Abstract
Antineoplastic drugs (ADs) are essential tools in cancer treatment, but their cytotoxicity poses a risk to workers involved in their handling. In a hospital environment fundamental strategies for minimising exposure involve proper use of safety cabinets and closed-circuit transfer devices, along with personnel training and increased awareness of risks. However, medical gloves remain the first line of defence. In this respect the evaluation of glove materials and best choices can improve hospital safety management and prevent potential hazards and long-term consequences. The aim of this study was to assess contamination of gloves in samples taken from AD administration and preparation units of nine Italian hospitals and to raise awareness of the importance of evaluating chemico-physical properties of gloves. Our findings show that 33 % of the analysed gloves were positive for at least one AD, with contaminations ranging from 0.6 to 20,729 pg/ cm2. We proposed the alert glove values (AGVs) for each AD as a limit value for contamination assessment and good practice evaluation. Our findings also point to multiple AD contamination (43 % of positive findings in preparation units), calculated as total AGV (AGV-T), and confirm that gloves should be replaced after 30 min of AD handling, based on cumulative permeation and area under the curve (AUC), to maintain safety and limit dermal exposure.
KEY WORDS: alert glove values, glove contamination, glove permeation
Sažetak
Antineoplastični lijekovi (AD) imaju raširenu i ključnu primjenu u liječenju karcinoma, ali zbog svoje citotoksičnosti predstavljaju rizik za zdravstvene radnike koji njima rukuju. U bolničkom okružju osnovne strategije za smanjenje izloženosti obuhvaćaju pravilnu primjenu zatvorenih sustava za rukovanje i prijenos tih lijekova te obuku osoblja i povećanje svijesti o rizicima. Ipak, prva crta obrane i dalje su medicinske rukavice. U tom smislu, ocjena materijala rukavica i izbor onih najboljih može unaprijediti sigurnost i spriječiti moguće opasnosti i dugoročne posljedice. Cilj je ovog istraživanja bio ocijeniti onečišćenje uzoraka rukavica uzetih nakon pripreme i primjene antineoplastičnih lijekova u devet talijanskih bolnica te ukazati na važnost ocjene fizikalno-kemijskih svojstava takvih rukavica. Pokazalo se da je 33 % analiziranih rukavica bilo pozitivno na barem jedan antineoplastični lijek, a onečišćenje se kretalo u rasponu od 0,6 do 20,729 pg/cm2. Naš novi parametar, koji smo nazvali kritične vrijednosti rukavica (izv. alert glove values, krat. AGV), izračunan za svaki pojedini antineoplastični lijek kao 95. percentil, može dobro poslužiti za usporedbu ocjena onečišćenja. Naši rezultati također upozoravaju na onečišćenje s više antineoplastičnih lijekova odjednom (43 % pozitivnih uzoraka u pripremnim jedinicama), koje je izračunano kroz ukupni AGV, te na potrebu da se rukavice zamijene 30 minuta nakon rukovanja antineoplastičnim lijekom s obzirom na kumulativnu propusnost i površinu ispod krivulje kako bi se sačuvala sigurnost i ograničila izloženost putem kože.
KLJUČNE RIJEČI: alert glove values, AGV, propusnost, kontaminacija
Just considering the healthcare sector, 12.7 million healthcare workers in the European Union (EU) (57 % of whom are nurses) are exposed to hazardous medicinal products (HMPs) (1) at work, antineoplastic drugs (ADs) in particular. Ample and conclusive scientific evidence illustrates that many HMP substances have carcinogenic, mutagenic, and reprotoxic effects (2). In addition, over 30 years of research point to higher cancer mortality among healthcare workers than the general population (3–5), and two to three times higher risk of malignancies and miscarriage in day-hospital nurses responsible for handling HMPs (6–8).
In June 2019, the European Parliament and Council issued the third revision of the so called Carcinogen and Mutagens Directive (CMD) 2004/37/EC (9) to recognise and prioritise for the first time this important issue for healthcare workers and patients exposed to HMPs. In 2020, the European Commission conducted a study and consultation to further amend the CMD (5, 10, 11), which resulted in the last revision (Directive 2022/431/EU) (12) in March 2022 to be adopted by national laws in all EU member states by 5 April 2024. The Directive extends its scope to include category 1A and 1B carcinogenic, mutagenic, and reprotoxic substances listed by the EU Classification, Labelling and Packaging (CLP) Regulation (EC) 1272/2008 (13). Following this revision, the EU commissioned new guidelines for the safe management of HMPs (14), based on which, the European Trade Union Institute (ETUI) published a list of HMPs (15).
To prevent occupational exposure to ADs, healthcare personnel is required to have their risk assessed. As a matter of fact, the EU regulatory hierarchy of control stipulates that personal protective equipment is the last of the safety levels to be implemented, giving fundamental priority to measures that lie upstream of the problem. So, the revised Directive 2022/431 recommends that workers exposed to HMPs must receive specific training to prevent the risk of adverse health effects. Furthermore, carcinogen, mutagen, or reprotoxic substances must be manufactured and used in closed systems corresponding to those used in healthcare industry, since it is often not technically possible to replace or substitute ADs. These closed systems include biological safety cabinets, containment isolators, and closed system transfer devices (CSTDs). As for CSTDs, the Cochrane review (16) concludes that “the evidence is too uncertain to conclude that there are differences in exposure or financial benefits between CSTD plus safe handling versus safe handling alone”. Some advances were made in recent years in the field of AD compounding robotics, but the final steps are still handled by the hospital personnel, and scientific evidence (17, 18) confirms that contamination can still be found on prepared doses and on the gloves of operators. Furthermore, the increasing number of new treatment procedures, such as Hyperthermic IntraPEritoneal Chemotherapy (HIPEC), which involves filling the abdominal cavity with high concentration drug solutions and is performed by surgeons in the operating room, creates new risk scenarios which require particular attention.
To minimise risk, the occupational safety approach consists of a combination of occupational hygiene control methods, which follow a specific hierarchy. Specifically, environmental and biological monitoring are useful tools to identify contamination trends and corrective measures and to increase operators’ awareness. Until today, several studies (19–21) have reported ADs in urine samples of hospital personnel who prepare and administer them. Dermal absorption is considered a major exposure pathway (22) and can occur by direct contact with the drugs (manipulation of contaminated packaging and/or pharmacological solutions in intravenous bags) or indirect contact (touching of contaminated surfaces and/or biological fluids). In this context, wipe sampling of exposed surfaces helps to monitor the potential risk of dermal contact, whereas biological monitoring provides valuable information only in critical exposure situations (23, 24).
