Abstract
Introduction
Untreated brain aneurysms are usually surveilled with serial MR imaging and evaluated with 2D multiplanar measurements. The assessment of aneurysm growth may be more accurate with volumetric analysis. We evaluated the accuracy of a magnetic resonance angiography (MRA) segmentation pipeline for aneurysm volume measurement and surveillance.
Methods
A pipeline to determine aneurysm volume was developed and tested on two aneurysm phantoms imaged with time-of flight (TOF) MRA and 3D rotational angiography (3DRA). The accuracy of the pipeline was then evaluated by reconstructing 10 aneurysms imaged with contrast enhanced-MRA (CE-MRA) and 3DRA. This calibrated and refined post-processing pipeline was subsequently used to analyse aneurysms from our prospectively acquired database. Volume changes above the threshold of error were considered true volume changes. The accuracy of these measurements was analysed.
Results
TOF-MRA reconstructions were not as accurate as CE-MRA reconstructions. When compared to 3DRA, CE-MRA underestimated aneurysm volume by 7.8% and did not accurately register the presence of blebs. Eighteen aneurysms (13 saccular and 5 fusiform) were analysed with the optimized 3D volume reconstruction pipeline, with a mean follow-up time of 11 months. Artifact accounted for 10.2% error in volume measurements using serial CE-MRA. When this margin of error was used to assess aneurysms volume in serial imaging with CE-MRA, only two fusiform aneurysms changed in volume. The variations in volume of these two fusiform aneurysms were caused by intra-mural and intrasaccular thrombosis.
Conclusions
CE-MRA and TOF-MRA 3D volume reconstructions may not register minor morphological changes such as the appearance of blebs. CE-MRA underestimates volume by 7.8% compared to 3DRA. Serial CE-MRA volume measurements had a larger margin of error of approximately 10.2%. MRA-based volumetric measurements may not be appropriate for aneurysm surveillance.
Keywords: Aneurysm, brain, magnetic resonance angiography, technology, stroke
Introduction
Aneurysms that grow have a higher risk of rupture.1,2 Current methods of surveilling and assessing aneurysm growth rely on 2D measurements taken from noninvasive angiographic imaging. These methods have high intraobserver and interobserver variability.3,4 Volume measurements may allow the detection of subtle changes in aneurysm size and morphology that may be undetectable by conventional 2D size measurements. 5
Predictive indices for intracranial aneurysm growth and rupture using volume and computational techniques are based on accurate 3D reconstructions from angiographic data. 6 The quality of 3D reconstructions is limited by image resolution, movement artifact and variable thresholding. 7 3D rotational angiography (3DRA) is the gold standard, providing the highest spatial resolution compared to contrast enhanced-magnetic resonance angiography (CE-MRA), time-of-flight MRA (TOF-MRA) or computerized tomographic angiography (CTA).8,9 However, both 3DRA and CTA use ionizing radiation. MRA is less invasive and is the preferred method of aneurysm surveillance.10,11
The main aim of this study is to evaluate the feasibility of using volume measurements to determine aneurysm growth. Towards this aim we developed a pipeline to generate 3D aneurysm volumes. We compared MRA volumetric measurements with 3DRA. Finally, we tested this pipeline in a cohort of patients with CE-MRA follow-up imaging. Aneurysm measurements were evaluated for reproducibility, and changes in volume and size.
Methods
After institutional review board approval, the [University of Iowa] aneurysm database was screened for analysis. Written informed consent was obtained at the time of imaging for data and imaging collection and processing. Aneurysms of at least 2 mm in size with good quality high-resolution 3T MRA were included. Detailed acquisition parameters are described in Supplementary Table 1. A reliable pipeline to determine the volume of brain aneurysms was developed with a two-step validation and calibration process. The first aim was to validate the segmentation protocol and 3D volume reconstruction pipeline in two saccular aneurysm phantoms imaged with 3DRA (gold standard) and time-of-flight MRA (TOF-MRA). The second step was aimed at comparing CE-MRA and 3DRA reconstructions of patients with intracranial aneurysms, to determine possible errors in MRA. Once the pipeline for segmentation and 3D volume reconstruction was developed and optimized, we proceeded to analyse a cohort of patients with baseline imaging and least one follow-up CE-MRA.
