Abstract
Background:
Legg–Calvé–Perthes disease is a self-limiting disorder that develops in children following interruption of the blood supply to the capital femoral epiphysis. This review outlines the current knowledge on the epidemiology, natural evolution, clinical spectrum, and management of the disease.
Methods:
The literature pertaining to these aspects of the disease were studied and summarized in this review.
Results:
Epidemiological studies suggest that environmental factors contribute to the causation of the disease. Incidence rates monitored over time indicate that the incidence of Legg–Calvé–Perthes disease is declining. The natural evolution followed on sequential plain radiographs enables division of the disease into Stages Ia, Ib, IIa, IIb, IIIa, IIIb, and IV. Reversible deformation of the capital occurs in Stages Ia–IIa simply on standing while irreversible deformation may occur in Stages IIb and IIIa. Treatment of Legg–Calvé–Perthes disease in Stages Ia–IIa aims to prevent the femoral head from getting deformed by containment and avoidance of weight-bearing. In Stages IIb and IIIa, treatment aims to remedy the effects of early irreversible deformation of the femoral head. In Stage IIIb and IV, treatment is directed to correcting the altered shape of the femoral head. The impression that these treatment methods are helpful is based on poor quality evidence.
Conclusion:
There is an urgent need to undertake Level I studies to establish the efficacy of currently treatment.
Level of evidence:
level V.
Keywords: Legg–Calvé–Perthes disease, social deprivation, declining incidence, containment, weight-relief
Introduction
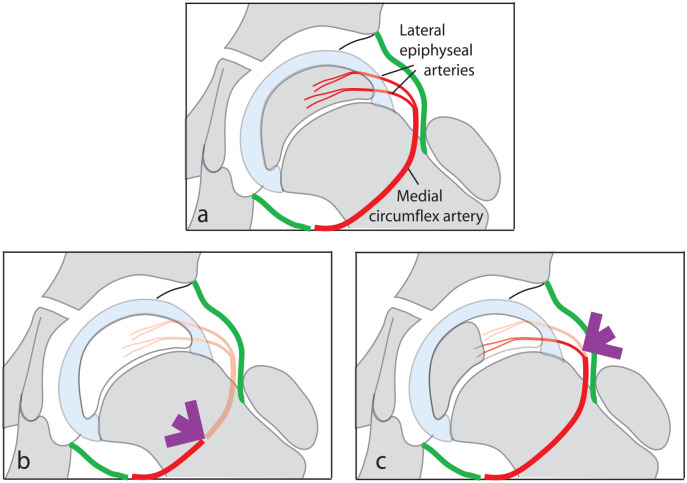
Legg–Calvé–Perthes disease (LCPD) is a self-limiting developmental disorder of the child’s hip characterized by avascular necrosis of the capital femoral epiphysis. The disease frequently develops between the ages of 4 and 7 years when the femoral epiphysis is supplied solely by the lateral epiphyseal branches of the medial circumflex artery. Consequently, occlusion of either the medial circumflex artery or the lateral epiphyseal vessels can result in avascular necrosis of part, or all, of the capital femoral epiphysis (Figure 1).
Figure 1.
Diagrammatic representation of the vascular supply to the capital femoral epiphysis in children between the ages of 4 and 7 years (a). Interruption of the medial circumflex artery (b) or the lateral epiphyseal vessels (c) can produce avascular necrosis of part or the entire epiphysis.
Vascular basis for causation of LCPD
Angiographic studies of Atsumi demonstrated that occlusion of the lateral epiphyseal arteries occurs in children with LCPD. 1 This and several other studies have provided adequate evidence to establish that it is a localized vascular episode that leads to LCPD.1 –9 The precise cause of vascular disruption that leads to femoral epiphyseal avascular necrosis, however, remains unknown.
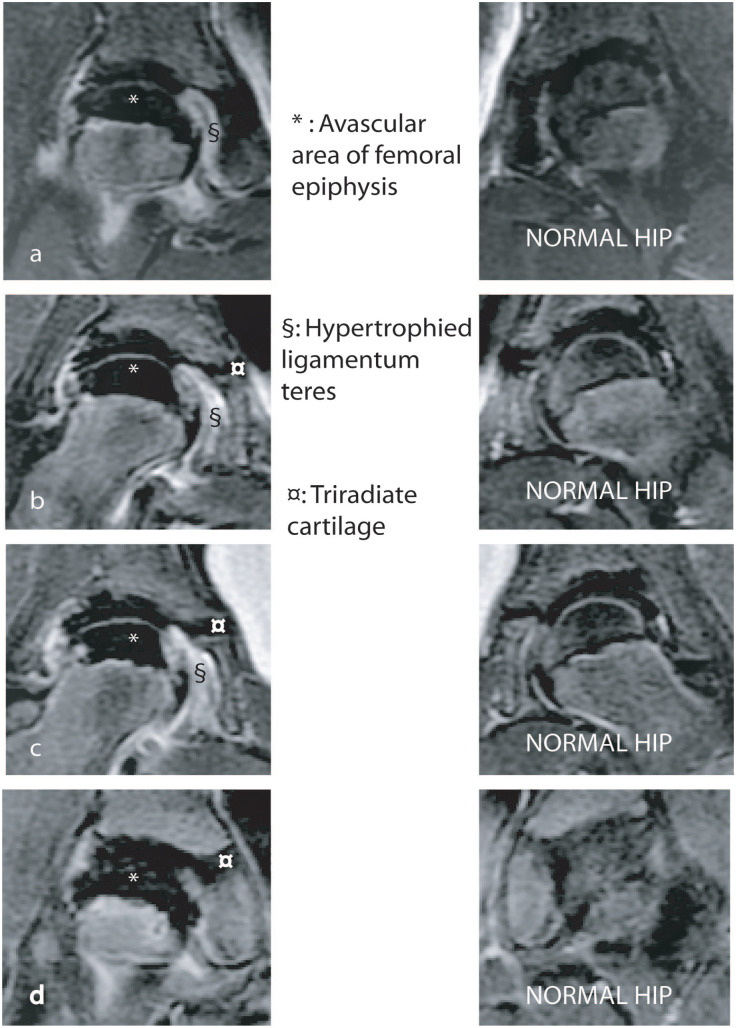
The extent of the femoral epiphysis rendered avascular by the vascular insult has an important bearing on the prognosis and treatment of the disease, and for this reason, the area of epiphyseal infarction should be quantified. Traditionally, this was done by estimating the portion of the femoral epiphysis that was sclerotic on plain antero-posterior and lateral radiographs and classifying the disease depending on whether the entire epiphysis, more than half the epiphysis and half or less than half the femoral epiphysis, was sclerotic as described by Catterall. 10 The disease, often, must evolve beyond the early stage before this subjective classification can be reliably applied and its reproducibility is not very high. 11 More recently, attempts have been made to use magnetic resonance imaging (MRI) to delineate and quantify the region of the epiphysis that is not perfused. 12 The technique referred to as “perfusion MRI” involves acquiring gadolinium enhanced images with fat suppression (Figure 2).12 –16 The perfusion MRI can be performed at the onset of symptoms with the potential advantage of facilitating early decision-making and intervention.
Figure 2.
Perfusion MRI scan of the hips of a child with LCPD of the right hip. Four sequential cuts through the LCPD hip are shown (a–d) and corresponding cuts through the normal hip are shown on the right. The avascular region appears black (*) and occupies over 95% of the epiphysis.
Biomechanical factors contributing to LCPD
Some theories suggest that in addition to the vascular insult, biomechanical factors may also contribute to the onset of symptoms and progression of the disease.17 –19
Epidemiology
LCPD may affect children between 2 and 14 years of age, with the peak age of onset around 5 or 6 years. 20 Males are affected five times more commonly than females. The disease predominantly affects white children from Northern Europe. 21 Among affected Indian children, the epidemiology is slightly different, with a slightly later age at disease onset. 22
Geographic distribution
There is a significant local, national, and international variation in the frequency of LCPD. The incidence varies between 0.2 and 19.1 per 100,000 0- to 14-year-olds per year, which equates to a lifetime risk between 1:400 and 1:35,000. 23 Equatorial regions have a low incidence of disease, while Northern Europe has the highest incidence. The geographic variation may be related to racial factors as black African children have a very low rate of disease as compared to Caucasian children.
Marked differences in the rates of disease have been noted within countries. In the United Kingdom, Scotland had more than twice the frequency of disease than London (10.39 vs 4.6 per 100,000 0- to 14-year-olds per year). 21 In India, the incidence in Manipal (south-west) was 10 times that of Vellore (south-east) (4.4 vs 0.4 per 100,000 0- to 14-year-olds per year). 22 In Norway, rates were 3.6 in the northern county of Finnmark, compared to 16.7 per 100,000 per year in western county of Sogn og Fjordane. 24
The incidence varies considerably even within very small areas, with the disease frequency varying on a street-by-street basis. In Liverpool, England, a high incidence of disease was noted in the inner city,25 –27 which led to studies examining the relationship with socioeconomic deprivation.23,25,28 A very steep socioeconomic gradient was observed, with the most socioeconomically deprived households having four times more LCPD than the most affluent.26,29 –31
Trends over time
In Merseyside, UK, the incidence of LCPD halved from 14.2 to 7.4 cases per 100,000 per year between 1976 and 2009.25 –27 This period was associated with a progressive improvement in the standard of living, which may account for the change in incidence. Primary care records from the United Kingdom also demonstrated a steady fall in the rate of disease, with the greatest fall in the most socioeconomically deprived regions. 23 Declining incidence rates have been noted in Northern Ireland also. 32
Heritability
Data from large case series suggest the occurrence of disease is 0.5%–0.8% among parents of affected children and 2%–4% among siblings of affected children.33,34 A robust study of heritability used the Danish twin registry to compare the difference in rates of LCPD between identical and non-identical twins. 35 The study included 81 sets of twins, of whom at least 1 had LCPD. The disease concordance was very low, and there were no occurrences of LCPD in the co-twin of identical twins, suggesting that any familial aggregation is due to a shared environment rather than a genetic susceptibility.
Associations
Stature
A large cross-sectional anthropometric study, of 232 children, demonstrated global growth disturbance, termed “rostral sparing dwarfism.” 36 The affected children had a normal head size (i.e. “rostral sparing”), but the arms and legs had subtle disproportionate shorting; similar to that seen among neonates with intrauterine growth retardation. However, it was unclear if this was confounded by socioeconomic deprivation, which is known to be associated with both LCPD and abnormal growth. A follow-up study using siblings of affected children as controls (thereby matching for socioeconomic deprivation) confirmed a clear growth restriction, most notable in the feet of affected children. 37
Birth weight and birth length
A study from Sweden examined the association of birth weight and LCPD and demonstrated a “dose-response”; children with the lowest birth weight had the greatest risk of LCPD (normal birth (1.0—ref), low birth weight odds ratio ((OR) = 1.33), very low birth weight (OR = 3.32)). 38 A Norwegian study showed an association with birth length; 39 the risk of LCPD increased by 50% among children with birth lengths below 50 cm, compared to those over 50 cm.
Disease associations
An association with congenital abnormalities could suggest an intrauterine disease trigger. Catterall et al. 40 first found an association between LCPD and inguinal hernia and genitourinary malformations. These findings along with a strong association with Down’s syndrome were subsequently reconfirmed in Norway 39 and the United Kingdom. 41
A strong association has been demonstrated with behavioral abnormalities and hyperactivity.42,43 However, it is uncertain if this finding is a consequence of the pain and immobility caused by disease, rather than something related to the disease etiology.
An association between asthma and Perthes’ disease has also been identified. 41 However, although socioeconomic deprivation was adjusted for in the analysis, this association could be the result of residual confounding or a further unadjusted confounder, such as tobacco smoke exposure.