The choice of appropriate medical gloves is critical: the lower their permeability, the better (25). The most common medical glove materials that have replaced vinyl in AD handling are chloroprene and nitrile – a synthetic rubber copolymer made by combining acrylonitrile and butadiene (26). The latter account for 72 % of the total glove market and are anticipated to dominate it even more by 2030 (27–28).
However, nitrile gloves seem to vary in quality, even within batches of the same producer, which can result in substantial differences in their permeation rate (PR), breakthrough detection time (BDT), and breakthrough time (BT), as reported by some authors (29–34). The main factors associated with the observed variations concern their polymer properties (such as polymer thickness and uniformity, area density, modulus and tensile strength, carboxylation of the base polymer and volume fraction) and composition (acrylonitrile content, inorganic fillers, extractable oil and oily plasticiser content). These characteristics are often not declared by producers, nor are detailed permeation data, which makes the selection of proper gloves difficult. In addition, even when permeation data are available, standardised quality control tests run by producers do not satisfactorily predict permeation, which limits the use of chemical compatibility charts for the selection of the most suitable glove. Therefore, to facilitate the choice, some authors have proposed indicators such as cumulative permeation, calculated by multiplying permeation rate and the time passed after the breakthrough time (29), which indicates the mass of substance that has passed through the glove during exposure, or Phalen’s area under the permeation curve (AUC) (35), which further focuses on exposure time and is best used to compare different glove materials at different times (36).
The aim of our study was to evaluate the contamination of 30 ADs on outer surfaces of gloves in Italian hospitals and propose a new index, which we have termed alert glove value (AGV), based on the 95th percentile of contamination values. In addition, the cumulative permeation and the AUC of commonly used nitrile gloves was evaluated as a tool to facilitate the selection and use of nitrile gloves when handling ADs.
MATERIALS AND METHODS
Sample collection and preparation
To evaluate AD contamination, we collected 174 pairs of medical nitrile gloves from the preparation (89 pairs) and administration (85 pairs) units of nine Italian hospitals, each handling more than 20,000 treatments a year. Hospital staff had received all necessary training and been kept abreast with the use of safety equipment and closed-system devices, research updates, emergency care protocols, and specific cleaning.
During sampling, the AD preparation and administration staff were instructed to remove used gloves (by turning them inside out) and collect them every 30 min. The average thickness of the gloves ranged between 0.05 and 0.12 mm. Each pair of gloves was then stored in a separate transport bag and sent to the laboratory for analysis.
Each glove was first inspected for the presence of punctures, and then filled with 40 mL of a 50:50 (v/v) mass spectrometry-grade water-methanol desorbing solution (both components purchased from Biosolve Chimie SARL, Dieuze, France). The gloves were then sealed with plastic clips and agitated for 30 s to obtain an exhaustive AD extraction. The extract was then transferred to a 60 mL glass vial and placed in a Genevac EZ-2 personal solvent evaporator (Genevac Ltd, Ipswich, UK) until completely dry. The dried sample was resuspended with 2 mL of desorbing solution containing the internal standard and transferred into a 2 mL glass vial through a 0.2 µm GHP Acrodisc 13-mm filter (Pall Corporation, New York, USA) (Figure 1).
Figure 1.
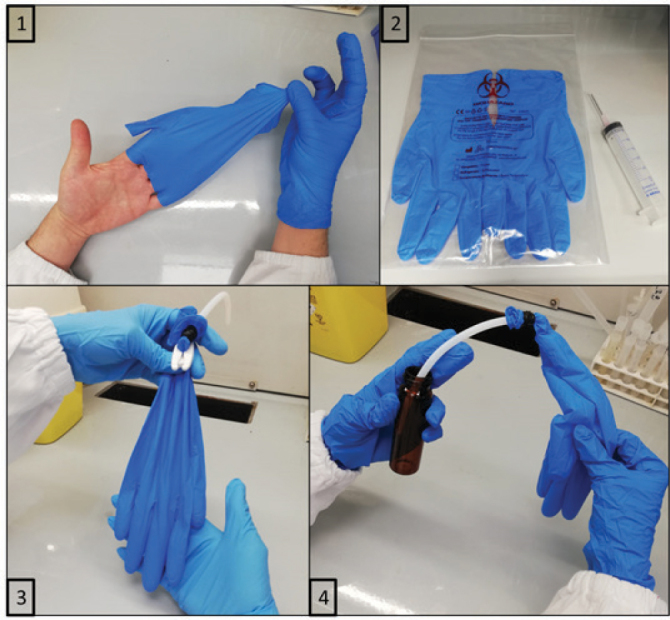
Nitrile glove sample collection (panels 1 & 2) and extraction procedure (panels 3 & 4)
Analytical methods
For calibration we used pharmaceutical preparations instead of analytical standards to get a more accurate response of the compound, taking into account the variability of analyte signals in the presence of excipients. The drug products dacarbazine, daunorubicin, docetaxel, doxorubicin, epirubicin, etoposide, idarubicin, iphosphamide, irinotecan, melphalan, methotrexate, paclitaxel, topotecan, vinblastine, vincristine, vinorelbine, and 5-fluorouracil were purchased from Teva Pharmaceutical Industries Ltd. (Petah Tiqwa, Israel). Cyclophosphamide was obtained from Baxter (Deerfield, IL, USA), mitomycin C, pemetrexed, carboplatin, cisplatin, cytarabine, and gemcitabine (GEM) from Accord Healthcare Inc. (Durham, NC, USA), oxaliplatin from Sun Pharmaceutical Industries Ltd. (Milan, Italy), busulfan from American Reagent Inc. (Shirley, NY, USA), fotemustine from Les Laboratoire Servier (Suresnes, France), raltitrexed from Pfizer Italia S.r.l. (Milan, Italy), vindesine from EG S.P.A (Milan, Italy), 5-azacytidine from Zentiva (Prague, Czech Republic), and tamoxifen from Merck KGaA (Darmstadt, Germany).