Optimization of the segmentation and 3d volume reconstruction pipeline
1) 3d volume reconstruction in phantoms
The Vascular Modeling Toolkit (VMTK) was used to perform semi-automatic 3D reconstructions based on methods previously published by Ramachandran et al.12–15 Parent vessels and aneurysm sacs were segmented using the colliding fronts and fast-marching algorithms respectively. Standardized parameters were used for iterations, curvature, and smoothing. Surface reconstructions were registered using the iterative closest point algorithm and superimposed to visually evaluate the fidelity of the registration. 16
Two ruptured saccular aneurysms from patients were segmented from 3DRA imaging and printed to scale to produce phantoms (Biomodex, Quincy, MA). A continuous flow pump was used to simulate flow through the phantom aneurysm and 3DRA images were acquired with a Siemens Artis Q biplane machine. A 100 ml of iodine contrast/water (60/40) mixture was used to acquire subtracted images. The phantoms were then imaged with a GE 3T MRI. (Supplementary Table 1). A Masterflex L/S High Performance Pump system (Cole-Parmer, Vernon Hills, IL) was used to continuously circulate 6 L of water through the tubing system and the aneurysm phantom model. Continuous flow was applied at a rate of 0.2 L/min, and TOF-MRA images were acquired. 3D volume reconstructions were created using 3DRA and TOF-MRA images.
2) In-vivo 3d volume reconstruction
To characterize the effect of pulsation/movement artifact on the 3D volume reconstruction pipeline, a cohort of ten patients was analysed. In this way, the pipeline was optimized with an in-vivo sample of aneurysms. 3D reconstructions of 3DRA were acquired with a Siemens Artis Q biplane machine and then co-registered with CE-MRAs. A Siemens 3T MRI was used to acquire CE-MRAs. (Supplementary Table 1). The volume of both 3DRA and CE-MRAs was calculated. Aneurysms were isolated at the neck and the parent vessel was excluded. Parent arteries were used as internal fiducials and recalibration was performed in case of misalignment. The average percent error of volume measurements between 3DRA and CE-MRA was calculated by defining 3DRA as the gold standard. Measurements of common 2D morphological metrics such as aneurysm size, size ratio, and aspect ratio were manually taken from source imaging of 3DRA and CE-MRA in the same projection.
Test of the 3d volume reconstruction pipeline in the surveillance of patients
1) 2d multiplanar size measurements
Aneurysms > 2 mm in diameter were prospectively imaged with serial 3T CE-MRA (Siemens) between April 2018 and June 2021 (Supplementary Table 1). Aneurysms were measured using the standard 2D multiplanar method by two investigators. Measurements were performed in the same slices to limit sampling errors. Aneurysm neck width, sac height, and largest diameter were measured in the cardinal planes of CE-MRA at baseline and follow-up. 17 Only the largest diameter was measured for fusiform aneurysms. Growth was defined as an increase in aneurysm dimension greater than 1 mm. A senior neuroendovascular surgeon adjudicated definitive measurements when there were differences between the two investigators of > 1 mm.
2) 3d volume measurements
Aneurysms and parent vessels were reconstructed in 3D from baseline and follow-up imaging using the protocol outlined above. Anatomical landmarks such as bifurcations were used when the neck of the aneurysm was not very apparent. Volume was measured for baseline and follow-up reconstructions after the aneurysm was isolated. The petrous segment of the ICA contralateral to the aneurysms was used to determine the threshold of artifact not attributed to vascular changes (Supplementary Figure 1). The average percent difference in the reconstructions of the petrous segments between baseline and follow-up imaging was calculated. The petrous segment of the carotid artery was used as a disease-free arterial segment to correct for the presence of motion artifact and minor differences in intensity. 7 This thresholding step was used to determine the margin of error above which true changes in aneurysm volume could be documented.
Statistical analysis
Normally distributed data was described using the average (µ) and standard deviation (σ), and non-normally distributed data was described with median and interquartile range (IQR). Independent comparisons between non-normally distributed data were conducted using Mann-Whitney U Tests and Spearman’s correlations. Similar comparisons between normally distributed data were conducted using Student’s T-Tests and Pearson’s correlations. Reliability analysis of continuous data was conducted using intraclass correlations. All statistical analysis was conducted using SPSS Statistics 27.0 (IBM Corp., Armonk, NY).