Smoking
The marked variation in incidence, related to socioeconomic deprivation, suggests a major environmental influence in the disease onset. This, combined with the association with low birth weight, has led to suggestion that tobacco smoke exposure may be important in the disease etiology. Using the Swedish inpatient register, a case–control study of 852 cases and 4432 controls, demonstrated a relationship between LCPD and parental smoking, with a dose-response (adjusted OR = 1.4 < 10 cigarettes/day and OR = 2.0 > 10 cigarettes/day). 44 Other studies confirm this association, including one using a biological measure of current exposure (urinary cotinine; a metabolite of nicotine), 45 and another which also confirmed an association with wood stove smoke exposure. 30 All these studies attempted to adjust for socioeconomic deprivation, although there is the possibility that the strength of the association between tobacco smoke and socioeconomic deprivation is so strong that statistical adjustment is insufficient due to a phenomenon called “residual confounding.”
Etiological hypotheses
Given the early age of onset, absence of a genetic influence, association with congenital malformations and very strong association with socioeconomic deprivation, it seems that the key etiological determinant is environmental, which must occur either during the prenatal period or very early in life. Hypotheses postulated to explain these findings include nutritional deficiency and thrombophilia.
A nutritional deficiency examined has been manganese, because of disproportionate growth abnormality and epiphyseal dysplasia in animals with the deficiency of this element. A case–control study appeared to support this association, 46 although a second study did not support the findings nor did supplementation after disease onset have any influence on disease. 47
Thrombophilia has been the more recent major focus of etiological investigations. If a thrombophilic tendency were important in the disease, it would be directly on the mechanistic pathway of disease (i.e. one would expect a clot to cause the epiphyseal infarct). We would also expect a very high proportion of children with LCPD to demonstrate this abnormality compared to a risk factor not directly on the mechanistic pathway such as social deprivation which is a risk factor although on its own is not a plausible cause of LCPD. However, there are weak associations to a range of thrombophilic traits and LCPD, with findings that are far from being consistent.48,49 So, studies of coagulopathy appear to exclude a major association. The etiological factor responsible for causation of LCPD, therefore, remains elusive.
Natural evolution of LCPD
The self-limiting nature of LCPD is evident on two fronts: first, the blood supply to the femoral epiphysis gets restored and second, the necrotic avascular bone is resorbed and replaced by healthy new bone over a period of 2–4 years.
Revascularization of the femoral epiphysis
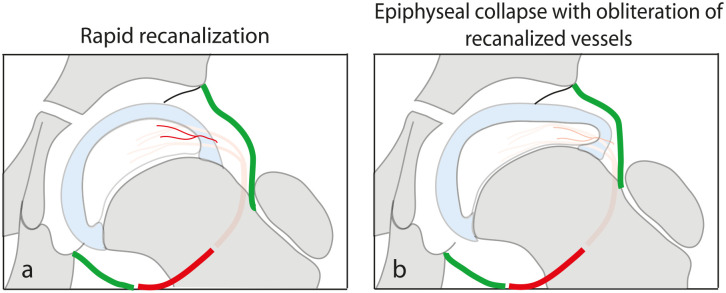
Angiographic,7 –9 radioisotope, 19 and more recent MRI studies12,14 confirm that revascularization of the femoral epiphysis occurs spontaneously. Based on radioisotope studies, Conway 50 demonstrated evidence of very early revascularization of the lateral part of the epiphysis which he attributed to recanalization of the occluded vessels (Figure 3(a)). If these recanalized vessels remain patent, they contribute to rapid restoration of normal epiphyseal blood supply. However, if epiphyseal collapse occurs, the recanalized vessels may get obliterated (Figure 3(b)) leading to considerable delay in the process of revascularization of the epiphysis. In rare instances, revascularization may be incomplete leading to osteochondritis dissecans of the femoral head. 51 Revascularization may also be incomplete when the onset of the disease is in adolescence. 52
Figure 3.
Very soon after the vascular insult, there is an attempt to restore the epiphyseal blood supply by recanalization of the occluded vessels (a). These recanalized blood vessels, however, can get obliterated if the epiphysis collapses (b).
Reconstitution of the necrotic femoral epiphysis
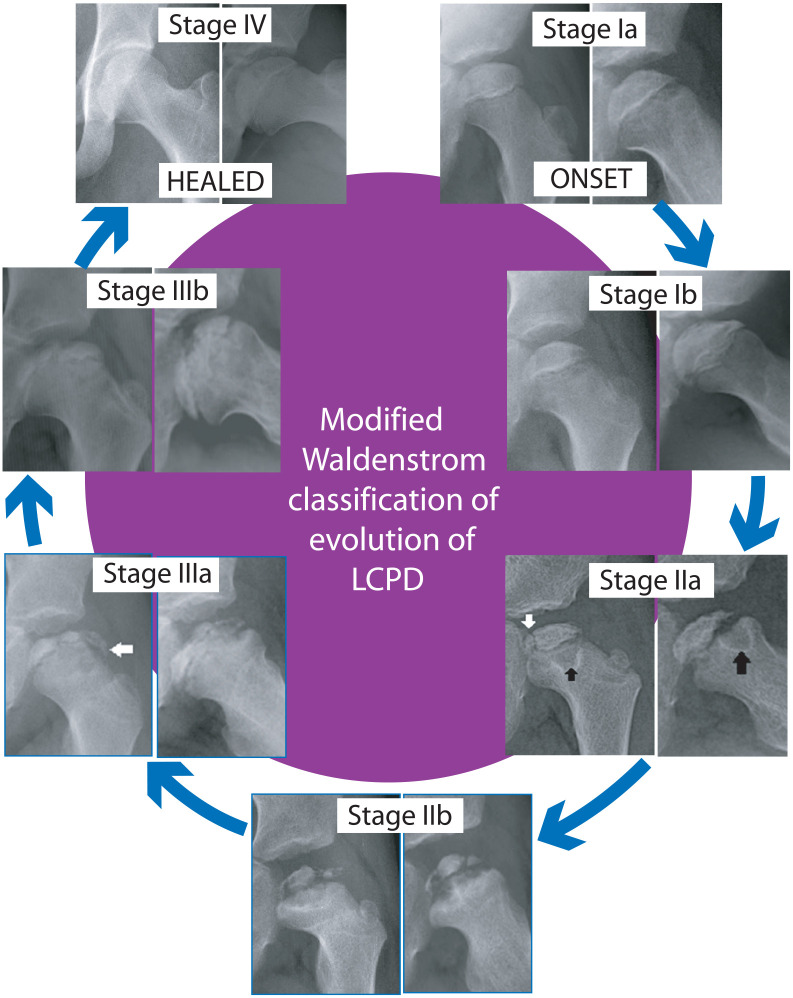
Healing of injured or diseased bone involves an interplay of osteoclastic resorption of damaged bone and new bone deposition by osteoblasts, and these cellular responses proceed in tandem in most clinical situations. In LCPD, however, there is a distinct paucity of an osteoblastic response in the early part of the disease.53,54 At the same time, osteoclastic resorption of dead bone proceeds at a brisk pace resulting in weakening of bone trabeculae of the femoral ossific nucleus and fragmentation of the epiphysis. As the disease evolves, new woven bone is laid down and eventually this bone is converted to lamellar bone. This sequence of repair and reconstitution of the femoral epiphysis can be followed on plain radiographs enabling classification of disease evolution into discrete stages (Stage Ia, Ib, IIa, IIb, IIIa, IIIb, and IV; Figure 4).55 –57 This classification (the modified Waldenström classification) is reproducible and consequently suitable for treatment planning. 57
Figure 4.
Stages of evolution of LCPD. The original Waldenström classification had four stages; stage of avascular necrosis (I), stage of fragmentation (II), stage of reconstitution (III), and healed stage (IV). In the modified classification, Stages I, II, and III are subdivided into early (a) and late (b) parts of the respective stages. Fragmentation commences with a vertical fissure, perpendicular to the articular surface (vertical white arrow—Stage IIa). In Stage II, metaphyseal cysts (black arrows) may be seen. Appearance of “wooly” new bone at the periphery of the epiphysis signals the beginning of Stage IIIa (horizontal white arrow).
Pathogenesis of femoral deformity
The femoral epiphysis may get irreversibly deformed in children with LCPD and treatment of this self-limiting disease is warranted primarily to prevent this complication. A clear understanding of the pathogenesis and the timing of deformation is needed to plan appropriate preventive intervention.
Three patterns of epiphyseal deformation may occur. 58 First, there may be a loss of epiphyseal height with a concomitant increase in the width of the epiphysis. This is referred to as “mushrooming” 59 and the mushroomed epiphysis heals with metaphyseal widening and coxa magna (Figure 5).
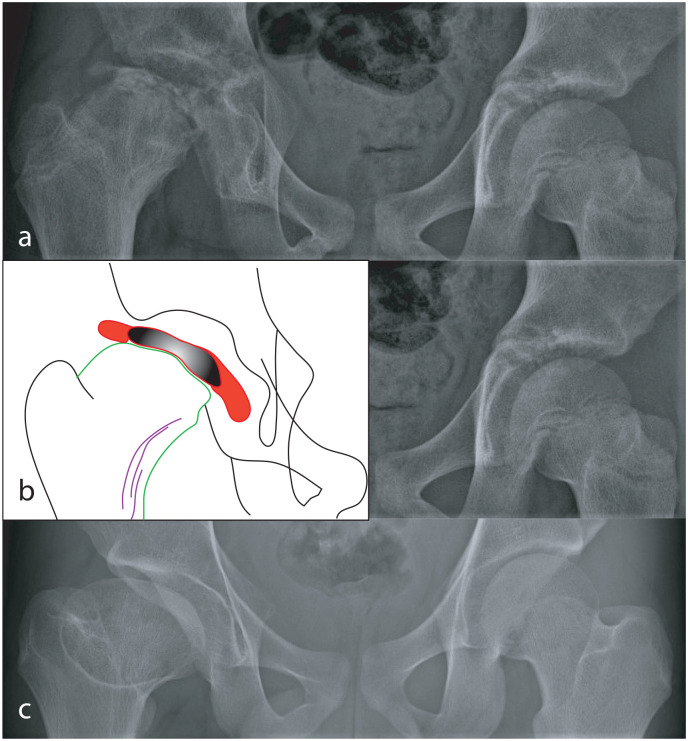
Figure 5.
Radiographs of the hips of a child with LCPD of the right hip that was not treated. In Stage III of the disease (a), the femoral epiphysis is severely flattened. The details of changes in the proximal femur are shown in the tracing (b); the femoral epiphysis, consisting of new bone that has formed (red) on either side of the necrotic part of the epiphysis (black), has “mushroomed” well beyond the margins of the metaphysis (green). In response to mushrooming of the epiphysis, there have been repeated attempts to increase the width of the metaphysis by remodeling of the neck (purple lines). The disease healed and at skeletal maturity, there is coxa magna, a short neck, and an aspherical femoral head (c).
Second, there may be loss of epiphyseal height without an increase in epiphyseal width (Figure 6(a)). This could occur if the broken fragments of bone trabeculae are compacted. 58 The height of the femoral epiphysis is permanently reduced even in hips where sphericity is retained (Figure 6(b)).
Figure 6.
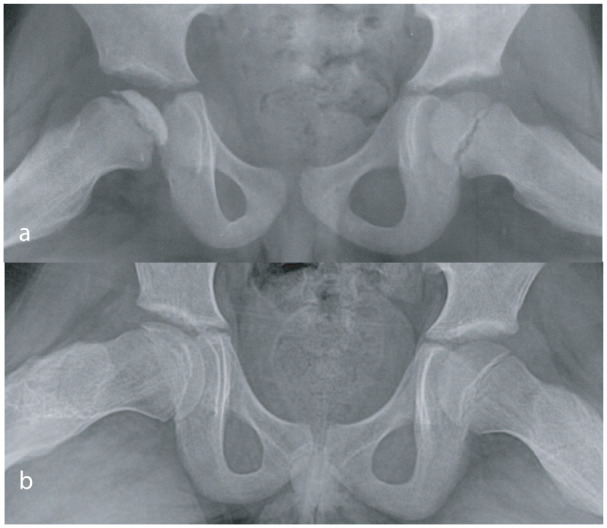
Appearance of the right hip at onset of the disease (a) and at healing (b). Uniform loss of epiphyseal height which persists till the disease heals.
Third, localized, focal collapse of the epiphysis may occur under the margin of the acetabulum (Figure 7).
Figure 7.
