculation was found to be effective in preventing infection. We conclude that PGG glucan reduces the risk of staphylococcal abscess formation. Neutrophil-activating agents are a novel means of prophylaxis against surgical infection and may be less likely than antibiotics to be affected adversely by the increasing antibiotic resistance of nosocomial pathogens.
Perioperative antibiotic prophylaxis has played an important role in the development of modern surgery by reducing the risk of postoperative infection (17, 24). In general, the prophylactic antibiotic regimen is selected to provide activity against the pathogen(s) most likely to be associated with the surgical procedure. The increasing prevalence of antibiotic-resistant organisms, including methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus epidermidis (MRSE), vancomycin-resistant enterococci, and extended-spectrum β-lactamase-producing gram-negative bacilli (11, 25, 27) threatens to reduce the efficacy of commonly used prophylactic agents such as the cephalosporins and vancomycin. A potential scenario for the future is higher surgical infection rates and reduced confidence in our ability to adequately treat the infections which develop. Alternative strategies for reducing the risk of perioperative infection are needed.
During the past decade, much has been learned about the immune response to bacterial pathogens. A variety of compounds with immunomodulating properties have been identified, some of which are immunosuppressive and others of which are immunostimulatory (9). Poly-[1-6]-β-d-glucopyranosyl-[1-3]-β-d-glucopyranose (PGG) glucan is a complex carbohydrate derived from Saccharomyces cerevisiae by a purification procedure that recovers the β-glucan polymer in a soluble form (12). It is synergistic with growth factors in stimulating hematopoietic proliferation and enhances neutrophil mobilization and migration as well as primes them to exhibit greater phagocytosis and a stronger oxidative burst in response to subsequent stimulation (4, 28). Overall, neutrophil microbicidal activity is increased by two- to threefold. These changes occur without an apparent direct effect on cytokine or eicosanoid production (4, 10, 26).
To test the hypothesis that PGG glucan might diminish the risk of staphylococcal infection, we determined its ability to prevent abscess formation in a small-inoculum guinea pig model.
MATERIALS AND METHODS
Source and in vitro characterization of S. aureus strains.
S. aureus 5030 and S. epidermidis 9021 were recovered from sternal wound infections complicating cardiac surgery. Susceptibilities to methicillin and PGG glucan (Betafectin; Alpha-Beta Technology, Inc., Worcester, Mass.) were determined by microdilution techniques (22) in Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.).
In vivo procedures.
Details of this small-inoculum prophylaxis model have been described previously (13–16). All in vivo experiments were approved by the Institutional Committee for Animal Care at the Nashville Veterans Affairs Medical Center. Guinea pigs were allowed to acclimate to the institution’s animal care facilities for at least 24 h prior to the initiation of the experimental protocol.
S. aureus strains were maintained at −70°C in tryptic soy broth (Difco) containing 10% glycerol. Bacterial colonies were harvested after overnight growth on tryptic soy agar (Difco) and suspended in phosphate-buffered saline (pH 6.0) to achieve a standard turbidity. Serial 10- and 2-fold dilutions were performed to prepare a range of inocula that produced abscesses from 0 to 100% of the time (ranges of 0.1 to 52 CFU for strain 5030 and 0.6 to 260 CFU for strain 9021). Each dilution was mixed in a 1:1 (vol/vol) ratio with sterile dextran microbeads (Cytodex; Sigma Chemical Co., St. Louis, Mo.). Back counts were performed in triplicate and averaged to determine the precise size of the bacterial inoculum.
On the day of in vivo experimentation, the dorsal hair was removed from albino Hartley guinea pigs (500 ± 50 g) of either sex (Harlan Sprague-Dawley, Indianapolis, Ind.), and a grid designating 12 sites was drawn. The potential space between fascia surrounding skin-related and trunk muscle groups underlying each site was inoculated with 0.2 ml of one of the bacterium-bead suspensions. After inoculation, the guinea pigs were returned to their cages. Three days later, the guinea pigs were sacrificed and the new-growth dorsal hair was removed by depilation. By using a biopsy punch and sterile technique, the microbeads, with or without adherent abscess material, were removed from each of the 12 sites and inoculated onto blood agar plates. The plates were incubated at 37°C for 24 h, and the presence or absence of bacterial growth was recorded.
