Abstract
Background
Urinary tract infection (UTI) is one of the most common bacterial infections in infants and children. Lower UTI is the most commonly presenting and in the majority of cases can be easily treated with a course of antibiotic therapy with no further complications. A number of antimicrobials have been used to treat children with lower UTIs; however is it unclear what are the specific benefits and harms of such treatments.
Objectives
This review aims to summarise the benefits and harms of antibiotics for treating lower UTI in children.
Search methods
We searched the Renal Group's Specialised Register (April 2012), CENTRAL (The Cochrane Library 2012, Issue 5), MEDLINE OVID SP (from 1966), and EMBASE OVID SP (from 1988) without language restriction. Date of last search: May 2012.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs in which antibiotic therapy was used to treat bacteriologically proven, symptomatic, lower UTI in children aged zero to 18 years in primary and community healthcare settings were included.
Data collection and analysis
Two authors independently assessed study quality and extracted data. Statistical analyses were performed using the random effects model and the results expressed as risk ratios (RR) with 95% confidence intervals (CI).
Main results
Sixteen RCTs, analysing 1,116 children were included. Conventional 10‐day antibiotic treatment significantly increased the number of children free of persistent bacteriuria compared to single‐dose therapy (6 studies, 228 children: RR 2.01, 95%CI 1.06 to 3.80). No heterogeneity was observed. Persistent bacteriuria at the end of treatment was reported in 24% of children receiving single‐dose therapy compared to 10% of children who were randomised to 10‐day therapy. There were no significant differences between groups for persistent symptoms, recurrence following treatment, or re‐infection following treatment. There was insufficient data to analyse the effect of antibiotics on renal parenchymal damage, compliance, development of resistant organisms or adverse events. Despite the inclusion of 16 RCTs, methodological weakness and small sample sizes made it difficult to conclude if any of the included antibiotics or regimens were superior to another.
Authors' conclusions
Although antibiotic treatment is effective for children with UTI, there are insufficient data to answer the question of which type of antibiotic or which duration is most effective to treat symptomatic lower UTI. This review found that 10‐day antibiotic treatment is more likely to eliminate bacteria from the urine than single‐dose treatments. No differences were observed for persistent bacteriuria, recurrence or re‐infection between short and long‐course antibiotics where the antibiotic differed between groups. This data adds to an existing Cochrane review comparing short and long‐course treatment of the same antibiotic who also reported no evidence of difference between short and long‐course antibiotics.
Plain language summary
Antibiotics for lower urinary tract infection in children
Urinary tract infection (UTI) is one of the most common bacterial infections in infants and children. The most commonly presenting infection of the urinary tract is known as cystitis and in the majority of cases can be easily treated with a course of antibiotic therapy with no further complications. This review identified 16 studies investigating antibiotics for UTI in children. Results suggest that 10‐day antibiotic treatment is more likely to eliminate bacteria from the urine than single‐dose treatments; there was not enough data to draw conclusions about other treatment durations, or effectiveness of particular antibiotics. Although antibiotic treatment is effective for children with UTI, there are insufficient data to recommend any specific regimen.
Summary of findings
Summary of findings for the main comparison. Single‐dose versus short‐course (3‐7 days) antibiotics for treating lower urinary tract infection in children.
| Single‐dose versus short‐course (3‐7 days) antibiotics for treating lower urinary tract infection in children | ||||||
| Patient or population: children with lower urinary tract infection Settings: outpatient or emergency departments Intervention: single‐dose Comparison: short‐course (3‐7 days) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short‐course (3‐7 days) | Single‐dose | |||||
| Persistent bacteriuria Follow‐up: 1‐7 days | Study population | RR 1.3 (0.65 to 2.62) | 145 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 200 per 1000 | 260 per 1000 (130 to 524) | |||||
| Medium risk population | ||||||
| 245 per 1000 | 318 per 1000 (159 to 642) | |||||
| Recurrence | Study population | RR 1.5 (0.43 to 5.26) | 145 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 100 per 1000 | 150 per 1000 (43 to 526) | |||||
| Medium risk population | ||||||
| 85 per 1000 | 128 per 1000 (37 to 447) | |||||
| Re‐infection | Study population | RR 0.16 (0.02 to 1.26) | 45 (1 study) | ⊕⊝⊝⊝ very low3,4 | ||
| 250 per 1000 | 40 per 1000 (5 to 315) | |||||
| Medium risk population | ||||||
| 250 per 1000 | 40 per 1000 (5 to 315) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Neither study reported allocation concealment or blinding; one reported adequate randomisation method, but the other did not; ITT was used in both studies, but one had losses to follow‐up > 10%. 2 Small number of participants (≤ 50/group) and wide CI that crosses 1 3 Allocation concealment and blinding not reported. Random numbers table and ITT analyses used and no losses to follow‐up. 4 Very small numbers of patients (45)
Summary of findings 2. Single‐dose versus conventional 10‐day antibiotic treatment for treating lower urinary tract infection in children.
| Single‐dose versus conventional 10‐day antibiotic treatment for treating lower urinary tract infection in children | ||||||
| Patient or population: children with lower urinary tract infection Settings: outpatient and/or emergency department Intervention: single‐dose Comparison: conventional 10‐day treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional 10‐day treatment | Single‐dose | |||||
| Persistent bacteriuria | Study population | RR 2.01 (1.06 to 3.8) | 228 (6 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 104 per 1000 | 209 per 1000 (110 to 395) | |||||
| Medium risk population | ||||||
| 126 per 1000 | 253 per 1000 (134 to 479) | |||||
| Persistent symptoms | Study population | RR 0.29 (0.03 to 2.5) | 30 (1 study) | ⊕⊝⊝⊝ very low3,4 | ||
| 214 per 1000 | 62 per 1000 (6 to 535) | |||||
| Medium risk population | ||||||
| 214 per 1000 | 62 per 1000 (6 to 535) | |||||
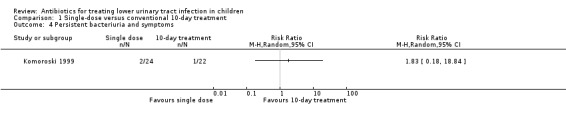
| Persistent bacteriuria and symptoms | Study population | RR 1.83 (0.18 to 18.84) | 46 (1 study) | ⊕⊝⊝⊝ very low5,6 | ||
| 45 per 1000 | 82 per 1000 (8 to 848) | |||||
| Medium risk population | ||||||
| 46 per 1000 | 84 per 1000 (8 to 867) | |||||
| Recurrence | Study population | RR 1.38 (0.55 to 3.5) | 79 (2 studies) | ⊕⊝⊝⊝ very low7,8 | ||
| 158 per 1000 | 218 per 1000 (87 to 553) | |||||
| Medium risk population | ||||||
| 154 per 1000 | 213 per 1000 (85 to 539) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Only 1/6 studies reported randomisation method; no study reported allocation concealment; 1/6 studies reported blinding; 1/6 studies reported ITT analyses. 2 Number of patients < 250 across all groups 3 Randomisation method, allocation concealment, and blinding not reported, ITT analysis not used. 4 Very small numbers of patients (31) 5 Randomisation method and allocation concealment not reported. Open label study. ITT analysis not used and losses to follow‐up > 10% 6 Very small numbers of patients (59) 7 Neither study reported allocation concealment. One reported using a random numbers table, the other reported blinding. Neither study used ITT analysis. 8 Number of participants is small, < 25 in each group across both studies. CI are wide and include 1.
