Abstract
Purpose
To illustrate the characteristics of acute ostial vertebral artery (VA) and basilar artery (BA) tandem occlusions. The endovascular treatment strategy for ostial VA-BA tandem occlusion was reported.
Materials and Methods
We conducted a retrospective analysis of patients with ostial VA-BA tandem occlusion who underwent endovascular treatment in our center between November 2018 and February 2022. We preferred to recanalize the dominant vertebral artery with priority. The imaging characteristics, treatment strategy, clinical outcomes, and complications were analyzed.
Results
In total, 9 patients with ostial VA-BA tandem occlusion were enrolled in this study. All the VA-BA tandem occlusion was caused by acute occlusion of the dominant VA. Endovascular revascularization was performed through the occluded dominant VA in 8 patients and was performed through contralateral non-dominant VA in 1 patient. Successful recanalization (mTICI 2b/3 grade) was achieved in all 9 patients, and 5 patients (55.5%) achieved functional independence with a mRS score of 0–2 at 90 days.
Conclusions
In this case series, the occurrence of ostial VA-BA tandem occlusions was mainly caused by acute occlusion of the dominant VA. Endovascular revascularization of ostial VA-BA tandem occlusions through occluded dominant VA was feasible and recommended.
Keywords: Posterior circulation, acute ischemic stroke, tandem occlusion, endovascular treatment
Introduction
Tandem vertebrobasilar stroke was defined as basilar artery (BA) occlusion with stenosis/occlusion of the extracranial vertebral artery (VA). 1 Ostial VA atherosclerotic stenosis complicated by concomitant acute atherothrombotic embolus was a source for emboli leading to BA occlusion. 2 The selection of appropriate treatment for the ostial VA-BA occlusions depended on the vertebrobasilar anatomy, side branch compensation, and the size of thrombosis.
In current literature, two endovascular approaches were reported in the revascularization of acute ostial VA-BA tandem occlusions: access via the contralateral patent VA (clean-road path), or through the ipsilateral occluded VA (dirty-road path).3–6
However, studies showed that one of the VAs was dominant in three-quarters of the population. 7 The significance of the dominant VA in the treatment of tandem vertebrobasilar occlusions had not been highlighted enough, no matter “clean” or “dirty”.
In this study, we first illustrated all possible ostial VA-BA tandem lesions patterns based on vascular anatomy. Then we reviewed the characteristics, endovascular strategies, and clinical prognoses of ostial VA-BA tandem occlusions at our center, advocating to recanalize the dominant VA with priority.
Materials and methods
Study population
A retrospective analysis was conducted on stroke patients with large vessel occlusion within 24 h of symptom onset who underwent endovascular treatment in our center between November 2018 and February 2022. In this study, we included patients with acute tandem ostial VA-BA occlusion. Patients with atherosclerotic stenosis of VA segments 2–4 and BA or isolated embolic BA occlusion were excluded. The study was approved by the ethics committee of our hospital. The institutional review board waived the need for patient consent. Informed consent forms for the endovascular procedure were acquired from patients or a family member.
Recorded clinical and imaging data included National Institutes of Health Stroke Scale (NIHSS) score at admission, occlusion sites, use of intravenous and/or arterial tissue plasminogen activator, stent used for the proximal arterial occlusion, time from stroke onset to groin puncture, time from groin puncture to intracranial recanalization, and cerebrovascular risk factors.
Anatomy of Va and tandem ostial Va- Ba occlusions
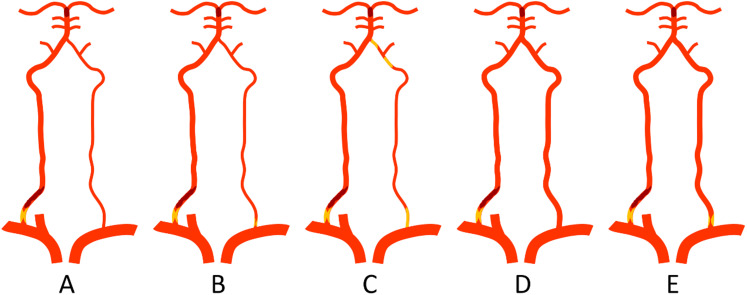
Posterior circulation hypoperfusion was demonstrated on CT perfusion, and BA occlusion was demonstrated on CT angiography. Bilateral subclavian artery angiography and/or bilateral common carotid artery angiography were performed to confirm the ostial VA occlusion, demonstrate the collateral circulation, and determine the dominant and non-dominant side of VAs. According to the anatomy of bilateral VAs (equivalent-sized or dominant vs. non-dominant VAs), five possible types of ostial VA- BA tandem occlusions were illustrated in Figure 1.