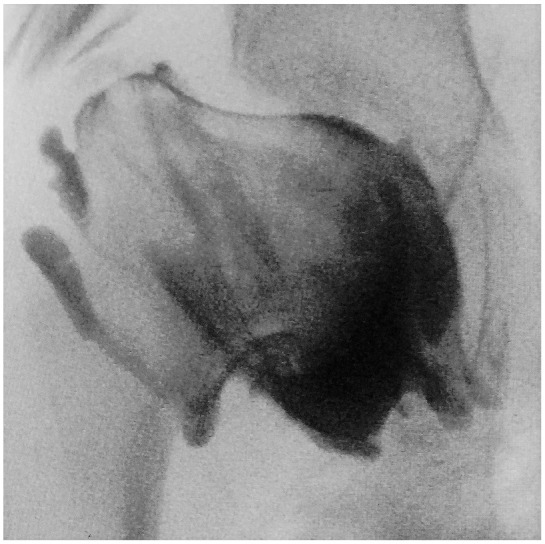
Arthrogram of the hip of a child with LCPD demonstrating localized collapse of the femoral epiphysis under the acetabular margin.
The mechanisms involved in causation and the propensity for femoral head deformation vary as the disease evolves.
Reversible femoral head deformation in the early stages of LCPD (Stages Ia, Ib, and IIa)
It has been estimated that the femoral head is subjected to compressive forces several times the individual’s body weight while standing, walking, running, or jumping.60,61 Although they were unaware of the magnitude of forces acting on the hip, surgeons in the latter half of the last century had assumed that compressive loading deforms the femoral epiphysis in LCPD and advocated restrictions on weight-bearing.62,63 However, until recently, no one had demonstrated that in LCPD, the femoral head gets deformed during normal childhood activity. Recent advances in imaging techniques enabled acquisition of MRI scans of the hips of children in the upright and recumbent positions and in 2020, an elegant pilot study demonstrated that in the early stages of LCPD (Stages Ia, Ib, and IIa), the femoral epiphyseal cartilage and the ossific nucleus deform in response to loading on bearing weight. 64 A mean 7.2% increase in the width and a 12.4% decrease in height of the femoral epiphysis occurred on standing (Figure 8) and the dimensions of the epiphysis were restored to the pre-loading state on lying down. This pattern of reversible deformation, akin to plastic deformation typical of soft tissues of the human body, was noted in all hips in the early stages of the disease. Epiphyseal deformation on weight-bearing was less evident in the later stages of the disease and was negligible in the normal femoral epiphysis. 64 These observations suggest that soon after infarction of the epiphysis, its mechanical properties are markedly altered leading to deformity through simply bearing weight on the limb.
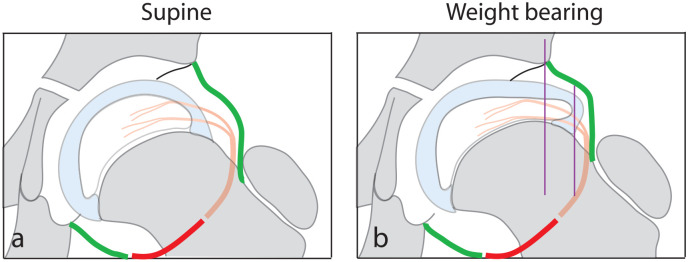
Figure 8.
Diagrammatic representation of the unloaded hip (a) and on bearing weight (b) demonstrating reversible deformation of the femoral epiphysis on weight-bearing in the stage of avascular necrosis of LCPD. Increased width of the epiphysis results in extrusion of its lateral part (purple lines). Reduction of epiphyseal height also occurs.
It is uncertain whether repeated reversible deformation of the epiphysis on cyclical loading of the LCPD hip while walking, running, or jumping in this early stage of the disease leads to permanent deformation. It is also unclear if the avascular epiphysis exhibits creep characteristics with further deformation on standing for a longer duration of time.
Irreversible femoral head deformation in Stages IIb and IIIa of LCPD
In Stage IIb of the disease, the bone trabeculae are weak and prone to crumble if subjected to compressive loading because the anabolic response of the osteoblasts does not keep pace with osteoclastic resorption.53,54 Collapse of the epiphysis at this stage is irreversible and permanent.55,56
New bone laid down in Stage IIIa is woven bone which is prone to deformation as the trabeculae of woven bone are aligned haphazardly and not aligned to counter deforming forces. 65 In Stage IIIb, the propensity for further deformation of the epiphysis is no longer present as the new bone has remodeled into lamellar bone with trabeculae aligned to resist compressive forces.65,66
Thus, the susceptibility of the femoral head to get deformed begins early in the disease and continues until the disease evolves to Stage IIIb (Figure 9). It follows that any method of treatment of LCPD that aims to prevent femoral head deformation should be instituted soon after onset of the disease and should continue through to Stage IIIb. 67
Figure 9.
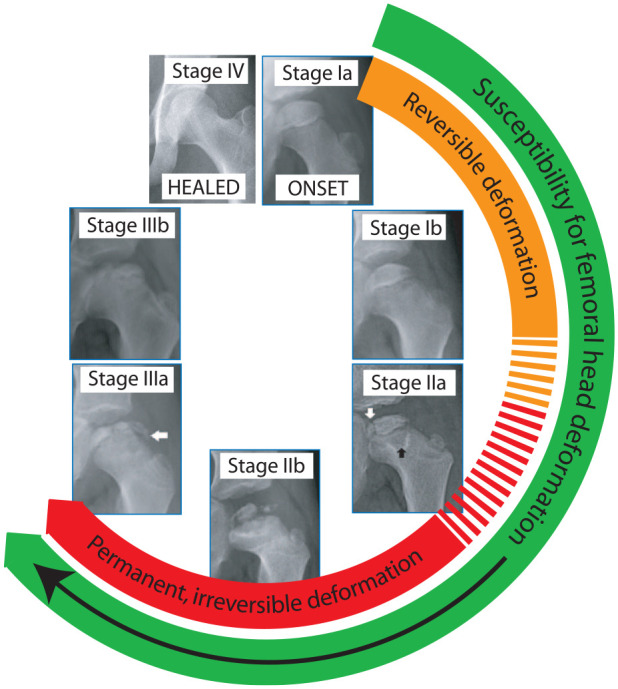
The femoral epiphysis may get deformed between Stages Ia and IIIa of LCPD.
Extrusion
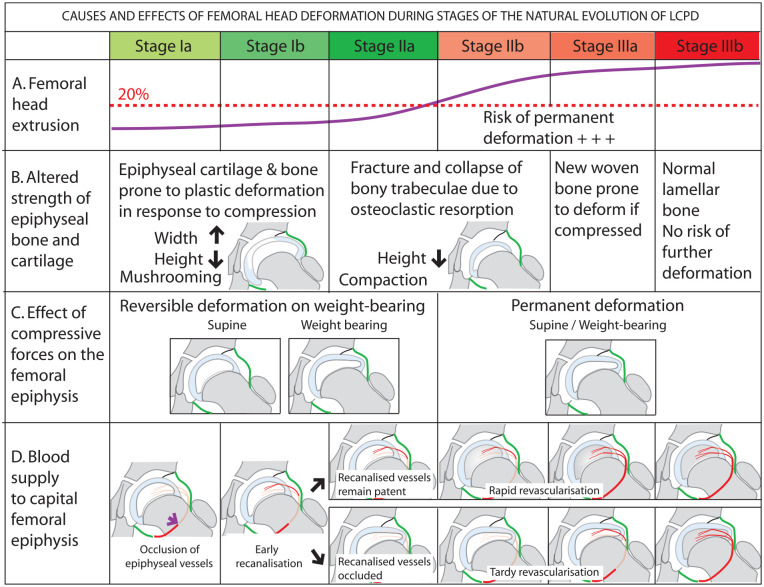
The lateral and anterior part of the femoral epiphysis tends to extrude beyond the confines of the acetabulum in LCPD,55,56 and there is evidence to show that lateral extrusion is associated with a poor outcome, particularly if more than 20% of the femoral head protrudes outside the acetabular margin.68,69 Hypertrophy of the articular cartilage of both the acetabulum and the femur and hypertrophy of the ligamentum teres initiate early extrusion and in untreated children, extrusion increases as the disease evolves (Figure 10(a)). 70 The progressive increase of extrusion is the modest and gradual in the early part of the disease (Stages Ia, Ib, and IIa); thereafter, an abrupt increase in extrusion occurs in Stage IIb. 55 Thus, at Stage IIb, extrusion often exceeds 20%, and at the same time, the epiphyseal bone is weak because of the imbalance between osteoclastic resorption and new bone formation (Figure 10(b)). A combination of both these factors makes the femoral head most vulnerable for deformation when subjected to compressive stress at this point in the evolution of the disease. Figure 10 summarizes the causes and effects of femoral head deformation in LCPD as the disease evolves.
Figure 10.
Diagrammatic representation of factors contributing to femoral head deformation during different stages of the evolution of LCPD (a–c) and the effect of femoral head deformation on revascularization of the epiphysis (d).
Clinical spectrum
“Typical” LCPD
The clinical presentation of typical LCPD has been well described in the literature;10,71 the child presents with a limp and pain in the hip or knee, without any associated constitutional symptoms. Typically, moderate restriction of abduction and internal rotation of the hip is noted. A plain radiograph of the hip early in the disease may not show any abnormality, but most radiographs show an area of sclerosis of the femoral capital epiphysis. Synovitis with a mild effusion is characteristically seen in the early part of the disease. Pain usually decreases, and the limitation of movement improves in a few weeks of restricted activity. Recurrence of pain and restriction of motion indicates the onset of complications like “hinge abduction.” 10 The disease heals after 2–4 years; generally, the shorter the duration of the disease, the better the outcome. The pattern of presentation and evolution of the disease is similar in children from varied ethnic backgrounds.55,56,71 –74
LCPD in the adolescent
In a small proportion of children with LCPD, the onset is in adolescence and the disease progression in these children is quite different from that of typical LCPD. 52 Three patterns of disease evolution have been described; they are the late-onset pattern, segmental collapse pattern, and the destructive pattern. The process of revascularization is very slow and often incomplete in the first two types and almost non-existent in the destructive pattern. The prognosis is poor in most adolescents with LCPD; in particular, the outcome is uniformly poor in hips with the destructive pattern. 52
The “discoid” epiphysis
A small subset of children with LCPD present with a completely sclerotic epiphysis (Catterall Group IV—whole head involvement) that is uniformly flattened (Herring C) even before fragmentation has commenced. 58 The appearance of the epiphysis is like a discus, and hence, it is referred to as a “discoid” epiphysis. 58 The prognosis is poor since the entire epiphysis is avascular and epiphyseal collapse is severe; only about 25% of these children end up with spherical heads even among those treated early.
Management
Management of LCPD may be classified as preventive intervention, remedial intervention and salvage depending on when during the evolution of the disease treatment is instituted. 67
Preventive intervention (Stages Ia, Ib, and IIa)
Preventive intervention aims to preserve the spherical shape of the femoral head by protecting it from factors that predispose to its deformation. Since irreversible deformation tends to occur in Stage IIb of the disease, it is important that treatment commences by Stage IIa. The importance of intervention early in the disease was emphasized in the results of a study that set out to determine the optimal time for preventive intervention. 75 The OR of the disease healing with an aspherical femoral head was 16.58 when the treatment was initiated in Stage IIb or later. 75 Three treatment approaches merit closer scrutiny; these are containment, weight-relief, and trochanteric growth arrest.
Containment
It is unclear who introduced the concept of “containment,” but over 50 years ago, attempts were made to reverse extrusion by non-operative and operative means. Realizing that extrusion (Figure 11(a)) can be corrected by abducting the hip (Figure 11(b)), Gordan Petrie and Bitenc 76 achieved containment with a broomstick plaster cast while Anatol Axer 77 resorted to a proximal femoral varus osteotomy (Figure 11(c)). Robert Salter 78 advocated an alternative approach by reorienting the acetabulum to cover the extruded part of the epiphysis by performing an innominate osteotomy (Figure 11(d)).
Figure 11.
