Abstract
Utilised in a variety of consumer products, per- and polyfluoroalkyl substances (PFAS) are major environmental contaminants that accumulate in living organisms due to their highly hydrophobic, lipophobic, heat-resistant, and non-biodegradable properties. This review summarizes their effects on microbial populations in soils, aquatic and biogeochemical systems, and the human microbiome. Specific microbes are insensitive to and even thrive with PFAS contamination, such as Escherichia coli and the Proteobacteria in soil and aquatic environments, while some bacterial species, such as Actinobacteria and Chloroflexi, are sensitive and drop in population. Some bacterial species, in turn, have shown success in PFAS bioremediation, such as Acidimicrobium sp. and Pseudomonas parafulva.
KEY WORDS: bioremediation, environment, microbiome, PFAS, toxicity
Sažetak
Budući da se koriste u izradi raznih potrošačkih proizvoda, per- i polifluoroalkilne tvari (engl. per- and polyfluoroalkyl substances, krat. PFAS) veliki su zagađivači okoliša koji se nakupljaju u živim organizmima zbog svoje izrazite hidrofobičnosti, lipofobičnosti, otpornosti na toplinu i biološke nerazgradljivosti. Ovaj članak donosi sažeti pregled njihova djelovanja na populacije mikroba u tlu, vodnim i biogeokemijskim sustavima te na humanom mikrobiomu. Pojedini su mikrobi neosjetljivi na zagađenje PFAS-om, čak i napreduju, poput bakterije Escherichia coli i proteobakterija u tlu i vodi, a osjetljive su pojedine bakterijske vrste, poput rodova Actinobacteria i Chloroflexi, pa im se smanjuje populacija u takvom okružju. Neke su se, pak, bakterije pokazale uspješnima u bioremedijaciji, poput vrsta Acidimicrobium sp. i Pseudomonas parafulva.
KLJUČNE RIJEČI: bioremedijacija, mikrobiom, okoliš, PFAS, toksičnost
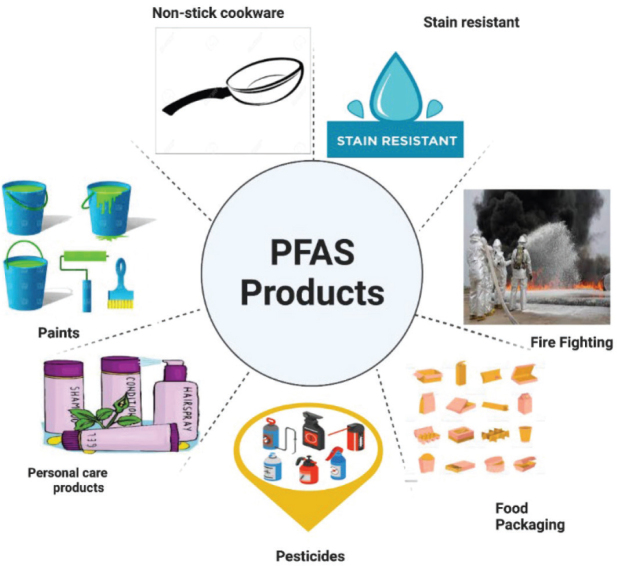
Per- and polyfluoroalkyl substances (PFAS) are a large group of over 9,000 fluorinated carbon compounds used in a wide range of industrial and consumer applications thanks to their heat resistance, non-degradability, and hydro- and oleo/lipophobicity. Industrial applications include aqueous film-fighting foams, coatings, and surfactants, whereas consumer applications include non-stick cookware, shampoos, cleaners, paints, and food packaging materials (1–4) (Figure 1). Their continued consumer and industrial use have made PFAS a major environmental and human health concern, as they contaminate soils, groundwater, rivers, lakes, drinking water, and the atmosphere.
Figure 1.
Most common sources of PFAS in the environment
They affect microbial communities across these diverse environments by disrupting their biogeochemical activities in water and soil, with a ripple effect on the organisms in the higher trophic level of the food chain, including humans and their microbiome (5–10).
This, in particular, affects their beneficial symbiotic role in animal and human metabolism through digestion and absorption of nutrients in the gut, neutralising drugs, and synthesis of vitamins that aid immune response (7), which may ultimately advance to the development of metabolic diseases (8). This evidence in mice models (7, 8) is driving research for comparable results in humans, although the knowledge of PFAS-induced microbial dysbiosis and metabolic syndrome in humans is limited and very much understudied.
Over the years, different environmental remediation strategies have been designed to degrade PFAS contaminants in the environment (11). This includes nanofiltration designs, reverse osmosis in household drinking water (12), and biodegradation. However, heavily fluorinated domains in the molecular structure of PFAS make them resistant to degradation and present a major challenge to environmental scientists, regulators, and government agencies in the development of active remediation methods (11). One possible avenue is microbial degradation, which has proved effective and cheap in the elimination of chlorinated compounds, gasoline spills, and most common industrial wastes (13).
The aim of this review is to take a closer look at the published and unpublished data on the complex relationship between PFAS and healthy microbiome in humans, microbial populations in the environment, and the utilisation of microbial species in bioremediation of PFAS in the environment.
PFAS: PROPERTIES AND CLASSIFICATION
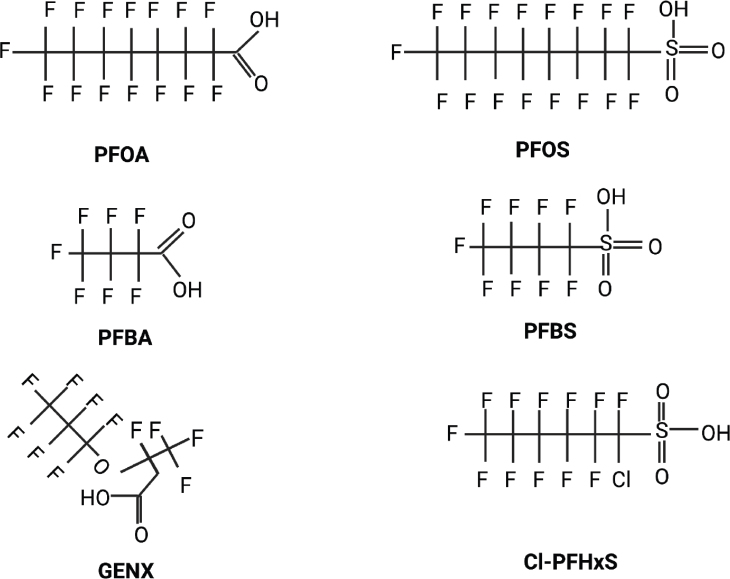
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are synthetic compounds with at least one carbon-fluorine bond (CF). Some of the most common PFAS include perfluoroalkyl acids (PFAAs), perfluoro octane sulphonate (PFOS), perfluorooctanoic acid (PFOA), and other fluoroalkyl substances (14, 15) (Figure 2).
Figure 2.
Chemical structure of some common long- and short-chain PFAS. PFOA – perfluorooctanoic acid (long-chain); PFOS – perfluorooctane sulphonate (long-chain); PFBS – perfluorobutane sulphonate (short-chain); PFBA – perfluorobutanoic acid (short-chain); GenX – hexafluoropropylene oxide dimer acid; PFHxS –1-chloro perfluorohexane sulphonate
Over the last 30 years, efforts have been made to characterise PFAS by their functional groups, from Banks et al. (14) in 1994 to the Organization for Economic Co-operation and Development/ United Nations Environment Program (OECD/UNEP) in 2019– 2021, which commissioned a report identifying 4730 substances (15, 16). Thanks to the further efforts of OECD, the European Chemical Agency (ECHA), and the US National Institutes of Health (NIH), by 2022 this number has grown to over 9,000 (17, 18).
PFASs are broadly classified as polymeric and non-polymeric, based on their chemical composition and the length of side chains (Table 1). The higher the number of carbon chains, the higher their hydrophobicity and bioaccumulation (19). Recent classification groups them in three classes: perfluoroalkyl substances (PerFAS), polyfluoroalkyl substances (PolyFAS), and fluorinated polymers. PerFAS are straight-chain, fluorinated aliphatic compounds with a fully fluorinated methyl or methylene carbon atom. PolyFAS are branched-chain fluorinated alkyl compounds. They may have a non-fluorinated aromatic ring with a fluorinated methyl or methylene carbon group and an aliphatic side chain or a fluorinated aromatic ring with a fluorinated methyl or methylene carbon group with an aliphatic side chain (19). Fluorinated polymers are further divided into three subclasses: fluoropolymers, perfluoropolyether, and side-chain fluorinated polymers.
