Abstract
CS-834 is a prodrug of the carbapenem R-95867, developed by Sankyo Co., Ltd., Tokyo, Japan. To investigate the possibility that CS-834 may be the first carbapenem usable in an oral dosage form, its in vitro antibacterial activity (as R-95867) and in vivo antibacterial activity were compared with those of cefpodoxime proxetil, cefditoren pivoxil, cefdinir, ofloxacin, imipenem, and amoxicillin. R-95867 had high levels of activity against methicillin-susceptible staphylococci and streptococci, including penicillin-resistant Streptococcus pneumoniae, as well as Neisseria gonorrhoeae, Moraxella catarrhalis, the members of the family Enterobacteriaceae (with the exception of Serratia marcescens), Haemophilus influenzae, and Bordetella pertussis; for all these strains, the MICs at which 90% of tested strains are inhibited (MIC90s) were 1.0 μg/ml or less. Against methicillin-resistant staphylococci, enterococci, Serratia marcescens, Brukholderia cepacia, Stenotrophomonas maltophilia, and Acinetobacter calcoaceticus, R-95867 showed activity comparable to or slightly less than that of imipenem, with MIC90s ranging from 2 to >128 μg/ml. The in vivo efficacy of oral CS-834 against experimental mouse septicemia caused by gram-positive and gram-negative bacteria was better than that of comparative drugs. In murine respiratory infection models, the efficacy of CS-834 reflected not only its potent in vitro activity but also the high levels present in the lungs.
Parenteral carbapenems such as imipenem-cilastatin, meropenem, and panipenem-betamipron have been developed and commercialized (1, 15). These carbapenems have a remarkably broad spectrum of activity against both gram-positive and gram-negative bacteria, including Pseudomonas aeruginosa, anaerobes, mycobacteria, and other microorganisms. The carbapenems’ remarkable spectrum of in vitro potency appears to result from their ability to penetrate the outer membrane of gram-negative bacilli, as well as their high affinity for certain penicillin-binding protein targets.
Since currently available carbapenems are parenteral drugs, however, the development of oral carbapenems would be of interest. Admittedly, expanded-spectrum cephalosporins stable toward many beta-lactamases from gram-negative bacteria are available in oral as well as parenteral dosage forms. However, members of the family Enterobacteriaceae such as Klebsiella pneumoniae, Klebsiella oxytoca, and Escherichia coli have acquired resistance to expanded-spectrum cephem antibiotics by producing extended-spectrum beta-lactamases (7, 8, 14).
The development of oral carbapenems has accordingly been moving toward human therapeutic application. Specifically, Sankyo Co. has synthesized and developed the first oral carbapenem, CS-834 (a prodrug of R-95867). This agent is chemically identified as (+)-[pivaloyloxymethyl-(4R,5S,6S)-6- [(R)-1-hydroxyethyl]-4-methyl-7-oxo-3[[(R)-5-oxopyrrolidin-3- yl]-thio]-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate]. To evaluate this drug’s potential, we compared the in vitro and in vivo activities of CS-834 (a prodrug of R-95867) with those of cefpodoxime proxetil (a prodrug of cefpodoxime), cefdinir, cefditoren pivoxil (a prodrug of cefditoren), ofloxacin, and imipenem.
MATERIALS AND METHODS
Drugs.
CS-834, R-95867, cefpodoxime, and cefpodoxime proxetil were supplied by Sankyo Co., Ltd. (Tokyo, Japan). The other antimicrobial agents were provided by the indicated manufacturers, as follows: cefdinir, Fujisawa Pharmaceutical Co., Osaka, Japan; cefditoren and cefditoren pivoxil, Meiji Seika Co. Ltd., Tokyo, Japan; ofloxacin, Daiichi Pharmaceutical Co. Ltd., Tokyo, Japan; and imipenem, Banyu Pharmaceutical Co., Tokyo, Japan.
Organisms.
The clinical isolates tested were obtained from hospitals in several areas of Japan during 1991 and 1996. All organisms had previously been identified by routine laboratory methods and had been stored at −80°C.
In vitro susceptibility tests.
The MICs for nonfastidious organisms were determined by the broth microdilution method in 0.1-ml volumes of cation-adjusted Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) (9). For fastidious organisms such as Streptococcus spp., Enterococcus spp., and Moraxella catarrhalis, cation-adjusted Mueller-Hinton broth supplemented with 5% lysed horse blood was used (10). For Haemophilus influenzae, cation-adjusted Mueller-Hinton broth was supplemented with 5% lysed horse blood plus 5 mg of yeast extract (Oxoid, Hampshire, England) per ml and 15 μg of NAD (Sigma Chemical Co., St. Louis, Mo.) per ml. For all strains, incubation was for 18 to 24 h at 35°C. Microdilution plates were inoculated with an automatic pin inoculator (MIC-2000; Dynatech Laboratories, Inc., Alexandria, Va.) so that the final inoculum was approximately 5 × 105 cfu/ml.
The MICs for Bordetella pertussis and Neisseria gonorrhoeae were determined by the agar dilution method with Bordet Gengou medium base (Difco) supplemented with 15% horse blood and 1% glycerol and GC II agar (BBL, Baltimore, Md.) supplemented with 1% hemoglobin and IsoVitaleX (BBL), respectively. The bacterial suspension was diluted with saline to a concentration of approximately 106 CFU/ml. A portion (about 5 μl) of the diluent was inoculated with an inoculation apparatus (Microplanter; Sakuma Seisakusho, Tokyo, Japan) onto agar plates containing graded concentrations of the drugs at a final inoculum size of 104 CFU/spot. Plates inoculated with B. pertussis and N. gonorrhoeae were incubated at 35°C for 48 h and in 5% CO2 at 35°C for 24 h, respectively.
The MIC was defined as the lowest concentration of drug resulting in the complete inhibition of visible growth.
In vivo activity.
Four-week-old male Slc/ICR mice (weight, 18 to 20 g; Sankyo Labo Service, Tokyo, Japan) were used in groups of 8 to 10 unless indicated otherwise. Test organisms were cultured overnight at 35°C on brain heart infusion agar (Eiken Chemical Co., Ltd., Tokyo, Japan) or blood agar. The organisms were suspended in saline containing 5% mucin (Difco) for bacteremic infection and in saline for local infection.