Administration of PGG glucan.
PGG glucan (Betafectin) was provided by Alpha-Beta Technology, Inc., in vials containing a 1-mg/ml solution, and the vials were maintained at 22°C until use. On the day prior to bacterial inoculation, each guinea pig was anesthetized with 40 mg of ketamine and 5 mg of xylazine per kg of body weight given intraperitoneally. The skin of the right neck was shaved, and a chlorhexidine solution (Western Medical Corp., Chicago, Ill.) was applied topically for antisepsis. A small incision was made, and the internal jugular vein was cannulated with a saline-filled polyethylene catheter (PE-50; Becton Dickinson Co., Sparks, Md.). An initial PGG glucan dose ranging from 0.015 to 4 mg/kg or a placebo (saline) was given to each guinea pig for 2 min via the jugular venous catheter. The catheter was flushed with 0.5 ml of saline followed by 0.2 ml of heparinized saline (500 U/ml; United Laboratories, Inc., Bensalem, Pa.). The distal end of the jugular cannula was tunneled through the subcutaneous tissue to exit the skin of the dorsal neck. The end of the protruding 3-cm portion of the catheter was clamped to prevent blood loss and was covered with gauze. After placement of the catheter, each guinea pig was housed separately. At the time of and on the day following bacterial inoculation, second and third doses, respectively, of PGG glucan or placebo were administered via the protruding distal end of the jugular catheter. Each guinea pig received the same PGG glucan dose that it had received initially, and 5 to 10 mg of sodium pentobarbital per kg was used for sedation. Control guinea pigs underwent all of the same surgical and anesthetic manipulations as the animals which received PGG glucan. The PGG glucan (Betafectin) lot was no. 2610034 for all experiments, excepting involving guinea pigs given a single PGG glucan dose 24 h following bacterial inoculation, which received lot no. 2610054.
Determination of PGG glucan levels in plasma.
During separate in vivo investigations, the pharmacokinetics of a single dose of 2 mg of PGG glucan per kg were determined. Jugular intravenous catheters were placed as described above. Blood samples of 0.5 ml each were obtained with syringes anticoagulated with heparin and 25-gauge needles via intracardiac puncture at times 15, 30, 60, and 90 min and 2, 3, 4, 6, and 8 h after intravenous administration of PGG glucan. After each sample was obtained, an equivalent volume of saline was administered subcutaneously to replace the volume loss. Intraperitoneal doses of sodium pentobarbitol (5 to 10 mg/kg) were given as needed to maintain sedation throughout the 8-h sampling interval after the effect of the ketamine-xylazine given at the time of jugular catheter placement had worn off. After the final blood sample from a guinea pig was collected, the animal was euthanized with a lethal intraperitoneal dose of sodium pentobarbital. Plasma was harvested from each blood specimen by centrifugation and was stored at −70°C until shipping to Alpha-Beta Technology, Inc. PGG glucan levels were determined by a pyrochrome assay based on the ability of PGG glucan to stimulate the activation of the horseshoe crab proteolytic coagulation cascade (Limulus amebocyte assay) in a concentration-dependent manner (21).
Statistical analysis.
Using binary logistic regression, an inoculum-response (dose-response) curve was calculated from the percentage of growth at various bacterial inoculum levels, and the differences between the inoculum-response data for the placebo versus each dosing level of PGG glucan for the treated animals were determined (19). The median infective dose (ID50) was determined by the equation exp(−intercept/slope of log of back count). This analysis was performed for each staphylococcal isolate-PGG glucan dose combination. All analyses were done with JMP, version 3.1.6, SAS Institute, Cary, N.C.
RESULTS
In vitro characteristics of the staphylococcal strains.
The MICs of methicillin for S. epidermidis 9021 and S. aureus 5030 were 16 and 32 μg/ml, respectively. As determined by broth microdilution techniques, neither isolate was inhibited in vitro by PGG glucan in concentrations of as much as 500 μg/ml.
PGG glucan levels.
