Abstract
Background
Anticoagulant treatment for intermittent claudication might improve functional capacity and prevent acute cardiovascular complications caused by peripheral obstructive arterial disease. This is an update of the review first published in 2001.
Objectives
To assess the effects of anticoagulant drugs (heparin, low molecular weight heparin (LMWH) and oral anticoagulants) in patients with intermittent claudication (Fontaine stage II) in terms of improving walking capacity (pain‐free walking distance or absolute walking distance), mortality, cardiovascular events, ankle/brachial pressure index, progression to surgery, amputation‐free survival and side effects of these drugs.
Search methods
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched May 2013) and CENTRAL (2013, Issue 4).
Selection criteria
All randomised trials of anticoagulants used to treat patients with intermittent claudication.
Data collection and analysis
Seven studies were included. Only three studies (two evaluating oral anticoagulants, one evaluating heparin) met the high quality methodological inclusion criteria and were included in the primary analysis. Four other studies were included in the sensitivity analysis. The authors extracted the data independently.
Main results
No new studies were included for this update. Seven studies with a combined total of 802 participants were included in this review. No significant difference was observed between heparin treatment and control groups for pain‐free walking distance or maximum walking distance at the end of treatment. There were no data to indicate that LMWHs benefit walking distance. Revascularisation or amputation‐free survival rates were reported in one study only with a five year follow‐up. No study reported a significant effect on overall mortality or cardiovascular events and the pooled odds ratios were not significant for these outcomes either. Major and minor bleeding events were significantly more frequent in the group treated with oral anticoagulants compared to control, with a non‐significant increase in fatal bleeding events. No major bleeding events were reported in the study evaluating heparin, while a non‐significant increase in minor bleeding events was reported.
Authors' conclusions
The benefit of heparin, LMWHs and oral anticoagulants for treatment of intermittent claudication has not been established while an increased risk of major bleeding events has been observed, especially with oral anticoagulants. There is no clear evidence to support the use of anticoagulants for intermittent claudication at this stage.
Plain language summary
Anticoagulants for intermittent claudication
Atherosclerosis is a disease of the arteries in which fatty deposits block the flow of blood. This can cause intermittent claudication, when cramping pain in the legs is brought on by exercise and relieved by rest. These fatty deposits can also cause serious blockages that lead to heart attacks and the need for amputation (surgical removal of the limb). Anticoagulants, such as heparin or warfarin, are drugs that prevent clotting and may help people with intermittent claudication. No new studies were included for this update. Seven studies with 802 participants were included in this review. The review of trials found that the benefit of heparin, LMWHs and oral anticoagulants for treatment of intermittent claudication has not been established while an increased risk of major bleeding events has been observed, especially with oral anticoagulants. There is no clear evidence to support the use of anticoagulants for intermittent claudication at this stage. More research is needed.
Background
Intermittent claudication (IC) is a symptom of lower limb atherosclerosis. The clinical manifestations of lower limb atherosclerosis depend on the extent and severity of the obstructive lesions and on the extent of the collateral circulation.
Four clinical stages are described according to Fontaine (Fontaine 1954), namely: (1) asymptomatic arterial insufficiency; (2) intermittent claudication, which is a symptom complex characterised by leg pain and weakness brought on by walking, with the disappearance of the symptoms following a brief rest; (3) pain at rest; (4) ulceration and gangrene.
Stages III and IV are also described as critical limb ischaemia in which the process endangers part, or all, of the extremity, and can lead to limb loss (amputation) (Wolfe 1997). The presence of lower limb atherosclerosis is an index of a diffuse atherosclerotic process and thus patients have an increased risk of cardiac and cerebrovascular complications (stroke and acute myocardial infarction) (Balkau 1994).
The aims of treating lower limb atherosclerosis are to:
improve functional capacity (increase walking distance);
inhibit the local progression of atherosclerotic lesions;
reduce cardiac and cerebrovascular morbidity and mortality.
Conservative treatment may ameliorate the symptoms of IC and is based upon modification of risk factors for atherosclerosis, regular exercise and pharmacologic intervention. Pharmacologic interventions such as antithrombotic agents, i.e. antiplatelet agents or anticoagulants, may prevent the progression of atherosclerotic lesions by interfering with thrombotic complications. Thrombus formation seems to be an important factor in progression of atherosclerotic disease and in the conversion of events from chronic to acute (Fuster 1992). The inhibition of thrombin formation provides a rationale for the use of anticoagulant drugs such as oral anticoagulants, heparin and low molecular weight heparins (LMWHs). The antithrombotic effect of these drugs could be beneficial in maintaining or improving functional capacity. A randomised trial has indicated a benefit of long term oral anticoagulants in improving survival rates after arterial reconstruction for lower limb atherosclerosis (Kretschmer 1992). Thus, treatment with anticoagulants might reduce the incidence of major cardiovascular complications of atherosclerosis‐related thrombosis in patients with IC.
The purpose of this review is to assess the evidence regarding the effectiveness and safety of anticoagulant drugs for the treatment of IC. This is an update of the review first published in 2001 (Cosmi 2001).
Objectives
To assess the effects of anticoagulant drugs (heparin, low molecular weight heparins (LMWHs), oral anticoagulants) in patients with intermittent claudication (Fontaine stage II) (Fontaine 1954) in terms of improving walking capacity (i.e. pain‐free walking distance or absolute walking capacity), mortality, cardiovascular events (acute myocardial infarction, sudden death and stroke), ankle/brachial pressure index, progression to surgery, amputation‐free survival and side effects of these drugs.
We wished to test the following a priori hypotheses: (1) Anticoagulants (either heparin or LMWHs or oral anticoagulants) are more efficacious than placebo in increasing pain‐free or maximum walking distance. (2) Anticoagulants (either heparin or LMWHs or oral anticoagulants) are more efficacious than alternative treatments in increasing pain‐free or maximum walking distance.
Methods
Criteria for considering studies for this review
Types of studies
Two types of studies were included: (1) Randomised controlled trials (RCTs), i.e. those trials with a randomised generation of allocation sequences such as the use of random number tables, computer random number generator, coin tossing. (2) Quasi‐randomised controlled trials (QRCTs), i.e. those trials with quasi‐randomised generation of allocation according to date of birth or case record number (Dickersin 1995).
Priority was given to double‐blind trials in which patients, care providers and outcome assessors were unaware of treatment allocation. Thus bias due to patient suggestion in claudication trials should be minimised (Waller 1989). For the principal analysis, a trial was regarded as double blinded if the term "double blind" was used to describe it or if it was stated that outcome assessors, care providers and patients were blinded to treatment allocation (Jadad 1996). If treatments were not allocated in a double‐blind fashion, the trials were considered eligible only in case of blinding of outcome assessors.
Types of participants
Patients with intermittent claudication (IC) Fontaine stage II (Fontaine 1954) regardless of the severity or duration of onset.
A diagnosis of IC was considered if based upon a typical history (Rose 1962). In addition, a physical examination (Criqui 1985), standardised assessment of walking distance (Siggaard 1968), abnormal decline of ankle blood pressure after exercise (Carter 1972), a decreased resting value of ankle/brachial systolic pressure ratio (Winsor 1950; Yao 1973), reactive hyperaemia (Hummel 1978), sonography or angiography were considered necessary for the diagnosis of lower limb atherosclerosis.
Trials including patients with inflammatory arteriopathy, thromboangiitis obliterans, acute ischaemia, pure neuropathic ulceration or attempted reconstruction and/or sympathectomy within the preceding three months were excluded.
Types of interventions
The following interventions were considered:
(1) all types of anticoagulant regimens versus placebo; (2) one anticoagulant drug versus another; (3) anticoagulant therapy versus an alternative treatment (such as antiplatelet agents, e.g. aspirin or haemorheological agents, e.g. pentoxifylline).
The type of therapy, dosage, target anticoagulant effect and duration of therapy were recorded. Usual treatments such as interventions targeting atherosclerosis risk factors were acceptable if applied to each group. The following anticoagulant drugs were considered; oral anticoagulants (warfarin, acenocoumarol, phenprocoumon) heparin and LMWHs.
Types of outcome measures
Primary outcomes
The two main outcome measures were:
maximum walking distance (absolute claudication distance), defined as the distance in metres, or time, walked until the patient stops as a result of claudication symptoms;
pain‐free walking distance (initial claudication distance), defined as the distance in metres, or time, walked until the onset of claudication symptoms, i.e. pain, cramps or severe fatigue.