(i) Bacteremic infection.
Mice were challenged intraperitoneally with a single 0.5-ml portion of bacterial suspension (9.5 × 106 CFU of Staphylococcus aureus Smith per mouse, 7.1 × 103 CFU of E. coli C11 per mouse, or 9.0 × 103 CFU of K. pneumoniae 3K25 per mouse). These inocula were sufficient to cause 100% mortality in untreated animals, with death occurring between 14 and 72 h after infection. Antimicrobial drugs were administered orally 1 h after infection. A 0.05-ml portion of a bacterial suspension of 4.8 × 106 CFU of Streptococcus pneumoniae TUH39 (MIC of penicillin G, 0.016 μg/ml) per mouse was instilled intranasally in ketamine-xylazine-anesthetized mice. These inocula caused 100% mortality in untreated animals at 72 to 116 h after infection. Antimicrobial drugs were administered orally two times a day for 3 days starting 20 h after intranasal instillation. The total number of mice surviving at each dose was recorded on day 7 after infection. The 50% effective dose (ED50) of each drug was calculated by the probit method, and the ED50 for the pneumococcal infection was expressed as one dose.
(ii) Local infection.
Mice under ketamine-xylazine anesthesia were infected by intranasal inoculation (inoculated volume, 0.05 ml) with S. pneumoniae TUM741 (MIC of penicillin G, 1.0 μg/ml; 5.1 × 105 CFU/ml), H. influenzae TUM8 (4.2 × 105 CFU/ml), or H. influenzae TUM36 (5.0 × 105 CFU/ml) (12, 16). Five-week-old female CBA/J mice (weight, 15 to 17 g; Japanese Charles River, Kanagawa, Japan) were used for pneumococcal infection (16). Oral administration of drugs commenced 2 days after infection and was continued for 3 days, with the drugs being given twice a day (at 12-h intervals) or three times a day (at 6-h intervals) for H. influenzae infection and three times a day (at 6-h intervals) or four times a day (at 4-h intervals) for S. pneumoniae infection.
Animals were sacrificed 20 h after the last administration of the test drug (in order to minimize the influence of the drug administered), and the infected tissues were dissected and homogenized. The number of viable organisms (number of CFU/pair of lungs) was determined by agar plating. Evaluation of efficacy was based on the proportional reduction of the bacterial counts in the infected tissues of treated animals compared with those in the infected tissues of untreated control animals. The statistical significance of the observed differences was determined by the Bonferroni multiple comparison test.
Pharmacokinetics in mice.
One day after infection with S. pneumoniae TUH39, groups of three mice each received an oral antibiotic at a single dose of 50 mg/kg of body weight. Samples of heart blood and lung and kidney tissues were obtained 5, 15, 30, 60, 120, 240, and 360 min after drug administration. The lungs and kidneys were slightly washed with saline in order to minimize contamination with blood. The levels of the biologically active form of the drug in serum and tissues were determined by a paper disk method, with E. coli NIHIJC-2 as the indicator organism for R-95867 and with Providencia stuartii IFO12930 as the indicator organism for cefdinir and cefditoren; the respective indicator organisms were incorporated into the medium (nutrient agar; Eiken).
RESULTS
In vitro antibacterial activity.
Table 1 presents the comparative in vitro activities of R-95867 and the other drugs tested against a variety of clinical isolates. R-95867 exhibited potent activity against methicillin-susceptible strains of both S. aureus and Staphylococcus epidermidis, with the MIC at which 90% of tested strains are inhibited (MIC90) being equal to or less than 0.5 μg/ml. Against these species, R-95867 was more active than cefpodoxime, cefditoren, or ofloxacin, but it was less active than imipenem and had almost the same activity as cefdinir. On the other hand, none of the test drugs showed more than minimal potency against methicillin-resistant staphylococci. For Streptococcus pyogenes and Streptococcus agalactiae, the MIC90s of R-95867 were 0.016 and 0.063 μg/ml, respectively. These values were superior to those for ofloxacin, comparable to those for cefpodoxime, cefdinir, and cefditoren, and inferior to those for imipenem. The MIC90 of R-95867 for penicillin-susceptible S. pneumoniae was 0.016 μg/ml, which was comparable to that for imipenem, but R-95867 demonstrated a potency at least eight times as great as those of the other comparative drugs. For penicillin-resistant strains of this organism, the MIC90 of R-95867 was 0.5 μg/ml; this value is comparable to those for cefditoren and imipenem but represents a potency four or more times as great as those of the other drugs tested.
TABLE 1.