Summary of findings 3. Short (3‐7 days) long‐course (10‐14 days) antibiotics for treating lower urinary tract infection in children.
| Short (3‐7 days) versus long‐course (10‐14 days) antibiotics for treating lower urinary tract infection in children | ||||||
| Patient or population: children with lower urinary tract infection Settings: paediatric department (1); not stated (3) Intervention: short‐course (3‐7 days) Comparison: long‐course (10‐14 days) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long‐course (10‐14 days) | Short‐course (3‐7 days) | |||||
| Persistent bacteriuria | Study population | RR 1.1 (0.68 to 1.77) | 265 (3 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 186 per 1000 | 205 per 1000 (126 to 329) | |||||
| Medium risk population | ||||||
| 185 per 1000 | 204 per 1000 (126 to 327) | |||||
| Recurrence | Study population | RR 1.14 (0.7 to 1.86) | 363 (4 studies) | ⊕⊝⊝⊝ very low3,4 | ||
| 127 per 1000 | 145 per 1000 (89 to 236) | |||||
| Medium risk population | ||||||
| 100 per 1000 | 114 per 1000 (70 to 186) | |||||
| Re‐infection | Study population | RR 0.88 (0.44 to 1.74) | 211 (2 studies) | ⊕⊝⊝⊝ very low4,5 | ||
| 147 per 1000 | 129 per 1000 (65 to 256) | |||||
| Medium risk population | ||||||
| 154 per 1000 | 136 per 1000 (68 to 268) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 No study reported blinding. One study was quasi‐RCT using alternation; the other two studies did not report randomisation method. One study reported allocation concealment and two studies reported ITT analyses. 2 Number of patients across groups was reasonably small (265) and CIs are wide and cross 1 3 No explanation was provided 4 CI crosses 1 5 Randomisation method and blinding were not reported in either study. Allocation concealment and ITT analysis was adequate in one of the two studies.
Summary of findings 4. 10‐day trimethoprim versus 10‐day trimethoprim+sulfamethoxazole for treating lower urinary tract infection in children.
| 10‐day trimethoprim versus 10‐day trimethoprim+sulfamethoxazole for treating lower urinary tract infection in children | ||||||
| Patient or population: children with lower urinary tract infection Settings: outpatients department Intervention: 10‐day trimethoprim Comparison: 10‐day trimethoprim+sulfamethoxazole | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 10‐day trimethoprim +sulfamethoxazole | 10‐day trimethoprim | |||||
| Persistent bacteriuria | Study population | RR 1.93 (0.38 to 9.76) | 59 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 69 per 1000 | 133 per 1000 (26 to 673) | |||||
| Medium risk population | ||||||
| 69 per 1000 | 133 per 1000 (26 to 673) | |||||
| Persistent symptoms | Study population | RR 4.84 (0.24 to 96.66) | 59 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Medium risk population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Recurrence | Study population | RR 2.9 (0.12 to 68.5) | 59 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Medium risk population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomisation method and allocation concealment were not reported. Investigator blind only. No ITT analysis and loss to follow‐up > 10% 2 Very small numbers of patients (59) and CI is very wide and crosses 1
Summary of findings 5. 10‐day cefadroxil versus 10‐day ampicillin for treating lower urinary tract infection in children.
| 10‐day cefadroxil versus 10‐day ampicillin for treating lower urinary tract infection in children | ||||||
| Patient or population: children with lower urinary tract infection Settings: not stated Intervention: 10‐day cefadroxil Comparison: 10‐day ampicillin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 10‐day ampicillin | 10‐day cefadroxil | |||||
| Persistent bacteriuria | Study population | RR 0.33 (0.01 to 7.62) | 32 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 62 per 1000 | 21 per 1000 (1 to 480) | |||||
| Medium risk population | ||||||
| 63 per 1000 | 21 per 1000 (1 to 480) | |||||
| Persistent symptoms | Study population | RR 0.33 (0.01 to 7.62) | 32 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 62 per 1000 | 21 per 1000 (1 to 480) | |||||
| Medium risk population | ||||||
| 63 per 1000 | 21 per 1000 (1 to 480) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomisation method, allocation concealment and blinding not reported. 2 Very small numbers of patients (32) and CIs are very wide and cross 1
Summary of findings 6. Single‐dose fosfomycin versus single‐dose netilmicin for treating lower urinary tract infection in children.
| Single‐dose fosfomycin versus single‐dose netilmicin for treating lower urinary tract infection in children | ||||||
| Patient or population: children with lower urinary tract infection Settings: outpatients department Intervention: single‐dose fosfomycin Comparison: single‐dose netilmicin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Single‐dose netilmicin | Single‐dose fosfomycin | |||||
| Persistent bacteriuria | Study population | RR 3.15 (0.68 to 14.64) | 135 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 31 per 1000 | 98 per 1000 (21 to 454) | |||||
| Medium risk population | ||||||
| 31 per 1000 | 98 per 1000 (21 to 454) | |||||
| Recurrence | Study population | RR 0.63 (0.26 to 1.56) | 135 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 156 per 1000 | 98 per 1000 (41 to 243) | |||||
| Medium risk population | ||||||
| 156 per 1000 | 98 per 1000 (41 to 243) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomisation method, allocation concealment and blinding not reported. 2 Number of patients is reasonably small (135) and CIs are very wide and crossed 1
Background
Urinary tract infection (UTI) is one of the most common bacterial infections in infants and children and the most common bacterial infection in infants under three months (Stanley 2005). Infection of the urinary tract presents in three ways; as covert bacteriuria on screening; non‐systemic symptoms and infection limited to the urethra and bladder (cystitis or lower UTI); or systemic symptoms and infection of the kidneys (pyelonephritis or upper UTI). Lower UTI is the most commonly presenting and in the majority of cases can be easily treated with a course of antibiotic therapy with no further complications. Some children, particularly young infants who are unable to describe how they feel, present with non‐specific symptoms making it difficult to diagnose as either lower or upper UTI, whereas older children present with more specific complaints such as dysuria or frequency. The prognosis of lower UTI in childhood is largely unknown. The relationship between UTI, renal scarring and vesicoureteric reflux (VUR) is unclear, as is the progression of lower UTI to pyelonephritis and subsequent damage to the kidneys. Current practice and treatment decisions are not underpinned by strong evidence and are largely the result of clinical judgment and the biological plausibility of future kidney damage.
It is difficult to obtain accurate estimates of the number of infants and children who will present with a lower UTI during childhood. Current practice in most countries is based on historical data from studies conducted in secondary and tertiary referral centres and renal registries. This fails to adequately describe the larger primary care population for whom very limited population‐based data, of robust quality, exists. Of the population‐based studies available, variable incidence rates, ranging from approximately 0.1% (Messi 1988) to 3% (Winberg 1974) have been presented. The cumulative incidence of childhood UTI is likely to be somewhere between 5% and 12% (Coulthard 1997; Hellstrom 1991).
A number of antimicrobials have been used to treat children with lower UTIs; however there is neither agreement on the most effective agent, nor the optimal dosage. This review aims to evaluate the benefits and harms of antibiotics used to treat lower childhood UTI by investigating the alleviation of symptoms and persistence of bacteriuria following treatment, recurrent symptomatic UTI and renal parenchymal damage.