Figure 1.
Illustration of 5 types of posterior tandem occlusions with distal embolic occlusion of the basilar artery. (A) A proximal stenosis/occlusion of the dominant right vertebral artery, with patency of left non-dominant vertebral artery. (B) A proximal stenosis/occlusion of the dominant right vertebral artery, with stenosis of the left non-dominant vertebral artery. (C) A proximal stenosis/occlusion of the dominant right vertebral artery, with chronic occlusion of left non-dominant vertebral artery (ostium or V2-V4 segments). (D) Equivalent-sized vertebral arteries, with a proximal stenosis/occlusion of the right vertebral artery. (E) Equivalent-sized vertebral arteries, with a proximal stenosis/occlusion of the right vertebral artery and stenosis of the left vertebral artery.
Endovascular treatment
All procedures were mostly performed under conscious sedation. BA mechanical thrombectomy via dominant VA (unaffected or affected) was preferred in our center. If the affected dominant VA was not applicable for endovascular treatment, mechanical thrombectomy was performed via contralateral VA. Successful reperfusion was defined as a modified Thrombolysis in Cerebral Infarction (mTICI) 2b or 3 at the end of the procedure.
Revascularization of affected dominant Va and Ba thrombectomy (dirty-road path approach)
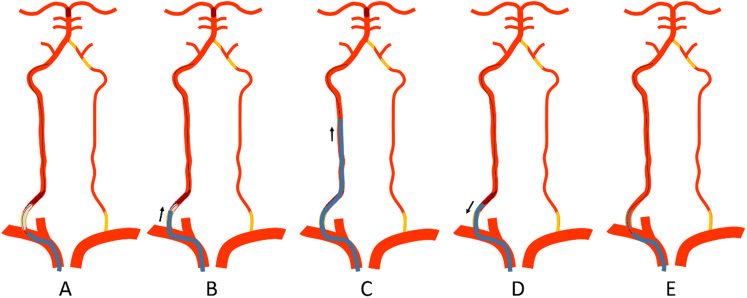
The “Distal-to-proximal” strategy was the preferred approach to treat this type of tandem occlusions. 8 A 6 Fr guiding sheath (90 cm, Flexor Shuttle, Cook Medical, US) or an 8 Fr guiding catheter (90 cm, Vista Brute Tip, Cordis, US) was placed at the subclavian artery. A 300 cm 0.014-inch microguidewire (Synchro-14, Boston Scientific/Target), a 0.021-inch microcatheter (Rebar, ev3, USA), and a 5 Fr MPA catheter (125 cm, Cordis) form a coaxial system, which was used to pass through the stenosis/occlusion segment. After confirmation of the true cavity with microcatheter angiography, the microguidewire was retained and the microcatheter was exited. A balloon (3 mm diameter) was introduced to dilate the stenotic segment, and the guiding catheter was advanced to the V2 segment through the stenotic segment over the partially inflated balloon. Then BA thrombectomy was performed with a stent retriever, contact aspiration, or a combination of stent retriever with aspiration. After confirming intracranial successful revascularization, the microguidewire was sent to the V2 segment, and the guiding catheter was pulled back with gentle aspiration to remove all possible residual intraarterial thrombi. A balloon-expandable/self-expandable stent was then implanted at the ostial vertebral artery over the microguidewire (Figure 2, Figure 3).
Figure 2.
Illustration of the “Distal-to-proximal” strategy for the posterior tandem occlusion through the occluded dominant vertebral artery (dirty-road path). (A) A guiding catheter was placed at the subclavian artery. After the microguidewire passed through the occluded segment, a balloon (3 mm diameter) was introduced to dilate the stenotic segment. (B-C) The guiding catheter was advanced to the V2 segment through the stenotic segment over the partially inflated balloon. Aspiration was performed first to remove residual thrombus. Then basilar artery thrombectomy was performed with a stent retriever, contact aspiration, or a combination of stent retriever with aspiration. (D) After successful revascularization of the basilar artery, the microguidewire was sent to the V2 segment, and the guiding catheter was pulled back with gentle aspiration to remove all possible residual intraarterial thrombi. (E) A balloon-expandable stent or a self-expendable stent was then implanted at the ostial vertebral artery over the microguidewire.