The analyses were carried out on a Shimadzu Nexera X2 liquid chromatography system coupled with a Shimadzu LCMS 8050 triple quadrupole mass spectrometer equipped with an electrospray ionisation (ESI) source (Shimadzu Corp., Kyoto, Japan). Liquid chromatography took two separate runs, one on a Cortecs® UPLC T3 (2.1×50 mm, 1.6 µm particle size) (Waters Corporation, Milford, MA, USA) and the other on an Agilent® Poroshell 120 HILIC-Z (2.1×100 mm, 2.7 µm particle size) (Agilent Technologies, Santa Clara, CA, USA) as described in detail earlier (37, 38). We added trofosfamide (MedChemExpress EU, Sollentuna, Sweden) as internal standard, and the two chromatographic runs were performed in sequence thanks to the automated switch of the Shimadzu CTO-20AC valve program.
A sample was considered positive for a drug if the value was above the methods’ limit of quantification (LOQ).
Determination of the alert glove value
The alert glove value (AGV) that we propose as a limit value for external glove contamination corresponds to the 95th percentile of the distribution of concentrations observed for each AD. It was calculated using Excel (Microsoft Office 365, Microsoft, Redmond, USA). Considering the difference in terms of exposure between administration and preparation units, we proposed two respective AGVs – AGVAdm and AGVPrep, – calculated from the analysis of the data obtained for the two roles separately. Furthermore, we summed up the quantities detected for every AD on each glove in order to evaluate the impact of a exposure to multiple ADs, and calculated the corresponding AGV, expressed as total AGV (AGV-T).
Cumulative permeation and area under the curve
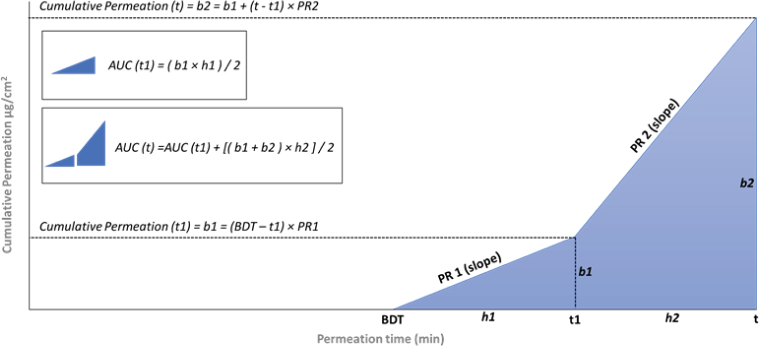
Exposure of at-risk hospital personnel was evaluated from Phalen’s AUC (36). The permeation rate (PR) and breakthrough detection time (BDT) for 5-fluorouracil, cyclophosphamide, carboplatin, etoposide, iphosphamide, and carmustine were extrapolated from Oriyama et al. (39). Cumulative permeation and the AUC at the time of exposure t were calculated for 0.05 mm thick nitrile gloves, using respective equations 1 and 2, as follows:
| 1 |
| 2 |
where: b1=(t1-BDT)×PR1; b2=b1+(t-t1)×PR2; h1=t1-BDT; h2=t- t1.
The AUC equation was modified to take into account variation in permeation rates with time by adding trapezoid surface sections to Phalen’s triangular AUC (36), as shown in Figure 2, and by replacing breakthrough time with breakthrough detection time (BDT) to gain a more accurate idea of real dermal exposure. Namely, breakthrough time (BT) is the time at which permeation rate surpasses 10 ng/(min cm2) as recommended by the American Society of Testing and Materials (ASTM) (33), whereas BDT is the time of the first analyte (AD in this case) detection and is more in line with the European Biosafety Network recommendation of 0.1 ng/cm2 (40) and the “as low as reasonably achievable” (ALARA) principle.
Figure 2.
Calculation of the cumulative permeation and area under the curve at different time points. AUC – area under the curve; b1 – cumulative permeation (t1); b2 – cumulative permeation (t); BDT – breakthrough detection time; PR1 – permeation rate (t1); PR2 – permeation rate (t)
RESULTS AND DISCUSSION
Of the 174 pairs of analysed nitrile gloves, 58 pairs were positive for at least one AD, 43 of which originated from preparation units, with contaminations ranging from 0.6 to 20,729 pg/cm2. Of the 30 drugs we tested the gloves for, seven can be found in concentrations which surpass the proposed 100 pg/cm2 limit for a total of 31 times.
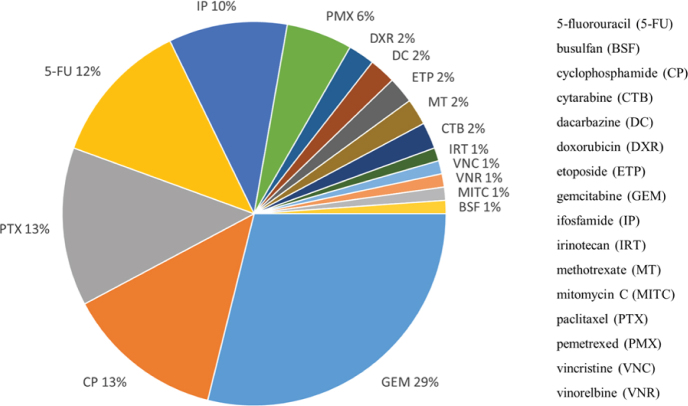
Figure 3 shows that, out of the 90 positive findings, contaminations with gemcitabine (26 pairs), cyclophosphamide (12 pairs), paclitaxel, (12 pairs), 5-fluorouracil (11 pairs), iphosphamide (9 pairs), and pemetrexed (5 pairs) were the most common.
Figure 3.
Prevalence of positive tests to antineoplastic drugs on collected nitrile gloves
Tables 1 and 2 show AD contamination and AGVs (expressed as pg/cm2), whereas Table 3 shows AGV-Ts and related data for administration and preparation units. The AGVs were greater than the instrumental LOQ for 5-fluorouracil in administration units and for 5-fluorouracil, gemcitabine, cyclophosphamide, paclitaxel, iphosphamide, pemetrexed, and carboplatin in preparation units. Preparation units seem to involve higher contamination, not only because of higher drug concentrations and frequency of use, but also for the presence of different pharmaceutical forms, such as powdered drugs, which involve higher risk of contamination.