Both distances to be assessed by treadmill exercise using pre‐specified criteria (Boyd 1949; Siggaard 1968).
Secondary outcomes
Secondary outcome measures included:
objective assessment of lower limb flow: ankle/brachial index;
proportion of patients for whom revascularisation procedure (percutaneous transluminal angioplasty or bypass surgery) was deemed necessary;
amputation‐free survival: survival rates with limb intact;
cardiovascular events: transient ischaemic attacks, stroke, unstable angina, acute myocardial infarction;
overall mortality;
proportion of patients experiencing adverse events: major and minor bleeding complications.
Search methods for identification of studies
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched May 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 4, part of The Cochrane Library, www.thecochranelibrary.com. See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
Searching other resources
Reference lists resulting from searches were scrutinised for further trial information.
Data collection and analysis
Selection of articles
Two authors (BC and EC) selected potentially eligible articles for inclusion in the review from data print‐outs. A study was considered potentially eligible if it was a prospective trial with at least two concurrent comparison groups in which patients suffering from IC were allocated to a regimen with anticoagulant drugs (as defined above). In order to assure clinical relevance of article selection, eligibility for inclusion was checked by a third author (SC). Additional information was sought when possible from the investigators of all trials that appeared to meet the inclusion criteria.
Methodological quality of trials
The methodological quality of each trial was assessed using a validated scale developed by Jadad, which includes appropriateness of randomisation and double blinding, a description of drop‐outs and withdrawals. This scale consists of three items which contribute to a maximum score of five points (two points each for randomisation and double blinding, and one point for withdrawals and drop‐outs), however, it assesses neither concealment of allocation, nor proportion and handling of drop‐outs and withdrawals. Three or more points are required for a trial to be judged as being of high quality (Jadad 1996). Quality score was also assessed according to the criteria suggested in the Cochrane Collaboration Handbook (Mulrow 1997).
Each trial was given an allocation score of A (clearly concealed), B (unclear if concealed) or C (clearly not concealed) and a summary score of A (low risk of bias), B (moderate risk of bias) or C (high risk of bias), according to the criteria indicated by the Cochrane Collaboration Handbook (Mulrow 1997). Trials scoring A were included and those scoring C were excluded. For trials scoring B, an attempt was be made to obtain more information by contacting the authors. Assessment was done by two independent authors (BC, EC) with the third author (SC) resolving any disagreement.
For each trial, the number of patients originally allocated to each treatment was extracted from the data and an intention‐to‐treat analysis was performed.
Data collection
Data were abstracted independently by two authors (BC and EC). Any discrepancies were resolved by the third author (SC). Primary authors were contacted to request additional information.
Statistical analyses
All of the analyses were based on the intention‐to‐treat data from the individual clinical trials.
Treatment effects were defined as the differences of the mean changes of pain‐free (PFWD) or maximum walking distances (MWD), from baseline to end of trial across treatment groups in metres. If time to onset of claudication symptoms or walking cessation only was provided, it was converted to walking distances in metres by multiplying the speed of the treadmill (metres per second) by the walking time (seconds). As a summary estimate of the treatment effect, the mean difference in metres (MDmetres) was calculated employing the inverse variance method, a fixed effect model described by Hald (Hald 1952), which summarises treatment effects in the same units as used in individual trials, i.e. metres. The major advantage of this is its comprehensibility, although the drawback is the underlying assumption that the outcome measures are standardised across trials, i.e. strictly speaking, individual trials must not only have the same units of measurement (metres), but also the same conditions of assessment such as intensity of walking tests, speeds and inclinations of the treadmills used. The MDmetres depends on the variance of changes of walking distances from baseline to end of trial in the individual treatment groups. If these measures were not provided, the approach by Follmann et al (Follmann 1992) was adopted and we relied mainly on the respective test‐statistics or the variances of individual baseline and end of trial walking distances. When the test statistic was not available, we computed it from the corresponding P value by using tables for the normal distribution.
To examine the effect of binary outcomes, such as mortality, odds ratios (OR) were computed using a random‐effects model.
When there was more than one assessment of the walking distance within the interval, the last one was taken for the analysis.
Funnel plots were examined (Light 1984), and possible asymmetry of the plots assessed adopting a regression approach (Egger 1997A). Although of limited power, a chi‐square‐test was used to assess heterogeneity of trials (Hedges 1985), with the significance level set at P = 0.1.
Heterogeneity between trials results was tested subjectively by clinical judgement of differences in patient populations and interventions and objectively using appropriate statistical tests. Where possible, trial results were pooled by meta‐analysis.
Agreement between authors regarding identification of potentially eligible trials and definitive article selection was determined using the kappa coefficient (Cohen 1960). Agreement regarding internal validity assessments was determined using the intra‐class correlation coefficient (Shrout 1979). Values above 0.60 were regarded as substantial (Landis 1977).
Results
Description of studies
Results of the search
See Figure 1.
1.
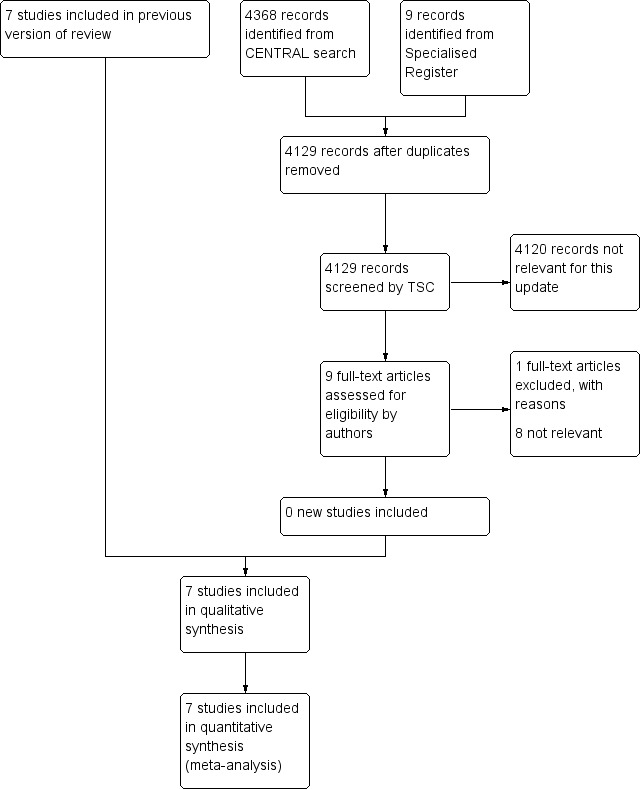
Study flow diagram
Included studies
No new studies were included for this update thus seven trials were included in the review. Two studies evaluated oral anticoagulants (de Smit 1987; Dettori [APIC] 1989); one study evaluated standard heparin (Antonicelli 1999); four studies evaluated LMWH (Calabrò 1993; Mannarino 1991; Palmieri 1988; Tesi 1989).
The studies evaluated a total of 802 participants; 201 participants (Antonicelli 1999), 36 participants (Calabrò 1993), 300 participants (de Smit 1987), 146 participants (Dettori [APIC] 1989), 44 participants (Mannarino 1991), 82 participants with only 55 evaluated (Palmieri 1988), and 20 participants (Tesi 1989).
In all trials the diagnosis of intermittent claudication (IC) was confirmed by clinical examination and objective criteria such as the ankle/brachial index (ABI) (Dettori [APIC] 1989; Tesi 1989), or ultrasound (Antonicelli 1999; de Smit 1987; Mannarino 1991; Palmieri 1988), or strain gauge plethysmography (Calabrò 1993), and/or arteriogram (Calabrò 1993; Mannarino 1991) (see 'Characteristics of included studies' table). Clinical judgement of heterogeneity indicated that the populations involved were similar with regards to age, sex and co‐morbidity, except for the study by de Smit (de Smit 1987) which included patients in Fontaine stages III and IV and the study by Palmieri (Palmieri 1988) in which the stage of disease was not specified. The baseline pain‐free walking distance (PFWD) was different among the studies, indicating a variation in severity of the disease. The treatment duration varied from a minimum of three months to a maximum of 18 months. The duration of follow up ranged from six months (Antonicelli 1999; Calabrò 1993; Mannarino 1991; Palmieri 1988; Tesi 1989) to one year (Dettori [APIC] 1989) and five years (de Smit 1987). Assessment of PFWD and maximum walking distance (MWD) was performed with walking tests of different intensities.