An existing Cochrane review investigates antibiotic therapy for acute pyelonephritis in children (Hodson 2007) and another compared short to standard duration oral antibiotic treatment for acute UTI in children (Michael 2003). The latter concluded that a 2‐4 day course of oral antibiotics appears to be as effective as 7‐14 days in eradicating lower tract UTI (cystitis) in children. Two additional reviews were identified (Keren 2002; Tran 2001) comparing the efficacies of single‐dose, short‐course and standard course antimicrobial therapy for childhood UTI. This review provides additional data in Cochrane format, particularly on the duration of antibiotic therapy.
Objectives
This review aimed to summarise the benefits and harms of antibiotics for treating bacteriologically proven, symptomatic, lower UTI in children.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) in which antibiotic therapy was used to treat lower UTI in children. The first period of randomised cross‐over studies was also included.
Types of participants
Inclusion criteria
-
Children aged zero to 18 years with bacteriologically proven UTI (at least one culture of a known urinary pathogen (bacterial culture > 105 cfu/mL) in a child with first time or recurrent UTI and who had at least one localised symptom of UTI (such as dysuria or frequency) treated with antibiotics in primary and community health care settings or an outpatient department were included.
Studies that primarily included children with acute pyelonephritis (at least one culture of a known urinary pathogen (bacterial culture > 105 cfu/mL) in a child with at least one symptom or sign of systemic illness such as fever, loin pain (or flank pain) or toxicity and additional diagnostic criteria as defined by the authors) were excluded. The symptom of fever is a controversial one; for the purposes of this review, we have excluded children who present with fever in an effort to differentiate between children with lower UTI and those with pyelonephritis.
Children found to have renal abnormalities during the study were included, however if they had known abnormalities prior to the study they were excluded. We included children with low grade (1‐2) reflux.
Studies including patients with lower UTI and either upper UTI or covert bacteriuria were included if the data for the patients with lower UTI could be extracted separately, otherwise these studies were excluded. Any urine collection method was acceptable, including urine collection pad or bag, clean‐catch method, catheter, or supra‐pubic aspiration.
Exclusion criteria
Children hospitalised for a condition not related to UTI.
Children with bacteriologically proven UTI and symptoms or signs of systemic illness (including fever, loin pain, toxicity).
Children with covert bacteriuria (non‐symptomatic).
Children with pre‐existing renal abnormalities or known underlying renal conditions (including high grade (3‐4) VUR, nephrotic syndrome and neurogenic bladder).
Children receiving prophylactic antibiotics for UTI.
Children receiving antibiotics for any other condition.
Immunosuppressed children.
Types of interventions
Antibiotic therapy (standard course) versus placebo, no therapy, a different antibiotic or alternative non‐antibiotic therapy.
Single‐dose (or single‐day therapy) versus standard dose.
Mode of administration (oral, intravenous or intramuscular)
Types of outcome measures
Persistent symptoms at completion of treatment.
Persistent, significant bacteriuria (> 105 cfu/mL) at completion of treatment.
Combinations of persistent bacteriuria (> 105 cfu/mL) and symptoms at completion of treatment.
Recurrent symptomatic UTI following treatment.
Symptomatic re‐infection following treatment.
Any renal parenchymal damage on DMSA, four to six months following UTI.
Compliance.
Adverse events.
Development of resistant organisms.
Any changes to antibiotic regimen.
Search methods for identification of studies
Electronic searches
The search strategy was comprehensive and was designed to cover two reviews being undertaken by the authors, this review and "Interventions for covert bacteriuria in children" (Fitzgerald 2012). We searched the following databases to identify relevant studies. Full details of the search strategies are reported in Appendix 1.
We searched the Cochrane Renal Group's Specialised Register (May 2012) through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
The Cochrane Renal Group’s Specialised Register contains studies identified from:
Quarterly searches of the Cochrane Central Register of Controlled Trials CENTRAL;
Weekly searches of MEDLINE OVID SP;
Handsearching of renal‐related journals & the proceedings of major renal conferences;
Searching of the current year of EMBASE OVID SP;
Weekly current awareness alerts for selected renal‐journals;
Searches of the International Clinical Trials Register (ICTRP) Search Portal & ClinicalTrials.gov
Studies contained in the specialised register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the 'Specialised Register' section of information about the Cochrane Renal Group.
Searching other resources
Reference lists of nephrology textbooks, review articles and relevant studies.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies relevant to the review. The titles and abstracts were screened independently by two authors assessed to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by the same authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were to be grouped together and the most recent or most complete dataset used. Any discrepancy between published versions was to be highlighted. Disagreements were resolved in consultation with a third author.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (seeAppendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. symptom resolution, persistent bacteriuria, recurrent infection) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). No continuous scales of measurement were used in the review.
Dealing with missing data
Any further information required from the original author was requested by written correspondence and any relevant information obtained in this manner was included in the review
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom (df), with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% corresponded to low, medium and high levels of heterogeneity.
Assessment of reporting biases
If possible, funnel plots were to be used to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
Data was pooled using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity (e.g. participants, interventions and study quality). Heterogeneity among participants could be related to age and renal pathology. Heterogeneity in treatments could be related to prior agent(s) used and the agent, dose and duration of therapy. Older and newer manuscripts evaluating the same antibiotic was also analysed as a subgroup. Subgroup analyses were also used to explore paediatric sub‐populations (infants, toddlers, children and adolescent groups).
Infants: under one year of age
Toddlers: one to under three years of age
Children: three to under 12 years of age
Adolescents: twelve to 18 years of age
Results
Description of studies
Results of the search
We initially identified 2316 potentially relevant reports of studies. After evaluating their titles and abstracts we excluded 1823 articles because they were not RCTs, included adult patients, or did not investigate antibiotics for UTI. The full‐text articles of the remaining 493 studies were evaluated, with a further 393 excluded, leaving 100 potentially relevant RCTs. After further assessment 16 studies met out inclusion criteria (Figure 1).
1.
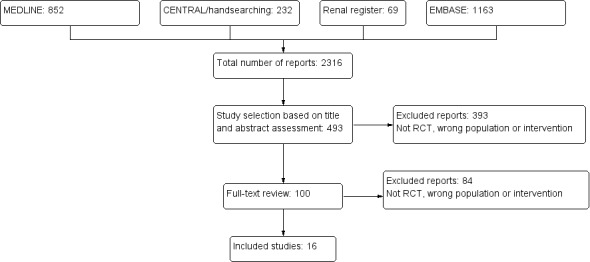
Study flow diagram show study selection process
Included studies
The 16 included studies enrolled 1116 children and young people aged between two weeks and 19 years. Most were published between 1981 and 1991, with two studies published between 1999 and 2001. The median sample size was 49 patients (range: 26 to 264). All included studies used the bacteriological definition of 105 cfu/mL for confirming UTI and included children with non‐systemic symptoms, the most common being dysuria and frequency.
Two studies compared single‐dose antibiotics with short‐course (3‐7 days) antibiotics.
Grimwood 1988 compared a single intramuscular gentamicin injection (3 mg/kg) with seven days of oral antibiotics sensitive to the organism cultured.
Lidefelt 1991 compared single‐dose trimethoprim (TMP) (6 mg/kg) with a five‐day course of TMP (3 mg/kg, twice daily).
Six studies investigated single‐dose antibiotics compared with conventional 10‐day courses.
Four studies (Avner 1983; Fine 1985; Shapiro 1981; Stahl 1984) compared single‐dose amoxicillin (1‐3 g) with 10‐day amoxicillin (125‐500 mg, thrice daily).