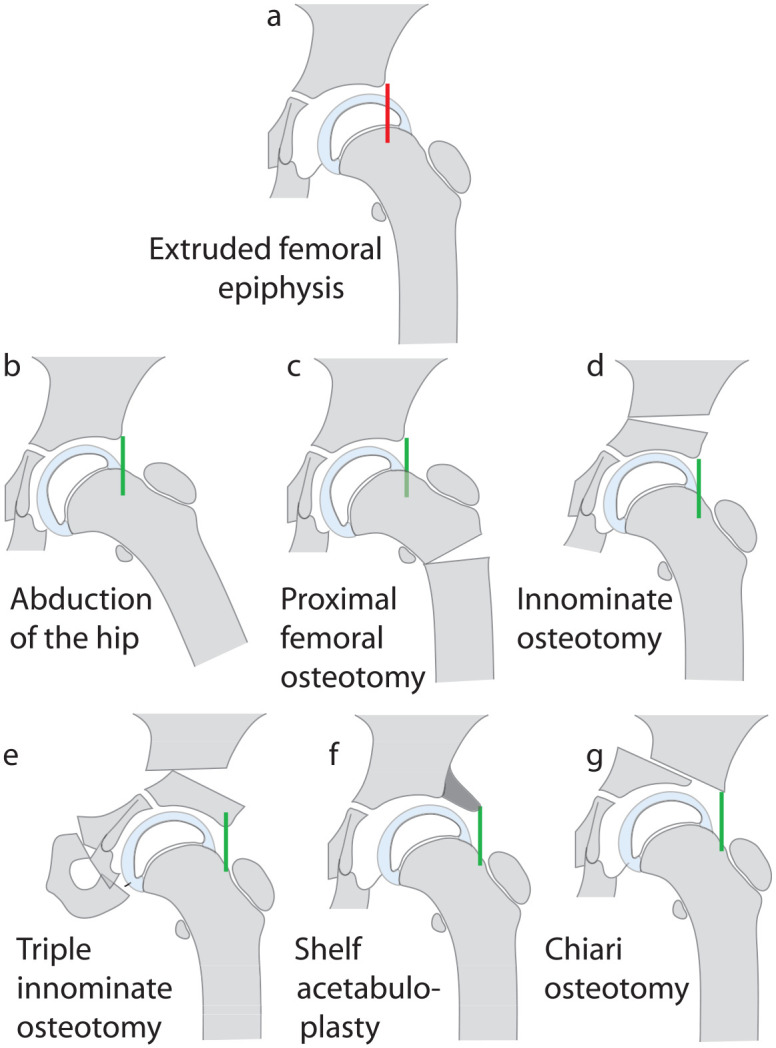
Diagrammatic representation of methods of treating femoral head extrusion in LCPD. The extruded femoral epiphysis (a) can be contained by abduction the hip in a broomstick cast or an abduction brace (b) or by a proximal femoral varus osteotomy (c). Containment can also be achieved by reorienting the acetabulum by a Salter innominate osteotomy (d) or a triple pelvic osteotomy (e). A shelf acetabuloplasty (f) and a Chiari osteotomy (g) provide a cover for the extruded part of the femoral epiphysis without attempting to reverse extrusion itself.
Proximal femoral osteotomy
Femoral varus osteotomy is the most widely used operation to achieve containment of the femoral head in LCPD (Figure 11(c)).79–93 The precise technique, including whether the osteotomy is an open-wedge or a closed wedge osteotomy, the nature of internal or external fixation, and the post-operative care, varies a great deal.
Concerns have been expressed regarding the extent of permanent shortening of the limb, the failure of the varus angulation to remodel with a persistent trendelenburg gait and valgus deformity of the knee secondary to the proximal femoral varus osteotomy. However, long-term studies have discounted each of these concerns; the mean shortening at skeletal maturity was 0.44 cm ± 0.68 (SD) in one study, 94 the neck shaft angle was restored to normal in most children in another study 95 and genu valgum was not encountered at skeletal maturity in a third study. 96 The advantages of a proximal femoral osteotomy are the simplicity of the operation and the ability to perform trochanteric epiphysiodesis, if required, through the same incision. The need of a second operation to remove the implant is one unavoidable disadvantage.
Innominate osteotomy
In many centers in North America, innominate osteotomy is the preferred option for achieving containment 97 with some surgeons performing a Salter osteotomy (Figure 11(d)) and others performing a triple innominate osteotomy.97 –107 Compared with the femoral varus osteotomy, a triple pelvic osteotomy may have some advantages; no shortening of the limb, no increased inclination of the epiphyseal plate or the risk of a trendelenburg gait. Unlike the Salter osteotomy, a triple pelvic does not increase the pressure on the femoral head and offers better freedom of reorientation of the acetabulum with the axis of rotation at the joint and not at the pubic symphysis (Figure 11(e)).108 –112
Shelf acetabuloplasty (labral support) and Chiari osteotomy
Yet another approach has been to create a shelf to cover the extruded part of the femoral head without reorienting either the proximal femur or the acetabulum (Figure 11(f) and (g)). The shelf operation, also referred to as a “labral support” procedure, is popular in some centers.113 –117 A similar concept of creating a cover for the extruded part of the femoral epiphysis is the rationale behind performing a Chiari osteotomy.118 –124
Combined femoral and innominate osteotomies
Combined femoral and innominate osteotomies have been practiced by a few surgeons.125 –128
Weight-relief
Animal studies have shown that weight-relief does have a beneficial effect on maintaining the shape of the femoral head following induced ischemic necrosis 129 and a few surgeons advocate weight-relief as an adjunct to operative or non-operative containment.130 –132 However, most surgeons recommend weight-relief only for the period of bone healing after an osteotomy, rather than prolonged weight-relief as a form of definitive treatment, because of concerns of adverse psychological effects on the child. 133
Duration of weight-relief
If weight-relief is included in the treatment protocol, what should the duration of weight-relief be? Since the propensity for femoral head deformation persists till the disease has reached Stage IIIb, it follows that weight-bearing should be avoided till Stage IIIb and this may entail around 24 months of weight-relief in children in whom treatment commenced at the onset of symptoms. 132
Trochanteric epiphysiodesis
One of the complications of LCPD is premature growth arrest of the proximal capital physis leading to a short femoral neck (coxa brevis) and what is erroneously referred to as “greater trochanteric overgrowth.” Unfortunately, there is no known sign during the evolution of the disease that will enable the surgeon to predict impending capital physeal arrest. Prophylactic trochanteric epiphysiodesis performed in conjunction with containment has been found to be effective in minimizing the frequency of trochanteric “overgrowth” in LCPD.94,134 –139
Decision-making for preventive intervention
Several factors have been identified as having important prognostic significance in LCPD. 68 The most important of them include, the age at onset of the disease, the extent of the epiphysis that is avascular, and the presence of epiphyseal extrusion; the greater each of these variables are, the greater the risk of permanent deformation of the femoral head.10,52,58,68,69 Consequently, these factors need to be taken into reckoning in treatment planning. Other signs that put the “head-at-risk” described by Catterall 10 are of prognostic significance but are seldom seen in Stage Ia, Ib, or IIa to include them in the treatment plan for preventive intervention. Similarly, often the extent of epiphyseal collapse on which Herring made recommendations for treatment may only be clear in Stage IIb.140 –142
Containment has been advocated for the older child and those with extrusion provided the disease has not progressed beyond Stage IIa.78,87 Pre-emptive “containment” is recommended in children over the age of 7 years at onset of the disease even in the absence of extrusion as extrusion invariably develops sooner or later in these older children.67,75,143
Remedial intervention (Stages IIb and IIIa)
Containment in Stage IIb may not prevent the femoral head from getting deformed, 75 and consequently, the role of surgical intervention is no more preventive in nature. The femoral head begins to get deformed at this point (Figure 12(a)). Recrudescence of pain and reduction of the range of motion of the hip (particularly abduction) may occur in Stage IIIa when a phenomenon initially recognized and termed “hinge abduction” by Catterall develops. 144 On attempting to abduct the hip the “bump” on the lateral aspect of the femoral head fails to slide under the acetabular rim but impinges against it and the medial joint space opens (Figure 12(b)). The center of rotation of the hip is no longer at the center of the femoral head but is at the acetabular margin.144 –146
Figure 12.
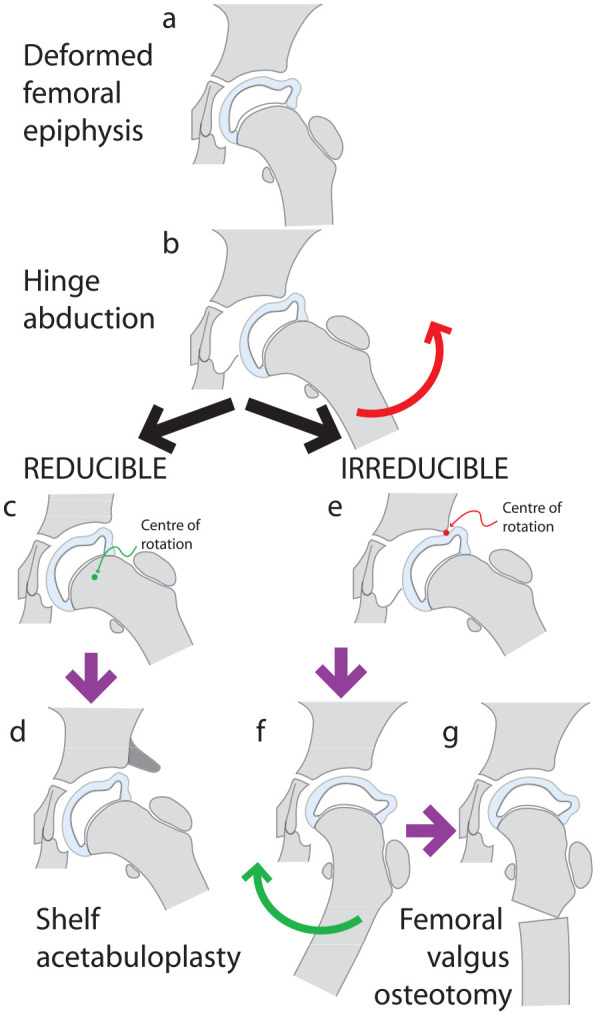
Diagram depicting hinge abduction. By Stage IIb or IIIa the femoral head may be deformed (a) and on attempting to abduct the hip (red arrow) the deformed femoral head may impinge on the acetabular margin and hinge on it such that the joint opens medially (b), creating a crescentic joint space. If the hinge abduction is reducible (c) a shelf acetabuloplasty is a recommended option (d). If the hinge abduction is irreducible (e), the hip is adducted (green arrow) and the position of maximum congruence is identified (f) and a proximal femoral valgus osteotomy is performed (g).
Initially, when examined under anesthesia, muscle spasm is overcome, the hinging resolves and the femoral head can be “reduced” into the acetabulum (Figure 12(c)). Increasing the volume of the acetabulum to accommodate the mushroomed femoral head has been shown to help relieve pain and prevent further hinging in hips with “reducible hinge abduction” (Figure 12(d)).145,146 Some hips do not reduce under anesthesia; adducting these hips with “irreducible hinge abduction” relieves pain and improves the congruence of the femoral head and acetabulum (Figure 12(e) and (f)). After confirming, with an arthrogram, the extent of adduction that restores maximum congruence of the femur and acetabulum, a proximal femoral osteotomy can be performed with the same degree of valgus angulation (Figure 12(g)).145 –153 While some surgeons may undertake this operation in Stage IIIa, 148 others prefer to defer it till Stage IIIb. 150
Decision-making for remedial intervention
The aims of treatment of LCPD in children presenting in Stages IIb and IIIa are to minimize the extent of femoral deformation and to relieve pain associated with hinge abduction. The factors that may influence treatment planning include the presence and severity of femoral deformity and the presence of reducible or irreducible hinge abduction. The treatment choices include femoral or pelvic osteotomy or a shelf procedure for hips with mild deformity and reducible hinge abduction and valgus osteotomy for irreducible hinge abduction. It needs to be emphasized that the chances of the femoral head retaining a spherical shape are low.68,75
Salvage (Stage IIIb and beyond)
Whatever femoral head deformity that is present at the onset of Stage IIIb will remain permanently with little chance of spontaneous remodeling. Consequently, treatment beyond this point is essentially the treatment of sequelae of LCPD.