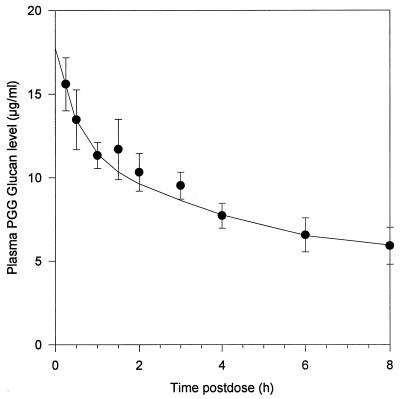
A single dose of 2 mg of PGG glucan per kg yielded a mean peak level of 16 μg/ml in plasma at 15 min postintravenous injection (Fig. 1). The terminal elimination half-life was 6.9 h.
FIG. 1.
Concentrations of PGG glucan in plasma in guinea pigs. A dose of 2 mg/kg was administered intravenously. The mean values and standard deviations of concentrations in plasma from 10 guinea pigs at each time interval are displayed.
Dose-ranging studies.
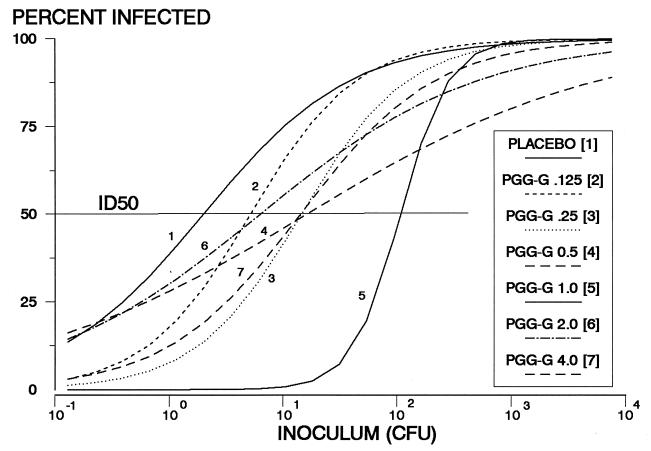
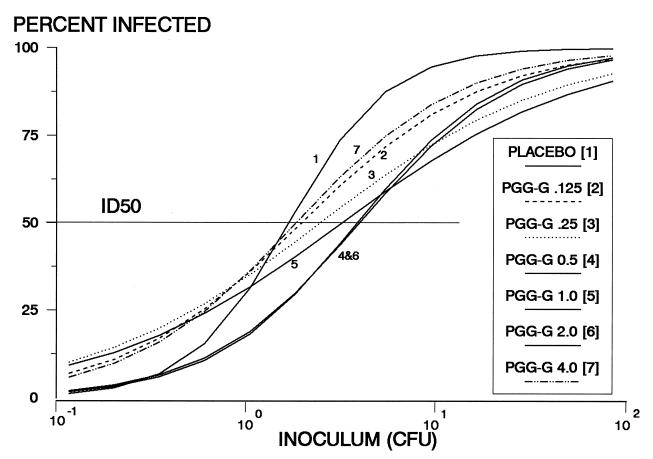
To determine whether PGG glucan reduces the risk of staphylococcal abscess formation and to determine the dose of PGG glucan with optimal efficacy, doses of PGG glucan ranging in twofold increments from 0.015 to 4 mg/kg were administered daily for 3 days to guinea pigs inoculated with S. epidermidis 9021. A benefit of PGG glucan prophylaxis was observed over almost the entire dose range (Fig. 2; Table 1). The greatest efficacy was associated with PGG glucan doses of 1 mg/kg and corresponded to a 60-fold increase in ID50 compared to that observed for control guinea pigs. Similar dose-response determinations with MRSA 5030 and doses ranging from 0.125 to 4 mg of PGG glucan per kg showed a maximal 2.5-fold increase in the ID50 with PGG glucan prophylaxis (Fig. 3; Table 1).
FIG. 2.
Semilogarithmic graphs of infection rate versus inoculum size (CFU) for animals inoculated with S. epidermidis 9021. Results of prophylaxis with doses between 0.125 and 4 mg of PGG glucan per kg are shown. An inoculum of 10−1 CFU indicates that on average only 1 guinea pig of 10 would be inoculated with bacteria. Each curve was constructed with the results of 100 to 150 lesions inoculated with S. epidermidis 9021 in guinea pigs receiving the indicated dose of PGG glucan or placebo. The ID50s (Table 1) were determined from the regression equation and correspond with the points on the x axis at which each curve intersects a horizontal line extending from the 50% infected mark on the y axis.