Table 1.
| Perfluoroalkyl Substances (PerFASs) | Acronym | Formula | Examples | |
|---|---|---|---|---|
| Non-polymeric PFAS | Perfluoroalkyl acids | PFAAs | CnF2n+1R | PFHxS, PFOA |
| Perfluoroalakane sulphonates | PFSAs | CnF2n+1SO3- | PFOS | |
| Perfluorocarboxylic acids | PFCAs | CnF2n+1COO- | C8-PFPA | |
| Perfluoroalkyl phosphonic acids | PFPAs | CnF2n+1(O)P(OH)O- | C8-PFPiA | |
| Perfluoroalkane sulphonamides | FASA | CnF2n+1SO2NH2 | FOSA | |
| Perfluoroalkyl ether acids | PFEAs | CnF2n+1O-CmF2m+1 | GenX | |
| Perfluoroalkyl sulphonamideotic acids | FASAAs | CnF2n+1SO2NHCH2COOH | FOSE, MeFOSA | |
| Polyfluoroalkyl Substances (PolyFASs) | ||||
| Fluorotelomer alcohols | FT | CnF2n+ 1CH2CH2OH | FTO | |
| Polyfluoroalkyl phosphoric acid esters | PAPs | (O)P(OH)3-X(OCH2CH2CnF2n+1)x | diPAP | |
| Fluorortelomer saturated aldehydes | FTALs | CnF2n+1CH2CHO | 8:2 FTAL | |
| Fluorotelomer unsaturated aldehydes | FTUALs | CnF2n+1CF=CHCHO | 4,8-Dioxa-3H-perfluorononoate | |
| Polymeric PFAAS | Fluoropolymers | FPs | PFTE | |
| Perfluoropolyethers | PFPEs | HOCH2O-(CmF2mO)n-CH2OH | PFPE-BP | |
| Side-chain fluorinated aromatics | sc-F | CnF2n+1-aromatic rings | Fluoriated methacrylate | |
The structure and chain lengths of PFAS confer different physiochemical and functional properties in regard to their transport, accumulation, and degradation in the environment. PFAS with an aliphatic fluorinated long carbon chain which ranges from C4–17 bioaccumulate more easily and resist biodegradation due to the strong C-F bonds (19).
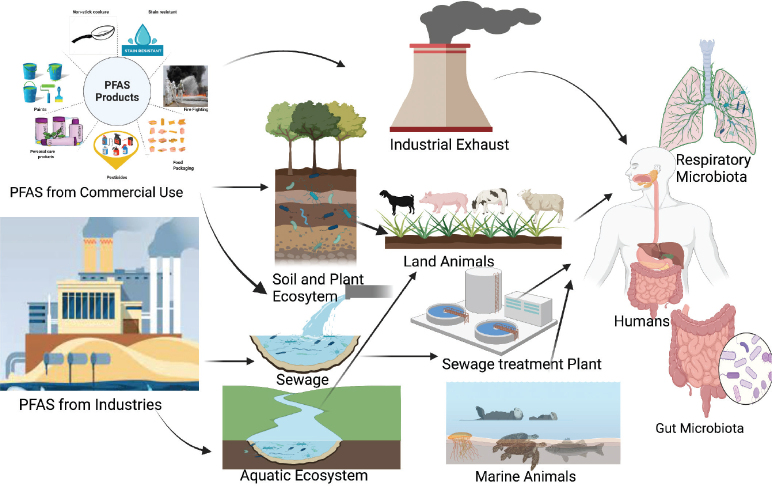
Substantial efforts have been made to restrict the production and ban the use of long-chain PFAS in commercial and consumer products, PFOS and PFOA in particular (20). However, precursors of PFOS and PFOA, perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkyl sulphonic acids (PFSAs), fluorotelomer alcohols (FTOHs), and shorter-chain PFAS such as GenX are still being produced and, therefore, still present pollution risk (Figure 3).
Figure 3.
PFAS transport across different ecosystems
PFAS AND THE HUMAN MICROBIOME
The human microbiome comprises 10–100 trillion microbes (9) which inhabit the entire human body, such as the gut, skin, and mouth. Its importance is obvious in different functions of the skin, intestinal homeostasis, nutrient absorption, and overall host health.
The major source of PFAS pollution in humans are industrial and municipal waste-water treatment plants (WWTPs). WWTPs also contribute directly to the release of PFAS into the atmosphere, freshwater, and soil through recycled wastewater and the release of contaminated sewage sludge as biosolid for fertilisation (21, 22). Over 300 chemicals from the environment have already been characterised in different human clinical samples (20), which has shifted focus of many microbiome projects on the diet and disruption of endogenous microbial populations and their diversity in humans (23). A nine-year study across the US (24) detected PFOS, PFOA, perfluorohexane sulphonate (PFHxS), and perfluorooctanoate (PFNA) in over 95 % of 7,876 participants. Another, more recent study (25) reported high levels of PFOA in overweight and obese 12–18 year-old children (25).
Another important source of air pollution are PFAS-containing materials such as upholstery, stain-resistant carpets, textiles, paints, and food packaging materials disposed of in landfills. There in the landfills, PFAS precursors degrade into more lightweight and volatile PFAS such as fluorotelomer alcohols (FTOHs) and end up in the atmosphere through aerobic and anaerobic processes causing air pollution (26, 27). However, no research data have specifically identified microbial species in the human oral/respiratory microbiome affected by airborne PFAS.
Long-chain perfluoroalkyl carboxylates (such as PFOA) and sulphonates (such as PFHxS) accumulate highly in plants and animals (30), whereas humans mostly accumulate PFAS through contaminated drinking water, non-stick cookware, food packaging, and food (31, 32). Food accounts for the bulk of exposure, which ranges about 16–99 % for PFOA, 81–199 % for PFOS, and 38–96 % for PFCAs and PFSAs (33). In addition, animals raised and plants grown in PFAS-contaminated areas have shown high concentrations of PFAS (34). This level of contamination is evident in the end products such as eggs, grain, milk and meat, and fruit and vegetables (34–37).
PFOA, PFOS, and GenX are reported to be highly toxic to the intestinal microbiome by murine model and ex vivo human studies of intestinal bacteria. The microbial abundance of Collinsella aerofaciens, a major bacterial species active in the metabolism of carbohydrates in the human gut is altered at varying concentrations of PFOA, PFOS, and GenX. However, Escherichia coli is insensitive to these chemicals even at concentrations higher than the reported tolerance of Collinsella sp. PFCA (39). A study of PFAS effects on Zebra fish (38) showed changes in the gut microbiome, inflammation, oxidative stress, impaired lipid metabolism, decreased levels of triglycerides and fatty acids, and compromised intestinal epithelial layer barrier. Our preliminary data show differences in the sensitivities of human microbiome bacteria to PFOA, PFOS, and GenX to (39), suggesting that the ingestion of these toxic chemicals from food and water can alter the gut microbiome and ultimately impair human metabolism. However, much more research is needed to evaluate the impact of PFAS on human microbiome and metabolic activity. In addition, identifying the genetic markers in the affected microbial species might help to elucidate biological functions and pathways affected by PFAS in humans.
PFAS and microbes in the environment
PFAS are present in all ecosystems (40–42) and can easily affect microbes native to these systems/habitats, whose major function is to maintain biotic processes such as nutrient cycling, decomposition of organic matter, and biodegradation of pollutants. Considering that microbes have a brief lifecycle and replicate rapidly due to simple genome, they quickly respond to environmental stress through genetic mutations and may be a good model to study PFAS toxicity. (43). Here we detail the effects of PFAS contamination on microbes in different environmental systems.
Aquatic ecosystems
PFAS have been found in a variety of aquatic systems, from rivers, lakes, and streams to groundwater, municipal sewage, treated drinking water, rainfall, and snow (44, 45). Volatile PFAS and their precursors, such as fluorotelomer carboxylic acids (FTCA), fluorotelomer unsaturated carboxylic acids (FTUCA), fluorotelomer sulphonate (FTSA), and perfluoroalkane sulphonamido acetic acid (FASAA) undergo a metabolic transformation in the atmosphere to produce short-chain PFSA and PFCA that end up in the aquatic environment. These short-chain PFAS are highly reactive with water (48), easily bioaccumulate in the aquatic food chain, and are toxic to many aquatic organisms (such as invertebrates and green algae) (49).