Results
3D volume reconstructions in phantoms imaged with 3DRA and TOF-MRA
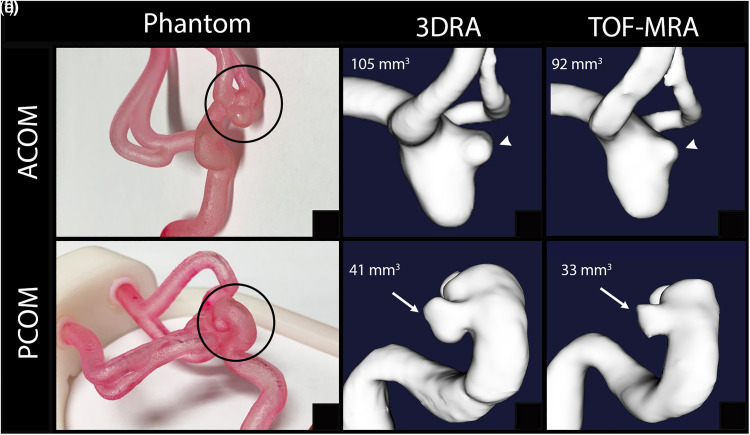
Two phantoms of patients with ruptured saccular aneurysms: 5 mm ACOM and 6 mm PCOM, were imaged with 3DRA and TOF-MRA (Figure 1). Morphological parameters were reliably reproduced with segmentations of the parent arteries and aneurysms. Manual measurements of aneurysm size from source imaging between 3DRA and TOF were the same. The size and aspect ratios of the ACOM aneurysm were 3.1 and 1.7 respectively on 3DRA imaging and 2.7 and 1.5 respectively on TOF imaging. The size and aspect ratios of the PCOM aneurysm were 0.66 and 0.62 respectively on 3DRA imaging and 0.66 and 0.52 respectively on TOF imaging. The volume difference between 3DRA and TOF-MRA reconstructions was 12.3% for the ACOM aneurysm and 20.2% for the PCOM aneurysm. TOF-MRA underestimated the aneurysm volume.
Figure 1.
3d reconstruction based on phantoms (circles in Panels a and d). TOF-MRA underestimates the volume compared to 3DRA. The TOF-MRA reconstruction of an ACOM aneurysm (Panel c) underestimates the size of the bleb compared to 3DRA (Panel b). In a PCOM aneurysm (Panels d-f), the aneurysm sac (arrows) is undersized on TOF-MRA (Panel f) compared to 3DRA reconstructions.
In-vivo 3d volume reconstructions of aneurysms imaged with 3DRA and Ce-MRA
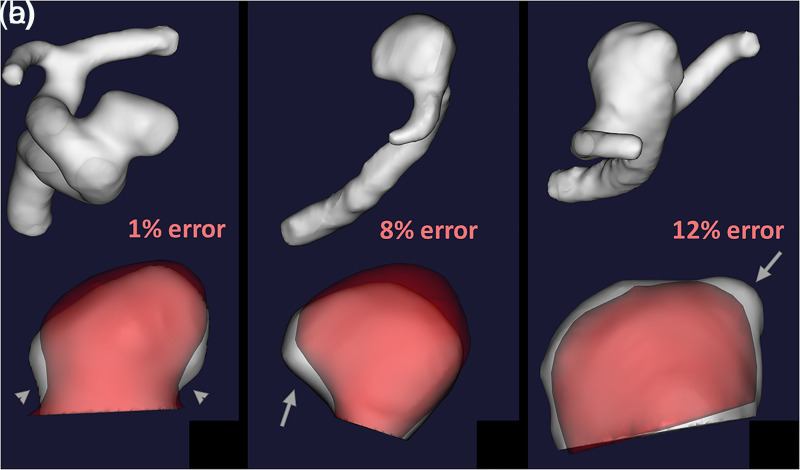
The 3D volume reconstruction pipeline previously tested in the two phantom aneurysms was now tested in patients with in-vivo imaging. Ten aneurysms with a median diameter of 6 mm were analysed with 3DRA and CE-MRA reconstructions (Table 1). The median (IQR) time between CE-MRA and 3DRA imaging was 6 (13) days. 2D measurements of aneurysm size, aspect ratio, and size ratio taken on 3DRA and CE-MRA source imaging were strongly correlated (Spearman’s rho = 0.973, 0.874, and 0.967 respectively) (Supplementary Table 2). The median (IQR) difference between volume measurements was 15 mm3 (8 mm3). The median (IQR) percent error between 3DRA and CE-MRA volumes was 7.8% (3.6%). Registration mismatch artifact accounted for 2.6% of this error. CE-MRA underestimated aneurysm volume compared to 3DRA (median difference = −12.8 mm3). Of the ten aneurysms analysed, seven (70%) had blebs. Two blebs (28.5%) documented on 3DRA were not visualized on CE-MRA, which led to errors in the quantification of volume (Figure 2).
Table 1.
In-vivo volume measurements of ten aneurysms.
| Location | Bleb | Span (d) | Diameter (mm) | 3DRA Volume (mm3) | MRA Volume (mm3) | Difference (mm3) | % Error |
|---|---|---|---|---|---|---|---|
| MCA | N | 13 | 5.1 | 216.3 | 234.0 | 17.7 | 8.19% |
| BA | N | 1 | 6.0 | 426.9 | 392.7 | −34.2 | 8.00% |
| BA | N | 0 | 7.5 | 323.9 | 311.5 | −12.4 | 3.84% |
| PCOM | Y | 15 | 7.0 | 209.2 | 195.9 | −13.2 | 6.33% |
| PCOM | Y | 0 | 7.8 | 486.3 | 467.4 | −18.9 | 3.90% |
| MCA | Y | 14 | 4.8 | 141.7 | 124.5 | −14.3 | 12.2% |
| MCA | Y | 2 | 5.7 | 268.7 | 247.3 | −21.4 | 7.96% |
| ICA | Y | 10 | 4.0 | 173.3 | 174.3 | 1.06 | 0.61% |
| MCA | Y | 32 | 4.0 | 69.53 | 59.63 | −9.90 | 14.2% |
| ACOM | Y | 1 | 6.0 | 109.3 | 117.5 | 8.24 | 7.54% |
| Median | 6 | 5.9 | 212.8 | 215.0 | −12.8 | 7.75% | |
| IQR | 13 | 1.9 | 160.5 | 158.5 | 8.6 | 3.64% | |
Span is the time between scans (d = days).