Table 1.
Glove contamination and calculated alert glove values (AGVs) in AD administration units expressed as pg/cm2
| Administration unit | |||||||
|---|---|---|---|---|---|---|---|
| Positive gloves (N) | Gloves with contamination >100 pg/cm2 (N) | Average contamination (pg/cm2) | Highest contamination (pg/cm2) | 90th percentile (pg/cm2) | AGV Adm (95th percentile) (pg/cm2) | LOQ (pg/cm2) | |
| 5-FU | 5 | 2 | 784 | 3244 | <LOQ | 3.3 | 0.15 |
| GEM | 3 | 1 | 496 | 1385 | <LOQ | <LOQ | 0.28 |
| IRT | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.06 |
| CP | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.03 |
| DXR | 1 | 0 | 7 | 8 | <LOQ | <LOQ | 0.12 |
| DC | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.04 |
| EPI | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.11 |
| ETP | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.21 |
| MT | 1 | 0 | 5 | 5 | <LOQ | <LOQ | 0.02 |
| PTX | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.19 |
| DTX | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 2.59 |
| TMX | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.08 |
| TPT | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.03 |
| VNC | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.16 |
| VNB | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.44 |
| VNR | 1 | 0 | 16 | 16 | <LOQ | <LOQ | 0.06 |
| FTM | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.04 |
| MITC | 1 | 0 | 2 | 2 | <LOQ | <LOQ | 0.02 |
| IDC | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.62 |
| IP | 4 | 0 | 23 | 52 | <LOQ | <LOQ | 0.04 |
| CTB | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.06 |
| MP | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.18 |
| BSF | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.09 |
| PMX | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.09 |
| RTX | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.08 |
| VND | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 5.71 |
| 5-AZ | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.70 |
| CisPt | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 29.58 |
| CarboPt | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 2.73 |
| OxaliPt | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.35 |
LOQ – instrumental limits of quantification for each substance. 5-FU – 5-fluorouracil; BSF – busulfan; CP – cyclophosphamide; CarboPt – carboplatin; CisPt – cisplatin; OxaliPt – oxaliplatin; CTB – cytarabine; DC – dacarbazine; DNR – daunorubicin; DTX – docetaxel; DXR – doxorubicin; EPI – epirubicin; ETP – etoposide; GEM – gemcitabine; FTM – fotemustine; IDC – idarubicine; IP – iphosfamide; IRT – irinotecan; MP – melphalan; MT – methotrexate; MITC – mitomycin C; PTX – paclitaxel; PMX – pemetrexed; TMX – tamoxifen; RTX – raltitrexed; TPT – topotecan; VNB – vinblastine; VNC – vincristine; VND – vindesine; VNR – vinorelbine
Table 2.
Glove contamination and calculated alert glove values (AGVs) in AD preparation units expressed as pg/cm2
| Preparation unit | |||||||
|---|---|---|---|---|---|---|---|
| Positive gloves (N) | Gloves with contamination >100 pg/cm2 (N) | Average contamination (pg/cm2) | Highest contamination (pg/cm2) | 90th percentile (pg/cm2) | AGVPrep (95th percentile) (pg/cm2) | LOQ (pg/cm2) | |
| 5-FU | 6 | 3 | 3667 | 20729 | <LOQ | 13.6 | 0.15 |
| GEM | 23 | 10 | 1292 | 12767 | 163.6 | 1985.2 | 0.28 |
| IRT | 1 | 0 | 36 | 36 | <LOQ | <LOQ | 0.06 |
| CP | 12 | 3 | 319 | 2934 | 13.8 | 40.6 | 0.03 |
| DXR | 1 | 0 | 39 | 39 | <LOQ | <LOQ | 0.12 |
| DC | 2 | 0 | 7 | 7 | <LOQ | <LOQ | 0.04 |
| EPI | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.11 |
| ETP | 2 | 0 | 35 | 56 | <LOQ | <LOQ | 0.21 |
| MT | 1 | 0 | 50 | 50 | <LOQ | <LOQ | 0.02 |
| PTX | 12 | 5 | 170 | 487 | 34 | 97.8 | 0.19 |
| DTX | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 2.59 |
| TMX | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.08 |
| TPT | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.03 |
| VNC | 1 | 0 | 46 | 46 | <LOQ | <LOQ | 0.16 |
| VNB | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.44 |
| VNR | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.06 |
| FTM | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.04 |
| MITC | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.02 |
| IDC | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.62 |
| IP | 5 | 1 | 84 | 395 | <LOQ | 0.6 | 0.04 |
| CTB | 2 | 1 | 2602 | 5190 | <LOQ | <LOQ | 0.06 |
| MP | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.18 |
| BSF | 1 | 0 | 18 | 18 | <LOQ | <LOQ | 0.09 |
| PMX | 5 | 4 | 421 | 1175 | <LOQ | 19.3 | 0.09 |
| RTX | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.08 |
| VND | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 5.71 |
| 5-AZ | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.70 |
| CisPt | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 29.58 |
| CarboPt | 6 | 1 | 0 | 414 | <LOQ | 11.2 | 2.73 |
| OxaliPt | 0 | 0 | 0 | 0 | <LOQ | <LOQ | 0.35 |
LOQ – instrumental limits of quantification for each substance. 5-FU – 5-fluorouracil; BSF – busulfan; CP – cyclophosphamide; CarboPt – carboplatin; CisPt – cisplatin; OxaliPt – oxaliplatin; CTB – cytarabine; DC – dacarbazine; DNR – daunorubicin; DTX – docetaxel; DXR – doxorubicin; EPI – epirubicin; ETP – etoposide; GEM – gemcitabine; FTM – fotemustine; IDC – idarubicine; IP – iphosfamide; IRT – irinotecan; MP – melphalan; MT – methotrexate; MITC – mitomycin C; PTX – paclitaxel; PMX – pemetrexed; TMX – tamoxifen; RTX – raltitrexed; TPT – topotecan; VNB – vinblastine; VNC – vincristine; VND – vindesine; VNR – vinorelbine
Table 3.