Excluded studies
For this update there was one additional excluded study (WAVE 2007) making a total of eight excluded studies (Allegra 1994; Andreozzi 1993; Cina 1996; Deutschinoff 1987; Montesi 1991; Serrao 1991; Simoni 1993; WAVE 2007).
Among the studies evaluating standard heparin, four studies were excluded because, although randomised, they were neither double blinded nor had a blinded outcome assessment (Allegra 1994; Andreozzi 1993; Cina 1996; Montesi 1991). Among the studies evaluating LMWH, two studies were excluded because they scored C on the Cochrane scale (Serrao 1991; Simoni 1993). Among the studies evaluating oral anticoagulants, one study was excluded because it was not appropriately randomised (Deutschinoff 1987) and one study was excluded (Wave 2007) because although randomised, it included patients with peripheral arterial disease both of the lower limbs and of other sites such as carotid or subclavian artery and it was not possible to retrieve data on the subgroup of patients with IC.
Risk of bias in included studies
Among the included studies, four studies scored B on the Cochrane scale (Calabrò 1993; Mannarino 1991; Palmieri 1988; Tesi 1989) and an attempt was made to obtain further information from the primary authors. However, no details were obtained due to the length of time since publication. These four studies were excluded from the primary analysis but included in the sensitivity analysis. Only three studies which scored A were included in the primary analysis (Antonicelli 1999; de Smit 1987; Dettori [APIC] 1989). These studies were double blinded except for one evaluating oral anticoagulants which was single blind trial but had blinded outcome assessment (Dettori [APIC] 1989).
Effects of interventions
Pain‐free walking distance
Pain‐free walking distance was evaluated in two studies (Antonicelli 1999; Dettori [APIC] 1989) with walking tests of different intensities. In the study by Dettori [APIC] 1989 the difference in walking distance could not be calculated from the data and no further information was provided by the primary author. However, this study reported a significant increase in the proportion of patients in the acenocoumarol group whose performance on the treadmill improved after one year, compared with patients taking placebo.
In the study by Antonicelli 1999, after 18 months of treatment, the PFWD increased from 196 metres to 283 metres in the group on standard heparin, and from 196 metres to 247 metres in the placebo group. The mean difference (MD) and a fixed‐effect model were used to test the significance of the results. The mean change within the two groups before and after treatment was analysed and no statistically significant difference between the two groups was found (MD 36.63 m; 95% CI ‐7.30 to + 80.56; P = 0.10).
A sensitivity analysis was attempted but it was not possible to calculate the mean change in metres before and after treatment in the lower quality studies reporting on PFWD (Calabrò 1993; Mannarino 1991; Palmieri 1988) as indicated in the 'Characteristics of included studies' table. These studies all reported a significant increase in pain‐free walking time with treatment when compared with placebo.
Maximum walking distance
Maximum walking distance was evaluated in one study (Antonicelli 1999). After 18 months of treatment, MWD increased from 266 metres to 388 metres in the group on standard heparin and from 289 metres to 342 metres in the placebo group. The mean difference and a fixed‐effect model were used to test the significance of the results. The mean change within the two groups before and after treatment was analysed and no statistically significant difference between the two groups was found (MD 66.60 m; 95% CI ‐2.30 to +135.50; P = 0.06).
A sensitivity analysis was attempted but it was not possible to calculate the mean change in metres before and after treatment in the lower quality studies reporting on maximum walking distance (Calabrò 1993; Tesi 1989) as indicated in the 'Characteristics of included studies' table. These studies reported a significant increase in MWD with treatment when compared with placebo.
Ankle/brachial index (ABI)
Ankle/brachial index (ABI) was evaluated in one study both at rest and after effort (Dettori [APIC] 1989). The mean difference and a fixed‐effect model were used to test the significance of the results. The mean difference between the two groups at the end of the treatment was analysed rather than the mean change within the two groups before and after treatment. No significant difference was observed in ABI either at rest (between the oral anticoagulant treated group and the pentoxifylline treated group) or after effort (between the oral anticoagulant treated group and the placebo treated group, or the pentoxifylline treated group). A significant difference in ABI was observed only at rest between the group treated with oral anticoagulant and the placebo group (MD 0.10; 95% CI 0.01 to 0.19; P = 0.02).
It was not possible to calculate the mean change in ABI before and after treatment in the lower quality studies reporting on ABI (Mannarino 1991; Tesi 1989) as indicated in the characteristics of included studies table. In the study by Tesi 1989, a significant increase in ABI was reported after treatment plus exercise when compared with control, while Mannarino 1991 reported no effect of treatment on ABI either at rest or after exercise, when compared with placebo.
Revascularisation, amputation‐free survival
One study (de Smit 1987) reported a significant effect of treatment on progression of peripheral and vascular disease during a five‐year follow up when compared with placebo. However, no specification of type and event rates of complications were given (e.g. cardiovascular events, revascularisation, amputation‐free survival). No other study reported data on revascularisation and amputation‐free survival.
Cardiovascular events
Cardiovascular events were reported by Dettori (Dettori [APIC] 1989) and by Antonicelli (Antonicelli 1999). Neither of the individual studies reported a significant benefit of treatment. The test for heterogeneity was not significant when the results of the studies were pooled. A non‐significant difference between the anticoagulant group and the control group was observed (pooled OR 2.22; 95% CI: 0.64 to 7.71). Among the low quality studies that reported on this outcome, no events were observed in either the treated groups or the control groups.
Mortality
Overall mortality was reported in four studies (Antonicelli 1999; de Smit 1987; Dettori [APIC] 1989; Tesi 1989). None of the individual studies reported a significant benefit of treatment. The test for heterogeneity was not significant when the results were pooled. A non‐significant difference between anticoagulants and control was observed (14/338 (4.1%) with treatment versus 6/329 (1.8%) with control; pooled OR 2.27; 95% CI 0.83 to 6.15). No events were reported by Tesi 1989, either in the treatment group or the control group.
Major and fatal bleeding events
Major bleeding events were reported in all studies. The results of studies evaluating oral anticoagulants were pooled separately from those evaluating heparin and LMWHs.
The pooled OR for major bleeding events was 11.40 (95% CI 1.45 to 89.87) in the groups treated with oral anticoagulants (event rate: 11/227 (4.8%)) compared with control (event rate: 0/219). This difference was mainly due to the effect of the study by de Smit (de Smit 1987 (1992 paper)) (OR 16.77; 95% CI 0.96 to 293) in which a higher therapeutic range (internationalised normalised ratio (INR) 2.8 to 4.8) was used than in the study by Dettori (Dettori [APIC] 1989) (INR 2.0 to 4.5) with an OR for bleeding events of 7.50 (95% CI 0.38 to 148).
No major bleeding events were reported in the studies evaluating heparin and LMWHs.
Fatal bleeding events were reported in two studies evaluating oral anticoagulants (de Smit 1987; Dettori [APIC] 1989) with a non‐significant difference between the treatment and control groups (event rate: 4/227 (1.7%) versus 0/219; pooled OR 5.00; 95% CI 0.58 to 43.22).
No fatal bleeding events were reported in the studies evaluating heparin and LMWH.
Minor bleeding events
Minor bleeding events were reported in one study evaluating oral anticoagulants (de Smit 1987) with an OR of 8.78 (95% CI: 3.34 to 23.06) for bleeding due to oral anticoagulants when compared with the control treatment. Minor bleeding events were also reported in one study evaluating heparin (Antonicelli 1999) with a non‐significant difference between the treatment and control groups (OR 5.15; 95% CI 0.24 to 108.69).
No minor bleeding events were reported in studies evaluating LMWH.
Discussion
Anticoagulant drugs such as heparin, LMWHs and oral anticoagulants may have a role in the treatment of patients with intermittent claudication (IC). Their antithrombotic action might influence the progression of disease and the acute complications of thrombosis superimposed on chronic atherosclerotic lesions.