Comparative in vitro activities of R-95867 and reference drugs against clinical isolates
| Organism (no. of strains) | Drug | MIC (μg/ml)a
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Staphylococcus aureus | ||||
| Methicillin-susceptible strains (58) | R-95867 | 0.032–8 | 0.125 | 0.25 |
| Cefpodoxime | 1–8 | 2 | 4 | |
| Cefdinir | 0.032–16 | 0.5 | 0.5 | |
| Cefditoren | 0.25–8 | 0.5 | 1 | |
| Ofloxacin | 0.125–32 | 0.5 | 1 | |
| Imipenem | 0.008–0.25 | 0.016 | 0.032 | |
| Methicillin-resistant strains (37) | R-95867 | 0.5–64 | 32 | 64 |
| Cefpodoxime | 1–>128 | >128 | >128 | |
| Cefdinir | 0.125–>128 | 128 | >128 | |
| Cefditoren | 1–>128 | 64 | 128 | |
| Ofloxacin | 0.25–128 | 8 | 128 | |
| Imipenem | 0.063–64 | 16 | 64 | |
| Staphylococcus epidermidis | ||||
| Methicillin-susceptible strains (51) | R-95867 | 0.032–8 | 0.063 | 0.5 |
| Cefpodoxime | 0.25–16 | 0.5 | 8 | |
| Cefdinir | 0.032–4 | 0.063 | 0.5 | |
| Cefditoren | 0.063–4 | 0.25 | 2 | |
| Ofloxacin | 0.063–128 | 0.25 | 8 | |
| Imipenem | ≤0.004–0.25 | 0.016 | 0.125 | |
| Methicillin-resistant strains (23) | R-95867 | 0.5–>128 | 8 | 64 |
| Cefpodoxime | 4–>128 | 16 | >128 | |
| Cefdinir | 0.125–>128 | 4 | >128 | |
| Cefditoren | 2–>128 | 4 | 128 | |
| Ofloxacin | 0.125–>128 | 8 | >128 | |
| Imipenem | 0.063–>128 | 0.5 | 64 | |
| Streptococcus pyogenes (45) | R-95867 | 0.008–0.5 | 0.016 | 0.016 |
| Cefpodoxime | ≤0.004–2 | 0.016 | 0.016 | |
| Cefdinir | ≤0.004–1 | 0.016 | 0.016 | |
| Cefditoren | ≤0.004–1 | 0.008 | 0.016 | |
| Ofloxacin | 0.5–2 | 1 | 2 | |
| Imipenem | ≤0.004–0.125 | ≤0.004 | ≤0.004 | |
| Streptococcus agalactiae (27) | R-95867 | 0.063 | 0.063 | 0.063 |
| Cefpodoxime | 0.032–0.063 | 0.032 | 0.063 | |
| Cefdinir | 0.032–0.063 | 0.063 | 0.063 | |
| Cefditoren | 0.032–0.063 | 0.032 | 0.032 | |
| Ofloxacin | 1–2 | 2 | 2 | |
| Imipenem | 0.016–0.032 | 0.016 | 0.016 | |
| Streptococcus pneumoniae | ||||
| Penicillin-susceptible strains (36) | R-95867 | 0.008–0.032 | 0.008 | 0.016 |
| Cefpodoxime | 0.008–1 | 0.032 | 0.25 | |
| Cefdinir | 0.032–2 | 0.125 | 0.5 | |
| Cefditoren | ≤0.004–0.25 | 0.016 | 0.125 | |
| Ofloxacin | 1–4 | 1 | 2 | |
| Imipenem | ≤0.004–0.016 | 0.008 | 0.008 | |
| Penicillin-resistant strains (32) | R-95867 | 0.032–0.5 | 0.25 | 0.5 |
| Cefpodoxime | 0.063–16 | 2 | 2 | |
| Cefdinir | 0.125–16 | 4 | 8 | |
| Cefditoren | 0.032–1 | 0.5 | 0.5 | |
| Ofloxacin | 1–2 | 2 | 2 | |
| Imipenem | 0.016–0.5 | 0.5 | 0.25 | |
| Enterococcus faecalis (28) | R-95867 | 2–128 | 8 | 32 |
| Cefpodoxime | 1–>128 | 32 | >128 | |
| Cefdinir | 0.5–>128 | 4 | 128 | |
| Cefditoren | 2–>128 | 32 | >128 | |
| Ofloxacin | 2–>128 | 2 | 64 | |
| Imipenem | 0.5–128 | 1 | 4 | |
| Enterococcus faecium (30) | R-95867 | 4–>128 | 128 | >128 |
| Cefpodoxime | 128–>128 | >128 | >128 | |
| Cefdinir | 16–>16 | >128 | >128 | |
| Cefditoren | 16–>128 | >128 | >128 | |
| Ofloxacin | 2–>128 | 32 | 128 | |
| Imipenem | 1–>128 | >128 | >128 | |
| Neisseria gonorrhoeae (27)b,c | R-95867 | 0.016–1 | 0.063 | 1 |
| Cefpodoxime | ≤0.004–4 | 0.063 | 4 | |
| Cefdinir | ≤0.004–1 | 0.032 | 1 | |
| Cefditoren | ≤0.004–8 | 0.063 | 4 | |
| Ofloxacin | 0.008–8 | 0.25 | 8 | |
| Imipenem | 0.25–4 | 1 | 4 | |
| Moraxella catarrhalis (42) | R-95867 | 0.016–0.125 | 0.063 | 0.125 |
| Cefpodoxime | 0.063–2 | 0.5 | 1 | |
| Cefdinir | 0.125–0.5 | 0.25 | 0.5 | |
| Cefditoren | 0.016–1 | 0.125 | 0.5 | |
| Ofloxacin | 0.063–0.5 | 0.125 | 0.125 | |
| Imipenem | 0.008–0.125 | 0.063 | 0.125 | |
| Escherichia coli (30) | R-95867 | 0.008–0.016 | 0.016 | 0.016 |
| Cefpodoxime | 0.125–8 | 0.25 | 0.5 | |
| Cefdinir | 0.063–8 | 0.25 | 0.5 | |
| Cefditoren | 0.063–2 | 0.25 | 0.25 | |
| Ofloxacin | 0.063–0.125 | 0.063 | 0.125 | |
| Imipenem | 0.063–0.25 | 0.125 | 0.125 | |
| Citrobacter freundii (38) | R-95867 | 0.008–2 | 0.016 | 0.125 |
| Cefpodoxime | 0.5–>128 | 2 | >128 | |
| Cefdinir | 0.125–>128 | 1 | 128 | |
| Cefditoren | 0.125–>128 | 1 | 64 | |
| Ofloxacin | 0.063–128 | 0.25 | 4 | |
| Imipenem | 0.125–0.5 | 0.25 | 0.25 | |
| Klebsiella pneumoniae (30) | R-95867 | 0.016–0.125 | 0.016 | 0.032 |
