Abstract
Some methicillin-resistant strains of Staphylococcus aureus are defective in the production of major surface components such as protein A, clumping factor, or other important adhesins to extracellular matrix components which may play a role in bacterial colonization and infection. To evaluate the impact of methicillin resistance (mec) determinants on bacterial adhesion mediated by fibrinogen or fibronectin adhesins, we compared the in vitro attachment of two genetically distinct susceptible strains (NCTC8325 and Newman) to protein-coated surfaces with that of isogenic methicillin-resistant derivatives. All strains containing an intact mec element in their chromosomes were found to be defective in adhesion to fibrinogen and fibronectin immobilized on polymethylmethacrylate coverslips, regardless of the presence or absence of additional mutations in the femA, femB, or femC gene, known to decrease expression of methicillin resistance in S. aureus. Western ligand affinity blotting or immunoblotting of cell wall-associated adhesins revealed similar contents of fibrinogen- or fibronectin-binding proteins in methicillin-resistant strains compared to those of their methicillin-susceptible counterparts. In contrast to methicillin-resistant strains carrying a mec element in their genomes, methicillin-resistant strains constructed in vitro, by introducing the mecA gene on a plasmid, retained their adhesion phenotypes. In conclusion, the chromosomal insertion of the mec element into genetically defined strains of S. aureus impairs the in vitro functional activities of fibrinogen or fibronectin adhesins without altering their production. This effect is unrelated to the activity of the mecA gene.
Bacterial adhesion to host cells or tissues is an important step in the initiation of infection. This process also plays an important role in bacterial colonization of medical devices coated with various cellular and extracellular host components. Staphylococcus aureus expresses specific surface proteins called adhesins (11, 12, 22, 33) allowing it to interact specifically with plasma or extracellular matrix proteins associated with normal tissues or adsorbed on biomedical devices. The most important proteins promoting adhesion of S. aureus are fibronectin (18, 46, 47, 49, 50), fibrinogen (6, 18, 29), collagen (33–35), vitronectin (7, 26), laminin (18, 27), thrombospondin (17), bone sialoprotein (53), and elastin (52).
Recent molecular studies of major S. aureus adhesins allowed identification and characterization of the genes coding for the fibrinogen-binding protein ClfA (clumping factor) (29, 30), the collagen adhesin (34, 36), and two distinct but related fibronectin-binding proteins (FnBPs) encoded by closely linked but separately transcribed genes called fnbA and fnbB (10, 13, 23). An important aspect of these molecular studies was to demonstrate the functional significance of S. aureus adhesins by the production of specific mutants expressing defective in vitro and in vivo attachment to their respective host proteins (13, 14, 29, 32, 35, 51). Thus, both fnb genes must be inactivated to eliminate bacterial interactions with fibronectin (13).
Clinical methicillin-resistant S. aureus (MRSA) isolates have acquired an additional 30- to 50-kb DNA element of unknown origin that integrates into a specific chromosomal site (21). This DNA element carries among yet-uncharacterized genes the methicillin resistance (mec) determinant coding for mecA, the structural gene for the low-affinity penicillin-binding protein, PBP2a or PBP2′, that mediates methicillin resistance. The level of methicillin resistance is dependent on the genetic background of each strain and is modulated by a number of chromosomal genes termed fem (15) or aux (8) factors. These factors are involved directly or indirectly in cell wall biosynthesis (3), whereby methicillin resistance is reduced by femC or femD inactivation (15) or completely abolished by inactivating either femA or femB (43) without affecting PBP2a production.
We lack precise information for MRSA strains about the molecular mechanisms of their attachment to various host tissues or implanted biomaterials. Detailed knowledge of molecular mechanisms of bacterial adhesion and colonization might help to explain why some strains of MRSA are more epidemic than others. To address this question, we compared the adhesion characteristics of isogenic methicillin-resistant derivatives of two well-defined laboratory strains of S. aureus. Strains were tested for in vitro adhesion to fibrinogen- and fibronectin-coated artificial surfaces and expression of adhesins by Western ligand affinity blotting or immunoblotting. The potential contribution of active or inactive fem factors which modulate the phenotypic expression of methicillin resistance was also evaluated.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains or plasmids useda
| Strain or plasmid | Relevant genotype | Phenotype | Source and/or reference |
|---|---|---|---|
| Strains | |||
| BB255 | 8325; parent strain | Mcs | (2) |
| BB859 | mec | Mcr Emr | This study; by transformation of mec(EK872)b into BB255 |
| BB270 | 8325, mec | Mcr | By transduction of mec(EK142)c into BB255 (1) |
| BB308 | 8325, mec Ω2003(femA::Tn551) | Mcs Emr | BB270 insertionally inactivated by Tn551 (28) |
| BB331 | 8325, Ω2003(femA::Tn551) | Mcs Emr | This study; by transduction of Ω2003(femA::Tn551) into BB255 |
| BB814 | 8325, mec Ω2006(femB::Tn551) | Mcs Emr | BB270 insertionally inactivated by Tn551 (16) |
| BB589 | 8325, mec Ω2005(femC::Tn551) | Mcs Emr | BB270 insertionally inactivated by Tn551 (15) |
| BB906 | 8325, Ω2005(femC::Tn551) | Mcs Emr | BB255 insertionally inactivated by Tn551 (15) |
| Newman | Parent strain | Mcs | |
| BB1003 | Newman, mec | Mcr | This study; transformation of Newman with DNA of strain BB270 |
| BB749 | 8325, pBBB79 | Mcr Cmr | Transduction of pBBB79 into BB255 (39) |
| BB702 | 8325, pBBB21 | Mcr Cmr | Transduction of pBBB21 into BB255 (44) |
| Plasmids | |||
| pBBB21 | pCA44 mecA insert (44) | AmprEscherichia coli, Cmr Mcr in S. aureus | Recombinant plasmid carrying a 6.2-kb insert from chmosomal DNA of Staphylococcus epidermidis carrying mecA (44) |
| pBBB79 | pGC2 mecA insert | Ampr in E. coli, Cmr Mcr in S. aureus | 4.2-kb HindIII fragment carrying mecA subcloned from pBBB21 (44) into the HindIII site of pGC2 (39) |
Abbreviations: Amp, ampicillin; Em, erythromycin; Cm, chloramphenicol; Mc, methicillin.