Our review was limited by our inability to obtain further information about methodological issues and data for several studies, due to the length of time since publication of the original trial reports. Thus, the primary analysis was based on only three high quality studies. Two of these studies evaluated oral anticoagulants and one study evaluated standard heparin. All the high quality studies were placebo‐controlled randomised trials. One study evaluating fixed‐dose subcutaneous heparin was double blinded. One study evaluating oral anticoagulants was single blinded due to the necessity of adjusting oral anticoagulant dosage on the basis of international normalised ratio (INR) results (Dettori [APIC] 1989), while the other study (de Smit 1987) was declared to be double blinded but no indication of dose adjustment was found in the placebo treated group on the basis of a sham INR.
In all studies IC was clinically diagnosed and patients were similar with regard to baseline characteristics such as age, sex and co‐morbidity. However there was variation between patients' walking capacity, and thus of severity of disease, at baseline. One study evaluating oral anticoagulants also included Fontaine stage III and IV patients. The intensity of the walking test varied between studies and the baseline and follow up walking distances varied.
All studies lacked defined criteria for assessment of severity of bleeding events and for the diagnosis of cardiovascular events such as stroke, unstable angina and myocardial infarction.
The effect of anticoagulants on pain‐free and maximum walking distance was properly evaluated in only two studies (Antonicelli 1999; Dettori [APIC] 1989), but we could not extract or obtain data from the study evaluating oral anticoagulants (Dettori [APIC] 1989). No significant difference was observed between heparin treatment and control groups for either pain‐free walking distance or maximum walking distance at the end of treatment.
No study reported on revascularisation or amputation‐free survival rates. No study reported a significant effect on either overall mortality or cardiovascular events. The pooled odds ratios were not significant for either outcome.
We analysed data on bleeding events separately for oral anticoagulants and heparin and LMWHs. Major and minor bleeding events were significantly more frequent in the groups treated with oral anticoagulants when compared with the control groups, with a non‐significant increase in fatal bleeding events. This could be attributed to the high therapeutic range used in one of the studies (de Smit 1987) (INR 2.8 to 4.8). Neither major nor minor bleeding events were reported in the studies evaluating LMWHs (Calabrò 1993; Mannarino 1991; Palmieri 1988; Tesi 1989). No major bleeding events were reported in the study evaluating heparin, but a non‐significant increase in minor bleeding events was reported.
The WAVE study (Wave 2007), which we could not include in the review, randomised patients with peripheral arterial disease (PAD) to combination therapy with an antiplatelet agent and an oral anticoagulant agent (INR 2.0 to 3.0) or to antiplatelet therapy alone. This study showed that the composite primary outcome of myocardial infarction, stroke, or death from cardiovascular causes occurred in 132 of 1080 patients receiving combination therapy (12.2%) and in 144 of 1081 patients receiving antiplatelet therapy alone (13.3%) (relative risk (RR), 0.92; 95% confidence interval (CI) 0.73 to 1.16; P = 0.48), while the secondary outcome myocardial infarction, stroke, severe ischaemia, or death from cardiovascular causes occurred in 172 patients receiving combination therapy (15.9%) as compared with 188 patients receiving antiplatelet therapy alone (17.4%) (RR 0.91; 95% CI 0.74 to 1.12; P = 0.37). Life‐threatening bleeding occurred in 43 patients receiving combination therapy (4.0%) as compared with 13 patients receiving antiplatelet therapy alone (1.2%) (RR 3.41; 95% CI 1.84 to 6.35; P < 0.001).
A meta‐analysis on the role of anti‐thrombotic drugs in the medical management of intermittent claudication has been published (Girolami 1999). The authors' conclusions were similar to ours. A clear benefit of anticoagulants for IC could not be established due to the low methodological quality of the available studies. The authors of the Wave 2007 study also conducted a meta‐analysis of nine trials involving 4889 patients with PAD in several stages also including IC evaluating oral anticoagulants (WAVE 2006). Oral anticoagulants may reduce mortality and graft occlusion but increase major bleeding compared with no treatment. Compared with aspirin, oral anticoagulants do not appear to reduce mortality (odds ratio (OR) 1.04, 95% CI 0.55 to 1.29), although the CI are wide, or graft occlusion (OR 0.91, 95% CI 0.77 to 1.06), and major bleeding is increased (OR 1.96, 95% CI 1.43 to 2.69). Compared with aspirin, oral anticoagulants used together with aspirin appears to increase mortality (OR 1.57, 95% CI 1.16 to 2.12); may reduce graft occlusion (OR 0.84, 95% CI 0.62 to 1.12), and major bleeding is increased (OR 2.13, 95% CI 1.27 to 3.57).
Authors' conclusions
Implications for practice.
The benefit of heparin, low molecular weight heparins and oral anticoagulants for treatment of intermittent claudication has not been established, while an increased risk of major bleeding events has been observed, especially with oral anticoagulants. There is no clear evidence to support the use of anticoagulants for intermittent claudication at this stage.
Implications for research.
Further trials of anticoagulants for intermittent claudication are required to determine their effectiveness. These trials should be methodologically adequate in terms of sample sizes, drug dosages, duration of follow up, and clinically relevant outcomes such as rate of cardiovascular events, amputation‐free survival, revascularisation procedures and mortality. The evaluation of functional improvements should be based on mean changes in pain‐free and maximum walking distances. Bleeding events should also be assessed on the basis of standardised criteria.
Feedback
Anticoagulant feedback
Summary
Feedback received on this review, and other reviews and protocols on anticoagulants, is available on the Cochrane Editorial Unit website at http://www.editorial‐unit.cochrane.org/anticoagulants‐feedback.
What's new
| Date | Event | Description |
|---|---|---|
| 20 March 2014 | New search has been performed | New searches carried out. No new included studies identified. One new study excluded. |
| 20 March 2014 | New citation required but conclusions have not changed | New searches carried out. No new included studies identified. One new study excluded. Minor copy edit changes made. No change to conclusions. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 14 February 2011 | Amended | Link to anticoagulant feedback added |
| 11 September 2008 | Amended | Converted to new review format. |
| 23 February 2005 | New search has been performed | Review updated without change. Searches re‐run and no new trials found. |
| 14 May 2003 | New search has been performed | One additional excluded study added. There are no changes to the conclusions of the review. |
Acknowledgements
The Cochrane Peripheral Vascular Diseases Review Group assisted with the literature search for this review.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Arteriosclerosis] this term only | 894 |