Due to high hydrophobicity, long-chain PFAS often have an even greater potential for bioaccumulation in aqueous environments and organisms (50). They are often released directly into surface waters from fluorine chemical plants or WWTPs (51). In Fuxin, China, the levels of PFOA have been reported to reach up to 524 ng/L in the groundwater and 668 ng/L in the river (52), which is way above the recommended regulatory standards.
Carbon build-ups in aquatic ecosystems contribute substantially to the control of metabolic activities of environmental microbiomes (53) and to the growth of planktonic bacteria and protozoa. However, PFAS reduce the amount of biogenic and total dissolved carbon in water, which adversely affects many microbial communities and nutrient cycling and composition in aquatic habitats (54, 55). PFOS and PFNA have also been reported to biomagnify in the food web of aquatic animals (56).
Exposure to PFAS significantly affects the balance of bacterial population. There are reports of reduced bacterial diversity and drops in the populations of Actinobacteria and Bacteriodetes (57) in favour of Verrucomicrobia, Proteobacteria (58, 59), and Photobacterium phosphoreum in surface water (60, 61). These studies also report that perfluoro butanoic acid (PFBA) interferes with the transfer of nutrients in bacteria (60), while perfluorotetradecanoic acid (PFTeDA) moves up the food chain from heterotrophic bacteria to amoebae (protozoa) (59). Some genera, such as Bacteroidetes, Proteobacteria, and especially Acidobacteria, have been reported resistant to PFAS concentrations of up to 20 mg/L (62) (Table 2).
Table 2.
Impact of PFAS and its compounds on aquatic and soil microbes
| Microbes/Genus | Response to PFAS exposure | PFAS compound | References |
|---|---|---|---|
| Sediminibacterium, Opitutus, Luteolibacter, Microcystis | Increase | PFAS | 48 |
| Photobacterium phosphoreum | Increase | PFCA, PFOS, PFOA | 58, 61 |
| Actinobacteria and Bacteriodetes | Decrease | PFAS | 106 |
| Verrucomicrobia and Proteobacteria | Increase | PFAS | 106 |
| Proteobacteria and Chloroflexi | Decrease | PFOS | 62 |
| Desulfococcus and GOUTA19 | Abundant/ Increase | PFAS, PFOS, PFOA | 77, 79 |
The molecular mechanism of PFAS toxicity to microbes involves oxidative stress, which damages the cell membrane and DNA, rendering some bacteria inactive (63, 64).
Sediments provide a habitat for a wide variety of benthic organisms, and much of their biomass includes microorganisms important for ecological processes such as biodegradation, biogeochemical cycling, and bioremediation (66–70).
Pollutants such as PFAS seem to have a major role in structuring microbial communities and their function in aquatic sediments (71, 72). Long-chained PFAS tend to build up over time (65, 73), which, once a threshold is crossed, may have irreversible negative effects on microbial populations and the structure of the benthic zone (74–77). In such conditions, tolerant and sulphate-reducing strains like the Desulfococcus genus and GOUTA19, become dominant microbial communities, and diversity drops (77–79).
PFAS in drinking water and health effects
Reports about the occurrence and toxicity of PFAS in drinking water come from all over the world (28, 48, 80). Average concentrations range between <10 ng/L and 1200 ng/L (81–85). PFAS occurrence in drinking water has been linked to several sources such as nearby fluorochemical production plants, PFAS-based firefighting foam (86), WWTPs, and surface runoff (87). Even at low concentrations (ng/L) PFAS in drinking water may have deleterious effects on human health as PFAS tend to build up in the liver and affect liver function (88). PFAS contamination of drinking water at levels above the United States Environmental Protection Agency (US EPA) health-based reference level of 70 ng/L (89) have also been associated with pregnancy-induced high blood pressure, hypertension, and preeclampsia as well as testicular and kidney cancers. Continued testing of drinking water and implementation of regulatory measures is therefore essential for keeping PFAS concentrations below this limit.
Soil ecosystems
Soil takes the lead as a sink for persistent chemical pollutants released into the environment from sources such as fluoride plants, aqueous film-forming foam from fire training sites, and sludge and biosolids from WWTPs, landfills, and incineration plants (6, 90–92). PFAS accumulate in soil over time and persist there for a long time, affecting micro and macro-organisms, which are abundant and serve to decompose organic matter, recycle biogeochemicals and nutrients, and remediate soil of contaminants (5, 43).
Soils are mostly contaminated by PFOS, because of their widespread and long-time application (94), but all PFAS change the structure of soil and, consequently, the distribution and function of microbial communities (5, 75, 93, 94). A recent study relying on a next generation sequencing technology (95) showed that PFOA in low concentrations affect bacterial diversity in soil less than PFOS. As for PFAS toxicity in soil microbial species, the mechanisms include membrane disruption, oxidative stress, and DNA damage-induced inactivation and/or death in Escherichia coli (96). In Pseudomonas putida PFOA is more toxic when combined with either chromium or tetra butyl ammonium (97).
PFAS contamination usually affects the diversity and composition of soil microbial communities. Long-chained PFAS and PFAS with the sulphonic group are more toxic to soil microbial flora than short-chained PFAS with carboxylic groups (97). There are reports, sometimes apparently controversial, that some important bacterial groups are eliminated, such as Cyanobacteria (75, 78), or depleted, such as Chloroflexi, Haliangium, Latescibacteria, and some species of the genus Acidobacteria (6, 35, 75). Higher PFC concentrations are also known to impede the growth and metabolism of the Bacillus spp. (95) and Sphingomonas paucimobilis (104, 105).
Other groups seem to thrive, such as Firmicutes, some Acidobacteria, and Actinobacteria (75). Comparative studies of bacterial communities affected by PFAS contamination point to potential changes in soil nutrients (5, 97, 98), total carbon, and pH (99). Elevated PFOA concentrations in sediments seem to boost some populations of Proteobacteria (100–102), such as Pseudomonas spp., which may be owed to their ability to defluorinate fluorotelomer alcohols by removing multiple CF2 groups to form shorter-chain PFCAs (102), which are less toxic (95, 103) and quicker to degrade (95). Besides Proteobacteria, Verrucomicrobia also seem to withstand PFAS contamination (106) (Table 2).
Greater abundance of some bacteria in contaminated soils provides an insight into which bacterial groups could be used to enhance PFAS biodegradation and bioremediation.
The effects of PFAS on soil organic content
As PFAS contamination affects the composition and diversity of microorganisms in soil, so does it affect its organic matter content (107, 108). Soil microorganisms are active in biogeochemical cycling and play an important role in pollutant degradation (109). The effect of PFAS on soil microbial populations may therefore affect the microbial diversity and ability to carry out geochemical processes in the soil (109, 110).
PFAS-induced changes in the abundance of fungi and bacteria may reflect on the organic content in soil and eventually carbon content available for cycling. However, possible connections with biogeochemical cycles have not been elucidated yet.
Zhalnina et al. (111) studied the effect of PFAS on the nitrogen cycle (nitrification and denitrification) and related cycle genes in 61 ammonia-oxidising microorganisms and reported that PFAS did not affect the ammonia monooxygenase gene abundance in bacteria but reduced it significantly in archaea. In fact, PFAS treatment seems to have favoured the growth of Acidovorax temperans in the soil. A. temperans is notorious for reducing the nitrate content in soil, and its proliferation implies nitrate depletion in PFAS-contaminated soils (Table 3). In contrast, nitrate- and sulphate-reducing bacterial genera, such as Acidobacteria, and Gammaproteobacteria, are inhibited by PFAS (35), leading to an increase in the level of nitrates and sulphates in the soil (35). Nitrates and sulphate concentrations and pH levels of contaminated soils influence nutrient availability, which, in turn, influences which plants will grow there.
Table 3.
Impact of PFAS on soil microbial communities and associated biogeochemical cycles
| PFAS | Impact on population | Bacteria groups impacted | Potential nutrient cycle associated | References |
|---|---|---|---|---|
| PFOS | Increase | Bacteriodetes | Nitrogen cycle | 34, 94, 127 |
| PFOS/PFOA | Increase | Alphaproteobacteria | Nitrogen cycle, Sulphur cycle, carbon cycle | 5, 75, |
| PFOA, PFOS | Increase | Gammaproteobacteria | Nitrogen cycle | 5, 75, 126 |
| PFOA, PFOS | Increase | Acidobacteria | Carbon cycle, nitrogen cycle | 5, 34, 75, 127, 128 |
| PFOS | Increase | Firmicutes | Nitrogen cycle | 75, 129, 130 |
| PFOA, PFOS | Increase/Decrease | Chloroflexi | Sulphur cycle | 5, 34, 75, 127, 131 |
| PFOS, PFOA | Increase/Decrease | Actinobacteria | Nitrogen cycle | 5, 75 |
| PFAS | Decrease | Thermoleophilia | Sulphur cycle | 5 |
| PFOS, PFAS | Decrease | Deltaproteobacteria | Sulphur cycle | 5, 75 |
As for the carbon cycle, PFAS are reported to inhibit glycoside hydrolases and interrupt carbohydrate metabolism and membrane transport in soil microbes (99). In addition to glycoside hydrolase inhibition, long-chain PFAS inhibit the activity of sucrase and urease (75).