EK872 is an MRSA clinical isolate from 1992.
EK142 is an MRSA clinical isolate from 1967.
Bacterial adhesion assay.
In vitro attachment of S. aureus to protein-coated polymer surfaces was measured by a previously described adhesion assay with polymethylmethacrylate (PMMA) coverslips coated in vitro with purified fibrinogen (29, 31) or fibronectin (13, 14). Human fibrinogen and fibronectin were purchased from Imco (Stockholm, Sweden) and Chemicon (Temecula, Calif.), respectively, as previously described (13, 29). Trace amounts of contaminating fibronectin were removed from fibrinogen by gelatin adsorption (38). To optimize adsorption of fibronectin from concentrations below 1 μg/ml, the PMMA coverslips were precoated with gelatin followed by rinsing in phosphate-buffered saline (PBS) as previously described (13, 14, 51).
The two-sided protein coating of PMMA was achieved by incubating for 60 min at 37°C native or gelatin-precoated coverslips with fibrinogen or fibronectin, respectively, at three different concentrations (0.25, 0.5, and 1 μg/ml) of either protein in PBS, followed by rinsing in PBS as previously described (13, 14, 29). PMMA surfaces were shown to be coated in a dose-dependent manner with amounts of human fibrinogen ranging from 41 to 145 ng per coverslip (28 to 101 ng/cm2) when radiolabeled fibrinogen (by reductive methylation with sodium boro[3H]hydride [42, 48]) was used. A similar linear coating of PMMA surfaces with [3H]fibronectin ranging from 46 to 190 ng per coverslip (31 to 128 ng/cm2) was recorded.
The adhesion characteristics of the different strains of S. aureus were evaluated by incubating the protein-coated coverslips with 4 × 106 CFU (8 × 104 cpm) of washed log-phase cultures, metabolically radiolabeled with [3H]thymidine during growth for 4 h at 37°C in Mueller-Hinton broth as previously described (average specific radioactivity, 0.02 cpm/CFU) (50). The protein-coated coverslips were incubated with radiolabeled bacteria for 60 min at 37°C in PBS with 1 mM Ca2+ and 0.5 mM Mg2+ supplemented with 5 mg of human serum albumin per ml, which prevented nonspecific adhesion of S. aureus (14, 29, 50). At the end of the attachment period, the fluids containing unbound bacteria were removed, the coverslips were rinsed, and radioactivity on the coverslips was counted as previously described (50). To compare under normalized conditions adhesion of the different strains whose cell-associated contents and viable counts differed slightly, the amount of radioactivity remaining on the coverslips divided by the number of radiolabeled bacteria initially added to the system (expressed as a percentage) was first evaluated. For each strain, the percentage of attached radiolabeled bacteria was used to estimate the number of adherent CFU per coverslip normalized to a fixed inoculum of 4 × 106 CFU/ml. Albumin-coated and gelatin-coated PMMA coverslips were used as controls of nonspecific adhesion to fibrinogen- and fibronectin-coated surfaces, respectively (13, 29, 50, 51).
Each experiment was performed at least three times, and the results were expressed as means ± standard errors of the means. Comparison of bacterial adhesion of methicillin-resistant mutants with their isogenic methicillin-susceptible parents was performed by one-way analysis of variance and t tests under analysis of variance for comparison of pairs of groups, with the Bonferroni correction for multiple comparisons (37). Statistical significance was evaluated for each of the three concentrations of fibrinogen or fibronectin immobilized on PMMA coverslips, and data were considered significant when P was <0.05 by using two-tailed significance levels.
Measurement of cell clumping.
Cell clumping was measured quantitatively in microtiter trays by a previously described procedure (29) with slight modifications. Suspensions of washed cells logarithmically grown for 4 h at 37°C were concentrated 100-fold in PBS. Cell clumping was tested by mixing 10 μl (108 CFU) of washed cells with 50 μl of fibrinogen (Imco), serially diluted twofold from a starting concentration of 160 μg/ml in PBS. The lowest concentration (range, 0.25 to 128 μg/ml) of fibrinogen which induced bacterial clumping was scored.
Preparation of cell wall-associated protein extracts.
Cell wall-associated proteins of S. aureus were prepared by growing organisms without shaking in 100 ml of Mueller-Hinton broth for 4 h at 37°C. After two washes in PBS, the bacterial cultures were suspended in 1 ml of PBS with 1 mM Ca2+ and 0.5 mM Mg2+ supplemented with 1.1 M sucrose and proteinase inhibitors (Complete; Boehringer, Mannheim, Germany). Protoplast formation was achieved by adding to the concentrated cell suspension 100 μg of lysostaphin (Ambicin; Applied Microbiology, Inc., Brooklyn, N.Y.) per ml for 5 min at 37°C. After removal of whole cells and debris by low-speed (2,000 × g, 10 min) centrifugation, the surface proteins solubilized from enzymatically digested cell walls were separated from intact protoplasts which were sedimented at 8,000 × g for 30 min. The cell wall-extracted proteins were precipitated from the protoplast-free supernatants with 10% trichloroacetic acid for 18 h at 4°C. After extraction of trichloroacetic acid with a 1:1 mixture of ethanol and chloroform followed by centrifugation at 12,000 × g for 15 min, the sedimented cell wall-associated proteins were suspended in a 100-μl volume of PBS and their concentrations were determined by the bicinchoninic acid assay (Pierce, Rockford, Ill.).