| #2 | MeSH descriptor: [Arteriolosclerosis] this term only | 0 |
| #3 | MeSH descriptor: [Arteriosclerosis Obliterans] this term only | 72 |
| #4 | MeSH descriptor: [Atherosclerosis] this term only | 407 |
| #5 | MeSH descriptor: [Arterial Occlusive Diseases] this term only | 766 |
| #6 | MeSH descriptor: [Intermittent Claudication] this term only | 720 |
| #7 | MeSH descriptor: [Ischemia] this term only | 764 |
| #8 | MeSH descriptor: [Peripheral Vascular Diseases] this term only | 554 |
| #9 | atherosclero* or arteriosclero* or PVD or PAOD or PAD | 17383 |
| #10 | (arter* or vascular or vein* or veno* or peripher*) near (occlus* or steno* or obstruct* or lesio* or block*) | 7355 |
| #11 | peripheral near/3 dis* | 3299 |
| #12 | (claudic* or hinken*) | 1451 |
| #13 | isch* or CLI | 17025 |
| #14 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 | 38493 |
| #15 | MeSH descriptor: [Anticoagulants] this term only | 3326 |
| #16 | MeSH descriptor: [Coumarins] explode all trees | 1537 |
| #17 | ((vitamin k or vit k) near/3 antagon*) | 225 |
| #18 | VKA | 54 |
| #19 | anticoagula* | 6117 |
| #20 | anti‐coagula* | 162 |
| #21 | warfarin* | 2179 |
| #22 | *coum* | 853 |
| #23 | Jantoven or Marevan or Lawarin or Waran or Warfant or Dindevan | 21 |
| #24 | phenindione | 50 |
| #25 | Sinthrome or Sintrom | 15 |
| #26 | Marcumar or Marcoumar or Falithrom | 26 |
| #27 | aldocumar or tedicumar | 7 |
| #28 | Rivaroxaban | 134 |
| #29 | BAY 59‐7939 | 25 |
| #30 | BAY 597939 | 1 |
| #31 | Dabigatran | 125 |
| #32 | Pradax* | 10 |
| #33 | Prazax* | 0 |
| #34 | apixaban | 70 |
| #35 | Ximelagatran or Exanta or Exarta or H 376/95 | 185 |
| #36 | AZD0837 | 10 |
| #37 | TTP889 | 2 |
| #38 | odiparcil | 6 |
| #39 | LY517717 | 9 |
| #40 | YM150 | 20 |
| #41 | DU‐176b | 13 |
| #42 | edoxaban or otamixaban | 33 |
| #43 | betrixaban | 12 |
| #44 | #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 | 7740 |
| #45 | MeSH descriptor: [Heparin] explode all trees | 3959 |
| #46 | hepar* or UFH | 7907 |
| #47 | calciparin* Ariven or Arteven or Calcilean or Certoparin or Depo‐Heparin or Eparina or Hed‐Heparin or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Multiparin or Novoheparin or Pabyrin or Parvoparin or Pularin or Thromboliquine or Vetren | 143 |
| #48 | LMWH or LMH | 850 |
| #49 | nadroparin* | 279 |
| #50 | fraxiparin* | 136 |
| #51 | enoxaparin | 1193 |
| #52 | Clexane or klexane or lovenox | 81 |
| #53 | dalteparin or Fragmin or ardeparin | 592 |
| #54 | normiflo or tinzaparin or logiparin | 231 |
| #55 | Innohep or certoparin* | 107 |
| #56 | sandoparin* | 31 |
| #57 | reviparin* | 110 |
| #58 | clivarin* | 50 |
| #59 | danaproid or danaparoid | 62 |
| #60 | antixarin or ardeparin* or bemiparin* | 64 |
| #61 | Zibor or cy 222 or embolex or monoembolex | 74 |
| #62 | parnaparin* or rd 11885 or RD1185 | 41 |
| #63 | tedelparin or Kabi‐2165 or Kabi 2165 | 68 |
| #64 | emt‐966 or emt‐967 or pk‐10 169 or pk‐10169 or pk10169 | 19 |
| #65 | fr‐860 or cy‐216 or cy216 | 80 |
| #66 | seleparin* or tedegliparin or seleparin* or tedegliparin* | 12 |
| #67 | wy90493 or "wy 90493" | 9 |
| #68 | kb101 or lomoparan or orgaran | 50 |
| #69 | fluxum or lohepa or lowhepa | 19 |
| #70 | op 2123 or parvoparin | 13 |
| #71 | ave 5026 or ave5026 | 12 |
| #72 | M118 or RO‐1 | 34 |
| #73 | #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 | 8584 |
| #74 | #44 or #73 | 13276 |
| #75 | #14 and #74 in Trials | 4368 |
Data and analyses
Comparison 1. Pain‐free walking distance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free walking distance in all studies | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1 Pain‐free walking distance, Outcome 1 Pain‐free walking distance in all studies.
Comparison 2. Maximum walking distance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum walking distance in all studies | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.

Comparison 2 Maximum walking distance, Outcome 1 Maximum walking distance in all studies.
Comparison 3. Ankle/brachial pressure index (ABI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ABI at rest: sintrom versus pentoxifylline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 ABI after effort: sintrom versus placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 ABI at rest: sintrom versus placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 ABI after effort: sintrom versus pentoxifylline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.

Comparison 3 Ankle/brachial pressure index (ABI), Outcome 1 ABI at rest: sintrom versus pentoxifylline.
3.2. Analysis.

Comparison 3 Ankle/brachial pressure index (ABI), Outcome 2 ABI after effort: sintrom versus placebo.
3.3. Analysis.

Comparison 3 Ankle/brachial pressure index (ABI), Outcome 3 ABI at rest: sintrom versus placebo.
3.4. Analysis.

Comparison 3 Ankle/brachial pressure index (ABI), Outcome 4 ABI after effort: sintrom versus pentoxifylline.
Comparison 4. Revascularisation and amputation‐free survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease progression at five years (peripheral disease) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
4.1. Analysis.
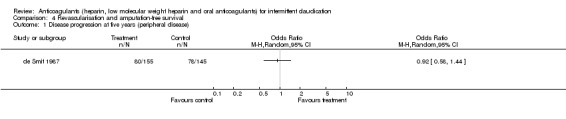
Comparison 4 Revascularisation and amputation‐free survival, Outcome 1 Disease progression at five years (peripheral disease).
Comparison 5. Cardiovascular events (TIA, stroke, UA, AMI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular events | 3 | 367 | Odds Ratio (M‐H, Random, 95% CI) | 2.22 [0.64, 7.71] |
| 2 Progression of vascular disease (events at five years) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
5.1. Analysis.
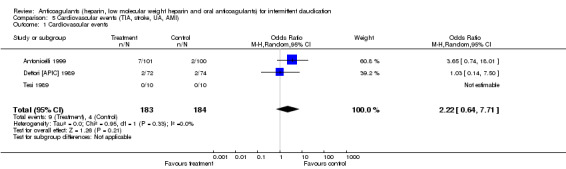
Comparison 5 Cardiovascular events (TIA, stroke, UA, AMI), Outcome 1 Cardiovascular events.
5.2. Analysis.
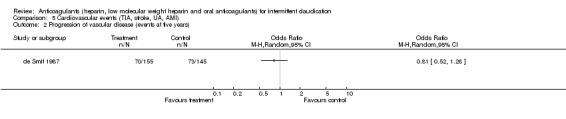
Comparison 5 Cardiovascular events (TIA, stroke, UA, AMI), Outcome 2 Progression of vascular disease (events at five years).
Comparison 6. Overall mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall mortality | 4 | 667 | Odds Ratio (M‐H, Random, 95% CI) | 2.27 [0.83, 6.15] |
6.1. Analysis.
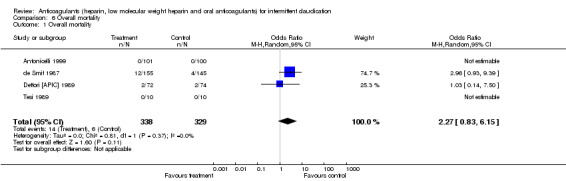
Comparison 6 Overall mortality, Outcome 1 Overall mortality.
Comparison 7. Major bleeding events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major bleeding events in all studies | 7 | 802 | Odds Ratio (M‐H, Random, 95% CI) | 11.40 [1.45, 89.86] |
| 2 Major bleeding events (oral anticoagulants) | 2 | 446 | Odds Ratio (M‐H, Random, 95% CI) | 11.40 [1.45, 89.86] |
7.1. Analysis.
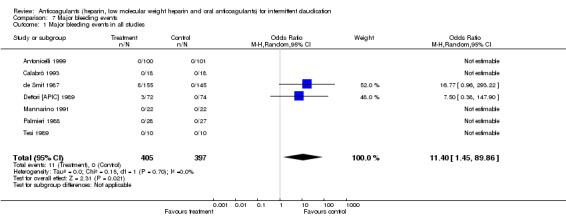
Comparison 7 Major bleeding events, Outcome 1 Major bleeding events in all studies.
7.2. Analysis.
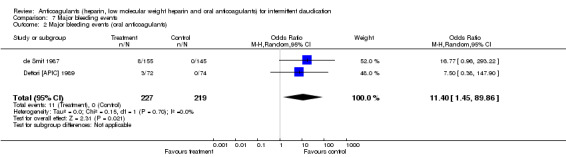
Comparison 7 Major bleeding events, Outcome 2 Major bleeding events (oral anticoagulants).
Comparison 8. Minor bleeding events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Minor bleeding events in all studies | 6 | 656 | Odds Ratio (M‐H, Random, 95% CI) | 8.36 [3.33, 20.99] |
| 2 Minor bleeding events (oral anticoagulants) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Minor bleeding events (standard heparin) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
8.1. Analysis.
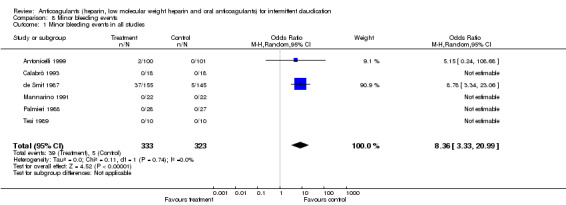
Comparison 8 Minor bleeding events, Outcome 1 Minor bleeding events in all studies.