Komoroski 1999 compared single intramuscular ceftriaxone (50 mg/kg) with 10‐day TMP‐sulfamethoxazole (TMP‐SMX) (4‐5 mg/kg, twice daily).
Wallen 1983 compared single‐dose intramuscular amikacin sulfate (7.5 mg/kg) with 10‐day sulfisoxazole 1(50 mg/kg/d in four divided doses).
We identified four studies comparing short duration antibiotics (3‐7 days) with conventional 10‐day courses using different antibiotics.
CSG 1991 compared 3‐day pivmecillinam (20‐40 mg/kg/d in two doses) with 10‐day sulfamethizole (40‐80 mg/kg/d in two doses).
Helin 1984 compared 3‐day cephalexin (25‐50 mg/kg/d in two doses) with 10‐day nitrofurantoin (3‐4 mg/kg/d in two or three doses).
Mitnik 1985 administered antibiotics to which the cultured organism was susceptible and treated three groups with three, five and 10 days of antibiotics. The data for the 3‐day group were included in the analyses.
Khan 1981 administered a range of antibiotics at random including ampicillin, sulfisoxazole and cephalexin in conventional doses given orally four times a day and compared 3‐day treatment with 10‐day treatment.
The Cochrane review by Michael 2003 compared short (2‐4 days) with long‐course (7‐14 days) antibiotics in children with UTI where the short and long‐course antibiotic were the same, identified nine studies (CSG 1991; Gaudreault 1992; Helin 1981; Johnson 1993; Kornberg 1994; Lohr 1981; Madrigal 1988; Wientzen 1979; Zaki 1986). We have not included these comparisons, however CSG 1991 was included in both reviews as this study presented antibiotic comparisons for both the same and different antibiotics.
Two studies compared different 10‐day regimens.
Ahmed 2001 compared TMP (monotherapy) with TMP‐SMX.
Malaka‐Zafirui 1984 compared cefadroxil (25 mg/kg, once daily) with ampicillin (50 mg/kg/d in four divided doses).
One study compared single‐dose regimens.
Principi 1990 compared oral fosfomycin trometamol (2 g) with intramuscular netilmicin (5 mg/kg).
In Sanchez 1990 (published only as an abstract), children were randomised to one of five antibiotics; amoxicillin, amoxicillin and clavulanic acid, cephalixin, TMP or co‐trimoxazole at standard doses for seven days.
Excluded studies
We excluded 84 articles.
Not RCT (6)
Included children with pre‐existing renal abnormalities (10)
Included children with pyelonephritis (18)
Included children with both pre‐existing abnormalities and pyelonephritis (2)
Included children with fever or signs of systemic illness (30)
Included prophylactic antibiotics (5)
Reported in a Cochrane review comparing long and short duration antibiotics (Michael 2003) (9)
Included children without bacteriologically proven UTI (1)
Significantly more patients were assigned to one group compared with the other suggesting non‐random allocation (1)
Four articles were excluded for other reasons (4) (see Characteristics of excluded studies)
Risk of bias in included studies
2.
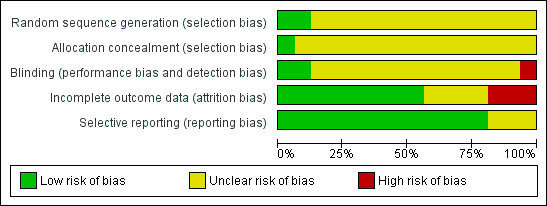
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
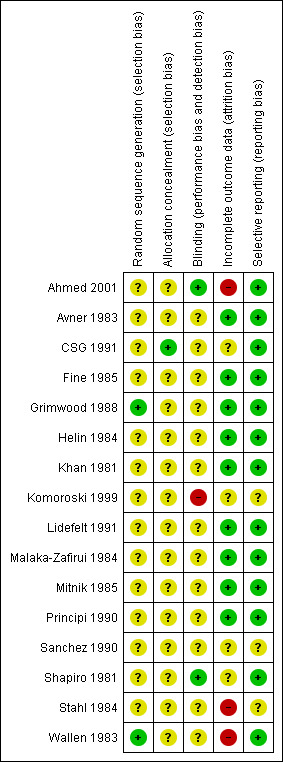
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
The quality of reporting of random sequence generation was poor. Two studies reported using random numbers tables to generate a random sequence (Grimwood 1988; Wallen 1983) and one study was quasi‐randomised using alternation (Khan 1981). CSG 1991 used a block randomisation method to ensure an equal number of patients in each group. Randomisation was in blocks of six within each of the ten participating departments. No details about the way the block randomisation was performed were reported. The remaining 12 studies did not report the randomisation method.
Allocation concealment
The quality of allocation concealment was very poor. CSG 1991 reported using sealed enveloped which were prepared by the manufacturer of the study drug. The remaining 15 studies did not report allocation concealment.
Blinding
The quality of blinding was also very poor. Shapiro 1981 reported blinding both patients and physicians; Ahmed 2001 reported blinding of the investigator; and Komoroski 1999 was reported to be open label. The remaining 13 studies did not reporting blinding.
Incomplete outcome data
In two studies it appeared that children were randomised to treatment before inclusion and exclusion criteria were applied.
CSG 1991: 26% of children randomised were lost to follow‐up for a variety of reasons including not fulfilling inclusion criteria; treatment discontinued before scheduled; and did not have repeat urine cultures within the allocated time. Nineteen boys were excluded from this study because of the small number and because they were not evenly distributed between groups.
Komoroski 1999: 37% of the children randomised were lost to follow‐up. Some urine cultures showed no significant growth; some urine samples were subject to laboratory or procedural errors; and some children did not return for follow‐up assessments.
Ahmed 2001 reported that only 52% of randomised patients were analysed, no reason for attrition were given.
Four other studies reported losses to follow‐up of less than 10% (Fine 1985; Shapiro 1981; Stahl 1984; Wallen 1983).
Sanchez 1990 was presented in an abstract and it was not clear whether patients were analysed in groups to which they were randomised.
In the remaining eight studies, all patients were analysed in groups to which they were randomised.
Selective reporting
Most studies reported all planned outcomes. Komoroski 1999 reported relapse and recurrence, but not in a format suitable for data extraction for this review. Sanchez 1990 was published only as an abstract and it was not clear whether all planned outcomes were reported.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Persistent bacteriuria at completion of treatment
Fourteen studies reported outcomes for persistent bacteriuria at completion of treatment: five studies completed follow‐up urine cultures between two and five days; and one study completed follow‐up urine cultures between 10 to 30 days.
Single‐dose versus conventional 10‐day treatment
Conventional 10‐day antibiotic treatment significantly increased the number of children free of persistent bacteriuria compared to single‐dose treatment (Analysis 1.1 (6 studies, 228 children): RR 2.01, 95% CI 1.06 to 3.80). No heterogeneity was observed (I² = 0%). The test for subgroup differences between the studies using amoxicillin in both arms and studies using other antibiotics did not show any difference (Chi² = 0.01, df = 1, P = 0.93, I² = 0%).
1.1. Analysis.
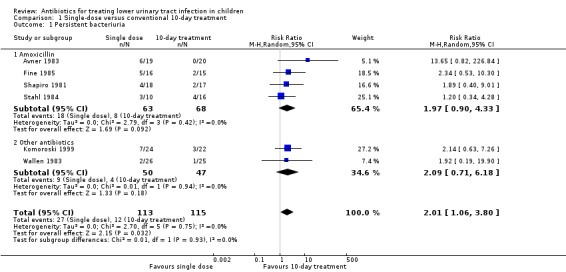
Comparison 1 Single‐dose versus conventional 10‐day treatment, Outcome 1 Persistent bacteriuria.