SDS-PAGE and Western ligand affinity blotting or immunoblotting.
Cell wall-associated protein amounts equivalent to 8 μg per strain were solubilized in sodium dodecyl sulfate (SDS) sample buffer and separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) with a 4 to 15% acrylamide gradient (25). Proteins were transferred to an Immobilon membrane (Millipore) by a liquid transblot system (Bio-Rad Laboratories). The membrane was blocked in 10 mM Tris-HCl (pH 8.0)–500 mM NaCl–0.1% Tween 20 (Fluka) (TBST) containing 2.5% bovine serum albumin.
The ClfA protein was detected by incubation with 2 μg of immunoglobulin G antibodies per ml in TBST, purified from rabbit serum against recombinant ClfA protein (30). After being rinsed several times in TBST, the filters were incubated with peroxidase-conjugated protein A (1:10,000 dilution; Amersham). Detection was by enhanced chemiluminescence (Amersham).
FnBPs were detected by Western ligand affinity blotting by incubation with pure human fibronectin (30 μg/ml) in TBST. The membrane was rinsed several times in TBST and incubated with monoclonal antibody 1936 raised against the N terminus of fibronectin (1:5,000; Chemicon) followed by peroxidase-conjugated anti-mouse immunoglobulin G (1:10,000). Detection was by enhanced chemiluminescence as described above.
Genetics methods.
Transformation of the mec determinant was done by the CaCl2 method, selecting for transformants growing on 5 μg of methicillin per ml. Transduction of chromosomally integrated Tn551 was with phage 80α with selection for transductants on 20 μg of erythromycin per ml (43).
RESULTS
Defective adhesion and clumping of S. aureus transformed with the methicillin resistance determinant.
To test the effects of the mec element on the adhesion properties of S. aureus for fibrinogen or fibronectin, two series of strains that varied in the presence or absence of the mec element were constructed. Two MRSA strains, EK142 (isolated in 1967) and EK872 (isolated in 1992), served as donors of the mec element. The recipients of these mec elements were strain BB255, a derivative of the laboratory strain NCTC8325, and S. aureus Newman. Both recipients are genetically distinct strains differing from each other by their chromosomal SmaI restriction patterns (data not shown). The mec elements were either transduced or transformed into these strains as described in Table 1. The sizes of both elements were approximately 30 kb as seen by the SmaI restriction patterns of the Mcr transformant BB859 (mecEK872) when compared with susceptible parent BB255 (data not shown). Both elements seemed identical at least over a region of 14 kb when probed with fragments covering part of the mec element, except for some restriction site polymorphism at the right chromosomal junction fragment (data not shown) and cotransformation of erythromycin resistance with mecEK872.
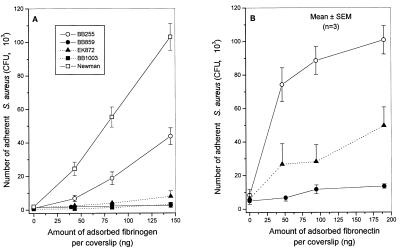
In vitro attachment of methicillin-resistant transformants of strain BB255 or Newman to increasing amounts of immobilized fibrinogen is shown in Fig. 1A. In contrast to the methicillin-susceptible parental strains, whose attachment was dose dependently promoted by fibrinogen, methicillin-resistant transformants of strain BB255 (BB859) or Newman (BB1003) showed completely defective attachment to fibrinogen (Fig. 1A), as illustrated by the lack of dose response to increasing amounts of fibrinogen and background levels of attached bacteria (≤103 CFU/coverslip). At the two higher levels of immobilized fibrinogen (83 and 145 ng/coverslip), the reductions in attachment of strains BB859 and EK872 were highly significant (P < 0.01) compared to that of strain BB255. This was also the case for the methicillin-resistant transformant (BB1003) of strain Newman, compared to its parent, which showed significant (P < 0.01) differences in adhesion at all levels of immobilized fibrinogen. Finally, the clinical MRSA strain EK872, which served as a donor of the mec element to strain BB859, also exhibited very low attachment to fibrinogen.
FIG. 1.
Adhesion to fibrinogen-coated (A) or fibronectin-coated (B) coverslips of methicillin-resistant transformants of S. aureus BB255 (closed symbols) and Newman (open symbols). Susceptible strain BB255, Mcr mutant strain BB859 derived from BB255; susceptible strain Newman, Mcr mutant strain BB1003 derived from Newman. Strain EK872 is the methicillin-resistant clinical isolate donor of the mec element to BB255. SEM, standard error of the mean.
Measurement of cell clumping confirmed the lack of the fibrinogen-binding ClfA protein-mediated agglutination in the methicillin-resistant transformants. Whereas the parental strain BB255 was clumped by 8 μg of fibrinogen per ml, strain BB859 remained clumping negative up to 128 μg/ml. The loss of clumping activity was even more impressive with the methicillin-resistant transformant of strain Newman, known to produce higher amounts of ClfA protein than strain 8325-4 or 8325 (29, 30). Whereas strain Newman was clumped by 0.5 μg of fibrinogen per ml, its methicillin-resistant transformant BB1003 lost clumping activity even in the presence of 128 μg of fibrinogen per ml.