Figure 2.
3DRA (white) and CE-MRA (red) 3D reconstructions are co-registered to show reconstruction errors. The neck of the supraclinoid ICA aneurysm was underestimated by CE-MRA (Panel A, arrowheads) which led to a 1% volumetric error. The MCA bifurcation aneurysm (Panel B) was poorly registered which led to an 8% volumetric error (arrow). In the second MCA bifurcation aneurysm (Panel C), the CE-MRA did not register a bleb (arrow), which led to a 12% volumetric error.
Test of the 3d volume reconstruction pipeline in the surveillance of patients imaged with Ce-MRA
After the 3D volume reconstruction pipeline was optimized in two phantom aneurysms and a cohort of ten patients with CE-MRA and 3DRA imaging, it was tested in patients with CE-MRA follow-up imaging.
Size measurements
Sixteen patients with 22 aneurysms were screened for the study. Four aneurysms were excluded due to poor image quality. The final cohort consisted of 16 patients with 18 aneurysms: 13 saccular and 5 fusiform. The (µ ± σ) follow-up time was 11 ± 7.2 months. Two fusiform aneurysms had intrasaccular thrombosis (Table 2, #14, #16). Changes in size measurements are reported in Supplementary Table 3. Fusiform aneurysms were larger in diameter (µ ± σ = 10.9 ± 4.7 mm) than saccular aneurysms (µ ± σ = 3.6 ± 1.0 mm). Fusiform aneurysms exhibited growth in the largest diameter (µ ± σ = 0.58 ± 0.57 mm), whereas saccular aneurysms did not change in size (µ ± σ = 0.00 ± 0.39 mm).
Table 2.
Size and volume measurements of surveilled aneurysms. .
| Diameter | Sac Height | Neck Width | Volume | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Location | Span (m) | BL (mm) | Δ (mm) | BL (mm) | Δ (mm) | BL (mm) | Δ (mm) | BL (mm3) | Δ (mm3) | Δ (%) |
| 1 | MCA | 15.8 | 2.2 | 0.2 | 1.6 | −0.1 | 1.2 | 0 | 18.5 | −0.8 | −4.3 |
| 2 | MCA | 2.8 | 2.4 | 0.1 | 1.6 | 0.1 | 2.4 | 0.1 | 32 | −1.8 | −5.6 |
| 3 | MCA | 19.5 | 2.5 | 0.7 | 3.2 | 0.8 | 1.2 | 0.6 | 36.7 | 0.7 | 1.9 |
| 4 | ICA | 13.6 | 2.7 | 0.5 | 2.5 | 0.2 | 2 | 0 | 80.3 | 0.9 | 1.1 |
| 5 | ICA | 6.9 | 3 | 0 | 2.3 | −0.4 | 3 | 0 | 119.8 | −6.5 | −5.4 |
| 6 | MCA | 17.5 | 3.2 | 0.1 | 4 | 0.3 | 1.9 | 0.1 | 42.5 | −3.5 | −8.2 |
| 7 | MCA | 1.6 | 3.6 | −0.4 | 3.3 | −0.5 | 3.3 | −0.6 | 49.9 | 5.1 | 10.1 |
| 8 | MCA | 2.7 | 3.8 | −0.2 | 3.4 | 0.2 | 3.8 | −0.6 | 62.9 | −0.7 | −1.1 |
| 9 | BA | 17.5 | 4 | −0.4 | 4 | −0.4 | 1.7 | 0.4 | 11 | 0.6 | 5.5 |
| 10 | ICA | 17.7 | 4.3 | 0 | 7 | −0.1 | 2.9 | −0.6 | 165.2 | −9.4 | −5.7 |
| 11 | PICA | 2.4 | 4.8 | −0.6 | 5 | −0.4 | 1.7 | −0.3 | 88.9 | 0.8 | 0.9 |
| 12 | ACOM | 13.6 | 5 | 0.4 | 5.8 | −0.5 | 4 | −0.2 | 63.9 | 4.1 | 6.4 |
| 13 | ICA | 7 | 5.2 | −0.4 | 2.8 | −0.6 | 2.3 | 1.1 | 186.5 | 1.3 | 0.7 |
| 14 | VB*† | 5.4 | 7.6 | 1.1 | 1913.9 | 440.9 | 23.0 | ||||
| 15 | BA* | 22.6 | 7.6 | 0.2 | 530.7 | −2.3 | −0.4 | ||||
| 16 | PCOM*† | 4.3 | 9.4 | 0.1 | 1072.5 | −513.3 | −47.9 | ||||
| 17 | BA* | 6.4 | 11.2 | 0.2 | 1503 | 73.9 | 4.9 | ||||
| 18 | ICA* | 19.7 | 18.9 | 1.3 | 2954.3 | 1837.9 | 62.2 | ||||
Span is the time between scans (m = months).