Impact of PFAS contamination on vegetation
Several studies have shown that PFAS accumulate in spring wheat, oats, potatoes, maize, perennial ryegrass, winter wheat, winter rye, canola, winter barley, carrots, and cucumber through soil (112–115). The last two studies (114, 115) point to the use of sewage effluent as fertiliser that contaminates the soil and then crops. Lechner and Knapp (114) further discourage the idea of air-to-plant transfer, as plants growing on soil not fertilised by sludge showed no increase in PFAS concentrations.
MICROBIAL BIOREMEDIATION OF PFAS
As we indicated earlier, strong covalent bonds between PFAS atoms make them resist biodegradation by microbial metabolism. Biodegradation has proven successful with oil and gas spills, chemical and industrial wastes, and other environmental pollutants. Although our current knowledge of microbial metabolism of fluorinated compounds is modest, enormous progress has been made towards microbial biodegradation of highly fluorinated alkyl compounds. Recent concepts involve direct targeting of specific PFAS molecules and regions that have fewer fluorine atoms, such as fluorobenzene, fluoroacetate, perfluorohexylethanol, and perfluorohexylsulphonate (61, 116–118). They also rely on combined knowledge of microbiology, enzyme biochemistry, and chemistry.
Bioremediation has a number of advantages over the classical physical and chemical remediation methods, including incineration, whose major drawback is the release of hazardous hydrogen fluoride and other toxic gases in the air. Other methods of PFAS elimination from water involve reverse osmosis, nanofiltration, and activated carbon filtration, yet these methods have not proven effective, safe, and reliable (120–122).
In contrast, biodegradation of PFOA and PFOS using activated sludge under low oxygen conditions has been reported successful (123). Success in defluorination has also been reported with fluoroacetate dehalogenase in the biodegradation of difluoractate, 2,3,3,3-tetrafluoropropionic acid, and trifluoroacetate (124). Microbial degradation of chlorides involves different mechanisms based on four types of reactions: oxidation/reduction, hydrolysis, substitution, and elimination. Bacterial species with reported success in PFAS bioremediation include Acidimicrobium sp. and Pseudomonas parafulva (Table 4).
Table 4.
Microbial species and mechanisms by which they biodegrade PFAS
| Bacterial species | Biodegradation mechanism | PFAS | References |
|---|---|---|---|
| Acidimicrobium sp. S. A6 | Defluorination | PFOA, PFOS | 124 |
| Synechocytis sp. PCC 6803 | Decarboxylation, 2x reductive & oxidative defluorination, trifluoromethyl loss | PFOA, PFOS | 132 |
| Pseudomonas parafulva S. YAB1 | Decarboxylation | PFOA | 133 |
| Pseudomonas aeruginosa S.HJ4 | C-C bond cleavage | PFOS | 10 |
| Pseudomonas plecoglossicida 2.4-D | Decarboxylation, desulphonation | PFOS | 134 |
| Gordonia sp. S. NB4-1Y | Desulphonation | FTSA, FTAB | 135 |
| Mycobacterium vaccae | Dechlorination | FTOH | 136 |
CONCLUSION
Physical, chemical, and biological methods are being explored to immobilise, eliminate, or degrade PFAS in the environment, and microbial remediation shows promise. It would be interesting to know how soil microbial bacteria adapted to PFAS can help in soil remediation, and especially how much PFAS they can take up and metabolise.
Another venue of action should involve more stringent regulations to minimise the use of PFAS and with it the threat it poses to the living organisms and humans.
Acknowledgements
Adenike Shittu wishes to thank Jill Zeilstra-Ryalls for her mentorship and guidance.
Footnotes
Conflicts of interest
None to declare.
REFERENCES
- 1.Scheringer M Long-range transport of organic chemicals in the environment Environ Toxicol Chem. 2009;28:677. doi: 10.1897/08-324R.1. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 2.Kim SK, Khim JS, Lee KT, Giesy JP, Kannan K, Lee DS, Koh CH Chapter 2 emission, contamination and exposure, fate and transport, and national management strategy of persistent organic pollutants in South Korea Dev Environ Sci. 2007;7:31. doi: 10.1016/S1474-8177(07)07002-7. . . ; : –. . doi: [DOI] [Google Scholar]
- 3.Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, Lunderberg DM, Lang JR, Peaslee GF Fluorinated compounds in U.S. fast food packaging Environ Sci Technol Lett. 2017;4:105. doi: 10.1021/acs.estlett.6b00435. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogue C PFAS targeted in legislation passed by US House of Representatives. 2021. . , [displayed 3 August 2023]. Available at https://cen.acs.org/environment/persistent-pollutants/PFAS.
- 5.Senevirathna STMLD, Krishna KCB, Mahinroosta R, Sathasivan A Comparative characterization of microbial communities that inhabit PFAS-rich contaminated sites: A case-control study J Hazard Mater. 2022;423:126941. doi: 10.1016/j.jhazmat.2021.126941. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Tsui M, Lam J, Wang Q, Hu C, Wai O, Zhou B, Lam P Contamination by perfluoroalkyl substances and microbial community structure in Pearl River Delta sediments Environ Poll. 2019;245:218. doi: 10.1016/j.envpol.2018.11.005. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 7.Peden-Adams MM, Keller JM, Eudaly JG, Berger J, Gilkeson GS, Keil DE Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate Toxicol Sci. 2008;104:144. doi: 10.1093/toxsci/kfn059. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 8.Lai KP, Ng AHM, Wan HT, Wong AYM, Leung CCT, Li R, Wong CKC Dietary exposure to the environmental chemical, PFOS on the diversity of gut microbiota, ssociated with the development of metabolic syndrome Front Microbiol. 2018;9:2552. doi: 10.3389/fmicb.2018.02552. . . ; : . doi: org/10.3389/fmicb.2018.02552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NIH HMP Working Group. Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C, Baker CC, Di Francesco V, Howcroft TK, Karp RW, Lunsford RD, Wellington CR, Belachew T, Wright M, Giblin C, David H, Mills M, Salomon R, Mullins C, Akolkar B, Begg L, Davis C, Grandison L, Humble M, Khalsa J, Little AR, Peavy H, Pontzer C, Portnoy M, Sayre MH, Starke-Reed P, Zakhari S, Read J, Watson B, Guyer M The NIH Human Microbiome Project Genome Res. 2009;19:2317. doi: 10.1101/gr.096651.109. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon BG, Lim HJ, Na SH, Choi BI, Shin DS, Chung SY Biodegradation of perfluorooctanesulfonate (PFOS) as an emerging contaminant Chemosphere. 2014;109:221. doi: 10.1016/j.chemosphere.2014.01.072. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 11.Lim X Can microbes save us from PFAS? ACS Cent Sci. 2021;7:3. doi: 10.1021/acscentsci.1c00013. . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang CY, Fu QS, Criddle CS, Leckie JO Effect of flux (transmembrane pressure) and membrane properties on fouling and rejection of reverse osmosis and nanofiltration membranes treating perfluorooctane sulfonate containing wastewater Environ Sci Technol. 2007;41:2008. doi: 10.1021/es062052f. . . ; : –. . doi: org/10.1021/es062052f. [DOI] [PubMed] [Google Scholar]
- 13.Wackett LP Nothing lasts forever: understanding microbial biodegradation of polyfluorinated compounds and per fluorinated alkyl substances Microb Biotechnol. 2022;15:773. doi: 10.1111/1751-7915.13928. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks RE, Smart BE, Tatlow JC . Organofluorine Chemistry: Principles and Commercial Applications. New York (NY): Springer; 1994. . . : ; . doi: [DOI] [Google Scholar]
- 15.Buck RC, Korzeniowski SH, Laganis E, Adamsky F Identification and classification of commercially relevant per- and poly-fluoroalkyl substances (PFAS) Integr Environ Assess Manag. 2021;17:1045. doi: 10.1002/ieam.4450. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Organization for Economic Co-operation and Development (OECD) Toward a new comprehensive global database of per- and polyfluoroalkyl substances (PFASs): Summary report on updating the OECD 2007 list of per- and polyfluoroalkyl substances (PFASs) . [displayed 24 August 2023]. Available at https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/5099062.