| Cefpodoxime | 0.063–32 | 0.125 | 0.125 | |
| Cefdinir | 0.063–64 | 0.125 | 0.125 | |
| Cefditoren | 0.125–8 | 0.25 | 0.25 | |
| Ofloxacin | 0.063–0.25 | 0.125 | 0.125 | |
| Imipenem | 0.125–0.5 | 0.125 | 0.25 | |
| Klebsiella oxytoca (38) | R-95867 | 0.008–0.032 | 0.016 | 0.032 |
| Cefpodoxime | 0.032–0.5 | 0.125 | 0.25 | |
| Cefdinir | 0.032–1 | 0.125 | 0.25 | |
| Cefditoren | 0.032–0.5 | 0.125 | 0.25 | |
| Ofloxacin | 0.063–0.5 | 0.125 | 0.125 | |
| Imipenem | 0.063–1 | 0.25 | 0.5 | |
| Enterobacter cloacae (29) | R-95867 | 0.016–2 | 0.125 | 1 |
| Cefpodoxime | 0.125–>128 | 1 | 64 | |
| Cefdinir | 0.063–>16 | 2 | >128 | |
| Cefditoren | 0.125–128 | 0.5 | 64 | |
| Ofloxacin | 0.063–16 | 0.063 | 0.25 | |
| Imipenem | 0.125–1 | 0.25 | 0.5 | |
| Serratia marcescens (30) | R-95867 | 0.063–16 | 0.125 | 2 |
| Cefpodoxime | 0.5–>128 | 1 | 16 | |
| Cefdinir | 2–>128 | 8 | >128 | |
| Cefditoren | 0.5–>128 | 1 | 128 | |
| Ofloxacin | 0.125–32 | 0.25 | 16 | |
| Imipenem | 0.25–2 | 0.25 | 0.5 | |
| Proteus vulgaris (42) | R-95867 | 0.016–0.125 | 0.063 | 0.125 |
| Cefpodoxime | 0.032–16 | 0.25 | 8 | |
| Cefdinir | 0.125–64 | 16 | 32 | |
| Cefditoren | 0.032–128 | 0.25 | 2 | |
| Ofloxacin | 0.063–2 | 0.125 | 0.25 | |
| Imipenem | 0.25–4 | 1 | 2 | |
| Proteus mirabilis (27) | R-95867 | 0.032–0.25 | 0.063 | 0.125 |
| Cefpodoxime | 0.032–0.125 | 0.063 | 0.125 | |
| Cefdinir | 0.063–0.25 | 0.125 | 0.125 | |
| Cefditoren | 0.032–0.25 | 0.125 | 0.125 | |
| Ofloxacin | 0.125–0.5 | 0.25 | 0.25 | |
| Imipenem | 0.5–8 | 2 | 4 | |
| Morganella morganii (32) | R-95867 | 0.016–1 | 0.125 | 0.25 |
| Cefpodoxime | 0.125–64 | 1 | 32 | |
| Cefdinir | 0.25–128 | 8 | 16 | |
| Cefditoren | 0.063–8 | 0.5 | 4 | |
| Ofloxacin | 0.063–128 | 0.063 | 0.25 | |
| Imipenem | 0.125–4 | 1 | 2 | |
| Providencia rettgeri (21) | R-95867 | 0.016–2 | 0.125 | 1 |
| Cefpodoxime | ≤0.004–8 | 0.032 | 4 | |
| Cefdinir | ≤0.004–128 | 0.063 | 8 | |
| Cefditoren | 0.016–64 | 0.125 | 16 | |
| Ofloxacin | 0.063–>128 | 0.5 | 64 | |
| Imipenem | 0.25–2 | 1 | 2 | |
| Pseudomonas aeruginosa (30) | R-95867 | 16–128 | 32 | 64 |
| Cefpodoxime | >128 | >128 | >128 | |
| Cefdinir | >128 | >128 | >128 | |
| Cefditoren | 32–>128 | 128 | >128 | |
| Ofloxacin | 0.5–>128 | 2 | 128 | |
| Imipenem | 0.5–16 | 1 | 16 | |
| Burkholderia cepacia (22) | R-95867 | 0.5–64 | 4 | 8 |
| Cefpodoxime | 16–>128 | 32 | 64 | |
| Cefdinir | 2–128 | 32 | 64 | |
| Cefditoren | 16–>128 | 32 | 64 | |
| Ofloxacin | 0.125–32 | 8 | 16 | |
| Imipenem | 0.125–32 | 16 | 32 | |
| Stenotrophomons maltophilia (32) | R-95867 | 4–>128 | >128 | >128 |
| Cefpodoxime | >128 | >128 | >128 | |
| Cefdinir | >128 | >128 | >128 | |
| Cefditoren | 32–>128 | >128 | >128 | |
| Ofloxacin | 0.5–16 | 2 | 4 | |
| Imipenem | 8–>128 | >128 | >128 | |
| Haemophilus influenzae (43) | R-95867 | 0.032–0.25 | 0.063 | 0.25 |
| Cefpodoxime | 0.032–0.5 | 0.063 | 0.25 | |
| Cefdinir | 0.25–2 | 0.5 | 1 | |
| Cefditoren | 0.008–0.063 | 0.016 | 0.032 | |
| Ofloxacin | 0.016–0.063 | 0.032 | 0.032 | |
| Imipenem | 0.125–1 | 0.5 | 1 | |
| Bordetella pertussis (51)c,d | R-95867 | 0.25–0.5 | 0.25 | 0.5 |
| Cefpodoxime | 8–16 | 16 | 16 | |
| Cefdinir | 32–128 | 64 | 64 | |
| Cefditoren | 0.25–0.25 | 0.25 | 0.25 | |
| Ofloxacin | 0.063–0.125 | 0.063 | 0.125 | |
| Imipenem | 0.5–2 | 1 | 1 | |
| Acinetobacter calcoaceticus (28) | R-95867 | 0.032–4 | 0.5 | 2 |
| Cefpodoxime | 0.5–>128 | 16 | >128 | |
| Cefdinir | 0.5–>128 | 4 | >128 | |
| Cefditoren | 1–>128 | 32 | >128 | |
| Ofloxacin | 0.032–4 | 0.25 | 0.5 | |
| Imipenem | 0.016–4 | 0.25 | 4 | |
The MICs were determined by the broth microdilution method with an inoculum of 104 CFU per well.
GC II Agar (BBL) supplemented with 1% hemoglobin and IsoVitaleX (Baltimore Biological Laboratories) was used to test N. gonorrhoeae isolates, which were incubated in 5% CO2 at 35°C for 24 h.
The MICs were determined by the agar dilution method with an inoculum 104 CFU per spot.
Bordet Gengou agar (Difco) supplemented with 15% horse blood and 1% glycerol was used, and the mixture was incubated at 35°C for 48 h.