With the refining of the technique of safe surgical dislocation of the hip, it has become possible to surgically reshape the misshapen femoral head.154 –157 At the same time, it is possible to identify and correct structural deformities of the proximal femur that cause impingement and damage the acetabulum.154 –157 The femoral abnormalities include intra-articular and extra-articular impingement, each of which can injure the acetabulum most frequently in the anterosuperior part of the acetabular rim between the 12 o’clock and 3 o’clock positions. 154
Decision-making for salvage
The aims of treatment at this stage are to halt the progression of joint damage brought on by the alteration in the shape of the femoral head and more importantly, to delay or prevent the onset of degenerative arthritis. The decision to address the problems by safe surgical dislocation or arthroscopically depends on the nature of abnormality and the expertise of the treating surgeon.
Outcome evaluation
Since the aim of preventive intervention is to preserve the sphericity of the femoral head by preventing femoral head deformation, the primary outcome measure should be the shape of the femoral head when the disease is fully healed and again at skeletal maturity. Till recently, the shape of the femoral head was assessed either by the Mose semi-quantitative method 158 or by the qualitative method of Stulberg et al. 159 from anteroposterior and frog lateral radiographs of the pelvis at skeletal maturity. Of five Stulberg classes, only Stulberg I and Stulberg II should be regarded as acceptable outcomes since the femoral head is spherical in these two classes only.
The Sphericity Deviation Score (SDS), which is a quantitative measure based on the engineering principle of “roundness error” estimation, has been shown to be reproducible and applicable both when the disease heals and at skeletal maturity.160 –162 A femoral head with a SDS value of 10 or below is regarded as a spherical head.160,161
Apart from the radiological outcome measure of femoral head sphericity, it is important that functional outcomes evaluating hip and lower limb function are also measured 163 as also patient-reported outcomes.164,165 However, there are strong correlations between the radiological outcome, functional outcome, and patient-reported outcomes of treatment of LCPD.164 –166
Effectiveness of preventive intervention
Unfortunately, there is no Level I study that evaluates preventive intervention in LCPD. Most studies are retrospective case series without controls. 97 A meta-analysis of this low-quality evidence suggested that containment may offer some benefit. 167
Two large, prospective studies from North America and Norway also suggest that containment surgery improves the chances of retaining a spherical femoral head particularly in older children.168,169 However, a recent prospective study from the United Kingdom casts some doubt on these findings, with no improvement demonstrated with surgical containment. 170
There are no robust data to indicate which method of surgical containment may yield the best results. Combined femoral and pelvic osteotomies have not proved to be more effective than isolated femoral or innominate osteotomies. 127
The results of the Norwegian prospective study 169 suggest that abduction braces are ineffective and should not be used to treat LCPD; similar views have been expressed by other authors. 171 However, impressive results have been reported with prolonged use of an A-frame 172 and with a Pogo stick brace, a non-weight-bearing abduction brace worn for up to 21 months. 173
Reappraisal of the role of weight-relief in the treatment of LCPD is warranted with experimental and clinical studies supporting the practice of avoiding weight-bearing till the disease has evolved till Stage IIIb.130 –132 A recent study compared the results in a group of children treated by containment and weight-relief for 6 months with results of a control group treated by identical containment and weight-relief till Stage IIIb. Seventy-five percent of hips protected from weight-bearing till Stage IIIb healed with spherical femoral heads while only 49% of hips protected from weight-bearing for 6 months were spherical when the disease healed. 132 The observation that prolonged weight-relief did not result in psychological problems in children lends further support this approach. 131 The results of some series that combined containment with weight-relief till Stage IIIb have been very good with 75% or more of hips healing with spherical femoral heads.132,173
Effectiveness of remedial intervention
Intervention in Stage IIb or IIIa of LCPD in children with symptomatic hinge abduction often results in relief of pain and improved range of motion of the hip, but the femoral head is seldom spherical when the disease heals.145 –151
Effectiveness of salvage intervention
Relief of pain, increased range of hip motion, and improvement in hip abductor muscle strength have been reported following surgery that improves the shape of the femoral head and removes intra-articular and extra-articular impingement.154 –157 However, adequate long-term follow-up of these patients are not yet available, and it remains to be seen if the aim of avoiding or significantly delaying total hip replacement is achieved by, so-called, “hip preservation surgery.”
Future directions
Since the imbalance between osteoclastic resorption and osteoblastic new bone formation has been implicated in the pathogenesis of femoral head deformation, attempts have been made to modulate these mechanisms. Bisphosphonates have been shown to suppress osteoclastic activity and bone morphogenetic protein to enhance new bone formation. Studies in experimental animals suggest that systemic administration of bisphosphonates may reduce the severity of femoral head deformation.174 –176 Since high systemic doses may be needed to reach and have a local effect on the avascular epiphysis, local administration of much small doses of bisphosphonates and bone morphogenetic protein into the epiphysis has been shown to be of benefit. 176 Clinical trials are currently underway and their results awaited. 177
The recent study that casts doubt on the effectiveness of containment surgery 170 clearly emphasizes the urgent need to embark on well-designed, prospective studies with adequate follow-up (ideally randomized controlled trials (RCTs)) to clarify the role of the current approach to treatment of LCPD. Realizing the need for robust prospective studies, the International Perthes Study Group (IPSG) has begun collecting data prospectively and undertaking studies on various aspects of LCPD. We hope that this international effort will answer several unanswered questions related to this enigmatic condition and improve its treatment outcomes in future.
Supplemental Material
Supplemental material, sj-pdf-1-cho-10.1177_18632521231203009 for Epidemiology, natural evolution, pathogenesis, clinical spectrum, and management of Legg–Calvé–Perthes by Benjamin Joseph, Hitesh Shah and Daniel C Perry in Journal of Children’s Orthopaedics
Footnotes
Author contributions: All the three authors contributed equally to the writing and reviewing of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Institutional Review Board (IRB) approval not obtained as this is a review of literature.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Atsumi T, Yamano K, Muraki M, et al. The blood supply of the lateral epiphyseal arteries in Perthes’ disease. J Bone Joint Surg Br 2000; 82(3): 392–398. [DOI] [PubMed] [Google Scholar]
- 2. Iwasaki K. The role of blood vessels within the ligamentum teres in Perthes’ disease. Clin Orthop Relat Res 1981(159): 248–256. [PubMed] [Google Scholar]
- 3. Inoue A, Freeman MA, Vernon-Roberts B, et al. The pathogenesis of Perthes’ disease. J Bone Joint Surg Br 1976; 58-B(4): 453–461. [DOI] [PubMed] [Google Scholar]
- 4. Inoue A, Ono K, Takaoka K, et al. A comparative study of histology in Perthes’ disease and idiopathic avascular necrosis of the femoral head in adults (IANF). Int Orthop 1980; 4(1): 39–46. [DOI] [PubMed] [Google Scholar]
- 5. Iwasaki K, Suzuki R, Okazaki T, et al. The haemodynamics of Perthes’ disease. An intraosseous venographic study combined with measurement of the intramedullary pressure. Int Orthop 1982; 6(3): 141–148. [DOI] [PubMed] [Google Scholar]
- 6. Rush BH, Bramson RT, Ogden JA. Legg-Calvé-Perthes disease: detection of cartilaginous and synovial change with MR imaging. Radiology 1988; 167(2): 473–476. [DOI] [PubMed] [Google Scholar]
- 7. LaMont RL, Muz J, Heilbronner D, et al. Quantitative assessment of femoral head involvement in Legg-Calvé-Perthes disease. J Bone Joint Surg Am 1981; 63(5): 746–752. [PubMed] [Google Scholar]
- 8. Théron J. Angiography in Legg-Calvé-Perthes disease. Radiology 1980; 135(1): 81–92. [DOI] [PubMed] [Google Scholar]
- 9. de Camargo FP, de Godoy RM, Jr, Tovo R. Angiography in Perthes’ disease. Clin Orthop Relat Res 1984(191): 216–220. [PubMed] [Google Scholar]
- 10. Catterall A. Legg-Calvé-Perthes syndrome. Clin Orthop Relat Res 1981; 158: 41–52. [PubMed] [Google Scholar]
- 11. Hardcastle PH, Ross R, Hamalainen M, et al. Catterall grouping of Perthes’ disease. An assessment of observer error and prognosis using the Catterall classification. J Bone Joint Surg Br 1980; 62-B(4): 428–431. [DOI] [PubMed] [Google Scholar]
- 12. Lamer S, Dorgeret S, Khairouni A, et al. Femoral head vascularisation in Legg-Calvé-Perthes disease: comparison of dynamic gadolinium-enhanced subtraction MRI with bone scintigraphy. Pediatr Radiol 2002; 32(8): 580–585. [DOI] [PubMed] [Google Scholar]
- 13. Sankar WN, Thomas S, Castañeda P, et al. Feasibility and safety of perfusion MRI for Legg-Calvé-Perthes disease. J Pediatr Orthop 2014; 34(7): 679–682. [DOI] [PubMed] [Google Scholar]
- 14. Kim HK, Wiesman KD, Kulkarni V, et al. Perfusion MRI in early stage of Legg-Calvé-Perthes disease to predict lateral pillar involvement: a preliminary study. J Bone Joint Surg Am 2014; 96(14): 1152–1160. [DOI] [PubMed] [Google Scholar]
- 15. Kim HK, Burgess J, Thoveson A, et al. Assessment of femoral head revascularization in Legg-Calvé-Perthes disease using serial perfusion MRI. J Bone Joint Surg Am 2016; 98(22): 1897–1904. [DOI] [PubMed] [Google Scholar]
- 16. Chong DY, Schrader T, Laine JC, et al. Reliability and validity of visual estimation of femoral head hypoperfusion on perfusion MRI in Legg-Calve-Perthes disease. J Pediatr Orthop 2021; 41(9): e780–e786. [DOI] [PubMed] [Google Scholar]
- 17. Ueo T, Tsutsumi S, Yamamuro T, et al. Biomechanical analysis of Perthes’ disease using the finite element method: the role of swelling of articular cartilage. Arch Orthop Trauma Surg (1978) 1987; 106(4): 202–208. [DOI] [PubMed] [Google Scholar]