- 17.Call for evidence, 2020. European Chemicals Agency (ECHA) . [displayed 3 August 2023]. Available at https://echa.europa.eu/-/five-european-states-call-for-evidence-on-broad-pfas-restriction.
- 18.Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS), 2022. NIH News in Health. . [displayed 3 August 2023]. Available at https://newsinhealth.nih.gov/2022/03/perfluoroalkyl-polyfluoroalkyl-substances-pfas.
- 19.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins Integr Environ Assess Manag. 2011;7:513. doi: 10.1002/ieam.258. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Report on Human Exposure to Environmental Chemicals, 2021. Centers for Disease Control and Prevention CDC. . [displayed 3 August 2023]. Available at https://www.cdc.gov/exposurereport/index.html.
- 21.Kyle AT, Soroosh M, Dana JG, Charles B, Jennifer H, Charles ES, Dickenson ERV Poly- and perfluoroalkyl substances in municipal wastewater treatment plants in the United States: seasonal patterns and meta-analysis of long-term trends and average concentrations ACS EST Water. 2022;2:690. doi: 10.1021/acsestwater.1c00377. . . ; : –. . doi: [DOI] [Google Scholar]
- 22.Lenka SP, Kah M, Padhy LP A review of the occurrence, transformation, and removal of poly- and perfluoroalkyl substances (PFAS) in wastewater treatment plants Water Res. 2021;199:117187. doi: 10.1016/j.watres.2021.117187. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 23.Tasnim N, Abulizi N, Pither J, Hart MM, Gibson DL Linking the gut microbial ecosystem with the environment: does gut health depend on where we live? Front Microbiol. 2016;8:1935. doi: 10.3389/fmicb.2017.01935. . ; : . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008 Environ Sci Technol. 2011;45:8037. doi: 10.1021/es1043613. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 25.Geiger SD, Yao P, Vaughn MG, Qian Z PFAS exposure and overweight/obesity among children in a nationally representative sample Chemosphere. 2021;268:128852. doi: 10.1016/j.chemosphere.2020.128852. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 26.Ahrens L, Bundschuh M Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: a review Environ Toxicol Chem. 2014;33:1921. doi: 10.1002/etc.2663. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 27.Morales-McDevitt ME, Becanova J, Blum A, Bruton TA, Vojta S, Woodward M, Lohmann R The air that we breathe: neutral and volatile PFAS in indoor air Environ Sci Technol Lett. 2021;8:897. doi: 10.1021/acs.estlett.1c00481. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui D, Li X, Quinete N Occurrence, fate, sources and toxicity of PFAS: what we know so far in Florida and major gaps Trends Anal Chem. 2020;130:115976. doi: 10.1016/j.trac.2020.115976. . . ; : . doi: [DOI] [Google Scholar]
- 29.Alderete TL, Song AY, Bastain T, Habre R, Toledo-Corral CM, Salam MT, Lurmann F, Gilliland FD, Breton CV Prenatal traffic-related air pollution exposures, cord blood adipokines and infant weight Pediatr Obes. 2018;13:348. doi: 10.1111/ijpo.12248. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindstrom AB, Strynar MJ, Libelo EL Polyfluorinated compounds: past, present, and future Environmental Sci Technol. 2011;45:7954. doi: 10.1021/es2011622. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 31.Poothong S, Papadopoulou E, Padilla-Sánchez JA, Thomsen C, Haug LS Multiple pathways of human exposure to poly- and perfluoroalkyl substances (PFASs): from external exposure to human blood Environ Int. 2020;134:105244. doi: 10.1016/j.envint.2019.105244. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 32.Domingo JL Health risks of GM foods: many opinions but few data Science. 2000;288:1748. doi: 10.1126/science.288.5472.1748. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 33.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects J Expo Sci Environ Epidemiol. 2019;29:131. doi: 10.1038/s41370-018-0094-1. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noorlander CW, van Leeuwen SP, Te Biesebeek JD, Mengelers MJ, Zeilmaker MJ Levels of perfluorinated compounds in food and dietary intake of PFOS and PFOA in the Netherlands J Agric Food Chem. 2011;59:7496. doi: 10.1021/jf104943p. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 35.Bao Y, Li B, Xie S, Huang J Vertical profiles of microbial communities in perfluoroalkyl substance-contaminated soils Ann Microbiol. 2018;68:399. doi: 10.1007/s13213-018-1346-y. . . ; : –. . doi: [DOI] [Google Scholar]
- 36.Guruge KS, Yeung LWY, Yamanaka N, Miyazaki S, Lam PKS, Giesy JP, Jones PD, Yamashita N Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA) Toxicol Sci. 2006;89:93. doi: 10.1093/toxsci/kfj011. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 37.Liu K, Wang X, Wei N, Song Z, Li D Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: implications for human health Environ Int. 2019;132:105127. doi: 10.1016/j.envint.2019.105127. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Reinhard M, Yin T, Nguyen TV, Tran NH, Gin KYH Multi-compartment distribution of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in an urban catchment system Water Res. 2019;154:227. doi: 10.1016/j.watres.2019.02.009. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 39.Shittu A . Toxicity Studies of Per- And Polyfluoroalkyl Substances (PFAS) [MSc thesis] Bowling Green: Bowling Green State University; 2021. . . : ; [displayed 3 August 2023]. Available at https://etd.ohiolink.edu/apexprod/rws_olink/r/1501/10?clear=10&p10_accession_num=bgsu1625018658596765. [Google Scholar]
- 40.Scher DP, Kelly JE, Huset CA, Barry KM, Hoffbeck RW, Yingling VL, Messing RB Occurrence of perfluoroalkyl substances (PFAS) in garden produce at homes with a history of PFAS-contaminated drinking water Chemosphere. 2018;196:548. doi: 10.1016/j.chemosphere.2017.12.179. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 41.Shahsavari E, Rouch D, Khudur LS, Thomas D, Aburto-Medina A, Ball AS Challenges and current status of the biological treatment of PFAS-contaminated soils Front Bioeng Biotechnol. 2021;8:602040. doi: 10.3389/fbioe.2020.602040. . . ; : . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi Y, Vestergren R, Nost TH, Zhou Z, Cai Y Probing the differential tissue distribution and bioaccumulation behavior of per- and polyfluoroalkyl substances of varying chain-lengths, isomeric structures and functional groups in crucian carp Environ Sci Technol. 2018;52:4592. doi: 10.1021/acs.est.7b06128. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 43.Yergeau E, Lawrence JR, Sanschagrin S, Waiser MJ, Korber DR, Greer CW Next-generation sequencing of microbial communities in the Athabasca River and its tributaries in relation to oil sands mining activities Appl Environ Microbiol. 2012;78:7626. doi: 10.1128/AEM.02036-12. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultz P, Shriver C, Tabanico JJ, Khazian AM Implicit connections with nature J Environ Psychol. 2014;24:31. doi: 10.1016/S0272-4944(03)00022-7. . . ; : –. . doi: [DOI] [Google Scholar]
- 45.Shahul Hamid F, Bhatti MS, Anuar N, Anuar N, Mohan P, Periathamby A Worldwide distribution and abundance of microplastic: how dire is the situation? Waste Manag Res. 2018;36:873. doi: 10.1177/0734242X18785730. . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 46.Butt CM, Muir DC, Mabury SA Biotransformation pathways of fluorotelomer-based polyfluoroalkyl substances: a review Environ Toxicol Chem. 2014;33:243. doi: 10.1002/etc.2407. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 47.Martin JW, Ellis DA, Mabury SA, Hurley MD, Wallington TJ Atmospheric chemistry of perfluoroalkanesulfonamides: kinetic and product studies of the OH radical and Cl atom initiated oxidation of N-ethyl perfluorobutanesulfonamide Environ Sci Technol. 2006;40:864. doi: 10.1021/es051362f. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 48.Wang YQ, Hu LX, Liu T, Zhao JH, Yang YY, Liu YS, Ying GG Per-and polyfluoralkyl substances (PFAS) in drinking water system: target and non-target screening and removal assessment Environment Int. 2022;163:107219. doi: 10.1016/j.envint.2022.107219. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 49.Hoke RA, Bouchelle LD, Ferrell BD, Buck RC Comparative acute freshwater hazard assessment and preliminary PNEC development for eight fluorinated acids Chemosphere. 2012;87:725. doi: 10.1016/j.chemosphere.2011.12.066. . . ; : –. . doi.: [DOI] [PubMed] [Google Scholar]