TABLE 1.
ID50s for staphylococcal strains in guinea pigs receiving different amounts of PGG glucana
| Dosing regimen of PGG glucan (mg/kg) | No. of CFU for the following strainsb
|
|
|---|---|---|
| 9021 (MRSE) | 5030 (MRSA) | |
| Placebo | 1.8 | 1.5 |
| 0.015 | 6.4 (<0.05) | ND |
| 0.03 | 8.2 (<0.01) | ND |
| 0.06 | 17.3 (<0.001) | ND |
| 0.125 | 4.8 (0.10) | 1.9 (0.45) |
| 0.25 | 14.1 (<0.01) | 2.4 (0.12) |
| 0.5 | 15.8 (<0.001) | 3.9 (<0.0005) |
| 1.0 | 115.1 (<0.0001) | 3.5 (<0.006) |
| 2.0 | 5.9 (<0.02) | 3.7 (<0.002) |
| 4.0 | 13.8 (<0.01) | 1.8 (0.45) |
ID50s were calculated from the results of the logistic regression equation. Three doses were given (the first 24 h prior to bacterial inoculation, the second on the day of inoculation, and the third at 24 h following inoculation).
Values in parentheses indicate the significance of the difference in the logistic regression analysis compared to placebo results with the same strain. ND, not determined.
FIG. 3.
Semilogarithmic graphs of infection rate versus inoculum size (CFU) for animals inoculated with S. aureus 5030. Results of prophylaxis with doses between 0.125 and 4 mg of PGG glucan per kg are shown. An inoculum of 10−1 CFU indicates that on average only 1 guinea pig of 10 would be inoculated with bacteria. Each curve was constructed with the results of 100 to 150 lesions inoculated with S. aureus 5030 in guinea pigs receiving the indicated doses of PGG glucan or placebo. ID50s are reported in Table 1.
Relationship of the timing of PGG glucan administration to efficacy.
Since the animals in the dose-ranging studies all received a three-dose regimen, we then determined whether all of the doses were necessary for activity by comparing the ID50s observed with the three-dose regimen (administered at −24, 0, and +24 h relative to the time of bacterial inoculation) to those found with two doses (−24 and 0 h) and with a single dose (−24 h). Although the single- and two-dose regimens reduced the risk of infection compared to the placebo, there was a clear benefit to receiving all three doses among the animals getting 1 or 2 mg of PGG glucan per kg (Table 2). Since these results suggested that PGG glucan given 1 day following bacterial inoculation could still reduce the risk of infection, an additional experiment in which a single 24-h postinoculation dose of PGG glucan was compared to placebo was done. ID50s for guinea pigs receiving the various regimens were 2.8 CFU for placebo and 25.4, 39.7, and 32.9 CFU for the 0.5-, 1-, and 2-mg/kg doses, respectively (P < 0.0001 for each PGG glucan dose versus placebo).
TABLE 2.
ID50s for MRSE 9021 in guinea pigs receiving single versus multiple doses of PGG glucan
| Dose of PGG glucan (mg/kg) | No. of CFU for the following dosing regimensa:
|
||
|---|---|---|---|
| −24 h | −24 and 0 h | −24, 0, and +24 h | |
| Placebo | ND | ND | 2.2b |
| 0.5 | 9.2 | 14.4 | 7.9 |
| 1.0 | 18.1c | 14.5c | 58.6c |
| 2.0 | 9.5d | 13.1d | 26.1d |
One-dose versus two-dose versus three-dose regimens are compared. The times at which doses were given relative to the time of bacterial inoculation are indicated. ND, not determined.
P < 0.0001 versus placebo for all regimens.
P < 0.0001, three doses versus two doses; P < 0.0001, three doses versus one dose.
P = 0.091, three doses versus two doses; P = 0.02, three doses versus one dose.