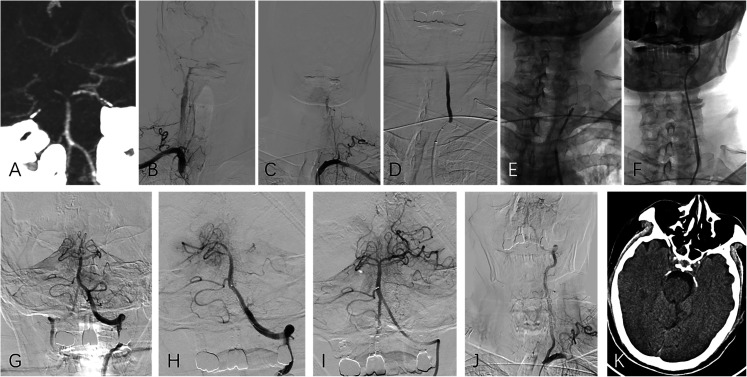
Figure 3.
(Case No. 8), dominant left vertebral artery occlusion with non-dominant right vertebral artery occlusion (type C tandem occlusion). (A) CT angiography showed basilar artery tip occlusion and dominant vertebral artery at left side. (B, C) Bilateral subclavian arteries angiography depicted left vertebral artery occlusion and a slender right vertebral artery. (D) A 300 cm 0.014-inch microguidewire, with a 0.021-inch microcatheter form a coaxial system and was used to pass through the stenosis/occlusion segment. (E, F) After confirmation of the true cavity with microcatheter angiography, a balloon (3 mm diameter) was introduced to dilate the stenotic segment, and the guiding catheter was then advanced to the V2 segment through the stenotic segment over the partially inflated balloon. (G-I) Basilar artery thrombectomy was performed with a combination of stent retriever and aspiration. (J) After confirming intracranial successful revascularization, the microguidewire was sent to the V2 segment, and the guiding catheter was pulled back with gentle aspiration to remove all possible residual intraarterial thrombi. A balloon-expandable stent was then implanted at the ostial vertebral artery over the microguidewire. (K) The follow-up CT showed a small infarction in the brain stem.
Occasionally, the 0.014-inch microguidewire could not provide enough support to pass through the occluded segment. A coaxial system consisting of a 5 Fr MPA catheter (125 cm, Cordis) and a 0.035-inch hydrophilic coated guidewire would be used to pass through the occluded segment.
BA thrombectomy through the unaffected vertebral artery (clean-road path approach)
In this case series, all unaffected VAs were non-dominant, which were not our first choice. Only if the attempt to revascularize the dominant VA fails, BA mechanical thrombectomy was performed via the non-dominant VA. The procedure was performed via the guiding catheter placed at the upper end of the V2 segment, and BA thrombectomy was performed with a stent retriever, contact aspiration, or a combination of stent retriever with aspiration.
Antiplatelet management
Patients received a bolus of intravenous tirofiban (5 µg/kg, Grand Pharma, China), glycoprotein IIb/IIIa inhibitor, to prevent thrombosis after stent implantation, followed by a maintenance dose of 0.1 µg/kg/min for 24 h. Twenty-four hours after the procedure, patients were given a loading dose of clopidogrel (300 mg) and aspirin (300 mg). Intravenous tirofiban was stopped 4 h after the initiation of dual antiplatelet therapy. Thereafter, patients continued to receive aspirin (100 mg/day) for 1 year and clopidogrel (75 mg/day) for 90 days. If hemorrhagic transformation was detected on at head CT scan 24 h after the operation, clopidogrel would be stopped. If hematoma was detected, the dual antiplatelet medication would be discontinued.
Clinical outcomes
Clinical outcomes mainly included: any hemorrhage transformation, symptomatic intracerebral hemorrhage, functional independence (mRS 0–2), and mortality at 90 days.