Among the tested drugs, only imipenem showed significant activity against Enterococcus faecalis while none exhibited appreciable activity against Enterococcus faecium. The MIC90s of both R-95867 and cefdinir for N. gonorrhoeae were 1 μg/ml, representing the highest activity of any agent tested. Against M. catarrhalis, the activity of R-95867 (MIC90, 0.125 μg/ml) proved to be comparable to those of ofloxacin and imipenem and superior to those of the cephems tested.
For E. coli, Citrobacter freundii, K. pneumoniae, K. oxytoca, Proteus vulgaris, Proteus mirabilis, and Morganella morganii, the MIC90s of R-95867 ranged from 0.016 to 0.25 μg/ml; these values were generally superior to those for the other agents tested. The MIC90s of R-95867 for Enterobacter cloacae, Serratia marcescens, and Providencia rettgeri were 1 or 2 μg/ml, and these levels of activity were roughly comparable to that of imipenem but greater than those of the comparative agents. R-95867 showed little activity against P. aeruginosa, which was also true of the comparative agents other than imipenem and ofloxacin. The agent was moderately active against Burkholderia cepacia (MIC90, 8 μg/ml), but all of the Stenotrophomonas maltophilia isolates were resistant to R-95867.
The MIC90s of R-95867 for H. influenzae and B. pertussis were 0.25 and 0.5 μg/ml, respectively, and these were generally superior to those for cefpodoxime, cefdinir, and imipenem but inferior to those for cefditoren and ofloxacin. For Acinetobacter calcoaceticus isolates the MIC90 of R-95867 was 2 μg/ml. This represented activity higher than those of cefpodoxime, cefdinir, cefditoren, or imipenem but lower than that of ofloxacin.
In vivo efficacy in mice. (i) Bacteremic infection.
The protective efficacy of CS-834 against experimental bacteremic infections caused by gram-positive and gram-negative bacteria in mice was compared to the protective efficacies of the reference drugs (Table 2). For infection with S. aureus Smith, the ED50 of CS-834 was 1.30 mg/kg; for the other drugs tested the ED50s were about 1.6 to 27.5 times larger. These values correspond to the respective MICs, which were 2 to 32 times larger for the comparative agents than for R-95867. For infection with E. coli C11, CS-834 was as effective as cefpodoxime proxetil and was more effective than cefdinir, cefditoren pivoxil, and ofloxacin. Against K. pneumoniae 3K25 infection, the ED50 of CS-834 was 6.42 mg/kg, which was comparable to that of cefpodoxime proxetil and which represented a greater effectiveness than those of the other agents tested. By contrast, the MICs of the tested agents for this organism were quite similar.
TABLE 2.
Protective effects of CS-834 and reference drugs against bacteremic infections in mice
| Organism | Challenge dose (CFU/ mouse) | Drug | MIC (μg/ml) | ED50 (mg/kg [95% confidence limit]) |
|---|---|---|---|---|
| S. aureus Smith | 9.5 × 106 | CS-834 | 0.063 | 1.30 (0.90–1.82) |
| Cefpodoxime proxetil | 2 | 17.67 (12.82–24.15) | ||
| Cefdinir | 0.125 | 2.10 (0.81–3.15) | ||
| Cefditoren pivoxil | 0.5 | 35.80 (25.16–59.84) | ||
| Ofloxacin | 0.25 | 10.35 (6.50–17.64) | ||
| E. coli C11 | 7.1 × 103 | CS-834 | 0.032 | 0.27 (0.18–0.35) |
| Cefpodoxime proxetil | 1 | 0.35 (0.29–0.42) | ||
| Cefdinir | 0.063 | 1.32 (1.04–1.65) | ||
| Cefditoren pivoxil | 0.25 | 0.94 (0.42–1.36) | ||
| Ofloxacin | 0.25 | 1.00 (0.70–1.30) | ||
| K. pneumoniae 3K25 | 9.0 × 103 | CS-834 | 0.016 | 6.42 (4.44–15.92) |
| Cefpodoxime proxetil | 0.125 | 7.36 (3.87–15.71) | ||
| Cefdinir | 0.125 | 43.30 (31.92–58.74) | ||
| Cefditoren pivoxil | 0.25 | 16.82 (6.52–25.22) | ||
| Ofloxacin | 0.125 | 38.50 (27.58–53.79) |
(ii) Local infection.
Against respiratory tract infections induced by inoculation with penicillin-susceptible S. pneumoniae TUH39, CS-834 was a little less effective than amoxicillin but was more effective than cefdinir and cefditoren pivoxil (Table 3). When the infection was induced by inoculation with penicillin-resistant S. pneumoniae, the mean number of bacteria recovered from the lungs of untreated mice was 7.51 ± 0.37 log CFU/animal (Table 4). Treatment with CS-834 at a dosage of 20 or 50 mg/kg three times a day for 3 days led to significant reductions (P < 0.05) in the numbers of bacteria recovered compared with the numbers of bacteria recovered from untreated animals or animals similarly treated with cefdinir, cefditoren pivoxil, or amoxicillin. However, treatment with 10 mg/kg did not significantly reduce the bacterial numbers in any comparison. The other agent demonstrating significant activity in this model was amoxicillin, which at a dose of 50 mg/kg produced significant reductions (P < 0.05) in the number of bacteria recovered compared with the numbers of bacteria recovered from control, cefdinir-treated, and cefditoren pivoxil-treated animals.
TABLE 3.
Therapeutic efficacies of CS-834 and reference drugs against murine respiratory tract infections with penicillin-susceptible S. pneumoniae TUH39a
| Drug | MIC (μg/ml) | ED50 (mg/kg [95% confidence limit]) |
|---|---|---|
| CS-834 | 0.016 | 1.78 (1.20–2.73) |
| Cefdinir | 0.25 | 19.79 (13.11–33.40) |
| Cefditoren pivoxil | 0.063 | 5.34 (3.40–7.49) |
| Amoxicillin | 0.032 | 1.03 (0.55–1.76) |
The drugs were administered orally two times a day for 3 days, and the ED50 was expressed as one dose. The challenge dose was 4.8 × 106 CFU/mouse.
TABLE 4.