8.2. Analysis.
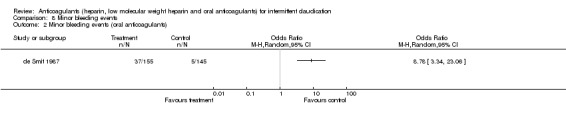
Comparison 8 Minor bleeding events, Outcome 2 Minor bleeding events (oral anticoagulants).
8.3. Analysis.

Comparison 8 Minor bleeding events, Outcome 3 Minor bleeding events (standard heparin).
Comparison 9. Fatal bleeding events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fatal bleeding events in all studies | 7 | 802 | Odds Ratio (M‐H, Random, 95% CI) | 5.00 [0.58, 43.22] |
| 2 Fatal bleeding events (oral anticoagulants) | 2 | 446 | Odds Ratio (M‐H, Random, 95% CI) | 5.00 [0.58, 43.22] |
9.1. Analysis.
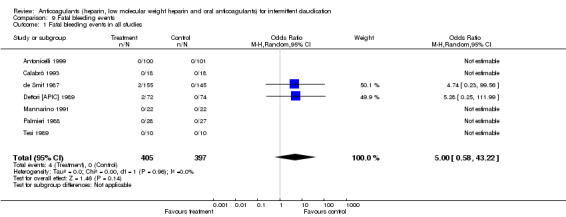
Comparison 9 Fatal bleeding events, Outcome 1 Fatal bleeding events in all studies.
9.2. Analysis.
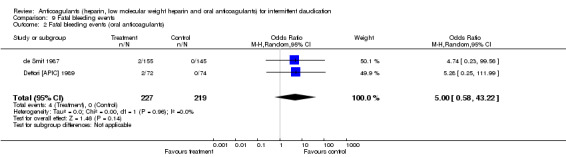
Comparison 9 Fatal bleeding events, Outcome 2 Fatal bleeding events (oral anticoagulants).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antonicelli 1999.
| Methods | Double‐blind, placebo‐controlled randomised trial with adequate sequence generation and adequate double‐blinding. Run‐in period: two weeks. Drop‐outs and study withdrawals: 65/201. Intention‐to‐treat: yes. Characteristics of study sample: no significant difference between the two groups at baseline. | |
| Participants | Country: Italy, 201 participants. Age: mean 67 years; males: 160; females: 41; diabetes mellitus: 69. Inclusion criteria: six month history of IC with mean walking distance equal or < 500 m with < 20% variability of walking test; PAOD confirmed by Doppler examination. Exclusion criteria: unstable PAOD, indication for surgery, any clinical condition limiting exercise, active peptic ulcer, untreatable or uncontrolled blood pressure, AMI or stroke within previous six months, any haemorrhagic condition, renal or hepatic failure, cancer, or auto‐immune disease. | |
| Interventions | Treatment group: 101 participants: calcium heparin 12,500 U s.c. o.d. + ASA 50 mg o.d.. Control group: 100 participants: placebo (provided in prefilled sterile syringes identical to treatment) + ASA 50 mg o.d.. Duration: three months, with a six month follow‐up (with ASA alone). Compliance: not evaluated. | |
| Outcomes | PFWD, MWD, , cardiovascular events (TIA, stroke, UA, AMI), overall mortality, bleeding events. | |
| Notes | Reasons for study withdrawals: 16 participants (8 in each group) for poor compliance; 21 participants (9 in treatment group, 12 in control group) for concurrent disease; 9 participants (5 in treatment group, 4 in placebo) for poor tolerability; 3 participants (1 in treatment group, 2 in placebo) for lack of efficacy; 5 participants (3 in treatment group, 2 in placebo group) for loss to follow‐up; 11 participants (5 in treatment group, 6 in placebo group) for reasons unrelated to study drug. Intensity of walking test: 2.6 km/h in the first minute and 4 km/h thereafter with 0% slope. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Calabrò 1993.
| Methods | Double‐blind, placebo‐controlled randomised trial, allocation concealment unclear, method of random sequence generation not stated. Run‐in period: none. Drop‐outs and study withdrawals: none. Intention‐to‐treat: yes. Characteristics of study sample: no significant difference between the two groups at baseline. | |
| Participants | Country: Italy, 36 participants (all male). Age: mean 64 years; diabetes mellitus: 11. Inclusion criteria: stage II PAOD (clinical, plethysmographic and/or angiographic diagnostic criteria). Exclusion criteria: severe liver failure, renal insufficiency, cancer, haemorrhagic diathesis, stroke in previous six months, patients taking heparin or haemorheological agents or fibrinolytic drugs of any kind, nicotinic acid derivatives or hypolipidemic drugs. | |
| Interventions | Treatment group: 18 participants: LMWH 15,000 U s.c. o.d. Control group: 18 participants: placebo (provided in prefilled sterile syringes identical to treatment) s.c. o.d. Duration: six months. Compliance: not evaluated. | |
| Outcomes | PFWD, MWD, ABI, major and minor bleeding events. | |
| Notes | Bar graphs only provided for PFWD and MWD. Intensity of walking test not stated. No further data provided by author due to length of time since publication. Significant increase in MWD reported in treatment group at end of treatment but no comparison with placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
de Smit 1987.
| Methods | Double‐blind, placebo‐controlled randomised trial with adequate double‐blinding. Method of random sequence generation not stated. Run‐in period: none. Drop‐outs and study withdrawals: four. Intention‐to‐treat: no. Characteristics of study sample: no significant difference between the two groups at baseline. | |
| Participants | Country: The Netherlands, 300 participants. Age: mean 59 years; males: 241; females: 59; diabetes mellitus: 20; smokers: 269; stage III and IV PAOD in 7% of included patients. Inclusion criteria: IC or aorto‐iliac surgery, PAOD confirmed by Doppler/exercise test. Exclusion criteria: age > 70 years; contraindication to oral anticoagulants; femoro‐popliteal, femoro‐crural, distal or extra‐anatomic bypass. | |
| Interventions | Treatment group: 155 participants: phenprocoumon (Marcumar), target INR range 2.8 to 4.8. Control group: 145 participants: placebo (indistinguishable tablets). Duration: five years. Compliance: not evaluated. | |
| Outcomes | Disease progression (Doppler exercise test), overall mortality and bleeding events. | |
| Notes | Significant difference in progression of peripheral and vascular disease reported between treatment and control. No specification of type of complications (e.g. cardiovascular events, revascularisation, amputation‐free survival) reported. No indication of adjustment of placebo on the basis of sham INR reported. Intensity of walking test not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Dettori [APIC] 1989.
| Methods | Single‐blind, placebo‐controlled randomised trial with blinded outcome assessment. Four treatment arms: sintrom, placebo, pentoxifylline, pentoxifylline + sintrom. Run‐in period: none. Drop‐outs and study withdrawals: 28. Intention‐to‐treat: no. Characteristics of study sample: no significant difference between groups at baseline. | |
| Participants | Country: Italy: 146 participants: Age: range of mean 58 to 62 years; males: 134; females: 12; diabetes mellitus: 21. Inclusion criteria: 12‐month history of IC with ABI < 0.90. Exclusion criteria: age > 75, effort angina or other diseases interfering with walking capacity, rest pain, ischaemic ulcers, gangrene, indication for surgery, previous vascular surgery, diseases necessitating oral anticoagulation or contra‐indication to oral anticoagulation, non‐atherosclerotic causes of IC, any clinical condition limiting exercise. | |
| Interventions | Treatment group (A): 36 patients: acenocoumarol (Sintrom) + placebo, target INR range 2.0 to 4.5. Treatment group (B): 36 patients: Sintrom + pentoxifylline 400 mg t.i.d. Control group (A): 37 patients: placebo (indistinguishable from Control group (B) tablets). Control group (B): 37 patients: pentoxifylline 400 mg t.i.d. Duration: one year. Compliance: evaluated (90%). | |
| Outcomes | PFWD, ABI, cardiovascular events (TIA, stroke, UA, AMI), overall mortality, bleeding events. | |
| Notes | PFWD expressed as geometric mean and range. No data provided by authors on pain‐free walking distance due to length of time since publication. Improvement > 25% of baseline significant in pentoxifylline and sintrom group. Reasons for withdrawal: 11 participants for negative end‐points of trial; 8 participants for medical problems unrelated to study medication; 4 participants for intolerance to pentoxifylline; 5 participants for refusal to attend regularly. Intensity of walking test: 3 km/h elevation 10%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Mannarino 1991.