Results
Baseline characteristics
Of the 9 patients with ostial VA-BA tandem occlusions, the mean baseline NIHSS score was 25 (range 8–37). Tissue plasminogen activator was administered in 6 (66.7%) patients. The mean time from stroke onset to groin puncture was 438 min (range 92–880), and the mean time from groin puncture to recanalization was 99.2 min (range 42–179) (Table 1). According to the illustration of Figure 1, there were 2 type A (case 2 and 7), 1 type B (case 4), and 6 type C (case 1, 3, 5, 6, 8, and 9) tandem occlusions. No type D or E tandem occlusion was identified. The symptom onset was considered caused by acute occlusion of the dominant VA in all 9 patients (refer to the images in appendix). The dominant VA was located at right side in 5 patients (55.6%).
Table 1.
The clinical data, treatment strategy, and patient clinical outcomes.
| Case No. | NIHSS at admission | occlusion | TTP (min) | TTR (min) | tPA | Contralateral vertebral artery | stent | mTICI | mRS at 3 months | cerebrovascular risk factors |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | D-LV1-BA | 780 | 140 | - | N-RV1 occlusion | BES, 5mm × 18mm | 3 | 2 | Hypertension, Smoking |
| 2 | 8 | D-RV1-BA | 420 | 117 | - | N-LVA clear | BES, 6mm × 18mm | 2b | 2 | Hypertension |
| 3 | 35 | D-RV1-BA | 105 | 97 | + | N-LV1 occlusion | BES, 6mm × 18mm | 2b | 5 | Smoking |
| 4 | 35 | D-RV1-BA | 290 | 179 | - | N-LV1 stenosis | BES, 5mm × 18mm | 2b | 6 | - |
| 5 | 8 | D-LV1-BA | 692 | 152 | + | N-RV1 occlusion | BES, 6mm × 18mm | 3 | 0 | Hypertension |
| 6 | 34 | D-LV1-BA | 397 | 49 | + | N-RV1 occlusion | BES, 6mm × 18mm | 3 | 5 | - |
| 7 | 9 | D-RV1-BA | 880 | 69 | + | N-LVA clear | SES, 6–8mm × 40mm | 2b | 1 | - |
| 8 | 35 | D-RV1-BA | 92 | 48 | + | N-LV1 occlusion | BES, 6mm × 18mm | 2c | 2 | Hypertension |
| 9 | 27 | D-LV1-BA | 286 | 42 | + | N-LVA occlusion | BES, 5mm × 18mm | 2c | 3 | Hypertension |
Note: No. = number, R = right, L = left, NIHSS = National Institutes of Health Stroke Scale, BA = basilar artery, V1 = first segment of the vertebral artery. D = dominant, min = minutes, TTT = time to puncture, TTR = time to recanalization, N = non-dominant, tPA = tissue plasminogen activator, BES = balloon-expandable stent, SES = self-expandable stent, mTICI = modified Treatment in Cerebral Ischemia score, mRS = modified Rankin Scale.
Endovascular treatment
All patients were proposed to be treated with dominant-side revascularization initially. It is noteworthy that the occluded dominant VA was recanalized in 8 patients, and only one patient (Case No.4) was treated through the non-dominant stenotic VA due to failure of passing through the occluded dominant VA with the 0.014-inch microguidewire. All the BA occlusions achieved good recanalization (mTICI 2b/3 grade) after mechanical thrombectomy.
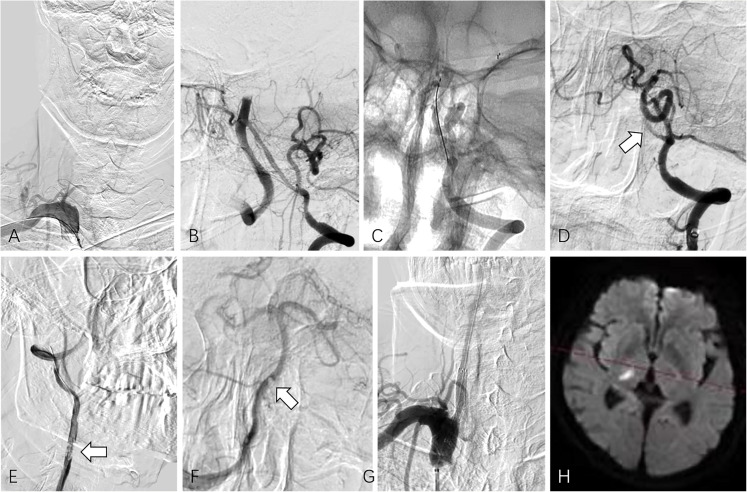
Of note, there is one patient (Case No.7), we first attempted to recanalize the occluded dominant VA with the 0.014-inch microguidewire, but failed. Intracranial thrombectomy was performed through the non-dominant VA, but the clot could not be retrieved through the non-dominant VA and was stuck at the site of the vertebral junction. Therefore, a 0.035-inch hydrophilic coated guidewire was used to pass through the occluded orifice of the dominant VA. Finally, successful revascularization was achieved (Figure 4).