- 50.Podder A, Sadmani AHMA, Reinhart D, Chang NB, Goel R Per and poly-fluoroalkyl substances (PFAS) as a contaminant of emerging concern in surface water: A transboundary review of their occurrences and toxicity effects J Hazard Mater. 2021;419:126361. doi: 10.1016/j.jhazmat.2021.126361. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 51.Phong Vo HN, Ngo HH, Guo W, Hong Nguyen TM, Li J, Liang H, Deng L, Chen Z, Hang Nguyen TA Poly- and perfluoroalkyl substances in water and wastewater: A comprehensive review from sources to remediation J Water Process Eng. 2020;36:101393. doi: 10.1016/j.jwpe.2020.101393. . . ; : . doi: [DOI] [Google Scholar]
- 52.Bao J, Liu W, Liu L, Jin Y, Dai J, Ran X, Zhang Z, Tsuda S Perfluorinated compounds in the environment and the blood of residents living near fluorochemical plants in Fuxin, China Environ Sci Technol. 2011;45:8075. doi: 10.1021/es102610x. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 53.Coskun ÖK, Vuillemin A, Schubotz F, Klein F, Sichel SE, Eisenreich W, Orsi WD Quantifying the effects of hydrogen on carbon assimilation in a seafloor microbial community associated with ultramafic rocks ISME J. 2022;16:257. doi: 10.1038/s41396-021-01066-x. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu JY, Hua ZL, Gu L Per-, poly-fluoroalkyl substances (PFASs) and planktonic microbiomes: Identification of biotic and abiotic regulations in community coalescence and food webs Environ Poll. 2022;302:119078. doi: 10.1016/j.envpol.2022.119078. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 55.Vila-Costa M, Cerro-Gálvez E, Martínez-Varela A, Casas G, Dachs J Anthropogenic dissolved organic carbon and marine microbiomes ISME J. 2020;14:2646. doi: 10.1038/s41396-020-0712-5. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miranda DA, Benskin JP, Awad R, Lepoint G, Leonel J, Hatje V Bioaccumulation of per- and polyfluoroalkyl substances (PFASs) in a tropical estuarine food web Sci Total Environ. 2021;754:142146. doi: 10.1016/j.scitotenv.2020.142146. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 57.Zhang DQ, Wang M, He Q, Niu X, Liang Y Distribution of perfluoroalkyl substances (PFASs) in aquatic plant-based systems: From soil adsorption and plant uptake to effects on microbial community Environ Poll. 2020;257:113575. doi: 10.1016/j.envpol.2019.113575. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 58.Zilles JL, Peccia J, Kim MW, Hung CH, Noguera DR Involvement of Rhodocyclus-related organisms in phosphorus removal in full-scale wastewater treatment plants Appl Environ Microbiol. 2002;68:2763. doi: 10.1128/AEM.68.6.2763-2769.2002. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu JY, Gu L, Hua ZL, Liang ZY, Chu KJ, He XX Per-, poly-fluoroalkyl substances (PFASs) pollution in benthic riverine ecosystem: Integrating microbial community coalescence and biogeochemistry with sediment distribution Chemosphere. 2021;281:130977. doi: 10.1016/j.chemosphere.2021.130977. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 60.Ahrens L Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate J Environ Monitor. 2011;13:20. doi: 10.1039/c0em00373e. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 61.Wang T, Lin Z, Yin D, Tian D, Zhang Y, Kong D Hydrophobicity-dependent QSARs to predict the toxicity of perfluorinated carboxylic acids and their mixtures Environ Toxicol Pharmacol. 2011;32:259. doi: 10.1016/j.etap.2011.05.011. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 62.Yu XL, Nishimura F, Hidaka T Impact of long-term perfluorooctanoic acid (PFOA) exposure on activated sludge process Water Air Soil Poll. 2018;229:134. doi: 10.1007/s11270-018-3760-y. . . ; : . doi: [DOI] [Google Scholar]
- 63.Yang M, Ye J, Qin H, Long Y, Li Y Influence of perfluorooctanoic acid on proteomic expression and cell membrane fatty acid of Escherichia coli Environ Poll. 2017;220:532. doi: 10.1016/j.envpol.2016.09.097. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 64.Fitzgerald NJ, Simcik MF, Novak PJ Perfluoroalkyl substances increase the membrane permeability and quorum sensing response in Aliivibrio fischeri Environ Sci Technol Lett. 2018;5:26. doi: 10.1021/acs.estlett.7b00518. . . ; : –. . doi: [DOI] [Google Scholar]
- 65.Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH Sources, fate and transport of perfluorocarboxylates Environ Sci Technol. 2006;40:32. doi: 10.1021/es0512475. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 66.Gibbons SM, Jones E, Bearquiver A, Blackwolf F, Roundstone W, Scott N, Hooker J, Madsen R, Coleman ML, Gilbert JA Human and environmental impacts on river sediment microbial communities PloS One. 2014;9(5):e97435. doi: 10.1371/journal.pone.0097435. . . ; ( ): . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer H, Pusch M Comparison of bacterial production in sediments, epiphyton and the pelagic zone of a lowland river Freshwater Biol. 2001;46:1335. doi: 10.1046/j.1365-2427.2001.00753.x. . . ; : –. . doi: [DOI] [Google Scholar]
- 68.Ducklow H Microbial services: challenges for microbial ecologists in a changing world Aquat Microb Ecol. 2008;53:13. doi: 10.3354/ame01220. . . ; : –. . doi: [DOI] [Google Scholar]
- 69.Reed HE, Martiny JB Microbial composition affects the functioning of estuarine sediments ISME J. 2013;7:868. doi: 10.1038/ismej.2012.154. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hiraoka S, Hirai M, Matsui Y, Makabe A, Minegishi H, Tsuda M, Rastelli E, Danovaro R, Corinaldesi C, Kitashi T, Tasumi E, Nishizawa M, Takai K, Nomaki H, Nunoura T Microbial community and geochemical analyses of trans-trench sediments for understanding the roles of hadal environments ISME J. 2020;14:740. doi: 10.1038/s41396-019-0564-z. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burton GA, Johnston EL Assessing contaminated sediments in the context of multiple stressors Environ Toxicol Chem. 2010;29:2625. doi: 10.1002/etc.332. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 72.Eggleton J, Thomas KV A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events Environ Int. 2004;30:973. doi: 10.1016/j.envint.2004.03.001. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 73.Chen H, Wang Q, Cai Y, Yuan R, Wang F, Zhou B, Chen Z Effect of perfluorooctanoic acid on microbial activity in wheat soil under different fertilization conditions Environ Pollut. 2020;264:114784. doi: 10.1016/j.envpol.2020.114784. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 74.De Vries P, Slijkerman DM, Kwadijk CJ, Kotterman MJ, Posthuma L, De Zwart D, Murk AJ, Foekema EM The toxic exposure of flamingos to per-and Polyfluoroalkyl substances (PFAS) from firefighting foam applications in Bonaire Mar Pollut Bull. 2017;124:102. doi: 10.1016/j.marpolbul.2017.07.017. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 75.Qiao W, Xie Z, Zhang Y, Liu X, Xie S, Huang J, Yu L Perfluoroalkyl substances (PFASs) influence the structure and function of soil bacterial community: Greenhouse experiment Sci Total Environ. 2018;642:1118. doi: 10.1016/j.scitotenv.2018.06.113. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 76.Sun W, Xiao E, Dong Y, Tang S, Krumins V, Ning Z, Sun M, Zhao Y, Wu S, Xiao T Profiling microbial community in a watershed heavily contaminated by an active antimony (Sb) mine in Southwest China Sci Total Environ. 2016;550:297. doi: 10.1016/j.scitotenv.2016.01.090. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 77.Zhang S, Merino N, Wang N, Ruan T, Lu X Impact of 6:2 fluorotelomer alcohol aerobic biotransformation on a sediment microbial community Sci Total Environ. 2017;575:1361. doi: 10.1016/j.scitotenv.2016.09.214. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 78.Kjeldsen KU, Joulian C, Ingvorsen K Oxygen tolerance of sulfate-reducing bacteria in activated sludge Environ Sci Technol. 2004;38:2038. doi: 10.1021/es034777e. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 79.Kleindienst S, Herbst FA, Stagars M, von Netzer F, von Bergen M, Seifert J, Peplies J, Amann R, Musat F, Lueders T, Knittel K Diverse sulfate-reducing bacteria of the Desulfosarcina/Desulfococcus clade are the key alkane degraders at marine seeps ISME J. 2014;8:2029. doi: 10.1038/ismej.2014.51. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Post GB Recent US state and federal drinking water guidelines for per- and polyfluoroalkyl substances Environ Toxicol Chem. 2021;40:550. doi: 10.1002/etc.4863. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 81.Jin YH, Liu W, Sato I, Nakayama SF, Sasaki K, Saito N, Tsuda S PFOS and PFOA in environmental and tap water in China Chemosphere. 2009;77:605. doi: 10.1016/j.chemosphere.2009.08.058. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 82.Pan CG, Liu YS, Ying GG Perfluoroalkyl substances (PFASs) in wastewater treatment plants and drinking water treatment plants: Removal efficiency and exposure risk Water Res. 2016;106:562. doi: 10.1016/j.watres.2016.10.045. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 83.Gebbink WA, van Asseldonk L, van Leeuwen SP Presence of emerging per-and polyfluoroalkyl substances (PFASs) in river and drinking water near a fluorochemical production plant in the Netherlands Environ Sci Technol. 2017;51:11057. doi: 10.1021/acs.est.7b02488. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McDonough CA, Choyke S, Barton KE, Mass S, Starling AP, Adgate JL, Higgins CP Unsaturated PFOS and other PFASs in human serum and drinking water from an AFFF-impacted community Environ Sci Technol. 2021;55:8139. doi: 10.1021/acs.est.1c00522. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 85.Chow SJ, Ojeda N, Jacangelo JG, Schwab KJ Detection of ultrashort-chain and other per- and polyfluoroalkyl substances (PFAS) in U.S. bottled water Water Res. 2021;201:117292. doi: 10.1016/j.watres.2021.117292. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 86.Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA, Higgins CP, Sunderland EM Detection of poly-and perfluoroalkyl substances (PFASs) in US drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants Environ Sci Technol Lett. 2016;3:344. doi: 10.1021/acs.estlett.6b00260. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lenka SP, Kah M, Padhye LP A review of the occurrence, transformation, and removal of poly-and perfluoroalkyl substances (PFAS) in wastewater treatment plants Water Res. 2021;199:117187. doi: 10.1016/j.watres.2021.117187. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 88.Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion Environ Res. 2005;99:253. doi: 10.1016/j.envres.2004.12.003. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 89.Environmental Protection Agency Announcement of final regulatory determinations for contaminants on the fourth drinking water contaminant candidate list Federal Register. 2021;86(40):12272. . . ; ( ): –. [displayed 7 August 2023]. Available at https://www.federalregister.gov/documents/2021/03/03/2021-04184/announcement-of-final-regulatory-determinations-for-contaminants-on-the-fourth-drinking-water. [Google Scholar]
- 90.Felizeter S, McLachlan MS, de Voogt P Uptake of perfluorinated alkyl acids by hydroponically grown lettuce (Lactuca sativa) Environ Sci Technol. 2012;46:11735. doi: 10.1021/es302398u. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 91.Kwok KY, Yamazaki E, Yamashita N, Taniyasu S, Murphy MB, Horii Y, Petrick G, Kallerborn R, Kannan K, Murano K, Lam PKS Transport of perfluoroalkyl substances (PFASs) from an arctic glacier to downstream locations: implications for sources Sci Total Environ. 2013;447:46. doi: 10.1016/j.scitotenv.2012.10.091. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 92.Schedin E Effect of organic carbon, active carbon, calcium ions and aging on the sorption of per- and polyfluoroalkylated substances (PFASs) to soil, 2013. . [displayed 7 August 2023]. Available at https://uu.diva-portal.org/smash/record.jsf?pid=diva2%3A668557&dswid=-655.
- 93.Cai Y, Chen H, Yuan R, Wang F, Chen Z, Zhou B Metagenomic analysis of soil microbial community under PFOA and PFOS stress Environ Res. 2020;188:109838. doi: 10.1016/j.envres.2020.109838. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 94.Xu R, Tao W, Lin H, Huang D, Su P, Gao P, Sun X, Yang Z, Sun W Effects of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) on soil microbial community Microb Ecol. 2022;83:929. doi: 10.1007/s00248-021-01808-6. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 95.Cai Y, Chen H, Yuan R, Wang F, Chenb Z, Zhou B Toxicity of perfluorinated compounds to soil microbial activity: effect of carbon chain length, functional group and soil properties Sci Total Environ. 2019;690:1162. doi: 10.1016/j.scitotenv.2019.06.440. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 96.Liu G, Zhang S, Yang K, Zhu L, Lin D Toxicity of perfluorooctane sulfonate and perfluorooctanoic acid to Escherichia coli: Membrane disruption, oxidative stress, and DNA damage induced cell inactivation and/or death Environ Poll. 2016;214:806. doi: 10.1016/j.envpol.2016.04.089. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 97.Chen H, Yao J, Wang F, Cai M, Liu H Toxicity of perfluorooctanoic acid to Pseudomonas putida in the aquatic environment J Hazard Mater. 2013;262:726. doi: 10.1016/j.jhazmat.2013.09.046. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 98.Li B, Bao Y, Xu Y, Xie S, Huang J Vertical distribution of microbial communities in soils contaminated by chromium and perfluoroalkyl substances Sci Total Environ. 2017;599–600:156. doi: 10.1016/j.scitotenv.2017.04.241. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 99.Cai Y, Wang Q, Zhou B, Yuan R, Wang F, Chen Z, Chen H A review of responses of terrestrial organisms to perfluorinated compounds Sci Total Environ. 2021;793:148565. doi: 10.1016/j.scitotenv.2021.148565. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 100.Gupta RS The phylogeny of proteobacteria-relationships to other eubacterial phyla ang eukaryotes FEMS Microbiol Rev. 2000;24:367. doi: 10.1111/j.1574-6976.2000.tb00547.x. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 101.Sun Y, Wang T, Peng X, Wang P, Lu Y Bacterial community compositions in sediment polluted by perfluoroalkyl acids (PFAAs) using Illumina high-throughput sequencing Environ Sci Poll Res Int. 2016;23:10556. doi: 10.1007/s11356-016-6055-0. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 102.Kim MH, Wang N, McDonald T, Chu KH Biodefluorination and biotransformation of fluorotelomer alcohols by two alkane-degrading Pseudomonas strains Biotechnol Bioeng. 2012;109:304. doi: 10.1002/bit.24561. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 103.Mahapatra CT, Damayanti NP, Guffey SC, Serafin JS, Irudayaraj J, Sepúlveda MS Comparative in vitro toxicity assessment of perfluorinated carboxylic acids J Appl Toxicol. 2017;37:699. doi: 10.1002/jat.3418. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 104.Wójcik A, Perczyk P, Wydro P, Broniatowski M Effects of water soluble perfluorinated pollutants on phospholipids in model soil decomposer membranes Biochim Biophys Acta Biomembr. 2018;1860:2576. doi: 10.1016/j.bbamem.2018.09.014. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 105.Yang S, Zhang X, Cao Z, Zhao K, Wang S, Chen M, Hu X Growth-promoting Sphingomonas paucimobilis ZJSH1 associated with Dendrobium officinale through phytohormone production and nitrogen fixation Microb Biotechnol. 2014;7:611. doi: 10.1111/1751-7915.12148. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang B, Wu X, Tai X, Sun L, Wu M, Zhang W, Chen X, Zhang G, Chen T, Liu G, Dyson P Variation in actinobacterial community composition and potential function in different soil ecosystems belonging to the Arid Heihe River Basin of Northwest China Front Microbiol. 2019;10:2209. doi: 10.3389/fmicb.2019.02209. . . ; : . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Delgado-Baquerizo M, Reich PB, Trivedi C, Eldridge DJ, Abades S, Alfaro FD, Bastida F, Berhe AA, Cutler NA, Gallardo A, García-Velázquez L, Hart SC, Hayes PE, He JZ, Hseu ZY, Hu HW, Kirchmair M, Neuhauser S, Pérez CA, Reed SC, Santos F, Sullivan BW, Trivedi P, Wang JT, Weber-Grullon L, Williams MA, Singh BK Multiple elements of soil biodiversity drive ecosystem functions across biomes Nat Ecol Evol. 2020;4:210. doi: 10.1038/s41559-019-1084-y. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 108.Kuzyakov Y Priming effects: Interactions between living and dead organic matter Soil Biol Biochem. 2010;42:1363. doi: 10.1016/j.soilbio.2010.04.003. . . ; : –. . doi: [DOI] [Google Scholar]