| Methods | Double‐blind, placebo‐controlled randomised trial, allocation concealment unclear, method of random sequence generation not stated. Run‐in period: none. Drop‐outs and study withdrawals: yes (2/44). Intention‐to‐treat: no. Not stated whether groups comparable at baseline. | |
| Participants | Country: Italy, 44 participants. Age: mean 66 years; males: 37; females: 7; co‐morbidity not stated. Inclusion criteria: stage II PAOD diagnosed on basis of clinical examination and Doppler velocimetry, and confirmed by angiography. Exclusion criteria: cardiac and lung failure; major liver, kidney or metabolic disease; infections and cancer; patients taking haemorheological or anticoagulant drugs; patients with contraindication to heparin therapy. | |
| Interventions | Treatment group: 22 participants: LMWH 15,000 U o.d. s.c. Control group: 22 participants: placebo in prefilled syringes. Duration: six months. Compliance: not evaluated. | |
| Outcomes | PFWD, ABI, major and minor bleeding events. | |
| Notes | Variation measure (SD?, SEM?) not specified for PFWD or ABI. No data provided by primary author due to length of time since publication. PFWD significantly improved in treatment group, but no comparison with placebo. Intensity of walking test: 12° slope/5 min/ 2 km /h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Palmieri 1988.
| Methods | Double‐blind, placebo‐controlled randomised trial, allocation concealment unclear, method of random sequence generation not stated. Run‐in period: none. Drop‐outs and study withdrawals: yes (27/82 for reasons not related to treatment but not stated). Intention‐to‐treat: no. Baseline characteristics of two groups not compared. | |
| Participants | Country: Italy, 82 participants: Age: mean 67 years; males: 52; females: 30. Inclusion criteria: PAOD (no stage defined) diagnosed on the basis of clinical examination and Doppler sonography. Exclusion criteria: renal or hepatic insufficiency, congestive heart failure, stage III hypertension, diabetes mellitus, thromboangiitis obliterans, Moenckeberg calcific arteriopathy, arteritis of non‐chronic degenerative aetiology, phlebopathy of the lower limbs. | |
| Interventions | Treatment group: 40 participants: LMWH 8,000 U s.c. o.d. Control: 42 participants: placebo (appearance not stated). Duration: six months. Compliance: not evaluated. | |
| Outcomes | PFWD, ABI, major and minor bleeding events. | |
| Notes | Bar graphs only provided for PFWD. No data provided by primary author. No comparison of change in walking distance between treatment group and control group. Intensity of walking test: 2 mph at 3 min interval. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Tesi 1989.
| Methods | Double‐blind, placebo‐controlled randomised trial, allocation concealment unclear, method of random sequence generation not stated. Run‐in period: none. Drop‐outs and study withdrawals: no. Intention‐to‐treat: yes. Baseline characteristics comparable between two groups. | |
| Participants | Country: Italy, 20 participants: Age: range 44 to 70 years; males: 11; females: 9; co‐morbidity not stated. Inclusion criteria: PAOD and IC diagnosed by clinical examination and ABI. Exclusion criteria: obesity, smoking, previous arterial surgery, AMI, angina pectoris. | |
| Interventions | Treatment group: 10 participants: LMWH 8,000 U s.c. o.d. Control group: 10 participants: placebo sc od (indistinguishable from treatment). Duration: six months. Compliance: evaluated. | |
| Outcomes | MWD, ABI, thrombotic complications, major and minor bleeding events. | |
| Notes | Bar graphs only provided for MWD. No data provided by primary author. Intensity of walking test not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Abbreviations: ABI: ankle/brachial index AMI: acute myocardial infarction ASA: acetylsalicylic acid IC: intermittent claudication INR: international normalised ratio LMWH: low molecular weight heparin MWD: maximum walking distance o.d.: once daily PAOD: peripheral arterial obstructive disease PFWD: pain free walking distance s.c.: subcutaneously SD: standard deviation SEM: standard error of the mean TIA: transient ischaemic attack t.i.d.: tris in diem (three times daily) UA: unstable angina
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allegra 1994 | No allocation concealment, no double‐blinding, score C. |
| Andreozzi 1993 | No double‐blinding, no appropriate randomisation. |
| Cina 1996 | No double‐blinding, no appropriate randomisation. |
| Deutschinoff 1987 | No appropriate randomisation. |
| Montesi 1991 | No double‐blinding, no appropriate randomisation, unblinded outcome assessment. |
| Serrao 1991 | No appropriate randomisation, non‐appropriate double‐blinding, allocation clearly unconcealed, method of random sequence generation not stated. |
| Simoni 1993 | No appropriate randomisation, no double‐blinding, unblinded outcome assessment. |
| WAVE 2007 | Data on the subgroup of patients with intermittent claudication were not available. |
Contributions of authors
Benilde Cosmi: selected potentially eligible articles, assessed trial quality, extracted data, updated review. Eleonora Conti: selected potentially eligible articles, assessed trial quality and extracted data. Sergio Coccheri: determined trial eligibility and resolved disagreements pertaining to eligibility, quality of trials and data extraction.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office
-
National Institute for Health Research (NIHR), UK.
The PVD Group editorial base is supported by a programme grant from the NIHR
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Antonicelli 1999 {published data only}
- Antonicelli R, Sardina M, Scotti A, Bonizzoni E, Paciaroni E on behalf of the CAP study group. Randomized trial of the effects of low‐dose calcium‐heparin in patients with peripheral arterial disease and claudication. American Journal of Medicine 1999;107(3):234‐9. [DOI] [PubMed] [Google Scholar]
Calabrò 1993 {published data only}
- Calabrò A, Piarulli F, Milan D, Rossi A, Coscetti G, Crepaldi G. Clinical assessment of low molecular weight heparin effects in peripheral vascular disease. Angiology 1993;44(3):188‐95. [DOI] [PubMed] [Google Scholar]
de Smit 1987 {published data only}
- Smit P, Urk H. Dutch oral anticoagulant trial. Acta Chir Austriaca 1992;24:5‐7. [Google Scholar]
- Smit P, Urk H. The effect of long term treatment with oral anticoagulants in patients with peripheral vascular disease. Arterielle Verschlusskrankheit and blutgerinnung. Hamburg, Germany: Roche, 1987; Vol. 122 Hamburger Symposion uber Blutgerinnung:211‐7.