Therapeutic efficacies of CS-834 and reference drugs against murine respiratory tract infection with penicillin-resistant S. pneumoniae TUM741
| Drug | MIC (μg/ml) | Dose (mg/kg) | Log CFU/lung (mean ± SE) |
|---|---|---|---|
| Control | 7.51 ± 0.37 | ||
| CS-834 | 0.5 | 50 | 3.13 ± 0.18a |
| 20 | 3.40 ± 0.52a | ||
| 10 | 6.61 ± 0.29 | ||
| Cefdinir | 8 | 50 | 7.33 ± 0.20 |
| 20 | 7.33 ± 0.26 | ||
| 10 | 7.84 ± 0.20 | ||
| Cefditoren pivoxil | 0.5 | 50 | 6.34 ± 0.38 |
| 20 | 6.19 ± 0.35 | ||
| 10 | 6.99 ± 0.15 | ||
| Amoxicillin | 1 | 50 | 4.56 ± 0.37b |
| 20 | 6.99 ± 0.15 | ||
| 10 | 7.78 ± 0.23 |
P < 0.05 versus control mice and mice treated with cefdinir, cefditoren pivoxil, and amoxicillin.
P < 0.05 versus control mice and mice treated with cefdinir and cefditoren pivoxil.
Among mice with respiratory infections caused by penicillinase-nonproducing H. influenzae TUM8, the number of bacteria in the lungs of untreated mice ranged from 5.09 to 5.5 log CFU/g (Table 5). Treatment with CS-834 or amoxicillin at a dose of 0.125 mg/kg led to significant reductions (P < 0.05) in the numbers of organisms in the lungs compared with the numbers of organisms in the lungs of untreated mice. Moreover, the bacterial numbers in the lungs of cefditoren pivoxil-treated mice were below the detectable limits. Among mice with pulmonary infections caused by penicillinase-producing H. influenzae TUM36, treatment with CS-834 led to significant reductions (P < 0.05) in the number of organisms recovered from the lungs compared with the numbers of organisms recovered from untreated, cefdinir-treated, and amoxicillin-treated mice. The numbers of bacteria in the lungs of cefditoren pivoxil-treated mice were again below the detectable limits.
TABLE 5.
Therapeutic efficacies of CS-834 and reference drugs against murine respiratory tract infections with H. influenzae
| Druga |
H. influenzae TUM8 (beta-lactamase nonproducing)
|
H. influenzae TUM36 (beta-lactamase producing)
|
||
|---|---|---|---|---|
| MIC (μg/ml) | Log CFU/g (mean ± SE) | MIC (μg/ml) | Log CFU/g (mean ± SE) | |
| Control | 5.19 ± 0.32 | 6.54 ± 0.32 | ||
| CS-834 | 0.125 | 3.09 ± 0.11b | 0.063 | 3.90 ± 0.29b |
| Cefdinir | 0.5 | 4.38 ± 0.32 | 0.25 | 6.07 ± 0.07 |
| Cefditoren pivoxil | 0.016 | UDc | 0.016 | UD |
| Amoxicillin | 1 | 3.73 ± 0.59b | 32 | 97.08 ± 0.45 |
The drugs were administered orally two times a day for 3 days at a dose of 0.125 mg/kg.
P < 0.05 versus control mice and mice treated with cefdinir and amoxicillin.
UD, under the lower limit of detectability.
Effect of cilastatin on the efficacy of CS-834.
The activity of the combination of CS-834 and the dehydropeptidase I (DHP-I) inhibitor cilastatin was sometimes, but not always, modestly superior to that of CS-834 alone (Table 6). For murine bacteremic infections caused by various strains, the ED50s of CS-834 alone were 1.1 to 1.7 times larger than those of CS-834–cilastatin. For respiratory infections caused by penicillin-susceptible S. pneumoniae TUH39, the addition of cilastatin reduced the ED50 by a factor of 2.3. Among mice with murine pulmonary infections caused by penicillin-resistant S. pneumoniae TUM741, the level of reduction of the number of organisms in the lungs following treatment with CS-834–cilastatin at a dose of 20 and 50 mg/kg was the same as that following treatment with CS-834 alone at the corresponding dose. However, in contrast to the lack of effect following treatment with CS-834 alone at a dose of 10 mg/kg, treatment with CS-834–cilastatin led to reductions in the numbers of organisms in the lungs that were significant (P < 0.05) compared with the reductions in the lungs of animals that were untreated or treated with CS-834, cefdinir, cefditoren pivoxil, or amoxicillin. Among mice with murine pulmonary infections due to penicillinase-nonproducing H. influenzae TUM8 or penicillinase-producing H. influenzae TUM36, the efficacies of CS-834–cilastatin were almost the same as those of CS-834 alone.
TABLE 6.
Effect of cilastatin on therapeutic efficacy of CS-834 against bacteremic and respiratory tract infections in mice
| Organisms | Challenge dose (CFU/mouse) | Drug | MIC (μg/ml) | Dose (mg/kg) | Therapeutic effect |
|---|---|---|---|---|---|
| S. aureus Smith | 9.5 × 106 | CS-834 | 0.063 | 1.30 (0.90–1.82)a | |
| CS-834–cilastatin | 0.76 (0.52–1.09)a | ||||
| E. coli C11 | 7.1 × 103 | CS-834 | 0.032 | 0.27 (0.18–0.35)a | |
| CS-834–cilastatin | 0.18 (0.09–0.25)a | ||||
| K. pneumoniae 3K25 | 9.0 × 103 | CS-834 | 0.016 | 6.42 (4.44–15.92)a | |
| CS-834–cilastatin | 5.66 (4.14–7.80)a | ||||
| S. pneumoniae TUH39, penicillin susceptible | 4.8 × 106 | CS-834 | 0.016 | 1.78 (1.20–2.73)a | |
| CS-834–cilastatin | 0.76 (0.49–1.42)a | ||||
| S. pneumoniae TUM741, penicillin resistant | 5.1 × 105 | Control | 7.51 ± 0.37b | ||
| CS-834 | 0.5 | 50 | 3.13 ± 0.18b,c | ||
| 20 | 3.40 ± 0.52b,c | ||||
| 10 | 6.61 ± 0.29b | ||||
| CS-834–cilastatin | 50 | 3.06 ± 0.21b,c | |||
| 20 | 3.76 ± 0.30b,c | ||||
| 10 | 4.76 ± 0.55b,d | ||||
| H. influenzae TUM8 (beta-lactamase nonproducing) | 4.2 × 105 | Control | 5.19 ± 0.32d | ||
| CS-834 | 0.125 | 20 | 3.09 ± 0.11c,e | ||
| CS-834–cilastatin | 20 | 3.11 ± 0.12c,e | |||
| H. influenzae TUM36 (beta-lactamase producing) | 1.0 × 105 | Control | 6.54 ± 0.32e | ||
| CS-834 | 0.063 | 20 | 3.90 ± 0.29c,e | ||
| CS-834–cilastatin | 20 | 3.60 ± 0.21c,e |
ED50 (milligrams per kilogram [95% confidence limit]).