In vitro attachment of the methicillin-resistant transformant of strain BB255 (BB859) to increasing amounts of immobilized fibronectin is shown in Fig. 1B. Compared to the methicillin-susceptible parent whose attachment was dose dependently promoted by fibronectin, the methicillin-resistant transformant showed a major reduction in attachment to fibronectin, which was highly significant (P < 0.001) at all levels of immobilized fibronectin. Figure 1B also shows that the clinical MRSA strain EK872, which was the donor of the mec element to strain BB859, exhibited a moderate attachment to fibronectin, which was significantly (P < 0.01) lower than that of strain BB255.
The methicillin-resistant transformant of strain Newman could not be tested for defective attachment to surface-bound fibronectin because of the weak affinity of the parental strain itself to this immobilized protein, as previously described (51).
Influence of fem mutations on adhesion properties of methicillin-susceptible or methicillin-resistant strains.
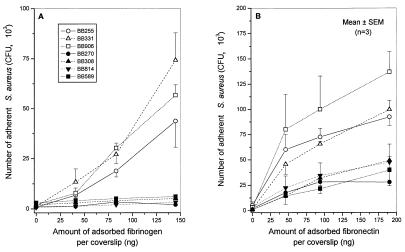
Dose-dependent attachment of various fem-inactivated mutants of either strain BB255 or its mec determinant-bearing transductant BB270 to fibrinogen- and fibronectin-coated coverslips is shown in Fig. 2A and B, respectively. Both femA and femC mutants of the methicillin-susceptible strain BB255 showed dose-response profiles of adhesion to solid-phase fibrinogen (Fig. 2A) or fibronectin (Fig. 2B) that were similar to or slightly, but not significantly, higher than those of their parent. On the other hand, femA, femB, or femC mutants of strain BB270 were all as defective in attachment to either fibrinogen or fibronectin as was the methicillin-resistant parent. The differences between the group of strain BB270 and its fem mutants (BB308, BB814, and BB589) and the group of BB255 and its fem mutants (BB331 and BB906) were highly significant (P < 0.01) at the two higher levels of immobilized fibrinogen (83 and 145 ng/coverslip) or fibronectin (94 and 190 ng/coverslip).
FIG. 2.
Adhesion to fibrinogen-coated (A) or fibronectin-coated (B) coverslips of fem mutants of either the methicillin-susceptible strain BB255 of S. aureus (open symbols) or its Mcr mutant BB270 (closed symbols). Susceptible strain BB255, femA mutant BB331 of strain BB255 and femC mutant BB906 of strain BB255; Mcr strain BB270, femA mutant BB308 of strain BB270, and femB mutant BB814 of strain BB270, and femC mutant BB589 of strain BB270. At points where bars for standard errors of the means (SEM) are not visible, the bars are smaller than the symbols.
Thus, the activity of fem factors played no significant role in the adhesion phenotypes of strain BB255 or its methicillin-resistant derivative BB270.
Expression of fibrinogen and fibronectin adhesins by methicillin-susceptible and methicillin-resistant strains.
Western immunoblotting or ligand affinity blotting of the ClfA protein or FnBPs, respectively, was performed to evaluate the level of expression of each adhesin in the wild-type and methicillin-resistant derivatives of strain Newman or BB255.
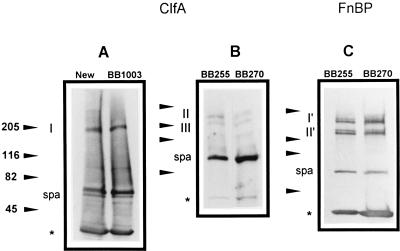
Fibrinogen adhesins were detected by Western immunoblotting with anti-ClfA protein antibodies. Figure 3A shows the presence of a predominant immunoreactive protein band (band I) of 180 to 190 kDa in parental strain Newman and its Mcr derivative BB1003 (lane 2). This band corresponds to the previously described native ClfA protein of 185 kDa (30). Unexpectedly, strains Newman and BB1003 exhibited equivalent amounts of cell wall-associated ClfA protein despite their widely different adhesion phenotypes.
FIG. 3.
Visualization of the ClfA protein (A and B) and FnBPs (C) by Western immunoblots and ligand affinity blots, respectively, in equivalent amounts (8 μg) of cell wall-associated protein extracts from methicillin-susceptible strains of S. aureus and their isogenic methicillin-resistant mutants. Positions of protein size markers (in kilodaltons) are shown at the left of each panel. Band I corresponds to native ClfA protein. Bands II and III represent truncated ClfA protein. Bands I′ and II′ correspond to native and truncated FnBPs, respectively. spa is identified as protein A. Asterisks indicate the fronts of the gels. (A) Lane 1, Newman; lane 2, Mcr strain BB1003 derived from Newman. (B) Lane 1, BB255; lane 2, Mcr strain BB270 derived from BB255. (C) Lane 1, BB255; lane 2, Mcr strain BB270.
Expression of the ClfA protein by strain BB255 is quite low compared to that by strain Newman. A previous study performed with S. aureus 8325-4, which is closely related to strain BB255, failed to detect immunoreactive proteins in whole-cell lysostaphin extracts (30). In contrast, whole-cell extracts of strain 8325-4 carrying the clfA gene of Newman on the multicopy plasmid pCF4 revealed proteins of 150 and 130 kDa which were assumed to be proteolytic fragments of the native 185-kDa ClfA protein (30). In this study, enrichment of cell wall-associated proteins allowed detection of immunoreactive proteins of 150 to 160 kDa (band II) and 130 to 140 kDa (band III) in strain BB255 and its methicillin-resistant derivatives. These sizes are similar to those reported for strain 8325-4(pCF4). Regardless of their different adhesion phenotypes, the methicillin-susceptible parental strain BB255 (Fig. 3B, lane 1), the methicillin-resistant transductant strain BB270 (Fig. 3B, lane 2), and transformant strain BB859 (data not shown) exhibited similar amounts of the proteolytic fragments of the cell wall-associated ClfA protein.