DISCUSSION
Clinical and animal studies suggest that in order for surgical prophylaxis to be effective, the inoculated bacteria must be susceptible to the prophylactic antibiotic (17, 24). The development and spread of antibiotic resistance among important nosocomial pathogens in recent years along with the potential for even more serious resistance patterns to emerge, e.g., vancomycin resistance in staphylococci, have important implications for the prevention of surgical infection and the effective use of antibiotic prophylaxis.
Another potential way of reducing the number of viable bacteria at the surgical site is by enhancing host defenses to facilitate bacterial killing. In this study, we have shown that the administration of an immunostimulatory carbohydrate, PGG glucan, reduces the susceptibility of guinea pigs to infection following the inoculation of virulent strains of S. aureus and S. epidermidis. Since PGG glucan has no direct antimicrobial effect upon staphylococci in vitro, the beneficial effect of PGG glucan appears to be due to an augmentation of host immune function.
The greatest benefit of PGG glucan, i.e., a 60-fold increase in ID50 compared to prophylaxis with placebo, was observed with a dose of 1 mg/kg given on the day before, on the day of, and on the day after inoculation with a strain of MRSE. The maximal increase in ID50s with the MRSA strain was only 2.5-fold. In both cases, the protection against infection observed with PGG glucan prophylaxis was much less than that reported previously with antimicrobial prophylaxis against antibiotic-susceptible strains of bacteria with the same animal model (13–16). For example, we have reported ID50s for MRSE 9021 and MRSA 8030 with vancomycin of >4,000,000 and 62,520 CFU, respectively (16). However, administration of an immune-enhancing agent might be effective against antibiotic-resistant strains and/or synergistic with antibiotic prophylaxis.
The dose-response effects reported in this investigation are remarkable in that the in vivo efficacy of PGG glucan peaked at about 1 mg/kg and was diminished with higher doses as well as with lower doses (Fig. 2 and 3) (Table 1). Similarly, dose-ranging experiments involving PGG glucan in a murine model of Escherichia coli-induced peritonitis found maximal protection at a dose of approximately 0.5 mg of PGG glucan per kg and found that doses higher and less than this amount were less effective (23). The biological reasons underlying the dose-response effect are unknown. However, it has also been shown with another immunomodulator, granulocyte colony-stimulating factor, in an animal model of infection that higher doses are less effective than doses within a certain therapeutic window (18).
The finding that PGG glucan administered 1 day after bacterial inoculation was still effective in reducing the risk of infection is intriguing. Animal studies and clinical trials in humans have shown that antibiotic prophylaxis in surgery is most effective when the agent is administered immediately before or at the time of the surgical procedure (5, 7, 20). Furthermore, since others have found that it takes several hours following PGG glucan administration for neutrophils to reach maximal chemiluminescence and microbicidal activity (3), our expectation was that any benefit of PGG glucan would be attributable primarily to the doses given on the day before and on the day of bacterial inoculation. The efficacy of PGG glucan when given 1 day postinoculation has mechanistic and potentially therapeutic implications. This suggests that the effect is not mediated solely by enhancing the early killing of bacteria at the time immediately after inoculation and that interventions initiated well after the time of surgery can reduce the risk of infection.
PGG glucan has been shown to confer protection in other models of surgical infection. The administration of PGG glucan 4 to 24 h before intraperitoneal inoculation of E. coli or S. aureus in rats reduces quantitative septicemia and mortality (23). It appears to act synergistically with antibiotics, with the combination providing enhanced protection against lethal challenge with E. coli or S. aureus compared with the use of antibiotics alone (29). Recent evidence suggests that the protective effect against lethal infection can be passively transferred from rats treated with PGG glucan to other rats by spleen cells, spleen cell lysates, peripheral blood leukocytes, or serum (6). The present study differs from the earlier animal work in that this model more closely imitates clean surgical procedures in which the bacterial inoculum is generally small and the end point is bimodal (i.e., either infected or not infected). Accordingly, whereas the previous studies demonstrate that PGG glucan can reduce the severity of infection or the mortality associated with infection, these results are unique in that they show that PGG glucan actually reduces the risk of developing infection.