Log CFU/mouse (mean ± standard error).
P < 0.05 versus control mice.
P < 0.05 versus control mice and CS-834.
Log CFU/gram (mean ± standard error).
Effect of drug administration interval on the efficacy of CS-834.
Among mice with pulmonary infections due to penicillin-resistant S. pneumoniae, the numbers of organisms in the lungs of mice administered CS-834–cilastatin four times a day at 4-h intervals, with each dose being 37.5 mg/kg (total daily dose, 150 mg/kg), were below detectable limits. This efficacy was greater than that seen when the same total daily dose was administered as three doses of 50 mg/kg at 6-h intervals. Treatment with CS-834–cilastatin at a dosage of 7.5 mg/kg four times per day led to significant reductions (P < 0.05) in the numbers of organisms in the lungs compared with the numbers in the lungs of untreated mice. Among mice with murine pulmonary infections due to penicillinase-nonproducing H. influenzae TUM8, the numbers of organisms in the lungs of mice administered 13.3 mg of CS-834–cilastatin per kg three times a day were below the detectable limits. This efficacy was better than that seen with administration of 20 mg/kg twice a day at 12-h intervals.
Drug levels in plasma and tissues of mice infected with S. pneumoniae.
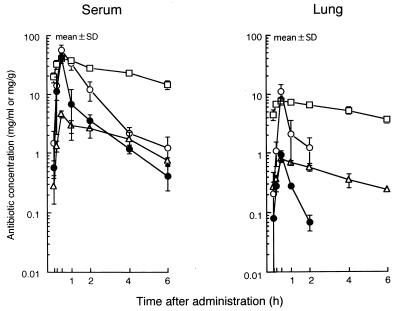
The levels of R-95867, R-95867–cilastatin, cefdinir, and cefditoren in the sera and lungs of mice infected with S. pneumoniae are presented in Fig. 1, and the calculated pharmacokinetics are presented in Table 7. For plasma, the area under the concentration-time curve (AUC), half-life (t1/2), and the maximum concentration (Cmax) of R-95867 were 32.0 μg · h/ml, 1.3 h, and 41.7 μg/ml, respectively; for the lungs, the AUC was 0.7 μg · h/g and the Cmax was 0.9 μg/ml. Cilastatin increased the AUC of R-95867 in plasma by a factor of 2.3 and the AUC of R-95867 in the lungs by a factor of 10.7. Similarly, the Cmax in plasma was increased by a factor 1.4 and that in lungs was increased by a factor of 12.2.
FIG. 1.
Levels of R-95867 after oral administration of CS-834 (50 mg/kg) to mice infected with S. pneumoniae TUM39 (n = 3). •, R-95867; ○, R-95867–cilastatin; ▵, cefdinir; □, cefditoren.
TABLE 7.
Values of pharmacokinetic parameters for CS-834, CS-834–cilastatin, and reference drugs
| Specimen | Drug | Cmax (μg/ml or μg/g) | AUC (μg · h/ml) | t1/2 (h) |
|---|---|---|---|---|
| Plasma | R-95867 | 41.7 | 32.0 | 1.3 |
| Cefdinir | 4.6 | 9.2 | 1.5 | |
| Cefditoren | 40.6 | 234.1 | 3.9 | |
| R-95867-cilastatin | 56.8 | 74.6 | 1.3 | |
| Lung | R-95867 | 0.9 | 0.7 | NDa |
| Cefdinir | 0.8 | 3.2 | 2.4 | |
| Cefditoren | 7.7 | 61.6 | 5.3 | |
| R-95867-cilastatin | 11.0 | 7.5 | ND |
ND, not determined.
DISCUSSION
Many types of antibacterial agents (for example, beta-lactams, macrolides, tetracyclines, and quinolones) are available as orally administered drugs. Nevertheless, it is well known that exposure to these antimicrobial agents has led to various types of drug-resistant organisms. Parenteral carbapenems have been developed because they are highly potent against gram-positive cocci and gram-negative bacilli, including P. aeruginosa. The recently introduced carbapenems include imipenem, which is highly resistant to the beta-lactamases produced by bacteria. However, it is not resistant to DHP-I derived from kidney and lung tissues and from the brush border of the alimentary tract (5). Therefore, the agent actually injected is a combination of imipenem and the DHP-I inhibitor cilastatin. On the other hand, meropenem is relatively resistant to human DHP-I and is therefore injected alone, even though it is not resistant to the DHP-I of experimental animals (4, 6). This result indicates that carbapenems’ stability to DHP-I differs with the source of the enzyme.
Since orally administered penems such as fropenem and ritipenem acoxil are relatively sensitive to DHP-I (2, 17), there is continuing interest in the development of novel oral carbapenems resistant to this enzyme. On the basis of a comparison of the values of the pharmacokinetic parameters for humans (13) and recovery in the urine of mice by coadministration with cilastatin (11) with those found in the present study, we speculate that the stability of R-95867 to DHP-I may be similar to that of meropenem. As a result, use of this new drug in humans may not require simultaneous administration of a DHP-I inhibitor.