*Denotes fusiform aneurysms, †denotes thrombosed aneurysms, BL = baseline, bold values indicate 2D length or volume change above the threshold (1 mm for length and 10.2% for volume).
2D measurements of aneurysm size showed high intraclass correlation. Largest diameter was the most reliable measurement (ICC = 0.972), followed by sac height (ICC = 0.913) and neck width (ICC = 0.742). Raters had moderate agreement in determination of aneurysm growth (Cohen’s kappa = 0.61). Both raters identified growth with 64% accuracy after consensus was achieved with the senior adjudicator.
Volume measurements
The median (IQR) volume was 84.6 (400.3) mm3 at baseline and 85.4 (400.3) mm3 at follow-up. The median (IQR) volume change was 0.65 (5.6) mm3. The median (IQR) percent volume change was 0.81(10.4)%. The average percent difference in petrous segment volume was 10.2%. True changes in volume, not attributable to noise or registration mismatch artifact were therefore defined as changes in volume greater than 10.2%. Based on a threshold of 10.2%, only two aneurysms (11%) increased in volume and one (5%) decreased in volume (Table 2, #14, #16, #18). Only fusiform aneurysms changed in volume (µ ± σ = 8.4 ± 0.39%) compared to saccular aneurysms (µ ± σ = −0.3 ± 0.05%).
Size and volume comparison
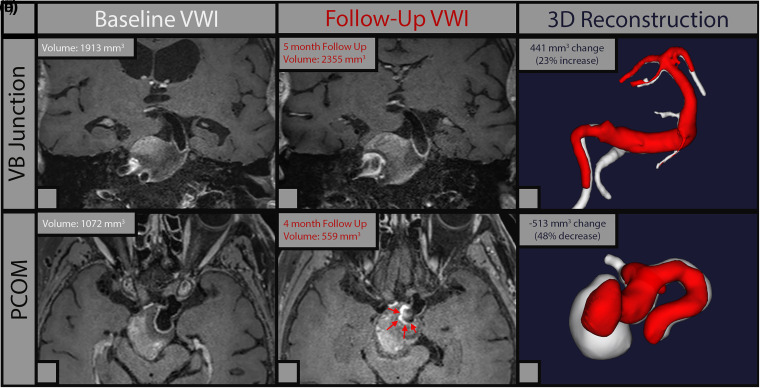
Of the three aneurysms that grew more than 1 mm in size as determined by 2D measurements, two showed parallel increases in volume, and both were fusiform aneurysms. (Supplementary Figure 2). The third aneurysm that apparently showed increased diameter at the neck on follow-up imaging was determined to be sampling artifact of the 2D measurement (Table 2, #13). In two aneurysms with intrasaccular thrombosis at baseline, measurements were taken from the patent lumen, and excluding the clot. The vertebrobasilar junction aneurysm that increased in luminal diameter by 1.1 mm also increased in volume by 440.9 mm3 (23%). Increase in luminal diameter mirrored a slight decrease in the thrombosed area visualized on T1 post contrast imaging. (Figure 3A). The posterior communicating fusiform aneurysm that decreased in diameter by 0.1 mm decreased in volume by 513 mm3 (47.9%). A decrease in volume mirrored increased intra-saccular thrombus formation visualized on T1 post contrast imaging. (Figure 3B).
Figure 3.
3d reconstructions of two thrombosed fusiform aneurysms. In the upper row volumetric reconstructions demonstrate that the vertebrobasilar aneurysm grew 23%. In this case the large saccular/intramural thrombus challenges the volume estimation of the true lumen. In the lower row, a large fusiform PCOM aneurysm developed more intrasaccular/intramural thrombus (arrows), with a 48% decrease in luminal volume visualized in the CE-MRA reconstructions (Panel F).