- 18. Rab GT, DeNatale JS, Herrmann LR. Three-dimensional finite element analysis of Legg-Calve-Perthes disease. J Pediatr Orthop 1982; 2(1): 39–44. [DOI] [PubMed] [Google Scholar]
- 19. Berthaume MA, Perry DC, Dobson CA, et al. Skeletal immaturity, rostral sparing, and disparate hip morphologies as biomechanical causes for Legg-Calvé-Perthes’ disease. Clin Anat 2016; 29(6): 759–772. [DOI] [PubMed] [Google Scholar]
- 20. Perry DC, Skellorn PJ, Bruce CE. The lognormal age of onset distribution in Perthes’ disease: an analysis from a large well-defined cohort. Bone Joint J 2016; 98-B(5): 710–714. [DOI] [PubMed] [Google Scholar]
- 21. Perry DC, Machin DMG, Pope D, et al. Racial and geographic factors in the incidence of Legg-Calve-Perthes disease: a systematic review. Am J Epidemiol 2012; 175(3): 159–166. [DOI] [PubMed] [Google Scholar]
- 22. Joseph B, Chacko V, Rao BS, et al. The epidemiology of Perthes disease in south India. Int J Epidemiol 1988; 17(3): 603–607. [DOI] [PubMed] [Google Scholar]
- 23. Perry DC, Bruce CE, Pope D, et al. Legg-Calvé-Perthes disease in the UK: geographic and temporal trends in incidence reflecting differences in degree of deprivation in childhood. Arthritis Rheum 2012; 64(5): 1673–1679. [DOI] [PubMed] [Google Scholar]
- 24. Wiig O, Terjesen T, Svenningsen S, et al. The epidemiology and aetiology of Perthes’ disease in Norway: a nationwide study of 425 patients. J Bone Joint Surg Br 2006; 88(9): 1217–1223. [DOI] [PubMed] [Google Scholar]
- 25. Perry DC, Bruce CE, Pope D, et al. Perthes’ disease: deprivation and decline. Arch Dis Child 2011; 96(12): 1124–1128. [DOI] [PubMed] [Google Scholar]
- 26. Hall AJ, Barker DJ, Dangerfield PH, et al. Perthes’ disease of the hip in Liverpool. Br Med J Clin Res Ed 1983; 287(6407): 1757–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Margetts BM, Perry CA, Taylor JF, et al. The incidence and distribution of Legg–Calvé–Perthes’ disease in Liverpool, 1982–95. Arch Dis Child 2001; 84(4): 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kealey WD, Moore AJ, Cook S, et al. Deprivation, urbanisation and Perthes disease in Northern Ireland. J Bone Joint Surg Br 2000; 82(2): 167–171. [PubMed] [Google Scholar]
- 29. Perry DC, Bruce CE, Pope D, et al. Perthes disease of the hip: socioeconomic inequalities and the urban environment. Arch Dis Child 2012; 97(12): 1053–1057. [DOI] [PubMed] [Google Scholar]
- 30. Daniel AB, Shah H, Kamath A, et al. Environmental tobacco and wood smoke increase the risk of Legg-Calvé-Perthes disease. Clin Orthop Relat Res 2012; 470(9): 2369–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johansson T, Lindblad M, Bladh M, et al. Incidence of Perthes’ disease in children born between 1973 and 1993. Acta Orthop 2017; 88(1): 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mullan CJ, Thompson LJ, Cosgrove AP. The declining incidence of Legg-Calve-Perthes’ disease in Northern Ireland: an epidemiological study. J Pediatr Orthop 2017; 37(3): e178–e182. [DOI] [PubMed] [Google Scholar]
- 33. Fisher RL. An epidemiological study of Legg-Perthes disease. J Bone Joint Surg Am 1972; 54(4): 769–778. [PubMed] [Google Scholar]
- 34. Gray IM, Lowry RB, Renwick DH. Incidence and genetics of Legg-Perthes disease (osteochondritis deformans) in British Columbia: evidence of polygenic determination. J Med Genet 1972; 9(2): 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Metcalfe D, Van Dijck S, Parsons N, et al. A twin study of Perthes disease. Pediatrics 2016; 137(3): e20153542. [DOI] [PubMed] [Google Scholar]
- 36. Burwell RG, Dangerfield PH, Hall DJ, et al. Perthes disease. An anthropometric study revealing impaired and disproportionate growth. J Bone Joint Surg Br 1978; 60-B(4): 461–477. [DOI] [PubMed] [Google Scholar]
- 37. Hall AJ, Barker DJ, Dangerfield PH, et al. Small feet and Perthes disease. A survey in Liverpool. J Bone Joint Surg Br 1988; 70(4): 611–613. [DOI] [PubMed] [Google Scholar]
- 38. Lindblad M, Josefsson A, Bladh M, et al. Risk factors during pregnancy and delivery for the development of Perthes’ disease, a nationwide Swedish study of 2.1 million individuals. BMC Pregnancy and Childbirth 2020; 20(1): 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wiig O, Terjesen T, Svenningsen S, et al. The epidemiology and aetiology of Perthes’ disease in Norway: a nationwide study of 425 patients. J Bone Joint Surg Br 2006; 88(9): 1217–1223. [DOI] [PubMed] [Google Scholar]
- 40. Catterall A, Lloyd Roberts GC, Wynne-Davies R. Association of Perthes’ disease with congenital anomalies of genitourinary tract and inguinal region. Lancet 1971; 297(7707): 996–997. [DOI] [PubMed] [Google Scholar]
- 41. Perry DC, Bruce CE, Pope D, et al. Comorbidities in Perthes disease: a case control study using the general practice research database. J Bone Joint Surg Br 2012; 94(12): 1684–1689. [DOI] [PubMed] [Google Scholar]
- 42. Perry DC, Pope D, Bruce CE, et al. Hyperactivity and the psychological burden of Perthes disease: a case-control study. J Pediatr Orthop 2013; 33(6): 644–649. [DOI] [PubMed] [Google Scholar]
- 43. Hailer YD, Nilsson O. Legg-Calvé-Perthes disease and the risk of ADHD, depression, and mortality: a registry study involving 4057 individuals. Acta Orthop 2014; 85(5): 501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bahmanyar S, Montgomery SM, Weiss RJ, et al. Maternal smoking during pregnancy, other prenatal and perinatal factors, and the risk of Legg-Calve-Perthes disease. Pediatrics 2008; 122(2): e459–e464. [DOI] [PubMed] [Google Scholar]
- 45. Perry DC, Thomson C, Pope D, et al. A case control study to determine the association between Perthes’ disease and the recalled use of tobacco during pregnancy, and biological markers of current tobacco smoke exposure. Bone Joint J 2017; 99-B(8): 1102–1108. [DOI] [PubMed] [Google Scholar]
- 46. Hall AJ, Margetts BM, Barker DJ, et al. Low blood manganese levels in Liverpool children with Perthes’ disease. Paediatr Perinat Epidemiol 1989; 3(2): 131–135. [DOI] [PubMed] [Google Scholar]
- 47. Perry CA. Perthes’ disease and blood manganese levels. Arch Dis Child 2000; 82(5): 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kenet G, Ezra E, Wientroub S, et al. Perthes disease and the search for genetic associations: collagen mutations, Gaucher disease and thrombophilia. J Bone Joint Surg Br 2008; 90(11): 1507–1511. [DOI] [PubMed] [Google Scholar]
- 49. Hayek S, Kenet G, Lubetsky A, et al. Does thrombophilia play an aetiological role in Legg-Calvé-Perthes disease. J Bone Joint Surg Br 1999; 81(4): 686–690. [DOI] [PubMed] [Google Scholar]
- 50. Conway JJ. A scintigraphic classification of Legg-Calvé-Perthes disease. Semin Nucl Med 1993; 23(4): 274–295. [DOI] [PubMed] [Google Scholar]
- 51. Bowen JR, Kumar VP, Joyce JJ, 3rd, et al. Osteochondritis dissecans following Perthes’ disease. Arthroscopic-operative treatment. Clin Orthop Relat Res 1986; 209: 49–56. [PubMed] [Google Scholar]
- 52. Joseph B, Mulpuri K, Varghese G. Perthes’ disease in the adolescent. J Bone Joint Surg Br 2001; 83(5): 715–720. [DOI] [PubMed] [Google Scholar]
- 53. Catterall A, Pringle J, Byers PD, et al. A review of the morphology of Perthes’ disease. J Bone Joint Surg Br 1982; 64(3): 269–275. [DOI] [PubMed] [Google Scholar]
- 54. Jonsater S. Coxa plana; a histo-pathologic and arthrografic study. Acta Orthop Scand Suppl 1953; 12: 5–98. [PubMed] [Google Scholar]
- 55. Joseph B, Varghese G, Mulpuri K, et al. Natural evolution of Perthes disease: a study of 610 children under 12 years of age at disease onset. J Pediatr Orthop 2003; 23(5): 590–600. [DOI] [PubMed] [Google Scholar]
- 56. Joseph B. Natural history of early onset and late-onset Legg-Calve-Perthes disease. J Pediatr Orthop 2011; 31(2, Suppl.): S152–S155. [DOI] [PubMed] [Google Scholar]
- 57. Hyman JE, Trupia EP, Wright ML, et al. Interobserver and intraobserver reliability of the modified Waldenström classification system for staging of Legg-Calvé-Perthes disease. J Bone Joint Surg Am 2015; 97(8): 643–650. [DOI] [PubMed] [Google Scholar]
- 58. Shah H, Singh KA, Joseph B. The “discoid epiphysis” —An uncommon presentation of Legg-Calvé-Perthes disease. J Pediatr Orthop 2022; 42(6): e570–e576. [DOI] [PubMed] [Google Scholar]
- 59. Crawford CJ, LaBerge M, Allen BL, Jr, et al. Growth profiles and articular cartilage characterization in a goat model of Legg-Calve-Perthes disease. J Invest Surg 1995; 8(6): 391–408. [DOI] [PubMed] [Google Scholar]
- 60. Bergmann G, Graichen F, Rohlmann A. Hip joint loading during walking and running, measured in two patients. J Biomech 1993; 26(8): 969–990. [DOI] [PubMed] [Google Scholar]
- 61. Heller MO, Bergmann G, Deuretzbacher G, et al. Musculo-skeletal loading conditions at the hip during walking and stair climbing. J Biomech 2001; 34(7): 883–893. [DOI] [PubMed] [Google Scholar]
- 62. Lauritzen J. Legg-Calvé-Perthes disease. Acta Orthop Scand 1971; 42(5): 456. [PubMed] [Google Scholar]
- 63. Lauritzen J. Legg-Calvé-Perthes disease—a comprehensive study. Acta Orthop Scand 1975; Supplement 159: 26–117. [DOI] [PubMed] [Google Scholar]
- 64. Aarvold A, Lohre R, Chhina H, et al. Dynamic deformation of the femoral head occurs on weightbearing in Legg-Calves-Perthes disease: a translational pilot study. Bone Jt Open 2020; 1(7): 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fuchs RK, Thompson WR, Warden SJ. Bone biology in bone repair biomaterials. 2nd ed. (ed KM Pawelec, JA Planell, Woodhead Publishing Series in Biomaterials). Sawston: Woodhead Publishing, 2019, pp. 15–52. [Google Scholar]
- 66. Morgan EF, Unnikrisnan GU, Hussein AI. Bone mechanical properties in healthy and diseased states. Annu Rev Biomed Eng 2018; 20: 119–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Joseph B, Price CT. Principles of containment treatment aimed at preventing femoral head deformation in Perthes disease. Orthop Clin North Am 2011; 42(3): 317–327, vi. [DOI] [PubMed] [Google Scholar]
- 68. Joseph B. Prognostic factors and outcome measures in Perthes disease. Orthop Clin North Am 2011; 42(3): 303–315, v. [DOI] [PubMed] [Google Scholar]
- 69. Green NE, Beauchamp RD, Griffin PP. Epiphyseal extrusion as a prognostic index in Legg-Calvé-Perthes disease. J Bone Joint Surg Am 1981; 63(6): 900–905. [PubMed] [Google Scholar]
- 70. Kamegaya M, Moriya H, Tsuchiya K, et al. Arthrography of early Perthes’ disease. Swelling of the ligamentum teres as a cause of subluxation. J Bone Joint Surg Br 1989; 71(3): 413–417. [DOI] [PubMed] [Google Scholar]
- 71. Chacko V, Joseph B, Seetharam B. Perthes’ disease in South India. Clin Orthop Relat Res 1986(209): 95–99. [PubMed] [Google Scholar]
- 72. Laine JC, Novotny SA, Tis JE, et al. Demographics and clinical presentation of early-stage Legg-Calvé-Perthes disease: a prospective, multicenter, international study. J Am Acad Orthop Surg 2021; 29(2): e85–e91. [DOI] [PubMed] [Google Scholar]