Single‐dose versus short‐course (3‐7 days) treatment
There was no significant difference in persistent bacteriuria between single‐dose and short‐course antibiotic treatment (Analysis 2.1 (2 studies, 145 children): RR 1.30, 95% CI 0.65 to 2.62; I² = 30%).
2.1. Analysis.
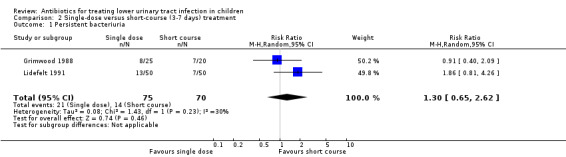
Comparison 2 Single‐dose versus short‐course (3‐7 days) treatment, Outcome 1 Persistent bacteriuria.
Short‐course (3‐7 days) versus long‐course (7‐10 days) treatment
There was no significant difference in persistent bacteriuria between short‐course and long‐course antibiotic treatment in three studies (Analysis 3.1 (3 studies, 265 children): RR 1.09, 95% CI 0.67 to 1.76; I² = 0%).
3.1. Analysis.
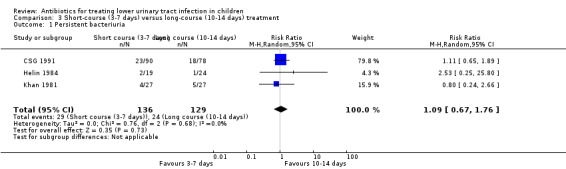
Comparison 3 Short‐course (3‐7 days) versus long‐course (10‐14 days) treatment, Outcome 1 Persistent bacteriuria.
Head‐to‐head studies
There were no significant differences in persistent bacteriuria between:
TMP (10 days) versus TMP‐SMX (10 days) (Analysis 4.1 (1 study, 59 children): RR 1.93, 95% CI 0.38 to 9.76);
cefadroxil (10 days) versus ampicillin (10 days) (Analysis 5.1 (1 study, 32 children): RR 0.33, 95% CI 0.01 to 7.62); and
fosfomycin (single‐dose) versus netilmicin (single‐dose) (Analysis 6.1 (1 study, 135 children): RR 3.15, 95% CI 0.26 to 1.56).
4.1. Analysis.
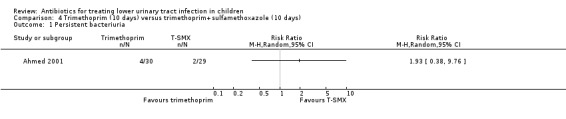
Comparison 4 Trimethoprim (10 days) versus trimethoprim+sulfamethoxazole (10 days), Outcome 1 Persistent bacteriuria.
5.1. Analysis.
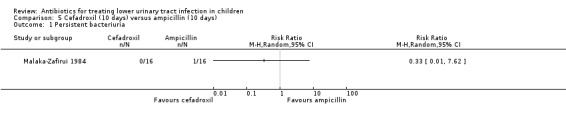
Comparison 5 Cefadroxil (10 days) versus ampicillin (10 days), Outcome 1 Persistent bacteriuria.
6.1. Analysis.
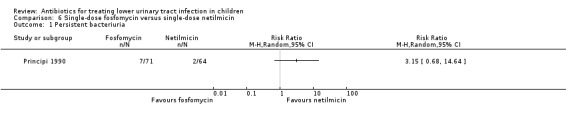
Comparison 6 Single‐dose fosfomycin versus single‐dose netilmicin, Outcome 1 Persistent bacteriuria.
Sanchez 1990 randomised children to one of five antibiotics: amoxicillin; amoxicillin + clavulanic acid; cephalexin; TMP; or co‐trimoxazole. Because of the small number of participants in each group (5 to 9) and the small number of events in each group (1 or 2) this data was unable to be included in meta‐analyses. Following treatment, the number of children free of bacteriuria in each group were 4/5 children who received amoxicillin, 2/7 children who received amoxicillin + clavulanic acid, 2/8 children who received cephalexin, 2/8 children who received TMP, and 2/9 children who received co‐trimoxazole.
Persistent symptoms at completion of treatment
Three studies reported outcomes for persistent symptoms following treatment and showed no differences between treated and untreated groups; all for different antibiotic comparisons and durations.
Single‐dose versus conventional 10‐day treatment
There was no significant difference between single‐dose treatment compared with conventional 10‐day treatment (Analysis 1.2 (1 study, 30 children): RR 0.29, 95% CI 0.03 to 2.50) where 1/16 of the single‐dose group had persistent symptoms compared with 3/14 of the 10‐day group (Fine 1985).
1.2. Analysis.
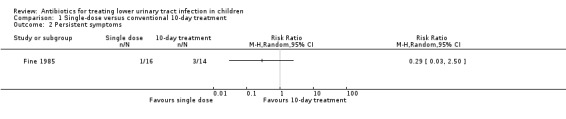
Comparison 1 Single‐dose versus conventional 10‐day treatment, Outcome 2 Persistent symptoms.
TMP (10 days) versus TMP‐SMX (10 days)
There was no significant difference between 10‐day TMP treatment compared with 10‐day TMP‐SMX treatment (Analysis 4.2 (1 study, 59 children): RR 4.84, 95% CI 0.24 to 96.66) where 2/30 of the TMP group had persistent symptoms compared with 0/29 of the TMP‐SMX group (Ahmed 2001).
4.2. Analysis.
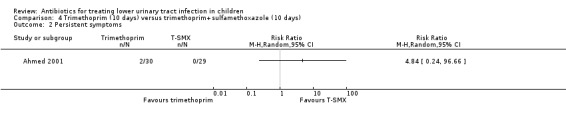
Comparison 4 Trimethoprim (10 days) versus trimethoprim+sulfamethoxazole (10 days), Outcome 2 Persistent symptoms.
Cefadroxil (10 days) versus ampicillin (10 days)
There was no significant difference in persistent symptoms between 10‐day cefadroxil treatment compared with 10‐day ampicillin treatment (Analysis 5.2 (1 study, 32 children): RR 0.33, 95% CI 0.01 to 7.62) where 0/16 of the cefadroxil group had persistent symptoms compared with 1/16 in the ampicillin group (Malaka‐Zafirui 1984).
5.2. Analysis.
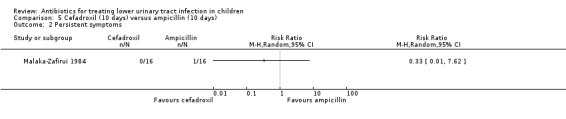
Comparison 5 Cefadroxil (10 days) versus ampicillin (10 days), Outcome 2 Persistent symptoms.
Recurrent symptomatic UTI following treatment
Ten studies reported outcomes for recurrence (with the same organism) following treatment for the initial infection; five studies reported recurrences within one month of antibiotic treatment; four studies reported recurrences between one and three months following antibiotic treatment; and one study reported recurrences at any time with a mean time of eight months.
Single‐dose versus short‐course (3‐7 days) treatment
There was no significant difference between single‐dose compared with short‐course (3‐7 days) treatment (Analysis 2.2 (2 studies, 145 children): RR 1.50, 95% CI 0.43 to 5.26; I² = 29%), where 11/75 (15%) of the single‐dose had recurrence compared with 7/70 (10%) of the short‐course group.