FnBPs were detected by Western ligand affinity blotting with fibronectin and antifibronectin monoclonal antibodies. Figure 3C shows the presence of two groups of FnBPs, whose sizes are 160 to 170 and 120 to 130 kDa, respectively. Each group is composed of similarly sized bands. The 160- to 170-kDa doublet (band I′) may represent the mixture of native FnBPA and FnBPB, whereas the 120- to 130-kDa doublet (band II′) may represent proteolytic fragments of the two FnBPs, according to their previously described patterns in the closely related strains 8325-4 (13) and ISP794 (4). Regardless of their different adhesion phenotypes, the methicillin-susceptible parental strain BB255 (Fig. 3C, lane 1), the methicillin-resistant transductant strain BB270 (Fig. 3C, lane 2), and transformant strain BB859 (data not shown) exhibited similar amounts of wall-associated FnBPs.
Control experiments showed that the 56-kDa protein band present in the Western blots is protein A as previously described (4).
Influence of the plasmid-located mecA gene on the adhesion phenotype.
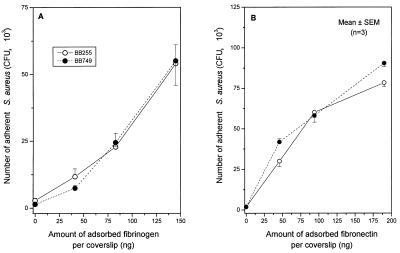
To evaluate whether the presence and/or activity of the mecA gene contributed to the defective attachment of S. aureus to fibrinogen or fibronectin, we analyzed a methicillin-resistant strain constructed in vitro, by introducing into strain BB255 the plasmid pBBB79 (39) having a 4.2-kb HindIII fragment carrying the mecA-encoding region on the shuttle vector pGC2 (39). Strain BB749 transduced with the mecA-carrying plasmid pBBB79 expressed heterogeneous resistance to methicillin as previously described (39). However, neither this heterogeneous Mcr strain nor the control transductant complemented with the shuttle vector pGC2 lacking the mecA insert (39) (data not shown) showed any reduction in attachment to fibrinogen (Fig. 4A) or fibronectin-coated coverslips (Fig. 4B) compared to that by the methicillin-susceptible parental strain BB255.
FIG. 4.
Adhesion to fibrinogen-coated (A) or fibronectin-coated (B) coverslips of the methicillin-susceptible strain BB255 of S. aureus (open symbols) or its methicillin-resistant transformant (closed symbols) carrying plasmid pBBB79 with a mecA insert leading to heterogeneous expression of methicillin resistance. SEM, standard error of the mean.
Additional experiments were performed with another methicillin-resistant transductant (BB702) of strain BB255, carrying the pBBB21 plasmid (44) with a larger 6.2-kb insert covering the mecA gene, the direct repeat (dru) element, and the insertion sequence-like element IS431. The methicillin-resistant strain BB702 also failed to demonstrate any significant reduction in attachment to fibrinogen or fibronectin compared to that of its methicillin-susceptible parent BB255 (data not shown).
These data indicated that expression of methicillin resistance in S. aureus by the low-affinity mecA-encoded PBP2′ did not contribute to the adhesion defects exhibited in vitro by methicillin-resistant mutants containing a chromosomally inserted mec element.
DISCUSSION
This study provides evidence that chromosomal insertion of methicillin resistance determinants into genetically defined susceptible strains of S. aureus alters functional surface expression of fibrinogen and fibronectin adhesins. Both mec elements, which were isolated independently, produced the same effect regardless of the genetic background (NCTC8325 or Newman) into which they were introduced. Since at least three genetically distinguishable mec elements have been described elsewhere (20, 21), it remains to be defined whether the adhesion defects observed in vitro are a general property of all chromosomally inserted mec elements or of only some of them.
The adhesion defects of methicillin-resistant transformants or transductants of S. aureus NCTC8325 or Newman were not influenced by their level of methicillin resistance and thus are unrelated to the presence or activity of the mecA locus. Furthermore, mutations affecting the femA, femB, or femC locus, which interfere with peptidoglycan precursor formation and lower expression of methicillin resistance, did not modify the defective adhesion phenotypes of strains bearing the mec determinant. Comparisons of the normal adhesion phenotypes exhibited by methicillin-resistant transductants carrying mecA on plasmids with the defective phenotypes of transductants or transformants having chromosomally inserted resistance determinants indicate that the genetic determinants of the adhesion defects may be found within the additional 30 kb of the mec-associated DNA or may be due to interruption of the chromosomal locus at the mec attachment site or to a polar effect of the mec element.
Several studies have described the defective in vitro expression of various surface proteins by clinical isolates MRSA. The most frequently reported defects in MRSA clinical isolates are lower contents of protein A and clumping factor (9, 19, 24, 41, 45). These phenotypic defects seem to occur more frequently in epidemic than in nonepidemic strains of MRSA (24, 41, 45). Unfortunately, most of the studies describing phenotypic alterations in MRSA clinical isolates failed to include for comparison an equivalent group of representative isolates of methicillin-susceptible S. aureus. Direct comparison of quantitative amounts of specific surface proteins expressed by methicillin-resistant versus methicillin-susceptible clinical isolates of S. aureus has not been reported and may be of limited significance in view of their different genetic backgrounds.