Figure 4.
(Case No. 7), dominant right vertebral artery occlusion with non-dominant left vertebral artery patency (type A tandem occlusion). (A) Selective angiogram of the right subclavian artery depicted right vertebral artery occlusion. Initially, attempts to pass the microguidewire through the occlusion segment of the right vertebral artery failed. (B) Left vertebral artery angiography showed the small size of the V4 segment of the left vertebral artery. (C) Mechanical thrombectomy for basilar artery with Solitaire AB stent retriever was made via the left vertebral artery for two times. (D) After each thrombectomy, angiography showed the occlusion of the left V4 segment (arrow). (E) A coaxial system consisting of a 5 Fr MPA catheter and a 0.035-inch hydrophilic coated guidewire was used to successfully pass through the occluded segment of the right vertebral artery, and an angiogram showed the residual intraarterial thrombi (arrow). (F) Right vertebral artery angiogram showed patency of basilar tip, and a filling defect was detected at the vertebrobasilar junction (arrow), which should be the thrombus stuck at the left vertebral artery. (G) After removal of residual thrombus by aspiration during catheter withdrawal, a self-expendable stent was implanted at the stenotic segment of the right vertebral artery over the microguidewire. (H) Follow-up MRI revealed a small infarction in the right thalamic region.
Clinical outcomes
There were no perforations, iatrogenic vessel dissections, or other technical complications. No hemorrhagic transformation of hematoma was detected at 24 h head CT scans in all patients. Five (55.5%) patients achieved functional independence with a mRS score of 0–2 at 90 days.
Discussion
In this case series report, all the VA-BA tandem occlusions were caused by acute occlusion of the dominant VAs. The occluded dominant VAs were recanalized in 8 patients. Successful recanalization (mTICI 2b/3 grade) was achieved in all patients, and functional independence was achieved in 5 patients (55.5%) at 90 days.
Although in Figure 1 we listed five occlusion patterns of tandem lesions based on the possible anatomy of the bilateral VAs, none of the 9 patients had codominant bilateral VAs. This may be related to the small sample size or may be related to the rarity of tandem occlusions that occurred in patients with codominant bilateral VAs. We reviewed the imaging data and found that all 9 VA-BA tandem occlusions occurred secondary to the acute occlusion of the orifice of the dominant VA with thrombosis. A short occlusion time meant it would not be difficult to pass through the occlusion segment, and the possibility of recanalization should be relatively high. Of our 9 cases, there were only 2 cases in whom thrombectomy through contralateral VA was possible, and only 1 of these 2 cases was suitable for BA thrombectomy finally. Although in previous studies the dirty path may be the dominant VA as well, these studies did not clearly indicate that the dominant VA may be the only option for successful vascularization.3–5,8 Some studies reported that the contralateral clean path can be the route of choice for thrombectomy and should be the first choice. 4 It is reasonable to achieve rapid BA reperfusion through the contralateral VA first, if the anatomical conditions are suitable for endovascular treatment. However, after opening the BA, whether to continue to recanalize the occluded dominant VA needs to be further explored.
The influence of anatomical configuration on mechanical thrombectomy results has been studied in details for acute middle cerebral artery (MCA) occlusions. Maus V et al. reported that the median diameter of the inferior trunk of MCA was larger than the superior trunk, and the choice of the inferior trunk for distal stent retriever placement in M1 occlusions is associated with a high rate of first-pass near-complete/complete reperfusion in embolectomy. 9 Shapiro M et al. stated that tortuous MCA configuration is associated with reduced stent-triever thrombectomy efficacy. The anatomy of vertebrobasilar artery may also has effect on mechanical thrombectomy. 10 Nishikata M et al. reported most patients had an opposite directional relationship between dominant VA and BA curvature, and BA usually bends to the opposite side of dominant VA. 11 Therefore, the direction of the dominant VA is more consistent with the direction of the BA than the non-dominant side. In addition, the median diameter of the dominant VA is larger than the non-dominant side. The choice of the dominant VA for distal stent retriever placement or aspiration catheter advancement in basilar artery occlusions recanalization may be associated with a higher rate of first-pass complete reperfusion in mechanical thrombectomy.