PGG glucan and other 1→3 β-glucans have been evaluated with high-risk surgical and trauma patients (1, 2, 8). In trials involving small numbers of patients, it appears to be well-tolerated. In addition, fewer infections have occurred among treated patients than controls, although the difference is attributable primarily to infection at sites other than the surgical wound (i.e., pneumonia, sepsis, or urinary tract infections). The value of PGG glucan in the surgical setting and particularly in the prevention of infections at the wound site will need to be ascertained by using large controlled clinical trials, which are currently in progress.
In summary, PGG glucan reduces the risk of wound infection in guinea pigs inoculated with staphylococci. Neutrophil-activating agents are a novel means of prophylaxis against surgical infection and may be less likely than antibiotics to be affected adversely by the increasing antibiotic resistance of nosocomial pathogens.
ACKNOWLEDGMENTS
This work was supported by grant NIH AI32126 and a grant from Alpha-Beta Technology, Inc.
We thank Trisha Deegan for instructing us on the methods on internal jugular vein cannulation in guinea pigs. PGG glucan levels were determined at Alpha-Beta Technology, Inc.
REFERENCES
- 1.Babineau T J, Marcello P, Swails W, Kenler A, Bistrian B, Forse R A. Randomized phase I/II trial of a macrophage-specific immunomodulator (PGG-glucan) in high-risk surgical patients. Ann Surg. 1994;220:601–609. doi: 10.1097/00000658-199411000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babineau T J, Hackford A, Kenler A, Bistrian B, Forse R A, Fairchild P G, Heard S, Keroack M, Caushaj P, Benotti P. A phase II multicenter, double-blind, randomized, placebo-controlled study of three dosages of an immunomodulator (PGG-glucan) in high-risk surgical patients. Arch Surg. 1994;129:1204–1210. doi: 10.1001/archsurg.1994.01420350102014. [DOI] [PubMed] [Google Scholar]
- 3.Bleicher P, Mackin W. Betafectin® PGG-glucan: a novel carbohydrate immunomodulator with anti-infective properties. J Biotechnology Healthcare Res Reg. 1995;2:207–222. [Google Scholar]
- 4.Brunke-Reese D, Gu Y, Crotty K, Fisette L, Mackin W M. Enhanced microbicidal activities of human peripheral blood monocytes and neutrophiles (PMN) after pre-treatment with PGG-glucan (Betafectin™) FASEB J. 1994;8:488. [Google Scholar]
- 5.Burke J F. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–168. [PubMed] [Google Scholar]
- 6.Cisneros R L, Gibson F C, Tzianabos A O. Passive transfer of poly-(1-6)-beta-glucotriosyl-(1-3)-beta-glucopyranose glucan protection against lethal infection in an animal model of intra-abdominal sepsis. Infect Immun. 1996;64:2201–2205. doi: 10.1128/iai.64.6.2201-2205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Classen D C, Evans R S, Pestotnik S L, Horn S D, Menlove R L, Burke J P. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281–286. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- 8.deFelippe J J, daRocha-Silva M, Maciel F M, Soares A de M, Mendes N F. Infection prevention in patients with severe multiple trauma with the immunomodulator beta 1-3 polyglucose (glucan) Surg Gynecol Obstet. 1993;177:383–388. [PubMed] [Google Scholar]
- 9.Frank M O, Mandell G L. Immunomodulators. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 450–458. [Google Scholar]
- 10.Gibson F C, III, Tzianabos A O, Onderdonk A B. The capsular polysaccharide complex of Bacteroides fragilis induces cytokine production from human and murine phagocytic cells. Infect Immun. 1996;64:1065–1069. doi: 10.1128/iai.64.3.1065-1069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold H S, Moellering R C. Antimicrobial-drug resistance. N Engl J Med. 1996;335:1445–1453. doi: 10.1056/NEJM199611073351907. [DOI] [PubMed] [Google Scholar]
- 12.Jamas, S., D. D. Easson, Jr., and G. Ostroff. June 1994. U.S. patent 5,322,841.