2.2. Analysis.
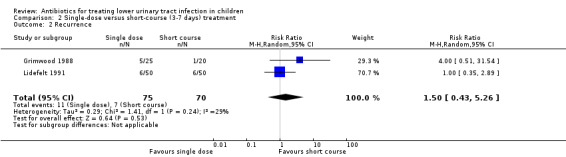
Comparison 2 Single‐dose versus short‐course (3‐7 days) treatment, Outcome 2 Recurrence.
Single‐dose versus conventional 10‐day treatment
There was no significant difference between single‐dose treatment compared to conventional 10‐day treatment (Analysis 1.3 (2 studies, 79 children): RR 1.38, 95% CI 0.55 to 3.50; I² = 0%), where 9/41 (22%) of the single‐dose group had recurrent symptomatic UTI compared with 6/38 (16%) in the 10‐day group.
1.3. Analysis.
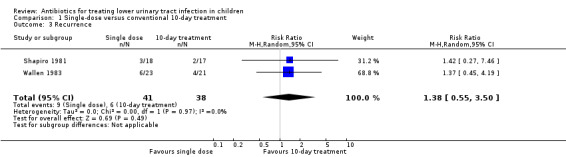
Comparison 1 Single‐dose versus conventional 10‐day treatment, Outcome 3 Recurrence.
Short‐course (3‐7 days) versus long‐course (7‐10 days) treatment
There was no significant difference between short‐course treatment compared with long‐course treatment (Analysis 3.2 (4 studies, 328 children): RR 1.25, 95% CI 0.74 to 2.13; I² = 0%). All four studies compared 3‐day to 10‐day treatment. Of these four studies, 25/163 (15%) of the short‐course group had recurrent symptomatic UTI compared with 21/165 (13%) of the long‐course group.
3.2. Analysis.
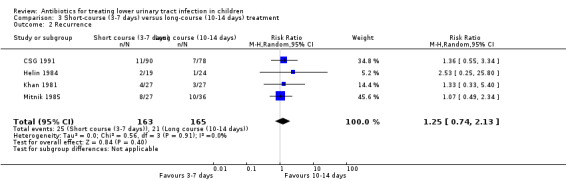
Comparison 3 Short‐course (3‐7 days) versus long‐course (10‐14 days) treatment, Outcome 2 Recurrence.
Head‐to‐head studies
There were no significant differences in recurrent symptomatic UTI between:
TMP (10 days) versus TMP‐SMX (10 days) (Analysis 4.3 (1 study, 59 children): RR 2.90, 95% CI 0.12 to 68.50), where 1/30 in the TMP (monotherapy) group had recurrent symptomatic UTI compared with 0/29 in the TMP‐SMX group; and
fosfomycin (single‐dose) versus netilmicin (single‐dose) (Analysis 6.2 (1 study, 135 children): RR 0.63, 95% CI 0.26 to 1.56) where 7/71 (10%) in the single‐dose fosfomycin group had recurrent symptomatic UTI compared with 10/64 (16%) in the single‐dose netilmicin group.
4.3. Analysis.
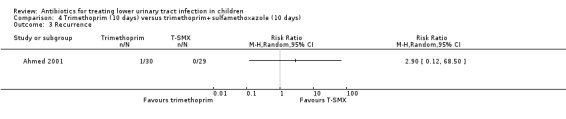
Comparison 4 Trimethoprim (10 days) versus trimethoprim+sulfamethoxazole (10 days), Outcome 3 Recurrence.
6.2. Analysis.
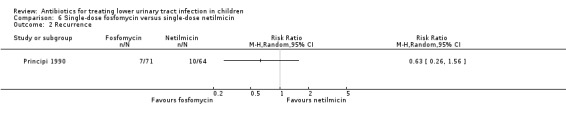
Comparison 6 Single‐dose fosfomycin versus single‐dose netilmicin, Outcome 2 Recurrence.
Re‐infection following treatment
Three studies reported outcomes for re‐infection (with a different organism) following antibiotic treatment for the initial infection; one study reported re‐infection occurring at more than one week, one study reported re‐infection at 1‐10 days and one study reported re‐infection at any time following antibiotic treatment, with a mean time of eight months.
Single‐dose versus short‐course (3‐7 days) treatment
There was no significant difference between single‐dose compared with short‐course treatment (Analysis 2.3 (1 study, 45 children): RR 0.16, 95% CI 0.02 to 1.26), where 1/25 (4%) of the single‐dose group had a re‐infection compared with 5/25 (20%) of the short‐course group.
2.3. Analysis.
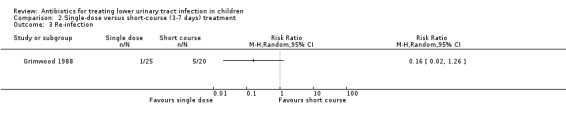
Comparison 2 Single‐dose versus short‐course (3‐7 days) treatment, Outcome 3 Re‐infection.
Short‐course (3‐7 days) versus long‐course (7‐10 days) treatment
There was no significant difference between short‐course treatment compared with long‐course treatment (Analysis 3.3 (2 studies, 211 children): RR 0.88, 95% CI 0.44 to 1.74; I² = 0%), where 14/109 (13%) of the short‐course group had a re‐infection compared with 15/102 (15%) of the long‐course group.
3.3. Analysis.
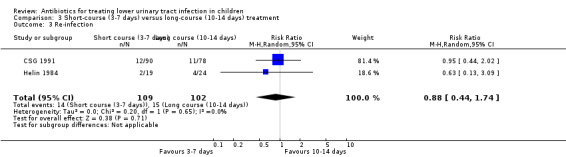
Comparison 3 Short‐course (3‐7 days) versus long‐course (10‐14 days) treatment, Outcome 3 Re‐infection.
Renal parenchymal damage
None of the included studies reported renal parenchymal damage. Two studies (Avner 1983, Stahl 1984) reported the use of micturating cystourethrogram or intravenous pyelogram following antibiotic therapy, however the studies were aimed to identify structural abnormalities and VUR and not renal parenchymal damage.
Compliance
Fine 1985 reported compliance with follow‐up assessments. Compliance at the first scheduled follow‐up appointment was 100% in the single‐dose treatment group compared to 60% in the 10‐day treatment group. Non‐returning patients often required several phone calls and letters before returning.
Three studies reported compliance with antibiotic treatment.
Fine 1985: 27% of the 10‐day treatment group reported perfect compliance with their medication; other patients finished their medication in more than 10 days, less than 10 days, and some had not finished their medication at the time of follow‐up.
Grimwood 1988: side effects prevented two children randomised to amoxicillin from completing their course of antibiotics.
Helin 1984: 'optimal patients compliance was assured in both groups'.
Development of resistant organisms
While many included studies reported the antibiotic sensitivity of organisms cultured prior to treatment, most did not report development of resistant organisms during the study period. Stahl 1984, comparing single‐dose with 10‐day amoxicillin, reported that in each of the three single‐dose patients who relapsed, the single‐dose failed to clear the urinary tract of a sensitive organism, and each of the four conventional therapy patients who relapsed developed organisms resistant to amoxicillin during therapy. All of these patients subsequently responded to additional or different antibiotic therapy.
Adverse events
Patients in five studies did not experience any side effects (Avner 1983; Helin 1984; Malaka‐Zafirui 1984; Wallen 1983).