R-95867 proved to be a broad-spectrum antibiotic that was slightly less active than imipenem against gram-positive cocci and more active than imipenem against the members of the family Enterobacteriaceae. In addition, R-95867 was more active than imipenem against H. influenzae and B. pertussis, although it lacked activity against P. aeruginosa. The data presented here suggest two major clinical indications for oral carbapenems: One might be for treatment of respiratory and urinary tract infections caused by members of the family Enterobacteriaceae. Another might be for treatment of infections due to S. pneumoniae; both penicillin-susceptible and penicillin-resistant strains proved to be susceptible to R-95867, although penicillin-resistant strains were less so (8).
Against murine bacteremic or lung infections involving various organisms, the efficacy of CS-834 was generally better than those of the reference drugs. CS-834–cilastatin was in general slightly more effective than CS-834 alone. The present study showed that administration of CS-834–cilastatin three times a day was more effective than administration of the combination twice a day in murine H. influenzae infection models. The same phenomenon was observed in a murine S. pneumoniae infection model. This result is in accord with the suggestion that the AUC above the MIC is the pharmacokinetic parameter that best indicates the efficacy of a beta-lactam (3).
Against a murine pulmonary infection caused by S. pneumoniae, the efficacy of cefditoren pivoxil proved to be lower than that which would have been predicted from the MIC. A feature of this model is penetration of the infecting organisms into the alveoli (16); thus, poor alveolar penetration by cefditoren pivoxil represents a possible explanation for its unexpectedly poor efficacy. Further studies examining the differences between the in vitro and in vivo activities of this drug should allow a better understanding of this discrepancy.
In conclusion, the studies described here indicate that CS-834 is a promising novel oral carbapenem for the treatment of infections caused not only by gram-negative bacteria but also by gram-positive bacteria, including penicillin-resistant S. pneumoniae. The results of clinical trials are awaited with interest.
ACKNOWLEDGMENT
We thank W. A. Thomasson for expert editorial assistance.
REFERENCES
- 1.Asahi Y, Miyazaki S, Yamaguchi K. In vitro and in vivo antibacterial activities of BO-2727, a new carbapenem. Antimicrob Agents Chemother. 1995;39:1030–1037. doi: 10.1128/aac.39.5.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett I, Broom N, Coleman K, Coulton S, Edwards P, Fracois I, Griffin D, Osborne N, Woodall P. 6-(Substituted methylene) penems, potent broad spectrum inhibitors of bacterial beta-lactamase. IV. Kidney stability, serum binding and additional biological evaluation of racemic derivatives. J Antibiot. 1991;44:338–343. doi: 10.7164/antibiotics.44.338. [DOI] [PubMed] [Google Scholar]
- 3.Fantin B, Leggett J, Ebert S, Craig W. Correlation between in vitro and in vivo activity of antimicrobial agents against gram-negative bacilli in a murine infection model. Antimicrob Agents Chemother. 1991;35:1413–1422. doi: 10.1128/aac.35.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukasawa M, Sumita Y, Harabe E T, Tanino T, Nouda H, Kohzuki T, Okuda T, Matsumura H, Sunagawa M. Stability of meropenem and effect of 1β-methyl substitution on its stability in the presence of renal dehydropeptidase I. Antimicrob Agents Chemother. 1992;36:1577–1579. doi: 10.1128/aac.36.7.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gnarpe H, Belsheim J, Blomqvist C, Lundback A. Stimulation of granulocyte functions in vitro by imipenem and the renal enzyme inhibitor MK 0791. Antimicrob Agents Chemother. 1984;25:178–181. doi: 10.1128/aac.25.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hikida M, Kawashima K, Yoshida M, Mitsuhashi S. Inactivation of new carbapenem antibiotics by dehydropeptidase-I from porcine and human renal cortex. J Antimicrob Chemother. 1992;30:129–134. doi: 10.1093/jac/30.2.129. [DOI] [PubMed] [Google Scholar]
- 7.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A beta-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs C, Huang L, Bartowsky E, Normark S, Park J. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for beta-lactamase induction. EMBO J. 1994;13:4684–4694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Japanese Society for Chemotherapy. Method for the determination of minimum inhibitory concentration (MIC) of aerobic bacteria by microdilution method. Chemotherapy (Tokyo) 1990;38:102–105. [Google Scholar]
- 10.Japanese Society for Chemotherapy. Method for the determination of minimum inhibitory concentration (MIC) of fastidious bacteria and anaerobic bacteria by microdilution method. Chemotherapy (Tokyo) 1993;41:183–189. [Google Scholar]
- 11.Miyauchi M, Endo R, Hisaoka M, Yasuda H, Kawamoto I. Synthesis and structure-activity relationships of a novel oral carbapenem, CS-834, J. Antibiot. 1997;50:429–439. doi: 10.7164/antibiotics.50.429. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki S, Nunoya T, Matsumoto T, Tateda K, Yamaguchi K. New murine model of bronchopneumonia due to cell-bound Haemophilus influenzae. J Infect Dis. 1997;175:205–209. doi: 10.1093/infdis/175.1.205. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima M, Umemura K, Ikeda Y, Kondou K. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. CS-834, a new oral carbapenem. V. Safety and pharmacokinetics in healthy male volunteer, abstr. F109; p. 119. [Google Scholar]
- 14.Sanders C. Chromosomal cephalosporinases responsible for multiple resistance to newer beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:573–593. doi: 10.1146/annurev.mi.41.100187.003041. [DOI] [PubMed] [Google Scholar]
- 15.Shimada J, Kawahara Y. Overview of a new carbapenem, panipenem/betamipron. Drugs Exp Clin Res. 1994;20:241–245. [PubMed] [Google Scholar]
- 16.Takashima K, Tateda K, Matsumoto T, Ito T, Iizawa Y, Nakao M, Yamaguchi K. Establishment of a model of penicillin-resistant Streptococcus pneumoniae pneumonia in healthy CBA/J mice. J Med Microbiol. 1996;45:319–322. doi: 10.1099/00222615-45-5-319. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji A, Sato H, Tamai I, Adachi H, Nishihara T, Ishiguro M, Ohmura N, Noguchi T. Physiologically based pharmacokinetics of a new penem, SUN5555, for evaluation of in vivo efficacy. Drug Metab Dispos. 1990;18:245–252. [PubMed] [Google Scholar]