Discussion
Surveillance of aneurysms is routinely conducted using 2D multiplanar measurements to determine changes in size and morphology. We hypothesized that these measurements would not capture subtle morphological changes, and that 3D volumetric measurements could better characterize these changes. By comparing MRA and 3DRA measurements in phantom and in-vivo aneurysms, we identified sources of error and optimized our 3D volume registration pipeline. Shortcomings detected during optimization of the pipeline were accounted at the time of analysing a group of aneurysms surveilled over time with CE-MRA. Despite the better accuracy of CE-MRA compared TOF-MRA, blebs and minor sac morphological changes were still missed by CE-MRA volumetric measurements. On average, CE-MRA volume measurements had a 10.2% margin of error. The surveillance of aneurysms using volumetric measurements with CE-MRA did not have enough accuracy to detect subtle morphological changes.
The pipeline used in this study for registration, segmentation, isolation, and quantification of aneurysm volumes was optimized in phantom and in-vivo aneurysms. 3DRA was used as the gold standard vascular imaging modality and was compared to TOF-MRA and CE-MRA. 4 Flow-related errors in volumetric reconstructions were detected in the two aneurysm phantoms. These errors were manually corrected in both 3DRA and TOF-MRA reconstructions. TOF-MRA measurements in the phantom models underestimated the “true volume” by an average of 16.3%. When we tested the optimized pipeline in a cohort of patients in-vivo, CE-MRA underestimated volume by 7.8% when compared to 3DRA. The use of contrast in MRA acquisitions improved image definition, leading to better reconstructions and more accurate volume estimations.
The American Stroke Association recommends TOF-MRA as the most appropriate first-line imaging modality for follow-up of unruptured intracranial aneurysms, since it does not require intravenous contrast and does not involve x-ray radiation. 18 Our results, however, suggest that CE-MRA performs better than TOF-MRA in determining aneurysm volume and morphology. Timmins et al. reported low interobserver reliability in measuring irregular aneurysms with TOF-MRA. 19 The authors reported even lower intraclass coefficient correlation for aneurysms that grew over time. In an analysis of forty-one aneurysms, Cirillo et al. described that CE-MRA was superior to TOF-MRA in detecting irregularities in large aneurysm. 20 In a cohort of 64 aneurysms, Zhu et al. determined that TOF-MRA had the largest measurement variance (CV = 15.5%) when compared to CE-MRA (CV = 7%). 21 Our results confirm these findings since the margin of error in quantifying volume was 16% and 7.8% from TOF-MRA and CE-MRA respectively, when compared to 3DRA. CTA has also been used in determining aneurysm volume with post-acquisition processing performed by commercially available softwares. 22 However, both CTA and 3DRA expose the patient to ionizing radiation.
The cohort of patients used for in-vivo optimization of the 3D volume registration pipeline provided interesting insights into the performance of CE-MRA compared to 3DRA. CE-MRA sub-registered areas more prone to rupture such as blebs (Figure 2C). The average size of blebs is 3 ± 1.4 mm, which challenges detection even through volumetric sampling.23,24 The average bleb volume is ∼ 5 mm3. In our analysis CE-MRA reconstructions underestimated aneurysm volume by ∼13 mm3, which would include average-sized blebs. Volume-based surveillance of aneurysms using CE-MRA may not capture subtle morphological changes such as the appearance of blebs, known areas of aneurysm rupture. Aneurysms are usually surveilled using multiplanar MRA measurements of aneurysm size. In our surveillance cohort, measurements of the aneurysm neck had the lowest intraclass correlation compared to aneurysm sac height and maximal diameter, which is consistent with other reports.3,19 Moreover, raters had moderate agreement in determining aneurysm growth. There is growing evidence to suggest that volumetric measurement of aneurysms is more reliable and accurate than 2D multiplanar measurement in detecting changes in size. 25 However, as shown in this study, volumetric analysis of aneurysms is challenging and reconstruction errors may lead to inaccurate estimations. Timmins et al. identified higher reliability in volumetric analysis of aneurysm growth compared to planar measurements in a cohort of 84 unruptured aneurysms. 19 Our study did not document any significant volume change in saccular aneurysms, probably due to the short follow up time and the inclusion of small aneurysms. Standard 2D morphological metrics such as size and aspect ratios were strongly correlated between 3DRA and CE-MRA.
One important challenge in developing a 3D volume registration pipeline is the isolation of the aneurysm sac. Subtle variations in determining the angle of the neck of the aneurysm may lead to changes in sac volume. This was confirmed with the in-vivo validation cohort in which co-registration errors between 3DRA and CE-MRA reconstructions had a large impact on aneurysm volume (Figure 2 C, D and supplementary Figure 1A, B). In our surveillance cohort, small aneurysms that apparently changed in volume by more than 7.8% (margin of error determined by comparing CE-MRA and 3DRA) had indeed thresholding artifact and did not change in size. The thresholding artifact was detected by comparing the caliber of reference parent vessels between two imaging sessions (Supplementary Figure 1C, D). 3D volume reconstruction techniques must therefore be tailored to avoid these artifacts. Liu et al. provided a robust method of identifying reconstruction errors on CE-MRA and used a reference vessel segment for volume matching. 7 We used the petrous segment of the ICA for thresholding and account for variations in acquisition, signal intensity and movement artifact. The margin of error between two CE-MRA reconstructions was established at 10%. After correcting for these artifacts, large fusiform aneurysms were the only aneurysms with true volume changes in our cohort.