Cumulative AD contamination and total alert glove values (AGV-T) for administration and preparation units
| Administration (pg/cm2) | Preparation (pg/cm2) | |
|---|---|---|
| Positive gloves (N) | 15 | 44 |
| Average cumulative contamination (pg/cm2) | 369 | 1503 |
| Highest cumulative contamination (pg/cm2) | 3279 | 33518 |
| Cumulative 70th percentile (pg/cm2) | <LOQ | 150 |
| AGV-T (95th percentile) (pg/cm2) | 123 | 3422 |
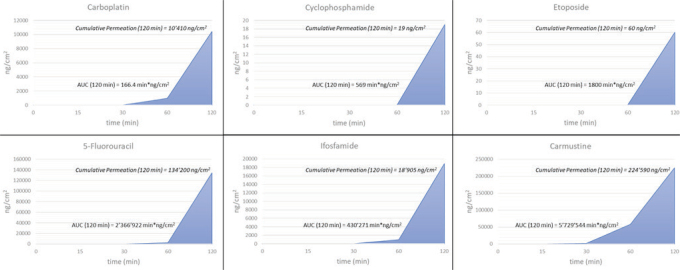
Figure 4 shows the calculated cumulative permeation and AUC for 0.05 mm thick nitrile gloves, and Figure 5 shows how carmustine permeation drops with glove thickness. These findings confirm that gloves should be replaced every 30 min when handling ADs, especially if their thickness is not greater than 0.05 mm. Thicker gloves are strongly recommended in preparation units, since both cumulative permeation and AUC strongly decrease with glove thickness. As several studies report (41–48), a significant factor for glove contamination with ADs in preparation units is direct contact with the external surface of a vial and/or primary packaging, which can reach as high as 344 ng for iphosphamide, 69,800 ng for cyclophosphamide, 272 ng for gemcitabine, 37 ng for cisplatin, 15,000 ng for methotrexate, 18,000 ng for 5-fluorouracil, 794 ng for carboplatin, and 1,890 ng for etoposide. In response to these reports, some manufacturers have shrink-wrapped drug vials and managed to reduce contamination by a factor between 1.5 and 2.0 (49).
Figure 4.
Calculated cumulative permeation and area under the curve (AUC) for carboplatin, cyclophosphamide, etoposide, 5-fluorouracil, iphosphamide, and carmustine after 120 min of exposure (assuming 0.05 mm glove thickness)
Figure 5.
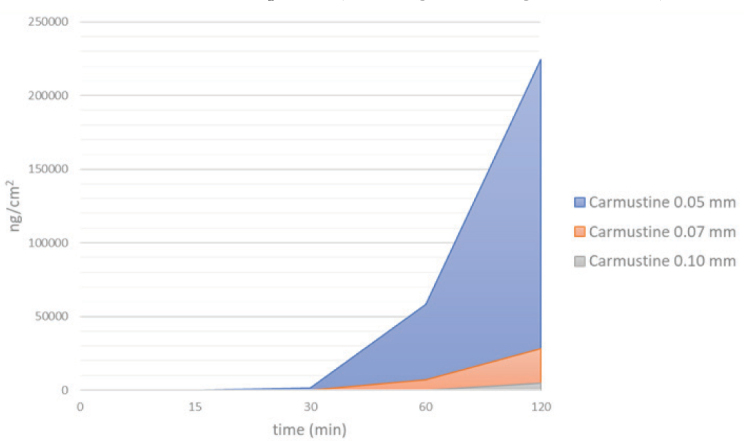
Comparison of carmustine permeation curves between nitrile gloves of different thickness
Concurrently, high surface contaminations can be encountered by the staff of administration units inside hospital wards, where accidental AD spills and biological fluids of treated patients can cause surface contamination, which can be encountered in 48 % of wipe samples (50, 51) and reach concentrations of 4.784 µg/cm2 (52).
Some authors (45, 53, 54) have already reported high AD glove contamination with cyclophosphamide (69,819 ng/pair), 5-fluorouracil (140,000 ng/pair), gemcitabine (2.4 ng/cm2), and methotrexate (1,632 ng/pair) in particular, suggesting possible AD spread within the pharmacy, posing a risk of indirect contamination for healthcare operators (55).
In this respect, we would also like to point out another important factor which contributes to glove permeation. Literature data show that 5-fluorouracil, cyclophosphamide, iphosphamide, and gemcitabine, which are used in greater quantities (56, 57), have low molecular weight (MW) (130, 261, 261, and 263 Da, respectively), and which, according to Oriyama et al. (39), increases the glove permeation rate. Besides low molecular weight, these and other authors point to a possible influence of other chemico-physical properties, such as high drug lipophilia (high logarithm of octanol-water partition coefficient, Log P) (39), which is still controversial (58), topological polar surface area, and hydrogen bond donor capacity(59).
As expected, the reported data on carmustine (Figure 5) confirm a direct correlation between glove thickness and permeation. Greater glove thickness or area density increases the breakthrough time (time before a chemical passes the barrier) and decreases the permeation rate (volume passing through a membrane) (34, 39, 60).
It is also important to remember that even when each AD is kept under a limit such as AGV, this does not rule out a cumulative effect from multiple exposure. This is why simultaneous evaluation of multiple AD contamination (such as AGV-T) should be fundamental. The data in Table 3 show a non-negligible cumulative exposure, especially in preparation units, where nearly half the positive gloves had multiple contaminations.
CONCLUSION
This study was focused on the risk posed by improper choice or use of medical gloves while handling ADs. We have taken this further by proposing AGV limit values and the evaluation of permeation risk for nitrile gloves.
Not all nitrile gloves provide equal chemical resistance to ADs, and their selection should be based on more than the usual information about thickness, tensile strength, and elongation at break, as these may in some cases poorly indicate permeation. Improved barrier performances can also be associated with higher area density, higher acrylonitrile content, higher carboxylation, lower polymer variation (improved uniformity), lower cumulative permeation, and AUC. These predictors should be included in future glove evaluations to reduce variability in performance and improve their quality. Some manufacturers have already proposed new ways to characterise gloves based on new instruments and surface characterisation techniques, such as skeletal density, rubber surface area, and glove surface topography (61).