In our center, a “distal-to-proximal” strategy was preferred to treat posterior tandem occlusions, which means opening the distal occlusion first followed by treatment of the proximal stenosis/occlusion. In this process, we used a 3 mm diameter balloon to dilate the stenotic segment first and then bridged the guiding catheter to the V2 segment. Since the stenosis segment was not fully expanded with a 3 mm diameter balloon, the guide catheter could block the blood flow and prevent thrombus distal migration. In addition, after successful opening the BA, during withdrawal of the guide catheter, maintaining a negative pressure suction to remove possible residual thrombus was also a key point in the procedure. In this case series, no perioperative distal thrombus migration occurred during revascularization via the occluded vertebral artery (a dirty pathway). However, if the residual thrombus could not be removed completely, a filter placed in the distal cervical VA could minimize the risk of re-embolisation during the treatment of the proximal stenosis. It is recommended to place the filter prior to the retraction of the guiding catheter from the VA into the subclavian artery. 12 With our experience accumulation, if the 0.014-inch microguidewire could not provide enough support to pass through the occluded segment, we preferred to switch to the 0.035-inch guidewire without hesitation to probe the VA opening, which was associated with a high success rate and no procedure-related complication.
In this case series, we did not use antegrade strategy to open the occluded VA, which means a balloon-expandable stent being deployed to the ostial VA first before intracranial occlusion thrombectomy. 4 We believed that this strategy had the following shortcomings: (1) once the stent was placed at the opening of the VA, the guiding catheter may be blocked at the subclavian artery, which may further affect the opening of the BA. (2) restoring the blood flow at the opening of the VA first may lead to the migration of residual thrombus in the VA to the distal end. (3) if the VA blood flow was restored, it would cause a “water hammer” effect on the occlusion site of the BA, resulting in a tighter occlusion. (4) if the blood flow was restored, the chance of fragmented thrombus distal migration would increase during the BA thrombectomy.
In emergency situations, we initiated antiplatelet therapy with tilofiban, a GP IIb/IIIa inhibitor. Tirofiban is a reversible antagonist of fibrinogen that binds to the GP IIb/IIIa receptor on platelets. 13 When administered according to the regimen of 25 µg/kg followed by a 0.15 µg/kg/min maintenance infusion, >90% inhibition of platelet aggregation is attained within 10 min. Due to its special pharmacokinetics, the IIb/IIIa inhibitors may let the procedure performed anytime rather than waiting for hours after administration of oral dual antiplatelet therapy in emergency setting. Our previous study showed that a bolus dose of tirofiban (5 μg/kg) followed with intravenous tirofiban infusion (0.10 μg/kg/min) could prevent thromboembolic events effectively without an increase in hemorrhagic complications. 14 Tirofiban has a half-life of approximately 2 h. Following discontinuation, ex vivo platelet aggregation returns to near baseline in 4–8 h in approximately 90% of patients. 15 Owing to antiplatelet drugs taking some time to reach therapeutic levels, patients in this study were loaded with dual antiplatelet therapy 4 h before discontinuation of tirofiban.
In previous studies, posterior circulation tandem occlusions were associated with mortality rates of 20–42.9% and good outcomes (mRS 0-2) in only 28.6–53.3%.3–5,12,16,17
In our study, five patients (55.5%) had good outcomes with a mRS score of 0–2 at 90 days and the mortality rate was 11.1%, which are similar to previously literature.3–5,12,16,17
This study has several limitations. First, this was a single-center empirical retrospective analysis, the sample size was relatively small. Second, we only used a “distal-to-proximal” strategy and there was no control group comparison.
Conclusion
In this case series, all the VA-BA tandem occlusions were caused by acute occlusion of the dominant VAs. Endovascular revascularization of acute vertebrobasilar artery tandem occlusion through occluded dominant VA (dirty-road path) could be performed successfully without technical complications.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Interventional Medicine Research Fund of Jiangsu Medical Association, National Natural Science Foundation of China, (grant number SYH-3201140-0024, 81971613).