Liu et al. reported episodes of “sudden growth” in a cohort of 112 aneurysms with a mean follow up time of 4 years. 7 van der Kamp et al. described a similar process in which aneurysms that grow quickly over short periods of time are more prone to rupture. 2 In our cohort, such growth was observed in two large fusiform aneurysms, whereas small saccular aneurysms remained stable. It is known that large fusiform aneurysms behave different from saccular aneurysms and may progress rapidly. 26 Interestingly, volumetric analysis of these aneurysms determined changes in the intra-luminal saccular thrombus. In one case the thrombus receded, and the aneurysm volume increased 23% (Figure 3A-C). The thrombus in the second aneurysm progressed and the luminal saccular volume decreased by 48% (Figure 3D-F). Volumetric determination of the aneurysm sac in this subset of aneurysms with intra-saccular thrombosis may be a useful tool in documenting aneurysm enlargement and/or intramural thrombosis. These changes may not be apparent with 2D planar measurements. Moreover, surveillance of fusiform aneurysms is usually challenging as by definition these aneurysms do not have a discernable neck and subtle changes along the tubular structure may not be appreciated on 2D imaging. Volumetric measurement of thrombosed or fusiform aneurysms may therefore provide further insight that 2D measurements may not capture.
This study is limited by the small and heterogenous sample size. Aneurysms less than 2 mm were excluded from the study due to limits on image resolution. Due to the short follow-up time, aneurysmal growth may not have been documented as frequently as other series.5,7 There is also selection bias, because aneurysms included in the study were surveilled, and were therefore less likely to grow and change. The method used for segmentation, isolation and reconstruction of aneurysms relied on accurate registration of 3D reconstructions. Since there were larger co-registration errors of 3DRA and MRA reconstructions, the threshold for detecting true changes in aneurysmal volume may have been overestimated. Finally, the surveillance cohort did not undergo a 3DRA since our institutional policy is to survey untreated aneurysms with CE-MRA. A comparison between 3DRA and CE-MRA in this cohort would have been optimal. However, the pipeline was already validated in a cohort of ten patients who underwent 3DRA and CE-MRA.
Conclusion
Volumetric analysis in the surveillance of aneurysms is highly dependent on the image modality and segmentation quality. Volumetric analysis using traditional methods of aneurysm surveillance such as TOF-MRA and CE-MRA may not register subtle changes in aneurysm morphology such as the appearance of blebs, better captured on 3DRA. This poses a safety issue in aneurysm surveillance. Volumetric surveillance of aneurysms using CE-MRA may be especially useful in fusiform and thrombosed aneurysms.
Footnotes
Author contributions: Conception and study design: DH, EAS. Data Acquisition: AR, RP, AV, RS. Analysis and interpretation of results: AR, RP, AV, RS. Drafting of the manuscript: AR, RP and EAS. Critical revision of the study: all authors. Final approval of the version to be published: EAS.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the The Bee Foundation, Society of Vascular Interventional Neurology, National Institutes of Health, (grant number 2019 Brain Aneurysm Research Grant, Pilot Research Grant, 1S10RR028821-01).
Ethical approval statement: After institutional review board approval (No. 20181813), the University of Iowa aneurysm database was screened for analysis. Written informed consent was obtained at the time of imaging for data and imaging collection and processing.