In conclusion, the selection and use of gloves should rely on the following principles: i) favour gloves that meet performance standards and provide information on permeation rate, breakthrough time, and degradation; ii) favour thicker gloves, as they generally provide greater protection, except if thickness affects handling (in some situations, double-gloving may be recommended); iii) when data are available, calculate the AUC for the substance under consideration; iv) workers should be trained to inspect gloves prior to and during use; v) gloves should be replaced every 30 min during AD handling; vi) gloves should be replaced immediately after damage or spillage; vii) hands should be washed after glove removal to eliminate potential traces of ADs; and viii) use proposed limit values, such as AGVs, to improve occupational safety and AD contamination control.
REFERENCES
- 1.Inclusion of Hazardous Medicinal Products within the scope of the Carcinogens and Mutagens Directive. European Trade Union Institute. . [displayed 20 March 2023]. Available at https://www.stopcanceratwork.eu/wp-content/uploads/2020/10/ETUI-Briefing-Note-HMP-CMD4.pdf.
- 2.Musu T, Vogel L Cancer and work: understanding occupational cancers and taking action to eliminate them, 2018. . [displayed 23 March 2023]. Available at https://policycommons.net/artifacts/2067248/cancer-and-work/2821452/
- 3.Petralia SA, Dosemeci M, Adams EE, Zahm SH Cancer mortality among women employed in health care occupations in 24 U.S. States, 1984–1993 Am J Ind Med. 1999;36:159. doi: 10.1002/(SICI)1097-0274(199907)36:1<159::AID-AJIM23>3.0.CO;2-K. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 4.Ratner PA, Spinelli JJ, Beking K, Lorenzi M, Chow Y, Teschke K, Le ND, Gallagher RP, Dimich-Ward H Cancer incidence and adverse pregnancy outcome in registered nurses potentially exposed to antineoplastic drugs BMC Nurs. 2010;9:15. doi: 10.1186/1472-6955-9-15. . . ; : . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Biosafety Network Protecting health workers from hazardous products. . [displayed 23 March 2023]. Available at https://www.europeanbiosafetynetwork.eu/protecting-health-workers-from-hazardous-products/
- 6.Skov T, Maarup B, Olsen J, Rørth M, Winthereik H, Lynge E Leukaemia and reproductive outcome among nurses handling antineoplastic drugs Occup Environ Med. 1992;49:855. doi: 10.1136/oem.49.12.855. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin S, Larson E Chemotherapy-handling practices of outpatient and office-based oncology nurses Oncol Nurs Forum. 2003;30:575. doi: 10.1188/03.ONF.575-581. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 8.Lawson CC, Rocheleau CM, Whelan EA, Lividoti Hibert EN, Grajewski B, Spiegelman D, Rich-Edwards JW Occupational exposures among nurses and risk of spontaneous abortion Obstet Anesth Digest. 2013;33:36. doi: 10.1097/01.aoa.0000426099.31929.92. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Directive (EU) 2019/983 of the European Parliament and of the Council of 5 June 2019 amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. [displayed 11 June 2023]. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32019L0983.
- 10.HOSPEEM-EPSU position in view of the European Commission study supporting the assessment of different options concerning the protection of workers from exposure to hazardous medicinal products, including cytotoxic medicinal products. European Public Service Unions (EPSU) . [displayed 23 March 2023]. Available at https://www.epsu.org/sites/default/files/article/files/HOSPEEM-EPSU-position-Carcinogens-and-Mutagens-Directive_0.pdf.
- 11.Study supporting the assessment of different options concerning the protection of workers from exposure to hazardous medicinal products, including cytotoxic medicinal products. Publications Office of the EU. . [displayed 23 March 2023]. Available at https://op.europa.eu/en/publication-detail/-/publication/f43015ec-a24f-11eb-b85c-01aa75ed71a1/language-en.
- 12.Directive (EU) 2022/431 of the European Parliament and of the Council of 9 March 2022 amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. [displayed 19 April 2023]. Available at https://eur-lex.europa.eu/eli/dir/2022/431/oj.
- 13.Regulation (EC) no 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. [displayed 11 June 2023]. Available at https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32008R1272.
- 14.Online workshops: Guidance document for the safe management of hazardous medicinal products at work. European Biosafety Network. . [displayed 19 April 2023]. Available at https://www.europeanbiosafetynetwork.eu/online-workshops-guidance-document-for-the-safe-management-of-hazardous-medicinal-products-at-work/
- 15.The ETUI’s list of hazardous medicinal products (HMPs) including cytotoxics and based on the EU CLP classification system of Carcinogenic, Mutagenic and Reprotoxic (CMR) substances. European Trade Union Institute. . [displayed 20 March 2023]. Available at https://www.etui.org/publications/etuis-list-hazardous-medicinal-products-hmps.