Several different mechanisms explaining the defective functional activities of fibrinogen and fibronectin adhesins in methicillin-resistant transformants or transductants of S. aureus are possible: (i) decreased biosynthesis of ClfA protein and FnBPs in methicillin-resistant compared to methicillin-susceptible strains; (ii) normal biosynthesis but defective cell wall sorting of ClfA protein and FnBPs in the methicillin-resistant strains, leading to decreased amounts of cell wall-anchored adhesins (40); and (iii) functional inhibition of ClfA protein and FnBPs which are synthesized and cell wall anchored in normal amounts but prevented from interacting with fibrinogen and fibronectin, respectively, by some unknown mechanisms.
Determination of the amounts of fibrinogen and fibronectin adhesins by Western ligand affinity blotting or immunoblotting, respectively, required the development of more elaborate procedures than those previously available (13, 29, 30). Initial studies (not shown) performed with whole-cell lysostaphin extract of strain BB255 and its methicillin-resistant derivatives failed to detect significant levels of the ClfA protein in this group of strains. These findings were similar to those previously reported with the closely related strain 8325-4 (29, 30). The preparation of cell wall-associated protein extracts allowed detection of significant amounts of ClfA protein and FnBPs in this group of strains by Western immunoblotting. Both fibrinogen and fibronectin adhesins were present in equivalent amounts in the cell wall protein extracts of the parental and methicillin-resistant strains. Further support for these observations was obtained by comparing the relative contents of ClfA in strain Newman, a high producer of this protein, with those of its methicillin-resistant transformants. All these different strains yielded equivalent contents of the fibrinogen adhesin regardless of their methicillin resistance.
The mechanisms leading to defective adhesion phenotypes of the isogenic methicillin-resistant mutants of S. aureus are difficult to explain in view of the normal amounts of ClfA protein and FnBPs recovered on their surface. We have to speculate that anchoring of the functionally defective adhesins to peptidoglycan (40) may occur in a conformationally inactive way or that some additional cell wall or surface protein component(s) specifically expressed by methicillin-resistant mutants may interfere with adhesin functions. Arguments for the second hypothesis were provided by two recent studies indicating the presence of a 230-kDa cell wall protein in certain methicillin-resistant clinical isolates of S. aureus which was not detected on the surfaces of other staphylococci (19, 24). The presence of this 230-kDa protein was assumed to contribute to negative agglutination results in commercial assays used to identify S. aureus clinical isolates, although the molecular mechanisms by which this inhibition may occur are not elucidated (19). More-extensive molecular studies are needed to characterize the complete primary structure of the 230-kDa protein and to identify the genetic loci responsible for its production by some methicillin-resistant isolates but not others. The relationship of the 230-kDa protein with the native ClfA protein and FnBPs, whose apparent molecular masses on SDS-PAGE are close to 200 kDa, needs also to be clarified.
The epidemiological and clinical significance of functional defects found in vitro for two major adhesins in methicillin-resistant mutants of the laboratory strains 8325 and Newman of S. aureus is difficult to evaluate. Significant defects in adhesin expression should be expected to prevent the emergence and sometimes epidemic spreading of certain MRSA isolates. However, since the adhesin defects expressed in laboratory conditions by isogenic methicillin-resistant mutants of S. aureus are also present in a significant proportion of MRSA clinical isolates (9, 19, 24, 41, 45), we believe that the in vivo relevance of our experimental in vitro data deserves to be further explored in future studies. Adhesin expression by S. aureus strains expressing different antibiotic resistance patterns also needs to be studied in experimental conditions more closely approaching the complex in vivo environment. Indeed, recent studies indicate that expression of some adhesins is under the control of internal and external signals (4, 5). Expression of FnBPs is regulated to some extent by the global regulators sar and agr, which are themselves responsive to the growth phase (5). Expression of bacterial adhesins is also altered by the combined presence of antibiotics and antibiotic resistance determinants, as shown by a recent report which documented the increased expression of FnBPs by fluoroquinolone-resistant mutants of S. aureus exposed to subinhibitory concentrations of ciprofloxacin (4). Further studies are planned to evaluate the functional surface expression of fibrinogen and fibronectin adhesins by laboratory or clinical methicillin-resistant strains of S. aureus exposed to subinhibitory levels of various antimicrobials. They may potentially help to evaluate how S. aureus adhesins contribute to bacterial attachment and colonization under clinically relevant conditions of antibiotic pressure and resistance determinants.
ACKNOWLEDGMENTS
This work was supported by grants 3200-045810.95/1 (to D.P.L.) and 3100-042182.94 (to B.B.-B.) from the Swiss National Foundation and by Ciba-Geigy-Jubiläums-Stiftung (to P.F.), the Wellcome Trust (project grant 033403), and the Health Research Board of Ireland.
We thank M. Bento for technical assistance.