Data availability: Derived data supporting the findings of this study are available from the corresponding author EAS upon request
ORCID iDs: Ashrita Raghuram https://orcid.org/0000-0001-8205-0527
Edgar A. Samaniego https://orcid.org/0000-0003-2764-2268
References
- 1.Villablanca JP, Duckwiler GR, Jahan R, et al. Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: growth and rupture incidence and correlation with epidemiologic risk factors. Radiology 2013; 269: 258–265. [DOI] [PubMed] [Google Scholar]
- 2.van der Kamp LT, Rinkel GJE, Verbaan D, et al. Risk of rupture after intracranial aneurysm growth. JAMA Neurol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HJ, Yoon DY, Kim ES, et al. Intraobserver and interobserver variability in CT angiography and MR angiography measurements of the size of cerebral aneurysms. Neuroradiology 2017; 59: 491–497. [DOI] [PubMed] [Google Scholar]
- 4.Turan N, Heider RA, Roy AK, et al. Current perspectives in imaging modalities for the assessment of unruptured intracranial aneurysms: a comparative analysis and review. World Neurosurg 2018; 113: 280–292. [DOI] [PubMed] [Google Scholar]
- 5.Leemans EL, Cornelissen BMW, Said M, et al. Intracranial aneurysm growth: consistency of morphological changes. Neurosurg Focus 2019; 47: E5. [DOI] [PubMed] [Google Scholar]
- 6.Rajabzadeh-Oghaz H, Varble N, Shallwani H, et al. Computer-Assisted three-dimensional morphology evaluation of intracranial aneurysms. World Neurosurg 2018; 119: e541–e550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Haraldsson H, Wang Y, et al. A volumetric metric for monitoring intracranial aneurysms: repeatability and growth criteria in a longitudinal MR imaging study. AJNR Am J Neuroradiol 2021; 42: 1591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren Y, Chen GZ, Liu Z, et al. Reproducibility of image-based computational models of intracranial aneurysm: a comparison between 3D rotational angiography, CT angiography and MR angiography. Biomed Eng Online 2016; 15: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rooij WJ, Sprengers ME, de Gast AN, et al. 3D Rotational angiography: the new gold standard in the detection of additional intracranial aneurysms. AJNR Am J Neuroradiol 2008; 29: 976–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forbrig R, Ozpeynirci Y, Grasser M, et al. Radiation dose and fluoroscopy time of modern endovascular treatment techniques in patients with saccular unruptured intracranial aneurysms. Eur Radiol 2020; 30: 4504–4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard BM, Hu R, Barrow JW, et al. Comprehensive review of imaging of intracranial aneurysms and angiographically negative subarachnoid hemorrhage. Neurosurg Focus 2019; 47: 20. [DOI] [PubMed] [Google Scholar]
- 12.Ramachandran M, Laakso A, Harbaugh RE, et al. On the role of modeling choices in estimation of cerebral aneurysm wall tension. J Biomech 2012; 45: 2914–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran M, Retarekar R, Harbaugh RE, et al. Sensitivity of quantified intracranial aneurysm geometry to imaging modality. Cardiovasc Eng Technol 2013; 4: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piccinelli M, Veneziani A, Steinman DA, et al. A framework for geometric analysis of vascular structures: application to cerebral aneurysms. IEEE Trans Med Imaging 2009; 28: 1141–1155. [DOI] [PubMed] [Google Scholar]
- 15.Ford MD, Hoi Y, Piccinelli M, et al. An objective approach to digital removal of saccular aneurysms: technique and applications. Br J Radiol 2009; 82 Spec No 1: S55–S61. [DOI] [PubMed] [Google Scholar]
- 16.Besl PJ, McKay ND. A method for registration of 3-D shapes. Ieee T Pattern Anal 1992; 14: 239–256. [Google Scholar]
- 17.Dhar S, Tremmel M, Mocco J, et al. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery 2008; 63: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson BG, Brown RD, Amin-Hanjani S, et al. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 2015; 46: 2368–2400. [DOI] [PubMed] [Google Scholar]
- 19.Timmins KM, Kuijf HJ, Vergouwen MDI, et al. Reliability and agreement of 2D and 3D measurements on MRAs for growth assessment of unruptured intracranial aneurysms. AJNR Am J Neuroradiol 2021; 42: 1598–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cirillo M, Scomazzoni F, Cirillo L, et al. Comparison of 3D TOF-MRA and 3D CE-MRA at 3T for imaging of intracranial aneurysms. Eur J Radiol 2013; 82: e853–e859. [DOI] [PubMed] [Google Scholar]
- 21.Zhu C, Wang X, Eisenmenger L, et al. Surveillance of unruptured intracranial saccular aneurysms using noncontrast 3D-black-blood MRI: comparison of 3D-TOF and contrast-enhanced MRA with 3D-DSA. AJNR Am J Neuroradiol 2019; 40: 960–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escobar-de la Garma VH, Zenteno M, Padilla-Vazquez F, et al. Comparative analysis of aneurysm volume by different methods based on angiography and computed tomography angiography. Neurosurg Rev 2018; 41: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 23.Salimi Ashkezari SF, Detmer FJ, Mut F, et al. Blebs in intracranial aneurysms: prevalence and general characteristics. J Neurointerv Surg 2021; 13: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindgren AE, Koivisto T, Bjorkman J, et al. Irregular shape of intracranial aneurysm indicates rupture risk irrespective of size in a population-based cohort. Stroke 2016; 47: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 25.Chung J, Ko JH. An efficient method for aneurysm volume quantification applicable in any shape and modalities. J Korean Neurosurg Soc 2021; 64: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabotin RP, Varon A, Roa JA, et al. Insights into the pathogenesis of cerebral fusiform aneurysms: high-resolution MRI and computational analysis. J Neurointerv Surg 2021. [DOI] [PubMed] [Google Scholar]