CSG 1991: children randomised to 10‐day sulfamethizole reported no side effects. Two children randomised to 3‐day pivmecillinam developed urticarial rash; two children discontinued treatment due to vomiting and abdominal rash; one child had diarrhoea; and one child developed irritability and fatigue.
Lidefelt 1991: one child randomised to single‐dose TMP experienced vomiting.
Principi 1990: two children receiving single‐dose fosfomycin experienced mild and transient diarrhoea which disappeared spontaneously; one had nausea; and one had skin rash.
Data reported in Ahmed 2001 was part of a larger study on children with both UTI and otitis media. Across both TMP and TMP‐SMX groups, adverse events included vomiting, abdominal pain, diarrhoea and skin rash, although less than 5% of children experienced these symptoms.
Fine 1985: Candida vaginitis occurred in three patients receiving 10‐day amoxicillin.
Komoroski 1999: two sexually active females reported vaginal itching after receiving intramuscular ceftriaxone.
Local discomfort from injection sites was reported in two studies (Komoroski 1999; Principi 1990).
Side effects were not reported in six studies (Grimwood 1988; Khan 1981; Mitnik 1985; Sanchez 1990; Shapiro 1981; Stahl 1984).
Subgroup analyses
We were unable to perform the pre‐specified subgroup analyses. The majority of studies did not report results for different paediatric sub‐populations, so this analysis could not be undertaken. Comparing older and newer manuscripts was also not possible because there were not enough 'newer' manuscripts. With the exception of Ahmed 2001 (which was the only study to compare 10‐day TMP with 10‐day TMP‐SMX) and Komoroski 1999 (single‐dose versus 10‐day treatment), all studies compared were performed within a 10‐year period of each other. Because the method of randomisation was not sufficiently described for most of the studies, we did not perform a sensitivity analysis that excluded quasi‐RCTs. Data of randomisation, method of allocation concealment and blinding were not reported by the majority of studies and we were therefore unable to conduct sensitivity analyses comparing higher and lower quality studies.
Formal testing for publication bias using funnel plots was not possible because of the small number of studies identified.
Discussion
Summary of main results
UTIs are relatively common childhood illness and require antibiotic treatment to alleviate symptoms and clear infection from the urinary tract. This review was designed to include all randomised and quasi‐RCTs addressing all aspects of antibiotic treatment for children with lower UTI. Relatively few studies investigated the same comparisons, therefore meta‐analyses included only a small number of studies. Nevertheless, we did not identify many instances of heterogeneity. The included studies predominantly examined bacteriological outcomes (persistent bacteriuria, recurrence, re‐infection) rather than clinical outcomes (persisting symptoms) as measures of treatment efficacy. Amoxicillin or amoxicillin + clavulanic acid, cephalosporins, nitrofurantoin or TMP‐SMX were the most common antibiotics given in the included studies.
Compared to single‐dose therapy, 10‐day antibiotic treatment was more effective in eliminating bacteriuria (RR 2.01, 95% CI 1.06 to 3.80). When we limited our analysis to only include those studies using the same antibiotic (amoxicillin) in both treatment groups, the differences no longer remained statistically significant (RR 1.97, 95% CI 0.90 to 4.33). It is unclear whether this is attributable to bias considering most of our included studies were of poor quality and sample sizes were too small to detect differences; or whether just by chance. Nevertheless, the actual numbers of children with persistent bacteriuria, 18/63 (29%) in the single‐dose group compared to 8/68 (12%) of the 10‐day group, represent a clinically significant difference. No differences were observed for persistent bacteriuria, recurrence or re‐infection between short and long‐course antibiotics where the antibiotic differed between groups; this data adds to an existing Cochrane review (Michael 2003) comparing short and long‐courses of the same antibiotic who also reported no evidence of difference between short and long‐course antibiotics.
No comparisons showed any significant differences between groups for persistent symptoms, recurrence, or re‐infection following treatment for any other dosing regimens. In this review, there were not enough data to draw conclusions about these results; at this stage, the lack of significant differences is more likely due to the inclusion of small, poorer quality studies, rather than demonstrating equivalence between different antibiotic or dosing regimens.
Overall completeness and applicability of evidence
While there were 16 RCTs included in this review, the number of children analysed totalled 1116 (1331 randomised); only three studies included more than 100 children. The median sample size was small (49 children), and wide CIs around most of the effect estimates suggest that studies probably lacked the statistical power to identify such differences. Of the 16 studies included, 13 were conducted between 1981 and 1990. Diagnosis of bacteriuria since this time has not changed significantly, but not all of the antibiotics used in these studies remain available. In most of the meta‐analyses carried out, there were few studies included, preventing a thorough investigation of the sources of heterogeneity between study results. In particular, we could not explore the influence of specific sources of bias or methodological quality, and most importantly we could not offer results stratified by age subgroups. Adverse events were reported by some studies, but were not analysed by any included studies; this hindered our efforts to present a full picture of the benefits and harms of antibiotics treatment for lower UTI, which was our intended aim.
Quality of the evidence
The quality of the included studies for every comparison was 'very low' according to GRADE criteria (see Summary of findings tables). The lack of reporting of randomisation method, allocation concealment and blinding in most studies, and the large losses to follow‐up in three studies are likely to contribute to biases in the results reported. Despite the inclusion of 16 studies, their methodological weakness and small sample sizes made it difficult to conclude if any of the included antibiotics or regimens were superior to another.
It was unfortunate that no study specifically addressed whether the efficacy of therapies differed according to patient age; we had planned subgroup analyses to investigate this because there are claims that UTIs can lead to long‐term damage in younger children (Vernon 1997). In Fine 1985, 90% of the adolescents included were sexually active; this may have caused bias in that sexually active people (particularly women) are known to experience more frequent UTIs than those who abstain (Leibovici 1987). Four studies included young people over the age of 16 years: Shapiro 1981 included children aged 2‐18 years, but the mean age was 5.6 years; Stahl 1984 included children aged 2‐17 years, but the mean age was 4.75 years; Komoroski 1999 included children aged 0‐18 years, but 80% were younger than 16 years; Fine 1985 included female adolescents aged 12‐18 years with a mean age of 16.5 years. Although a lower tract UTI in an adolescent female is likely a very different condition than lower tract UTI in an infant or young child, post‐hoc sensitivity analysis, removing Fine 1985 did not alter the conclusions for persistent bacteriuria.
None of the included studies systematically collected data on the adverse effects of antibiotics; this made our original objective of summarising the benefits and harms of antibiotic treatment difficult. Although it is not possible to compare the benefits and harms of antibiotic therapy from the included studies, from the few adverse antibiotic effects reported it seems unlikely that the expected side‐effects course of antibiotics would be significant.
Data on resistant organisms were only reported in one study. There is concern regarding the increasing resistance to antibiotics, specifically to penicillins and cephalosporins. Increasing antibiotic resistance complicates the choice of empiric regimens and is likely to become an increasing problem, particularly considering the majority of health professionals prescribe antibiotics prior to knowing results of urine cultures. Utilizing local antimicrobial susceptibility data (e.g. hospital or laboratory data) to predict the antibiotics that would likely be active may be useful.
Optimal duration of antibiotic therapy in children has both cost and practical implications. Single‐dose treatment, if effective can increase compliance as the treatment can be administered at the healthcare site and is likely to be cheaper. These advantages are even more important in under‐resourced countries and in lower socio‐economic areas.