REFERENCES
- 1.Beck W D, Berger-Bächi B, Kayser F H. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J Bacteriol. 1986;165:373–378. doi: 10.1128/jb.165.2.373-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger-Bächi B. Insertional inactivation of staphylococcal methicillin resistance by Tn551. J Bacteriol. 1983;154:479–487. doi: 10.1128/jb.154.1.479-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger-Bächi B. Update on methicillin resistance mechanisms in staphylococci. Chemotherapy. 1996;42:19–26. [Google Scholar]
- 4.Bisognano C, Vaudaux P E, Lew D P, Ng E Y W, Hooper D C. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 1997;41:906–913. doi: 10.1128/aac.41.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung A L, Eberhardt K J, Chung E, Yeaman M R, Sullam P M, Ramos M, Bayer A S. Diminished virulence of a sar−/agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Invest. 1994;94:1815–1822. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung A L, Fischetti V A. The role of fibrinogen in staphylococcal adherence to catheters in vitro. J Infect Dis. 1990;161:1177–1186. doi: 10.1093/infdis/161.6.1177. [DOI] [PubMed] [Google Scholar]
- 7.Chhatwal G S, Preissner K T, Müller-Berghaus G, Blobel H. Specific binding of the human S protein (vitronectin) to streptococci, Staphylococcus aureus, and Escherichia coli. Infect Immun. 1987;55:1878–1883. doi: 10.1128/iai.55.8.1878-1883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Lencastre H, Tomasz A. Reassessment of the number of auxiliary genes essential for expression of high-level methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:2590–2598. doi: 10.1128/aac.38.11.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duckworth G J, Jordens J Z. Adherence and survival properties of an epidemic methicillin-resistant strain of Staphylococcus aureus compared with those of methicillin-sensitive strains. J Med Microbiol. 1990;32:195–200. doi: 10.1099/00222615-32-3-195. [DOI] [PubMed] [Google Scholar]
- 10.Flock J I, Froman G, Jonsson K, Guss B, Signäs C, Nilsson B, Raucci G, Höök M, Wadström T, Lindberg M. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 1987;6:2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster T J, McDevitt D. Surface-associated proteins of Staphylococcus aureus: their possible roles in virulence. FEMS Microbiol Lett. 1994;118:199–206. doi: 10.1111/j.1574-6968.1994.tb06828.x. [DOI] [PubMed] [Google Scholar]
- 12.Foster T J, McDevitt D. Molecular basis of adherence of staphylococci to biomaterials. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. Washington, D.C: American Society for Microbiology; 1994. pp. 31–44. [Google Scholar]
- 13.Greene C, McDevitt D, François P, Vaudaux P, Lew D P, Foster T J. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin binding proteins and studies on the expression of fnb genes. Mol Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- 14.Greene C, Vaudaux P E, François P, Proctor R A, McDevitt D, Foster T J. A low-fibronectin-binding mutant of Staphylococcus aureus 879R4S has Tn918 inserted into its single fnb gene. Microbiology. 1996;142:2153–2160. doi: 10.1099/13500872-142-8-2153. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson J, Strässle A, Hächler H, Kayser F H, Berger-Bächi B. The femC locus of Staphylococcus aureus required for methicillin resistance includes the glutamine synthetase operon. J Bacteriol. 1994;176:1460–1467. doi: 10.1128/jb.176.5.1460-1467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henze U, Sidow T, Wecke J, Labischinski H, Berger-Bächi B. Influence of femB on methicillin resistance and peptidoglycan metabolism in Staphylococcus aureus. J Bacteriol. 1993;175:1612–1620. doi: 10.1128/jb.175.6.1612-1620.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann M, Suchard S J, Boxer L A, Waldvogel F A, Lew D P. Thrombospondin binds to Staphylococcus aureus and promotes staphylococcal adherence to surfaces. Infect Immun. 1991;59:279–288. doi: 10.1128/iai.59.1.279-288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrmann M, Vaudaux P, Pittet D, Auckenthaler R, Lew D P, Schumacher-Perdreau F, Peters G, Waldvogel F A. Fibronectin, fibrinogen and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 19.Hildén P, Savolainen K, Tyynelä J, Vuento M, Kuusela P. Purification and characterisation of a plasmin-sensitive surface protein of Staphylococcus aureus. Eur J Biochem. 1996;236:904–910. doi: 10.1111/j.1432-1033.1996.00904.x. [DOI] [PubMed] [Google Scholar]
- 20.Hiramatsu K. Molecular evolution of MRSA. Microbiol Immunol. 1995;39:531–543. doi: 10.1111/j.1348-0421.1995.tb02239.x. [DOI] [PubMed] [Google Scholar]
- 21.Hiramatsu K, Kondo N, Ito T. Genetic basis for molecular epidemiology of MRSA. J Infect Chemother. 1996;2:117–129. doi: 10.3412/jsb.52.417. [DOI] [PubMed] [Google Scholar]
- 22.Höök M, Switalski L M, Wadström T, Lindberg M. Interactions of pathogenic microorganisms with fibronectin. In: Mosher D F, editor. Fibronectin. San Diego, Calif: Academic Press; 1989. pp. 295–308. [Google Scholar]
- 23.Jonsson K, Signäs C, Muller H P, Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991;202:1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuusela P, Hildén P, Savolainen K, Vuento M, Lyytikäinen O, Vuopio-Varkila J. Rapid detection of methicillin-resistant Staphylococcus aureus strains not identified by slide agglutination tests. J Clin Microbiol. 1994;32:143–147. doi: 10.1128/jcm.32.1.143-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Liang O D, Maccarana M, Flock J-I, Paulsson M, Preissner K T, Wadström T. Multiple interactions between human vitronectin and Staphylococcus aureus. Biochim Biophys Acta. 1993;1225:57–63. doi: 10.1016/0925-4439(93)90122-h. [DOI] [PubMed] [Google Scholar]