- 13.Kaiser A B, Kernodle D S, Parker R A. Low-inoculum model of surgical wound infection. J Infect Dis. 1992;166:393–399. doi: 10.1093/infdis/166.2.393. [DOI] [PubMed] [Google Scholar]
- 14.Kernodle D S, Kaiser A B. Comparative prophylactic efficacy of cefazolin and vancomycin in a guinea pig model of Staphylococcus aureus wound infection. J Infect Dis. 1993;168:152–157. doi: 10.1093/infdis/168.1.152. [DOI] [PubMed] [Google Scholar]
- 15.Kernodle D S, Kaiser A B. Efficacy of prophylaxis with β-lactams and β-lactam/β-lactamase inhibitor combinations against wound infection by methicillin-resistant and borderline-susceptible Staphylococcus aureus in a guinea pig model. Antimicrob Agents Chemother. 1993;37:702–707. doi: 10.1128/aac.37.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kernodle D S, Kaiser A B. Comparative prophylactic efficacies of ciprofloxacin, ofloxacin, cefazolin, and vancomycin in an experimental model of staphylococcal wound infection. Antimicrob Agents Chemother. 1994;38:1325–1330. doi: 10.1128/aac.38.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kernodle D S, Kaiser A B. Postoperative infections and antimicrobial prophylaxis. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2742–2756. [Google Scholar]
- 18.Lorenz W, Reimund K-P, Weitzel F, Celik I, Kurnatowski M, Schneider C, Mannheim W, Heiske A, Neumann K, Sitter H, Rothmund M. Granulocyte colony-stimulating factor prophylaxis before operation protects against lethal consequences of postoperative peritonitis. Surgery. 1994;116:925–934. [PubMed] [Google Scholar]
- 19.McCullagh P, Nelder J A. Generalized linear models. 2nd ed. London, United Kingdom: Chapman and Hall; 1989. [Google Scholar]
- 20.Miles A A, Miles E M, Burke J. The value and duration of defense mechanisms to the primary lodgement of bacteria. Br J Exp Pathol. 1957;38:79–86. [PMC free article] [PubMed] [Google Scholar]
- 21.Nakao A, Kato H, Kanbe T, Tanaka K, Tamura H, Tanaka S, Takagi H. Quantitative assay (1-3)-β-d-glucan in culture media of Candida albicans using the G-test. Eur J Surg Res. 1994;26:194–200. doi: 10.1159/000129336. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 2nd ed. Approved Standard M7-A2. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 23.Onderdonk A B, Cisneros R L, Hinkson P, Ostroff G. Anti-infective effect of poly-β1-6-glucotriosyl-β-1-3-glucopyranose glucan in vivo. Infect Immun. 1992;60:1642–1647. doi: 10.1128/iai.60.4.1642-1647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page C P, Bohnen J M A, Fletcher J R, McManus A T, Solomkin J S, Wittmann D H. Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch Surg. 1993;128:79–88. doi: 10.1001/archsurg.1993.01420130087014. [DOI] [PubMed] [Google Scholar]
- 25.Panlilio A L, Culver D H, Gaynes R P, et al. Methicillin-resistant Staphylococcus aureus in U. S. hospitals. Infect Control Hosp Epidemiol. 1992;13:582–586. doi: 10.1086/646432. [DOI] [PubMed] [Google Scholar]
- 26.Poutsiaka D D, Mengozzi M, Vannier E, Sinha B, Dinarello C A. Cross-linking of the β-glucan receptor on human monocytes results in interleukin-1 receptor antagonist but not interleukin-1 production. Blood. 1993;82:3695–3700. [PubMed] [Google Scholar]
- 27.Schaberg, D. R., D. H. Culver, and R. P. Gaynes. 1991. Major trends in the microbial etiology of nosocomial infection. Am. J. Med. 91(Suppl. 3B):72S–75S. [DOI] [PubMed]
- 28.Stashenko P, Wang C Y, Riley E, Wu Y, Ostroff G, Niederman R. Reduction of infection-stimulated periapical bone resorption by the biological response modifier PGG glucan. J Dent Res. 1995;74:323–330. doi: 10.1177/00220345950740010701. [DOI] [PubMed] [Google Scholar]
- 29.Tzianabos A O, Cisneros R L. Prophylaxis with the immunomodulator PGG glucan enhances antibiotic efficacy in rats infected with antibiotic-resistant bacteria. Ann N Y Acad Sci. 1996;797:285–287. doi: 10.1111/j.1749-6632.1996.tb52980.x. [DOI] [PubMed] [Google Scholar]