- 109.Londono N, Donovan AR, Shi H, Geisler M, Liang Y Effects of environmentally relevant concentrations of mixtures of TiO2, ZnO and Ag ENPs on a river bacterial community Chemosphere. 2019;230:567. doi: 10.1016/j.chemosphere.2019.05.110. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 110.Brusseau ML, Anderson RH, Guo B PFAS concentrations in soils: Background levels versus contaminated sites Sci Total Environ. 2020;740:140017. doi: 10.1016/j.scitotenv.2020.140017. . . ; : . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhalnina K, Dörr de Quadros P, Camargo FAO, Triplett EW Drivers of archaeal ammonia-oxidizing communities in soil Front Microbiol. 2012;3:210. doi: 10.3389/fmicb.2012.00210. . . ; : . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krippner J, Falk S, Brunn H, Georgii S, Schubert S, Stahl T Accumulation potentials of perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkyl sulfonic acids (PFSAs) in maize (Zea mays) J Agric Food Chem. 2015;63:3646. doi: 10.1021/acs.jafc.5b00012. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 113.Stahl T, Heyn J, Thiele H, Hüther J, Failing K, Georgii S, Brunn H Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plants Arch Environ Contam Toxicol. 2009;57:289. doi: 10.1007/s00244-008-9272-9. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 114.Lechner M, Knapp H Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plant and distribution to the different plant compartments studied in cultures of carrots (Daucus carota ssp. Sativus), potatoes (Solanum tuberosum), and cucumbers (Cucumis Sativus) J Agric Food Chem. 2011;59:11011. doi: 10.1021/jf201355y. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 115.Stahl T, Riebe RA, Falk S, Failing K, Brunn H Long-term lysimeter experiment to investigate the leaching of perfluoroalkyl substances (PFASs) and the carry-over from soil to plants: results of a pilot study J Agric Food Chem. 2013;61:1784. doi: 10.1021/jf305003h. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 116.Sinclair GM, Long SM, Jones OAH What are the effects of PFAS exposure at environmentally relevant concentrations? Chemosphere. 2020;258:127340. doi: 10.1016/j.chemosphere.2020.127340. . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 117.Zhang S, Szostek B, McCausland PK, Wolstenholme BW, Lu X, Wang N, Buck RC 6:2 and 8:2 fluorotelomer alcohol anaerobic biotransformation in digester sludge from a WWTP under methanogenic conditions Environ Sci Technol. 2013;47:4227. doi: 10.1021/es4000824. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 118.Davis CK, Webb RI, Sly LI, Denman SE, McSweeney CS Isolation and survey of novel fluoroacetate-degrading bacteria belonging to the phylum Synergistetes FEMS Microbiol Ecol. 2012;80:671. doi: 10.1111/j.1574-6941.2012.01338.x. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 119.Cordner A, Goldenman G, Birnbaum LS, Brown P, Miller MF, Mueller R, Patton S, Salvatore DH, Trasande L The true cost of PFAS and the benefits of acting now Environ Sci Technol. 2021;55:9630. doi: 10.1021/acs.est.1c03565. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sonmez Baghirzade B, Zhang Y, Reuther JF, Saleh NB, Venkatesan AK, Apul OG Thermal regeneration of spent granular activated carbon presents an opportunity to break the forever PFAS cycle Environ Sci Technol. 2021;55:5608. doi: 10.1021/acs.est.0c08224. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 121.Hogue C PFAS used in fracking fluids in US, report says C&EN Global Enterprise. 2021;99:17. . . ; : [displayed 8 August 2023]. Available at https://pubs.acs.org/doi/pdf/10.1021/cen-09926-polcon4. [Google Scholar]
- 122.Belkouteb N, Franke V, McCleaf P, Köhler S, Ahrens L Removal of per- and polyfluoroalkyl substances (PFASs) in a full-scale drinking water treatment plant: Long-term performance of granular activated carbon (GAC) and influence of flow-rate Water Res. 2020;182:115913. doi: 10.1016/j.watres.2020.115913. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 123.Schröder HF Determination of fluorinated surfactants and their metabolites in sewage sludge samples by liquid chromatography with mass spectrometry and tandem mass spectrometry after pressurised liquid extraction and separation on fluorine-modified reversed-phase sorbents J Chromatogr A. 2003;1020:131. doi: 10.1016/s0021-9673(03)00936-1. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 124.Huang S, Jaffé PR Defluorination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. Strain A6 Environ Science Technol. 2019;53:11410. doi: 10.1021/acs.est.9b04047. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 125.Inoue J, Oshima K, Suda W, Sakamoto M, Iino T, Noda S, Hongoh Y, Hattori M, Ohkuma M Distribution and evolution of nitrogen fixation genes in the phylum Bacteroidetes Microbes Environ. 2015;30:44. doi: 10.1264/jsme2.ME14142. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koirala A, Brözel VS Phylogeny of nitrogenase structural and assembly components reveals new insights into the origin and distribution of nitrogen fixation across bacteria and Archaea Microorganisms. 2021;9(8):1662. doi: 10.3390/microorganisms9081662. . . ; ( ): . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cao L, Xu W, Wan Z, Li G, Zhang F Occurrence of PFASs and its effect on soil bacteria at a fire-training area using PFOS-restricted aqueous film-forming foams iScience. 2022;25(4):104084. doi: 10.1016/j.isci.2022.104084. . . ; ( ): . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kalam S, Basu A, Ahmad I, Sayyed RZ, El-Enshasy HA, Dailin DJ, Suriani NL Recent understanding of soil acidobacteria and their ecological significance: a critical review Front Microbiol. 2020;11:580024. doi: 10.3389/fmicb.2020.580024. . . ; : . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Iwaloye OP . Metagenomics and Metatranscriptomics of Lake Erie Ice. [MSc thesis] Bowling Green: Bowling Green State University; 2021. . . : ; [displayed 8 August 2023]. Available at https://etd.ohiolink.edu/apexprod/rws_etd/send_file/send?accession=bgsu1626157167377268&disposition=inline. [Google Scholar]
- 130.Nelson MB, Martiny AC, Martiny JB Global biogeography of microbial nitrogen-cycling traits in soil Proc Natl Acad Sci U S A. 2016;113:8033. doi: 10.1073/pnas.1601070113. . . ; : –. . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Narsing Rao MP, Luo ZH, Dong ZY, Li Q, Liu BB, Guo SX, Nie GX, Li WJ Metagenomic analysis further extends the role of Chloroflexi in fundamental biogeochemical cycles Environ Res. 2022;209:112888. doi: 10.1016/j.envres.2022.112888. . . ; : . doi: [DOI] [PubMed] [Google Scholar]
- 132.Marchetto F, Roverso M, Righetti D, Bogialli S, Filippini F, Bergantino E, Sforza E Bioremediation of per- and poly-gluoroalkyl substances (PFAS) by Synechocystis sp. PCC 6803: a chassis for a synthetic biology approach Life (Basel) 2021;11(12):1300. doi: 10.3390/life11121300. . . ; ( ): . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yi LB, Chai LY, Xie Y, Peng QJ, Peng QZ Isolation, identification, and degradation performance of a PFOA-degrading strain Genet Mol Res. 2016;15(2):gmr.15028043. doi: 10.4238/gmr.15028043. . . ; ( ): . doi: [DOI] [PubMed] [Google Scholar]
- 134.Chetverikov SP, Sharipov DA, Korshunova TY, Loginov ON Degradation of perfluorooctanyl sulfonate by strain Pseudomonas plecoglossicida 2.4-D Appl Biochem Microbiol. 2017;53:533. doi: 10.1134/S0003683817050027. . . ; : –. . doi: [DOI] [Google Scholar]
- 135.Shaw DMJ, Munoz G, Bottos EM, Duy SV, Sauvé S, Liu J, Van Hamme JD Degradation and defluorination of 6:2 fluorotelomer sulfonamidoalkyl betaine and 6:2 fluorotelomer sulfonate by Gordonia sp. strain NB4-1Y under sulfur-limiting conditions Sci Total Environ. 2019;647:690. doi: 10.1016/j.scitotenv.2018.08.012. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]
- 136.Kim MH, Wang N, Chu KH 6:2 Fluorotelomer alcohol (6:2 FTOH) biodegradation by multiple microbial species under different physiological conditions Appl Microbiol Biotechnol. 2014;98:1831. doi: 10.1007/s00253-013-5131-3. . . ; : –. . doi: [DOI] [PubMed] [Google Scholar]