- 16.Gurusamy KS, Best LMJ, Tanguay C, Lennan E, Korva M, Bussières JF Closed-system drug-transfer devices plus safe handling of hazardous drugs versus safe handling alone for reducing exposure to infusional hazardous drugs in healthcare staff Cochrane Database Syst Rev. 2018;2018(3):CD012860. doi: 10.1002/14651858.CD012860.pub2. . . ; ( ): . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schierl R, Masini C, Groeneveld S, Fischer E, Böhlandt A, Rosini V, Paolucci D Environmental contamination by cyclophosphamide preparation: Comparison of conventional manual production in biological safety cabinet and robot-assisted production by APOTECAchemo J Oncol Pharm Pract. 2016;22:37. doi: 10.1177/1078155214551316. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 18.Krämer I, Federici M, Schierl R Environmental and product contamination during the preparation of antineoplastic drugs with robotic systems Pharm Technol Hosp Pharm. 2018;3:153. doi: 10.1515/pthp-2018-0018. . . ; : –. . doi: [DOI] [Google Scholar]
- 19.Ndaw S, Remy A Occupational exposure to antineoplastic drugs in twelve French health care setting: biological monitoring and surface contamination Int J Environ Res Public Health. 2023;20(6):4952. doi: 10.3390/ijerph20064952. . . ; ( ): . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labrèche F, Ouellet C, Roberge B, Caron NJ, Yennek A, Bussières JF Occupational exposure to antineoplastic drugs: what about hospital sanitation personnel? Int Arch Occup Environ Health. 2021;94:1877. doi: 10.1007/s00420-021-01731-w. . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 21.Leso V, Sottani C, Santocono C, Russo F, Grignani E, Iavicoli I Exposure to antineoplastic drugs in occupational settings: a systematic review of biological monitoring data Int J Environ Res Public Health. 2022;19(6):3737. doi: 10.3390/ijerph19063737. . . ; ( ): . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connor TH Hazardous anticancer drugs in health care Ann N Y Acad Sci. 2006;1076:615. doi: 10.1196/annals.1371.021. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 23.Dugheri S, Bonari A, Pompilio I, Boccalon P, Mucci N, Arcangeli G A new approach to assessing occupational exposure to antineoplastic drugs in hospital environments Arh Hig Rada Toksikol. 2018;69:226. doi: 10.2478/aiht-2018-69-3125. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 24.Mucci N, Dugheri S, Farioli A, Garzaro G, Rapisarda V, Campagna M, Bonari A, Arcangeli G Occupational exposure to antineoplastic drugs in hospital environments: potential risk associated with contact with cyclophosphamide-and ifosfamide-contaminated surfaces Med Pr. 2020;71:519. doi: 10.13075/mp.5893.00931. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 25.Dugheri S, Mucci N, Mini E, Cappelli G, Bucaletti E, Squillaci D, Trevisani L, Arcangeli G An update on permeation of protective medical gloves by antineoplastic drugs Sigurnost. 2022;64:341. doi: 10.31306/s.64.4.2. . . ; : –. . doi: [DOI] [Google Scholar]
- 26.Chemo Rated Nitrile Gloves According to Experts. Medrux. . [displayed 23 March 2023]. Available at https://medrux.com/chemo-rated-nitrile-gloves/
- 27.Nitrile Gloves Market Size, Share & Trends Analysis Report By Type (Powdered, Powder-Free), By Grade (Medical Grade, Industrial Grade, Food Grade), By Texture (Smooth, Micro Roughened, Aggressively Textured), By End-Use (Medical & Healthcare, Food & Beverage, Automotive, Oil & Gas, Construction, Chemical, Pharmaceutical, Metals & Machinery, Others) Based On Region, And Segment Forecasts, 2022–2028. Brand Mark Res. . [displayed 19 April 2023]. Available at https://brandessenceresearch.com/chemical-and-materials/nitrile-gloves-market.
- 28.History of Nitrile Gloves, 2021. MedSupply. . [displayed 23 March 2023]. Available at https://www.med-supply.net/blogs/product-specifications/nitrile-gloves-history.
- 29.Perkins JL, Pool B Batch lot variability in permeation through nitrile gloves Am Ind Hyg Assoc J. 1997;58:474. doi: 10.1080/15428119791012568. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 30.Phalen RN, Hee SSQ, Xu W, Wong WK Acrylonitrile content as a predictor of the captan permeation resistance for disposablenitrile rubber gloves J Appl Polym Sci. 2007;103:2057. doi: 10.1002/app.25349. . . ; : –. . doi: [DOI] [Google Scholar]
- 31.Brouwer DH, Aitken RJ, Oppl R, Cherrie JW Concepts of skin protection: considerations for the evaluation and terminology of the performance of skin protective equipment J Occup Environ Hyg. 2005;2:425. doi: 10.1080/15459620500220453. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 32.Mickelsen RL, Hall RC A breakthrough time comparison of nitrile and neoprene glove materials produced by different glove manufacturers Am Ind Hyg Assoc J. 1987;48:941. doi: 10.1080/15298668791385859. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 33.Performance of Protective Clothing. [displayed 20 April 2023]. Available at https://www.astm.org/stp900-eb.html.
- 34.Phalen RN, Dubrovskiy AV, Brown BC, Gvetadze AR, Bustillos M, Ogbonmwan J Chemical permeation of similar disposable nitrile gloves exposed to volatile organic compounds with different polarities Part 2. Predictive polymer properties J Occup Environ Hyg. 2020;17:172. doi: 10.1080/15459624.2020.1721511. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 35.Phalen RN, Wong WK Tensile properties and integrity of clean room and low-modulus disposable nitrile gloves: a comparison of two dissimilar glove types Ann Occup Hyg. 2012;56:450. doi: 10.1093/annhyg/mer116. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson DI, Phalen RN Review of the performance, selection, and use of gloves for chemical protection ACS Chem Health Saf. 2022;29:39. doi: 10.1021/acs.chas.1c00084. . . ; : –. . doi: [DOI] [Google Scholar]
- 37.Dugheri S, Mucci N, Squillaci D, Marrubini G, Bartolucci G, Melzi C, Bucaletti E, Cappelli G, Trevisani L, Arcangeli G Developing a fast ultra-high-performance liquid chromatography-tandem mass spectrometry method for high-throughput surface contamination monitoring of 26 antineoplastic drugs Separations. 2021;8:150. doi: 10.3390/separations8090150. . . ; : . doi: [DOI] [Google Scholar]
- 38.Dugheri S, Mucci N, Squillaci D, Bucaletti E, Cappelli G, Trevisani L, Valsecchi C, Consonni V, Gosetti F, Ballabio D, Arcangeli G Expanding antineoplastic drugs surface monitoring profiles: enhancing of zwitterionic hydrophilic interaction methods Separations. 2022;9:34. doi: 10.3390/separations9020034. . . ; : . doi: [DOI] [Google Scholar]
- 39.Oriyama T, Yamamoto T, Nara K, Kawano Y, Nakajima K, Suzuki H, Aoyama T Prediction of the permeability of antineoplastic agents through nitrile medical gloves by zone classification based on their physicochemical properties J Pharm Health Care Sci. 2020;6:23. doi: 10.1186/s40780-020-00179-3. . . ; : . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amendments to the carcinogens and mutagens directive on hazardous drugs and implications for change to the healthcare system in Europe to ensure compliance with its requirements. European Biosafety Network. . [displayed 20 March 2023]. Available at https://www.europeanbiosafetynetwork.eu/wp-content/uploads/2019/03/Amendments-to-CMD3-and-implications.pdf.