- 27.Lopez J D, Dos Reis M, Bretani R R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985;229:275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- 28.Maidhof H, Reinicke B, Blumel P, Berger-Bächi B, Labischinski H. femA, which encodes a factor essential for expression of methicillin resistance, affects glycine content of peptidoglycan in methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J Bacteriol. 1991;173:3507–3513. doi: 10.1128/jb.173.11.3507-3513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDevitt D, François P, Vaudaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 30.McDevitt D, François P, Vaudaux P, Foster T J. Identification of the ligand-binding domain of the surface-located fibrinogen receptor (clumping factor) of Staphylococcus aureus. Mol Microbiol. 1995;16:895–907. doi: 10.1111/j.1365-2958.1995.tb02316.x. [DOI] [PubMed] [Google Scholar]
- 31.McDevitt D, Vaudaux P, Foster T J. Genetic evidence that bound coagulase of Staphylococcus aureus is not clumping factor. Infect Immun. 1992;60:1514–1523. doi: 10.1128/iai.60.4.1514-1523.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreillon P, Entenza J M, Francioli P, McDevitt D, Foster T J, François P, Vaudaux P. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–4743. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patti J M, Allen B L, McGavin M J, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 34.Patti J M, Boles J O, Höök M. Identification and biochemical characterization of the ligand binding domain of the collagen adhesin from Staphylococcus aureus. Biochemistry. 1993;32:11428–11435. doi: 10.1021/bi00093a021. [DOI] [PubMed] [Google Scholar]
- 35.Patti J M, Bremell T, Krajewska-Pietrasik D, Abdelnour A, Tarkowski A, Rydén C, Höök M. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect Immun. 1994;62:152–161. doi: 10.1128/iai.62.1.152-161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patti J M, Jonsson H, Guss B, Switalski L M, Wiberg K, Lindberg M, Höök M. Molecular characterization and expression of a gene encoding Staphylococcus aureus collagen adhesin. J Biol Chem. 1992;267:4766–4772. [PubMed] [Google Scholar]
- 37.Rosner B. Fundamentals of biostatistics. 3rd ed. Belmont, Calif: Duxbury Press; 1990. [Google Scholar]
- 38.Ruoslahti E, Hayman E G, Pierschbacher M, Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82A:803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- 39.Ryffel C, Strässle A, Kayser F H, Berger-Bächi B. Mechanisms of heteroresistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:724–728. doi: 10.1128/aac.38.4.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneewind O, Fowler A, Faull K F. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 1995;268:103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 41.Schwarzkopf A, Karch H, Schmidt H, Lenz W, Heesemann J. Phenotypical and genotypical characterization of epidemic clumping factor-negative, oxacillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993;31:2281–2285. doi: 10.1128/jcm.31.9.2281-2285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spycher M O, Nydegger U E. Part of the activating cross-linked immunoglobulin G is internalized by human platelets to sites not accessible for enzymatic digestion. Blood. 1986;67:12–18. [PubMed] [Google Scholar]
- 43.Strandén A M, Ehlert K, Labischinski H, Berger-Bächi B. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J Bacteriol. 1997;179:9–16. doi: 10.1128/jb.179.1.9-16.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tesch W, Strassle A, Berger-Bächi B, O’Hara D, Reynolds P, Kayser F H. Cloning and expression of methicillin resistance from Staphylococcus epidermidis in Staphylococcus carnosus. Antimicrob Agents Chemother. 1988;32:1494–1499. doi: 10.1128/aac.32.10.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Wamel W J B, Fluit A C, Wadström T, Van Dijk H, Verhoef J, Vandenbroucke-Grauls C M J E. Phenotypic characterization of epidemic versus sporadic strains of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1995;33:1769–1774. doi: 10.1128/jcm.33.7.1769-1774.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaudaux P, Lew D P, Waldvogel F A. Host factors predisposing to and influencing therapy of foreign body infections. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. Washington, D.C: American Society for Microbiology; 1994. pp. 1–29. [Google Scholar]
- 47.Vaudaux P, Pittet D, Haeberli A, Huggler E, Nydegger U E, Lew D P, Waldvogel F A. Host factors selectively increase staphylococcal adherence on inserted catheters: a role for fibronectin and fibrinogen/fibrin. J Infect Dis. 1989;160:865–875. doi: 10.1093/infdis/160.5.865. [DOI] [PubMed] [Google Scholar]
- 48.Vaudaux P, Pittet D, Haeberli A, Lerch P G, Morgenthaler J J, Proctor R A, Waldvogel F A, Lew D P. Fibronectin is more active than fibrin or fibrinogen in promoting Staphylococcus aureus adherence to inserted intravascular catheters. J Infect Dis. 1993;167:633–641. doi: 10.1093/infdis/167.3.633. [DOI] [PubMed] [Google Scholar]
- 49.Vaudaux P, Suzuki R, Waldvogel F A, Morgenthaler J J, Nydegger U E. Foreign body infection: role of fibronectin as a ligand for the adherence of Staphylococcus aureus. J Infect Dis. 1984;150:546–553. doi: 10.1093/infdis/150.4.546. [DOI] [PubMed] [Google Scholar]
- 50.Vaudaux P, Waldvogel F A, Morgenthaler J J, Nydegger U E. Adsorption of fibronectin onto polymethylmethacrylate and promotion of Staphylococcus aureus adherence. Infect Immun. 1984;45:768–774. doi: 10.1128/iai.45.3.768-774.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaudaux P E, François P, Proctor R A, McDevitt D, Foster T J, Albrecht R M, Lew D P, Wabers H, Cooper S L. Use of adhesion-defective mutants of Staphylococcus aureus to define the role of specific plasma proteins in promoting bacterial adhesion to canine arteriovenous shunts. Infect Immun. 1995;63:585–590. doi: 10.1128/iai.63.2.585-590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woo Park P, Roberts D D, Grosso L E, Parks W C, Rosenbloom J, Abrams W R, Mecham R P. Binding of elastin to Staphylococcus aureus. J Biol Chem. 1991;266:23399–23406. [PubMed] [Google Scholar]
- 53.Yacoub A, Lindahl P, Rubin K, Wendel M, Heinegård D, Rydén C. Purification of a bone sialoprotein-binding protein from Staphylococcus aureus. Eur J Biochem. 1994;222:919–925. doi: 10.1111/j.1432-1033.1994.tb18940.x. [DOI] [PubMed] [Google Scholar]