ORCID iDs: Sheng Liu https://orcid.org/0000-0002-4058-4413
Haibin Shi https://orcid.org/0000-0003-1293-5789
Zhenyu Jia https://orcid.org/0000-0002-5134-9339
References
- 1.Baik SH, Jung C, Kim BMet al. et al. Mechanical thrombectomy for tandem vertebrobasilar stroke: characteristics and treatment outcome. Stroke 2020; 51: 1883–1885. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ali F, Barrow T, Duan L, et al. Vertebral artery ostium atherosclerotic plaque as a potential source of posterior circulation ischemic stroke: result from borgess medical center vertebral artery ostium stenting registry. Stroke 2011; 42: 2544–2549. [DOI] [PubMed] [Google Scholar]
- 3.Ecker RD, Tsujiura CA, Baker CBet al. et al. Endovascular reconstruction of vertebral artery occlusion prior to basilar thrombectomy in a series of six patients presenting with acute symptomatic basilar thrombosis. J Neurointerv Surg 2014; 6: 379–383. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JE, Leker RR, Gomori JM, et al. Emergent revascularization of acute tandem vertebrobasilar occlusions: endovascular approaches and technical considerations-confirming the role of vertebral artery ostium stenosis as a cause of vertebrobasilar stroke. J Clin Neurosci 2016; 34: 70–76. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Ma N, Zhang S, et al. Endovascular revascularisation of acute tandem vertebrobasilar artery occlusion: seven case series with literature reviews. Stroke Vasc Neurol 2018; 3: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siebert E, Bohner G, Zweynert S, et al. Revascularization techniques for acute basilar artery occlusion : technical considerations and outcome in the setting of severe posterior circulation steno-occlusive disease. Clin Neuroradiol 2019; 29: 435–443. [DOI] [PubMed] [Google Scholar]
- 7.Chang JY, Jung S, Jung Cet al. et al. Dominant vertebral artery status and functional outcome after endovascular therapy of symptomatic basilar artery occlusion. J Neuroradiol 2017; 44: 151–157. [DOI] [PubMed] [Google Scholar]
- 8.Xing PF, Zhang YW, Li ZF, et al. The “distal-to-proximal” strategy for the treatment of posterior circulation tandem occlusions: a single-centre experience. Neuroradiology 2020; 62: 867–876. [DOI] [PubMed] [Google Scholar]
- 9.Maus V, Brehm A, Tsogkas Iet al. et al. Stent retriever placement in embolectomy: the choice of the post-bifurcational trunk influences the first-pass reperfusion result in M1 occlusions. J Neurointerv Surg 2019; 11: 237–240. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro M, Raz E, Nossek Eet al. et al. Neuroanatomy of the middle cerebral artery: implications for thrombectomy. J Neurointerv Surg 2020; 12: 768–773. [DOI] [PubMed] [Google Scholar]
- 11.Nishikata M, Hirashima Y, Tomita Tet al. et al. Measurement of basilar artery bending and elongation by magnetic resonance cerebral angiography. Arch Gerontol Geriatr 2004; 38: 251–259. [DOI] [PubMed] [Google Scholar]
- 12.Piechowiak EI, Kaesmacher J, Zibold F, et al. Endovascular treatment of tandem occlusions in vertebrobasilar stroke: technical aspects and outcome compared with isolated basilar artery occlusion. J Neurointerv Surg 2020; 12: 25–29. [DOI] [PubMed] [Google Scholar]
- 13.Kim KS, Fraser JF, Grupke Set al. et al. Management of antiplatelet therapy in patients undergoing neuroendovascular procedures. J Neurosurg 2018; 129: 890–905. [DOI] [PubMed] [Google Scholar]
- 14.Shen G, Jia Z, Zhao Let al. et al. The safety and efficacy of a low dose of tirofiban for early complications during and after stent-assisted coiling of ruptured intracranial aneurysms: a propensity matching study. Clin Neurol Neurosurg 2022; 214: 107132. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Choi JH, Kang Met al. et al. Safety and efficacy of intravenous tirofiban as antiplatelet premedication for stent-assisted coiling in acutely ruptured intracranial aneurysms. AJNR Am J Neuroradiol 2016; 37: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baik SH, Park HJ, Kim JHet al. et al. Mechanical thrombectomy in subtypes of basilar artery occlusion: relationship to recanalization rate and clinical outcome. Radiology 2019; 291: 730–737. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg JH, Sweid A, Sajja K, et al. Posterior circulation tandem occlusions: classification and techniques. Clin Neurol Neurosurg 2020; 198: 106154. [DOI] [PubMed] [Google Scholar]