- 73. Kessler JI, Cannamela PC. What are the demographics and epidemiology of Legg-Calvé-Perthes disease in a large Southern California integrated health system? Clin Orthop Relat Res 2018; 476(12): 2344–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ailabouni R, Zomar BO, Slobogean BL, et al. The natural history of non-operatively managed Legg-Calvé-Perthes’ disease. Indian J Orthop 2022; 56(5): 867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Joseph B, Nair NS, Narasimha Rao K, et al. Optimal timing for containment surgery for Perthes disease. J Pediatr Orthop 2003; 23(5): 601–606. [DOI] [PubMed] [Google Scholar]
- 76. Petrie JG, Bitenc I. The abduction weight-bearing treatment in Legg-Perthes’ disease. J Bone Joint Surg Br 1971; 53(1): 54–62. [PubMed] [Google Scholar]
- 77. Axer A. Subtrochanteric osteotomy in the treatment of Perthes’ disease: a preliminary report. J Bone Joint Surg Br 1965; 47: 489–499. [PubMed] [Google Scholar]
- 78. Salter RB. Legg-Perthes disease: the scientific basis for the methods of treatment and their indications. Clin Orthop Relat Res 1980; 150: 8–11. [PubMed] [Google Scholar]
- 79. Puranen J, Heikkinen E. Intertrochanteric osteotomy in the treatment of Perthes’ disease. Acta Orthop Scand 1976; 47(1): 79–88. [DOI] [PubMed] [Google Scholar]
- 80. Trias A. Femoral osteotomy in Perthes disease. Clin Orthop Relat Res 1978(137): 195–207. [PubMed] [Google Scholar]
- 81. Laurent LE, Poussa M. Intertrochanteric varus osteotomy in the treatment of Perthes’ disease. Clin Orthop Relat Res 1980(150): 73–77. [PubMed] [Google Scholar]
- 82. Cordeiro EN. Femoral osteotomy in Legg-Calvé-Perthes disease. Clin Orthop Relat Res 1980(150): 69–72. [PubMed] [Google Scholar]
- 83. McElwain JP, Regan BF, Dowling F, et al. Derotation varus osteotomy in Perthes disease. J Pediatr Orthop 1985; 5(2): 195–198. [PubMed] [Google Scholar]
- 84. Hoikka V, Lindholm TS, Poussa M. Intertrochanteric varus osteotomy in Legg-Calvé-Perthes disease: a report of 112 hips. J Pediatr Orthop 1986; 6(5): 600–604. [DOI] [PubMed] [Google Scholar]
- 85. Karpinski MR, Newton G, Henry AP. The results and morbidity of varus osteotomy for Perthes’ disease. Clin Orthop Relat Res 1986(209): 30–40. [PubMed] [Google Scholar]
- 86. Hoikka V, Poussa M, Yrjönen T, et al. Intertrochanteric varus osteotomy for Perthes’ disease. Radiographic changes after 2-16-year follow-up of 126 hips. Acta Orthop Scand 1991; 62(6): 549–553. [DOI] [PubMed] [Google Scholar]
- 87. Joseph B, Srinivas G, Thomas R. Management of Perthes disease of late onset in southern India. The evaluation of a surgical method. J Bone Joint Surg Br 1996; 78(4): 625–630. [PubMed] [Google Scholar]
- 88. Friedlander JK, Weiner DS. Radiographic results of proximal femoral varus osteotomy in Legg-Calvé-Perthes disease. J Pediatr Orthop 2000; 20(5): 566–571. [DOI] [PubMed] [Google Scholar]
- 89. Than P, Halmai V, Shaikh S, et al. Long-term results of derotational femoral varus osteotomy in Legg-Calvé-Perthes disease: 26-year follow-up. Orthopedics 2003; 26(5): 487–491. [DOI] [PubMed] [Google Scholar]
- 90. Beer Y, Smorgick Y, Oron A, et al. Long-term results of proximal femoral osteotomy in Legg-Calvé-Perthes disease. J Pediatr Orthop 2008; 28(8): 819–824. [DOI] [PubMed] [Google Scholar]
- 91. Castañeda P, Haynes R, Mijares J, et al. Varus-producing osteotomy for patients with lateral pillar type B and C Legg-Calvé-Perthes disease followed to skeletal maturity. J Child Orthop 2008; 2(5): 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Copeliovitch L. Femoral varus osteotomy in Legg-Calve-Perthes disease. J Pediatr Orthop 2011; 31(2. Suppl.): S189–S191. [DOI] [PubMed] [Google Scholar]
- 93. Terjesen T, Wiig O, Svenningsen S. Varus femoral osteotomy improves sphericity of the femoral head in older children with severe form of Legg-Calvé-Perthes disease. Clin Orthop Relat Res 2012; 470(9): 2394–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shah H, Siddesh ND, Joseph B, et al. Effect of prophylactic trochanteric epiphysiodesis in older children with Perthes’ disease. J Pediatr Orthop 2009; 29(8): 889–895. [DOI] [PubMed] [Google Scholar]
- 95. Shah H, Siddesh ND, Joseph B. To what extent does remodeling of the proximal femur and the acetabulum occur between disease healing and skeletal maturity in Perthes disease? A radiological study. J Pediatr Orthop 2008; 28(7): 711–716. [DOI] [PubMed] [Google Scholar]
- 96. Tercier S, Shah H, Siddesh ND, et al. Does proximal femoral varus osteotomy in Legg-Calvé-Perthes disease predispose to angular mal-alignment of the knee? A clinical and radiographic study at skeletal maturity. J Child Orthop 2013; 7(3): 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Braito M, Wolf S, Dammerer D, et al. Global differences in the treatment of Legg-Calvé-Perthes disease: a comprehensive review. Arch Orthop Trauma Surg 2021; 141(1): 1–16. [DOI] [PubMed] [Google Scholar]
- 98. Barer M. Role of innominate osteotomy in the treatment of children with Legg-Perthes disease. Clin Orthop Relat Res 1978(135): 82–89. [PubMed] [Google Scholar]
- 99. Cotler JM, Donahue J. Innominate osteotomy in the treatment of Legg-Calvé-Perthes disease. Clin Orthop Relat Res 1980(150): 95–102. [PubMed] [Google Scholar]
- 100. Stevens PM, Williams P, Menelaus M. Innominate osteotomy for Perthes’ disease. J Pediatr Orthop 1981; 1(1): 47–54. [DOI] [PubMed] [Google Scholar]
- 101. Maxted MJ, Jackson RK. Innominate osteotomy in Perthes’ disease. A radiological survey of results. J Bone Joint Surg Br 1985; 67(3): 399–401. [DOI] [PubMed] [Google Scholar]
- 102. Robinson HJ, Jr, Putter H, Sigmond MB, et al. Innominate osteotomy in Perthes disease. J Pediatr Orthop 1988; 8(4): 426–435. [DOI] [PubMed] [Google Scholar]
- 103. Ishida A, Kuwajima SS, Laredo Filho J, et al. Salter innominate osteotomy in the treatment of severe Legg-Calvé-Perthes disease: clinical and radiographic results in 32 patients (37 hips) at skeletal maturity. J Pediatr Orthop 2004; 24(3): 257–264. [PubMed] [Google Scholar]
- 104. Sanchez Mesa PA, Yamhure FH. Percutaneous innominate pelvic osteotomy without the use of bone graft for femoral head coverage in children 2-8 years of age. J Pediatr Orthop B 2010; 19(3): 256–263. [DOI] [PubMed] [Google Scholar]
- 105. Thompson GH. Salter osteotomy in Legg-Calvé-Perthes disease. J Pediatr Orthop 2011; 31(2, Suppl.): S192–S197. [DOI] [PubMed] [Google Scholar]
- 106. Wenger DR, Pandya NK. Advanced containment methods for the treatment of Perthes disease: Salter plus varus osteotomy and triple pelvic osteotomy. J Pediatr Orthop 2011; 31(2, Suppl.): S198–S205. [DOI] [PubMed] [Google Scholar]
- 107. Stepanovich M, Upasani VV, Bomar JD, et al. Advanced containment with triple innominate osteotomy in Legg-Calve-Perthes disease: a viable option even in severe cases. J Pediatr Orthop 2017; 37(8): 563–569. [DOI] [PubMed] [Google Scholar]
- 108. Wenger DR, Pring ME, Hosalkar HS, et al. Advanced containment methods for Legg-Calvé-Perthes disease: results of triple pelvic osteotomy. J Pediatr Orthop 2010; 30(8): 749–757. [DOI] [PubMed] [Google Scholar]
- 109. Vukasinovic Z, Spasovski D, Vucetic C, et al. Triple pelvic osteotomy in the treatment of Legg-Calve-Perthes disease. Int Orthop 2009; 33(5): 1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pailhé R, Cavaignac E, Murgier J, et al. Triple osteotomy of the pelvis for Legg-Calve-Perthes disease: a mean fifteen year follow-up. Int Orthop 2016; 40(1): 115–122. [DOI] [PubMed] [Google Scholar]
- 111. Conroy E, Sheehan E, O’Connor P, et al. Triple pelvic osteotomy in Legg–Calve–Perthes disease using a single anterolateral incision: a 4-year review. J Pediatr Orthop B 2010; 19(4): 323–326. [DOI] [PubMed] [Google Scholar]
- 112. Hosalkar H, Munhoz da, Cunha AL, Baldwin K, et al. Triple innominate osteotomy for Legg-Calvé-Perthes disease in children: does the lateral coverage change with time? Clin Orthop Relat Res 2012; 470(9): 2402–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yoo WJ, Choi IH, Cho T-J, et al. Shelf acetabuloplasty for children with Perthes’ disease and reducible subluxation of the hip: prognostic factors related to hip remodelling. J Bone Joint Surg Br 2009; 91(10): 1383–1387. [DOI] [PubMed] [Google Scholar]
- 114. Ghanem I, Haddad E, Haidar R, et al. Lateral shelf acetabuloplasty in the treatment of Legg-Calvé-Perthes disease: improving mid-term outcome in severely deformed hips. J Child Orthop 2010; 4(1): 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Carsi B, Judd J, Clarke NM. Shelf acetabuloplasty for containment in the early stages of Legg-Calve-Perthes disease. J Pediatr Orthop 2015; 35(2): 151–156. [DOI] [PubMed] [Google Scholar]
- 116. Li WC, Xu RJ. Lateral shelf acetabuloplasty for severe Legg-Calvé-Perthes disease in patients older than 8 years: a mean eleven-year follow-up. Medicine (Baltimore) 2016; 95(45): e5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jacobs R, Moens P, Fabry G. Lateral shelf acetabuloplasty in the early stage of Legg-Calvé-Perthes disease with special emphasis on the remaining growth of the acetabulum: a preliminary report. J Pediatr Orthop B 2004; 13(1): 21–28. [DOI] [PubMed] [Google Scholar]
- 118. Klisić PJ. Treatment of Perthes’ disease in older children. J Bone Joint Surg Br 1983; 65(4): 419–427. [DOI] [PubMed] [Google Scholar]
- 119. Johnston CE, Siegel MS. Chiari osteotomy for severe deformity in Legg-Calvé-Perthes disease. Orthopedics 1983; 6(9): 1199–1203. [DOI] [PubMed] [Google Scholar]
- 120. Klisic P, Bauer R, Bensahel H, et al. Chiari’s pelvic osteotomy in the treatment of Legg-Calvé-Perthes disease. Bull Hosp Jt Dis Orthop Inst 1985; 45(2): 111–118. [PubMed] [Google Scholar]
- 121. Lack W, Feldner-Busztin H, Ritschl P, et al. The results of surgical treatment for Perthes’ disease. J Pediatr Orthop 1989; 9(2): 197–204. [PubMed] [Google Scholar]
- 122. Cahuzac JP, Onimus M, Trottmann F, et al. Chiari pelvic osteotomy in Perthes disease. J Pediatr Orthop 1990; 10(2): 163–166. [PubMed] [Google Scholar]
- 123. Bennett JT, Mazurek RT, Cash JD. Chiari’s osteotomy in the treatment of Perthes’ disease. J Bone Joint Surg Br 1991; 73(2): 225–228. [DOI] [PubMed] [Google Scholar]
- 124. Reddy RR, Morin C. Chiari osteotomy in Legg-Calve-Perthes disease. J Pediatr Orthop B 2005; 14(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 125. Napiontek M, Pietrzak S. Double osteotomy in the surgical treatment of Perthes’ disease: Dega’s transiliac osteotomy and subtrochanteric osteotomy. Ortop Traumatol Rehabil 2004; 6(6): 728–732. [PubMed] [Google Scholar]
- 126. Javid M, Wedge JH. Radiographic results of combined Salter innominate and femoral osteotomy in Legg-Calvé-Perthes disease in older children. J Child Orthop 2009; 3(3): 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mosow N, Vettorazzi E, Breyer S, et al. Outcome after combined pelvic and femoral osteotomies in patients with Legg-Calvé-Perthes disease. J Bone Joint Surg Am 2017; 99(3): 207–213. [DOI] [PubMed] [Google Scholar]