- 41.Mason HJ, Morton J, Garfitt SJ, Iqbal S, Jones K Cytotoxic drug contamination on the outside of vials delivered to a hospital pharmacy Ann Occup Hyg. 2003;47:681. doi: 10.1093/annhyg/meg078. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 42.Connor TH, Sessink PJM, Harrison BR, Pretty JR, Peters BG, Alfaro RM, Bilos A, Beckmann G, Bing MR, Anderson LM, DeChristoforo R Surface contamination of chemotherapy drug vials and evaluation of new vial-cleaning techniques: Results of three studies Am J Health Syst Pharm. 2005;62:475. doi: 10.1093/ajhp/62.5.475. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 43.Hilliquin D, Bussières J External contamination of antineoplastic drug containers from a Canadian wholesaler J Oncol Pharm Pract. 2020;26:423. doi: 10.1177/1078155219868525. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 44.Nygren O, Gustavsson B, Ström L, Friberg A Cisplatin contamination observed on the outside of drug vials Ann Occup Hyg. 2002;46:555. doi: 10.1093/annhyg/mef074. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 45.Sessink PJM, Boer KA, Scheefhals APH, Anzion RBM, Bos RP Occupational exposure to antineoplastic agents at several departments in a hospital. Environmental contamination and excretion of cyclophosphamide and ifosfamide in urine of exposed workers Int Arch Occup Environ Health. 1992;64:105. doi: 10.1007/BF00381477. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 46.Delporte JP, Chenoix P, Hubert PH Chemical contamination of the primary packaging of 5-Fluorouracil RTU solutions commercially available on the Belgian market Eur Hospital Pharm. 1999;5:119. . . ; : –. . [Google Scholar]
- 47.Osawa T, Naito T, Suzuki N, Imai K, Nakanishi K, Kawakami J Validated method using liquid chromatography-electrospray ionization tandem mass spectrometry for the determination of contamination of the exterior surface of vials containing platinum anticancer drugs Talanta. 2011;85:1614. doi: 10.1016/j.talanta.2011.06.059. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 48.Fleury-Souverain S, Nussbaumer S, Mattiuzzo M, Bonnabry P Determination of the external contamination and cross-contamination by cytotoxic drugs on the surfaces of vials available on the Swiss market J Oncol Pharm Pract. 2014;20:100. doi: 10.1177/1078155213482683. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 49.Schierl R, Herwig A, Pfaller A, Groebmair S, Fischer E Surface contamination of antineoplastic drug vials: comparison of unprotected and protected vials Am J Health Syst Pharm. 2010;67:428. doi: 10.2146/ajhp080621. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 50.Korczowska E, Jankowiak-Gracz H, Crul M, Tuerk J, Arnold D, Meier K 3PC-041 Surface contamination with cytotoxic drugs in European hospital wards Eur J Hosp Pharm. 2020;27(Suppl 1):A41. doi: 10.1136/ejhpharm-2020-eahpconf.88. . . ; ( ): . doi: [DOI] [Google Scholar]
- 51.Korczowska E, Crul M, Tuerk J, Meier K Environmental contamination with cytotoxic drugs in 15 hospitals from 11 European countries-results of the MASHA project Eur J Oncol Pharm. 2020;3(2):pe24. doi: 10.1097/OP9.0000000000000024. . . ; ( ): . doi: [DOI] [Google Scholar]
- 52.Lancharro PM, Iglesias NDCA, González-Barcala FJ, González JDM Evidence of exposure to cytostatic drugs in healthcare staff: a review of recent literature Farm Hosp. 2016;40:604. doi: 10.7399/fh.2016.40.6.9103. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 53.Hilliquin D, Tanguay C, Bussières J-F External contamination of commercial containers by antineoplastic agents: a literature review Eur J Hosp Pharm. 2020;27:313. doi: 10.1136/ejhpharm-2018-001705. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crauste-Manciet S, Sessink PJM, Ferrari S, Jomier J-Y, Brossard D Environmental contamination with cytotoxic drugs in healthcare using positive air pressure isolators Ann Occup Hyg. 2005;49:619. doi: 10.1093/annhyg/mei045. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 55.Cotteret C, Secretan P-H, Gilles-Afchain L, Rousseau J, Vidal F, Salguero-Hernandez G, Batista J, Valverde V, Guitton J, Cisternino S, Schlatter J External contamination of antineoplastic drug vials: an occupational risk to consider Eur J Hosp Pharm. 2022;29:284. doi: 10.1136/ejhpharm-2020-002440. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dugheri S, Mucci N, Bucaletti E, Squillaci D, Cappelli G, Trevisani L, Bonari A, Cecchi M, Mini E, Ghiori A, Tognoni D, Berti N, Alderighi F, Li Vigni N, Orlandi I, Arcangeli G Monitoring surface contamination for thirty antineoplastic drugs: a new proposal for surface exposure levels (SELs) Med Pr. 2022;73:383. doi: 10.13075/mp.5893.01288. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 57.Rubino FM, Verduci C, Buratti M, Fustinoni S, Campo L, Omodeo-Salè E, Giglio M, Iavicoli S, Brambilla G, Colombi A Assay of urinary α-fluoro-β-alanine by gas chromatography-mass spectrometry for the biological monitoring of occupational exposure to 5-fluorouracil in oncology nurses and pharmacy technicians Biomed Chromatogr. 2006;20:257. doi: 10.1002/bmc.559. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 58.Wallemacq PE, Capron A, Vanbinst R, Boeckmans E, Gillard J, Favier B Permeability of 13 different gloves to 13 cytotoxic agents under controlled dynamic conditions Am J Health Syst Pharm. 2006;63:547. doi: 10.2146/ajhp050197. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 59.Nalin M, Hug G, Boeckmans E, Machon C, Favier B, Guitton J Permeation measurement of 27 chemotherapy drugs after simulated dynamic testing on 15 surgical and examination gloves: A knowledge update J Oncol Pharm Pract. 2021;27:1395. doi: 10.1177/1078155220950423. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 60.Phalen RN, Wong WK Polymer properties associated with chemical permeation performance of disposable nitrile rubber gloves J Appl Polym Sci. 2015;132:41449. doi: 10.1002/app.41449. . . ; : . doi: [DOI] [Google Scholar]
- 61.Analysis of laboratory nitrile gloves: From pores to the surface. Relevant for: rubber, thin films, elastomers, atomic force microscopy, density testing, nanostructure analysis, surface roughness, surface area. Covalent Metrology. . [displayed 20 March 2023]. Available at https://covalentmetrology.com/wp-content/uploads/2021/05/D53IA031EN-B_ApplReport_RubberGlove.pdf.