Dettori [APIC] 1989 {published data only}
- Dettori AG, Pini M, Moratti A, Paolicelli M, Basevi P, Quintavalla R, et al. Acenocoumarol and pentoxifylline in intermittent claudication. A controlled clinical study. Angiology 1989;40(4 part I):237‐48. [PubMed] [Google Scholar]
Mannarino 1991 {published data only}
- Mannarino E, Pasqualini L, Innocente S, Orlandi U, Scricciolo V, Lombardini R, et al. Efficacy of low‐molecular weight heparin in the management of intermittent claudication. Angiology 1991;42:1‐7. [DOI] [PubMed] [Google Scholar]
Palmieri 1988 {published data only}
- Palmieri G, Ambrosi G, Agrati AM, Ferraro G, Marcozzi S. A new low molecular weight heparin in the treatment of peripheral arterial disease. International Angiology 1988;7(3 Suppl):41‐7. [PubMed] [Google Scholar]
Tesi 1989 {published data only}
- Tesi M, Bronchi GF, Carini A, Morfini M, Cinotti S, Filiberti E. Efficacy and safety of a new low molecular weight heparin in the medium‐term treatment of atherosclerotic arteriopathy of the lower limbs. Journal of Drug Development 1989;2(2):73‐82. [Google Scholar]
References to studies excluded from this review
Allegra 1994 {published data only}
- Allegra C, Carlizza A, Sardina M. Long term effects of low‐dose calcium‐heparin versus ASA in patient with peripheral arterial occlusive disease at lib Leriche Fontaine stage. Thrombosis and Haemostasis 1993;69(6):653 ‐ Abstract no 401. [Google Scholar]
- Allegra C, Pollari G, Carioti B, Sardina M. Thrombin and platelet inhibition with low‐dose calcium heparin in comparison with ASA in patients with peripheral arterial occlusive disease at Leriche‐Fontaine IIb class. International Journal of Clinical Pharmacology and Therapeutics 1994;32(12):654‐61. [PubMed] [Google Scholar]
Andreozzi 1993 {published data only}
- Andreozzi GM, Signorelli SS, Cacciaguerra G, Pino L, Martini R, Monaco S. Three‐month therapy with calcium‐heparin in comparison with ticlopidine in patients with peripheral arterial occlusive disease at Leriche‐Fontaine IIb class. Angiology 1993;44:307‐13. [DOI] [PubMed] [Google Scholar]
- Cacciaguerra G, Buttò G, Monaco S, Garagozzo G, Pasquale R, Zappalà D, et al. Effects of calcium heparin on walking performance in Leriche and Fontaine stage IIb peripheral obstructive arterial disease [Effetti dell'eparina calcica sulla performance deambulatoria di arteriopatici periferici allo stadio IIb di Leriche e Fontaine]. Minerva Angiologica 1991;16:71‐4. [Google Scholar]
Cina 1996 {published data only}
- Cina G, Vernich M, Campisi C, Cascone C, Ofria F, Leopardi N, et al. Physical training and low‐dose calcium heparin in patients suffering from chronic obliterating arteriopathy of the lower limbs with intermittent claudication [Training fisico ed eparina calcica a basse dosi in pazineti affetti da arteriopatia obliterante cronica degli arti inferiori con claudicatio intermittens]. Minerva Cardioangiologia 1996;44:179‐85. [PubMed] [Google Scholar]
Deutschinoff 1987 {published data only}
- Deutschinoff A, Grozdinsky L. Rheological and anticoagulant therapy of patients with chronic peripheral occlusive arterial disease (COAD). Angiology 1987;38(5):351‐8. [DOI] [PubMed] [Google Scholar]
Montesi 1991 {published data only}
- Montesi G, Arosio E, Zannoni M, Pancera P, Priante F, Ribul M, et al. Efficacy of calcium heparin and ASA on long‐term therapy of subjects with peripheral obstructive arterial disease [Efficacia di eparina calcica ed ASA nella terapia a lungo termine di soggetti affetti da arteriopatia obliterante degli arti inferiori]. Minerva Cardioangiologica 1991;16(2 suppl 1):148‐50. [Google Scholar]
Serrao 1991 {published data only}
- Serrao E, Mangialardi N. Treatment of peripheral arteriopathies with a new low weight heparin. Results of a double blind, controlled study. Panminerva Medica 1991;33:197‐204. [PubMed] [Google Scholar]
Simoni 1993 {published data only}
- Simoni G, Lucertini G, Decian F. Low molecular weight heparins: therapeutic insight in peripheral arterial occlusive disease. Clinical Trials and Meta‐Analysis 1993;28(3):137‐45. [Google Scholar]
WAVE 2007 {published data only}
- Warfarin Antiplatelet Vascular Evaluation Trial Investigators, Anand S, Yusuf S, Xie C, Pogue J, Eikelboom J, et al. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. New England Journal of Medicine 2007;357(3):217‐27. [NCT00125671] [DOI] [PubMed] [Google Scholar]
Additional references
Balkau 1994
- Balkau B, Vray M, Eschwege E. Epidemiology of peripheral arterial disease. Journal of Cardiovascular Pharmacology 1994;23 Suppl 3:S8‐16. [PubMed] [Google Scholar]
Boyd 1949
- Boyd AM, Hall Ratcliffe A, Jepson RP, James GWH. Intermittent claudication. A clinical study. Journal of Bone and Joint Surg British Volume 1949;31:325‐55. [PubMed] [Google Scholar]
Carter 1972
- Carter SA. Response of ankle systolic pressure to leg exercise in mild or questionable arterial disease. New England Journal of Medicine 1972;287(12):578‐82. [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Criqui 1985
- Criqui MH, Froneck A, Klauber MR, Barret‐Connor E, Gabriel S. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation 1985;71(3):516‐22. [DOI] [PubMed] [Google Scholar]
Dickersin 1995
- Dickersin K, Larson K. Establishing and maintaining an international register of RCTs. In: Sackett D, Oxman A editor(s). Cochrane Collaboration Handbook [updated 14 July 1995]. The Cochrane Library, Issue 3, 1996. Oxford: Update Software, 1996. [Google Scholar]
Egger 1997A
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769‐73. [DOI] [PubMed] [Google Scholar]
Fontaine 1954
- Fontaine VR, Kim M, Kieny R. Surgical treatment for peripheral vascular disease [Die chirurgische Behandelung der peripheren Durchblutungstorungen]. Helvetica Chirurgica Acta 1954;21:499‐533. [PubMed] [Google Scholar]
Fuster 1992
- Fuster V, Badimon L, Badimon JJ, Chesebro J. The pathogenesis of coronary artery disease and the acute coronary syndromes. New England Journal of Medicine 1992;326(5):310‐8. [DOI] [PubMed] [Google Scholar]
Girolami 1999
- Girolami B, Bernardi E, Prins MH, Cate JW, Prandoni P, Hettiarachchi R, et al. Antithrombotic drugs in the primary medical management of intermittent claudication: a meta‐analysis. Thrombosis and Haemostasis 1999;81(5):715‐22. [PubMed] [Google Scholar]
Hald 1952
- Hald A. The distribution of the mean. Statistical theory with engineering applications. New York: John Wiley, 1952:214‐52. [Google Scholar]
Hedges 1985
- Hedges LV, Olkin I. Statistical methods for meta‐analysis. Boston: Academic Press, 1985:153‐156. [Google Scholar]
Hummel 1978
- Hummel BW, Hummel BA, Mowbry A, Maixner W, Barnes RW. Reactive hyperemia vs treadmill exercise testing in arterial disease. Archives of Surgery 1978;113:95‐8. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Kretschmer 1992
- Kretschmer G, Herbst F, Prager M, Sautner T, Wenzl E, Berlakovich GA, et al. A decade of oral anticoagulant treatment to maintain autologous vein grafts for femoropopliteal atherosclerosis. Archives of Surgery 1992;127(9):1112‐5. [DOI] [PubMed] [Google Scholar]
Landis 1977
- Landis RJ, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159‐74. [PubMed] [Google Scholar]
Light 1984
- Light RJ, Pillemer DB. Summing up. The science of reviewing research. Cambridge: Harvard University Press, 1984:63‐72. [Google Scholar]
Mulrow 1997
- Mulrow CD, Oxman AD, editors. Cochrane Reviewers' Handbook [updated September 1997]. In: The Cochrane Library [database on CDROM]. The Cochrane Collaboration. Oxford: Update Software; 1997, issue 4.
Rose 1962
- Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bulletin of the World Health Organisation 1962;27:645‐58. [PMC free article] [PubMed] [Google Scholar]
Shrout 1979
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychology Bulletin 1979;86:420‐8. [DOI] [PubMed] [Google Scholar]
Siggaard 1968
- Siggaard‐Andersen J, Petersen FB. Intermittent claudication. A comparison between subjective and measured claudication walking distance. Angiology 1968;19(7):426‐34. [DOI] [PubMed] [Google Scholar]
Waller 1989
- Waller PC, Solomon SA, Ramsay LE. The acute effects of cigarette smoking on treadmill exercise distances in patients with stable intermittent claudication. Angiology 1989;40(3):164‐9. [DOI] [PubMed] [Google Scholar]
WAVE 2006
- The WAVE Investigators. The effects of oral anticoagulants in patients with peripheral arterial disease: rationale, design, and baseline characteristics of the Warfarin and Antiplatelet Vascular Evaluation (WAVE) trial, including a meta‐analysis of trials. American Heart Journal 2006;151(1):1‐9. [DOI] [PubMed] [Google Scholar]
Winsor 1950
- Winsor T. Influence of arterial disease on the systolic blood pressure gradients of the extremity. American Journal of Medical Science 1950;220:117‐26. [DOI] [PubMed] [Google Scholar]
Wolfe 1997
- Wolfe JH, Wyatt Y. Critical and subcritical ischemia. European Journal of Vascular and Endovascular Surgery 1997;13(6):578‐82. [DOI] [PubMed] [Google Scholar]
Yao 1973
- Yao JS. New techniques in objective arterial evaluation. Archives of Surgery 1973;106(4):600‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cosmi 2001
- Cosmi B, Conti E, Coccheri S. Anticoagulants (heparin, low molecular weight heparin and oral anticoagulants) for intermittent claudication. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD001999] [DOI] [PubMed] [Google Scholar]