- 128. Pisecky L, Großbötzl G, Stevoska S, et al. Short term radiological outcome of combined femoral and ilium osteotomy in pelvic reconstruction of the child. Children (Basel) 2022; 9(3): 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kim HK, Aruwajoye O, Stetler J, et al. Effects of non-weight-bearing on the immature femoral head following ischemic osteonecrosis: an experimental investigation in immature pigs. J Bone Joint Surg Am 2012; 94(24): 2228–2237. [DOI] [PubMed] [Google Scholar]
- 130. Peck JB, Greenhill DA, Morris WZ, et al. Prolonged non-weightbearing treatment decreases femoral head deformity compared to symptomatic treatment in the initial stage of Legg-Calvé-Perthes disease. J Pediatr Orthop B 2022; 31(3): 209–215. [DOI] [PubMed] [Google Scholar]
- 131. Do DH, McGuire MF, Jo CH, et al. Weightbearing and activity restriction treatments and quality of life in patients with Perthes disease. Clin Orthop Relat Res 2021; 479(6): 1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Shah H, Singh KA, Joseph B. Does prolonged weight relief increase the chances of a favourable outcome after containment for Perthes disease? J Pediatr Orthop 2023; 43(2): e144–e150. [DOI] [PubMed] [Google Scholar]
- 133. Wenger DR, Ward TW, Herring JA. Current concept review. Legg-Calvé-Perthes disease. J Bone Joint Surgery 1991; 73-A: 778–788. [PubMed] [Google Scholar]
- 134. Gage JR, Cary JM. The effects of trochanteric epiphysiodesis on growth of the proximal end of the femur following necrosis of the capital femoral epiphysis. J Bone Joint Surg Am 1980; 62(5): 785–794. [PubMed] [Google Scholar]
- 135. Evans IK, Deluca PA, Gage JR. A comparative study of ambulation-abduction bracing and varus derotation osteotomy in the treatment of severe Legg-Calvé-Perthes disease in children over 6 years of age. J Pediatr Orthop 1988; 8(6): 676–682. [DOI] [PubMed] [Google Scholar]
- 136. McCarthy JJ, Weiner DS. Greater trochanteric epiphysiodesis. Int Orthop 2008; 32(4): 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kitoh H, Kaneko H, Mishima K, et al. Prognostic factors for trochanteric overgrowth after containment treatment in Legg-Calvé-Perthes disease. J Pediatr Orthop B 2013; 22(5): 432–436. [DOI] [PubMed] [Google Scholar]
- 138. Stevens PM, Anderson LA, Gililland JM, et al. Guided growth of the trochanteric apophysis combined with soft tissue release for Legg-Calve-Perthes disease. Strategies Trauma Limb Reconstr 2014; 9(1): 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Eidelman M, Kotlarsky P. Does transepiphyseal drilling and closure of the greater trochanter in early Legg-Calve-Perthes disease improve natural history? Musculoskelet Surg 2023; 107: 279–285. [DOI] [PubMed] [Google Scholar]
- 140. Herring JA, Neustadt JB, Williams JJ, et al. The lateral pillar classification of Legg-Calvé-Perthes disease. J Pediatr Orthop 1992; 12(2): 143–150. [DOI] [PubMed] [Google Scholar]
- 141. Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part I: classification of radiographs with use of the modified lateral pillar and Stulberg classifications. J Bone Joint Surg Am 2004; 86(10): 2103–2120. [PubMed] [Google Scholar]
- 142. Rosenfeld SB, Herring JA, Chao JC. Legg-Calve-Perthes disease: a review of cases with onset before six years of age. J Bone Joint Surg Am 2007; 89(12): 2712–2722. [DOI] [PubMed] [Google Scholar]
- 143. Muirhead-Allwood W, Catterall A. The treatment of Perthes’ disease. The results of a trial of management. J Bone Joint Surg Br 1982; 64(3): 282–285. [DOI] [PubMed] [Google Scholar]
- 144. Quain S, Catterall A. Hinge abduction of the hip. Diagnosis and treatment. J Bone Joint Surg Br 1986; 68(1): 61–64. [DOI] [PubMed] [Google Scholar]
- 145. Reinker KA. Early diagnosis and treatment of hinge abduction in Legg-Perthes disease. J Pediatr Orthop 1996; 16(1): 3–9. [DOI] [PubMed] [Google Scholar]
- 146. Shore BJ, Miller PE, Zaltz I, et al. Determining hinge abduction in Legg-Calvé-Perthes disease: can we reliably make the diagnosis. J Pediatr Orthop 2019; 39(2): e95–e101. [DOI] [PubMed] [Google Scholar]
- 147. Freeman RT, Wainwright AM, Theologis TN, et al. The outcome of patients with hinge abduction in severe Perthes disease treated by shelf acetabuloplasty. J Pediatr Orthop 2008; 28(6): 619–625. [DOI] [PubMed] [Google Scholar]
- 148. Yoo WJ, Choi IH, Chung CY, et al. Valgus femoral osteotomy for hinge abduction in Perthes’ disease. Decision-making and outcomes. J Bone Joint Surg Br 2004; 86(5): 726–730. [DOI] [PubMed] [Google Scholar]
- 149. Patil S, Sherlock D. Valgus osteotomy for hinge abduction in avascular necrosis. J Pediatr Orthop B 2006; 15(4): 262–266. [DOI] [PubMed] [Google Scholar]
- 150. de Gheldere A, Eastwood DM. Valgus osteotomy for hinge abduction. Orthop Clin North Am 2011; 42(3): 349–54, vi–vii. [DOI] [PubMed] [Google Scholar]
- 151. Farsetti P, Benedetti-Valentini M, Potenza V, et al. Valgus extension femoral osteotomy to treat “hinge abduction” in Perthes’ disease. J Child Orthop 2012; 6(6): 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yoo WJ, Choi IH, Moon HJ, et al. Valgus femoral osteotomy for noncontainable Perthes hips: prognostic factors of remodeling. J Pediatr Orthop 2013; 33(6): 650–655. [DOI] [PubMed] [Google Scholar]
- 153. Kurup HV, Clarke NM. Sugioka transtrochanteric valgus osteotomy for hinge abduction in children. J Pediatr Orthop 2011; 31(7): 727–731. [DOI] [PubMed] [Google Scholar]
- 154. Albers CE, Steppacher SD, Ganz R, et al. Joint-preserving surgery improves pain, range of motion, and abductor strength after Legg-Calvé-Perthes disease. Clin Orthop Relat Res 2012; 470(9): 2450–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Leunig M, Ganz R. Relative neck lengthening and intracapital osteotomy for severe Perthes and Perthes-like deformities. Bull NYU Hosp Jt Dis 2011; 69(Suppl. 1): S62–S67. [PubMed] [Google Scholar]
- 156. Kim YJ, Novais EN. Diagnosis and treatment of femoroacetabular impingement in Legg-Calvé-Perthes disease. J Pediatr Orthop 2011; 31(2, Suppl.): S235–S240. [DOI] [PubMed] [Google Scholar]
- 157. Novais EN, Clohisy J, Siebenrock K, et al. Treatment of the symptomatic healed Perthes hip. Orthop Clin North Am 2011; 42(3): 401–417, viii [DOI] [PubMed] [Google Scholar]
- 158. Mose K. Methods of measuring in Legg-Calvé-Perthes disease with special regard to the prognosis. Clin Orthop Relat Res 1980; 150: 103–109. [PubMed] [Google Scholar]
- 159. Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg-Calvé-Perthes disease. J Bone Joint Surg Am 1981; 63(7): 1095–1108. [PubMed] [Google Scholar]
- 160. Shah H, Siddesh ND, Pai H, et al. Quantitative measures for evaluating the radiographic outcome of Legg-Calvé-Perthes disease. J Bone Joint Surg Am 2013; 95(4): 354–361. [DOI] [PubMed] [Google Scholar]
- 161. Siddesh ND, Shah H, Tercier S, et al. The sphericity deviation score: a quantitative radiologic outcome measure of Legg-Calvé Perthes disease applicable at the stage of healing and at skeletal maturity. J Pediatr Orthop 2014; 34(5): 522–528. [DOI] [PubMed] [Google Scholar]
- 162. Kang MS, Kak A, Prasadh JG, et al. Reliability of the modified method for sphericity deviation score using only the involved hip radiographs in the patients with Legg-Calve-Perthes disease. J Pediatr Orthop 2023; 43: e554–e560. [DOI] [PubMed] [Google Scholar]
- 163. Westhoff B, Zilkens C, Reith A, et al. Correlation of functional outcome and X-ray findings after Perthes disease. Int Orthop 2011; 35(12): 1833–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hailer YD, Penno E. Agreement of radiographic measurements and patient-reported outcome in 61 patients with Legg-Calvé-Perthes disease at mean follow-up of 28 years. J Pediatr Orthop B 2019; 28(2): 100–106. [DOI] [PubMed] [Google Scholar]
- 165. Luo W, Ali MS, Limb R, et al. Use of the PROMIS mobility score in assessing function in adolescents and adults previously affected by childhood hip disease. Bone Jt Open 2021; 2(12): 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ali MS, Khattak M, Metcalfe D, et al. Radiological hip shape and patient-reported outcome measures in healed Perthes’ disease. Bone Joint J 2023; 105-B(6): 711–716. [DOI] [PubMed] [Google Scholar]
- 167. Saran N, Varghese R, Mulpuri K. Do femoral or salter innominate osteotomies improve femoral head sphericity in Legg-Calvé-Perthes disease? A meta-analysis. Clin Orthop Relat Res 2012; 470(9): 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part II: prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am 2004; 86(10): 2121–2134. [PubMed] [Google Scholar]
- 169. Wiig O, Terjesen T, Svenningsen S. Prognostic factors and outcome of treatment in Perthes’ disease: a prospective study of 368 patients with five-year follow-up. J Bone Joint Surg Br 2008; 90(10): 1364–1371. [DOI] [PubMed] [Google Scholar]
- 170. Perry DC, Arch B, Appelbe D, et al. The British Orthopaedic Surgery Surveillance study: Perthes’ disease: the epidemiology and two-year outcomes from a prospective cohort in Great Britain. Bone Joint J 2022; 104-B(4): 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hardesty CK, Liu RW, Thompson GH. The role of bracing in Legg-Calve-Perthes disease. J Pediatr Orthop 2011; 31(2, Suppl.): S178–S181. [DOI] [PubMed] [Google Scholar]
- 172. Rich MM, Schoenecker PL. Management of Legg-Calvé-Perthes disease using an A-frame orthosis and hip range of motion: a 25-year experience. J Pediatr Orthop 2013; 33(2): 112–119. [DOI] [PubMed] [Google Scholar]
- 173. Kim WC, Hosokawa M, Tsuchida Y, et al. Outcomes of new pogo-stick brace for Legg-Calvé-Perthes’ disease. J Pediatr Orthop B 2006; 15(2): 98–103. [DOI] [PubMed] [Google Scholar]
- 174. Little DG, Kim HK. Potential for bisphosphonate treatment in Legg-Calve-Perthes disease. J Pediatr Orthop 2011; 31(2, Suppl.): S182–S188. [DOI] [PubMed] [Google Scholar]
- 175. Young ML, Little DG, Kim HK. Evidence for using bisphosphonate to treat Legg-Calvé-Perthes disease. Clin Orthop Relat Res 2012; 470(9): 2462–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Cheng TL, Murphy CM, Cantrill LC, et al. Local delivery of recombinant human bone morphogenetic proteins and bisphosphonate via sucrose acetate isobutyrate can prevent femoral head collapse in Legg-Calve-Perthes disease: a pilot study in pigs. Int Orthop 2014; 38(7): 1527–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Jamil K, Zacharin M, Foster B, et al. Protocol for a randomised control trial of bisphosphonate (zoledronic acid) treatment in childhood femoral head avascular necrosis due to Perthes disease. BMJ Paediatr Open 2017; 1(1): e000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cho-10.1177_18632521231203009 for Epidemiology, natural evolution, pathogenesis, clinical spectrum, and management of Legg–Calvé–Perthes by Benjamin Joseph, Hitesh Shah and Daniel C Perry in Journal of Children’s Orthopaedics