Potential biases in the review process
The literature search included major international databases and a fairly broad search strategy was utilized to ensure all relevant studies were identified. We assume that we identified all RCTs relevant to our review question; however many of the excluded studies were foreign language articles and there is the potential that a relevant study was overlooked in the translation process. Although we stated in out protocol that we would attempt to contact investigators for missing or additional data, 10/16 studies were over 20 years old and the most recent of the remaining six studies was published a decade ago and we were not able to make contact with any investigator.
Agreements and disagreements with other studies or reviews
A Cochrane review comparing short (2‐4 days) with standard (7‐14 days) duration antibiotic therapy of the same antibiotic in children with UTI found no significant differences between groups and concluded that short duration of treatment offers a reasonable option for children with lower tract UTI (Michael 2003). We excluded this comparison from our analysis to avoid duplication, however we did include the comparison with different antibiotics; the results did not differ to Michael 2003; there were no differences between 2‐4 day and 7‐10 day antibiotic treatment. A systematic review by Keren 2002 compared short (both single‐dose and 2‐4 days) with long (7‐10 days) treatment. Of the 17 included studies, no differences were found between groups for treatment failure or re‐infection. Subgroup analyses with one day, and 2‐4 day groups did not change these results. A systematic review by Tran 2001 reported that overall, short‐course therapy was not as effective as conventional long‐course. The review found insufficient evidence for single‐dose aminoglycoside treatment to draw conclusions.
A Cochrane review in non‐pregnant adult women with lower UTI found that three days of antibiotic therapy was similar to 5‐10 days in achieving symptomatic cure, while the longer treatment was more effective in obtaining bacteriological cure (Milo 2005). Our review did not have enough data on persisting symptoms at the completion of treatment to investigate the differences between bacteriological and symptomatic cure.
Authors' conclusions
Implications for practice.
This review adds to the evidence that short‐course therapy is an appropriate therapy for children with lower UTI. While 10 days of therapy was significantly more effective than single‐dose therapy, there were no differences in either this review or Michael 2003 between short and longer duration antibiotic therapy. Single‐dose therapy is not recommended in children with UTI; 10‐day treatment was significantly more effective in eliminating bacteriuria. No comparisons showed any differences between groups for persisting symptoms, recurrence, or re‐infection following treatment, however this is due to a paucity of RCTs rather than demonstrating equivalence. This review did not provide incontrovertible evidence about the optimal duration of antibiotic therapy or which antibiotic should be used.
Implications for research.
Adequately powered RCTs investigating single‐dose compared to standard (7‐14 day) therapy, and single‐dose compared to short duration (2‐4 days) therapy should be conducted to establish the effectiveness of single‐dose treatment. Additional studies comparing single‐dose with 10 days of amoxicillin are needed to investigate whether single‐dose increases persistent bacteriuria. Outcomes for further studies should include both clinical and bacteriological measures.
What's new
| Date | Event | Description |
|---|---|---|
| 22 July 2014 | Amended | Minor copy edit of study name |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 8, 2012
| Date | Event | Description |
|---|---|---|
| 10 July 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank:
Dr Julie Brown for her technical advice during the preparation of this review;
Narelle Willis of the Cochrane Renal Group for her assistance; and
the referees for their comments and feedback.
Appendices
Appendix 1. Electronic search strategy
| Database | Search terms used |
| CENTRAL |
|
| MEDLINE (OVID SP) |
|
| EMBASE (OVID SP) |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Single‐dose versus conventional 10‐day treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria | 6 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [1.06, 3.80] |
| 1.1 Amoxicillin | 4 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [0.90, 4.33] |
| 1.2 Other antibiotics | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [0.71, 6.18] |
| 2 Persistent symptoms | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Recurrence | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.55, 3.50] |
| 4 Persistent bacteriuria and symptoms | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
1.4. Analysis.
Comparison 1 Single‐dose versus conventional 10‐day treatment, Outcome 4 Persistent bacteriuria and symptoms.
Comparison 2. Single‐dose versus short‐course (3‐7 days) treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.65, 2.62] |
| 2 Recurrence | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.43, 5.26] |
| 3 Re‐infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Short‐course (3‐7 days) versus long‐course (10‐14 days) treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria | 3 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.67, 1.76] |
| 2 Recurrence | 4 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.74, 2.13] |
| 3 Re‐infection | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.44, 1.74] |
Comparison 4. Trimethoprim (10 days) versus trimethoprim+sulfamethoxazole (10 days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Persistent symptoms | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Recurrence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Cefadroxil (10 days) versus ampicillin (10 days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Persistent symptoms | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Single‐dose fosfomycin versus single‐dose netilmicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Recurrence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmed 2001.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Source of funding: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Less than half the randomised patients were analysed, no reason for losses to follow‐up given. |
| Selective reporting (reporting bias) | Low risk | Planned outcomes were all analysed |
Avner 1983.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised were analysed |
| Selective reporting (reporting bias) | Low risk | Planned outcomes were all analysed |
CSG 1991.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | To ensure an equal number of patients in each group, a block randomisation method was used. Randomisation was in blocks of 6 within each of the 10 participating departments. No details about the way the block randomisation was performed were reported. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by drawing consecutively numbered sealed envelopes prepared by the manufacturer |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 36 children did not fulfil inclusion criteria (26 bacteriuria not significant, 10 provided bag sample); treatment was discontinued in 6 children before scheduled; 32 children did not have urine cultures completed within 10 days from treatment; 2 children were not evaluated for other reasons; 19 boys were excluded because of the small number and because they were not evenly distributed between groups. The side effects of the 95 children who were not analysed were included as they received treatment. |
| Selective reporting (reporting bias) | Low risk | Planned outcomes were all analysed |
Fine 1985.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants were excluded from analyses because of early pregnancy; one participant did not turn up to the follow‐up appointments. |
| Selective reporting (reporting bias) | Low risk | Planned outcomes were all analysed |
Grimwood 1988.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised were analysed |
| Selective reporting (reporting bias) | Low risk | Planned outcomes were all analysed |
Helin 1984.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
|
|
| Outcomes |
|
|
| Notes | Source of funding: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised were analysed |
| Selective reporting (reporting bias) | Low risk | Planned outcomes were all analysed |
Khan 1981.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
Antimicrobial agents were 'chosen at random' for both groups and included ampicillin, sulfisoxazole and cephalexin in conventional doses given orally 4 times/day. |
|
| Outcomes |
|
|
| Notes | Source of funding: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Alternation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were analysed in groups to which they were assigned |
| Selective reporting (reporting bias) | Low risk | Planned outcomes were analysed. Data for re‐infection was presented across cystitis, pyelonephritis and asymptomatic bacteriuria and was not reported for cystitis alone. |
Komoroski 1999.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It appears that children were randomised to treatment before the inclusion/exclusion criteria were applied. 23 children had urine cultures that showed no significant growth; 8 children had a laboratory or procedural error occurred (e.g., urinalysis obtained but culture not done, organisms in culture not worked up); 3 children did not return for follow‐up assessment |
| Selective reporting (reporting bias) | Unclear risk | Relapse and recurrence were reported, but not in a format suitable for data extraction for this review. |
Lidefelt 1991.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were analysed in groups to which they were assigned |
| Selective reporting (reporting bias) | Low risk | Planned outcomes were analysed |
Malaka‐Zafirui 1984.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were analysed in group to which they were assigned |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were analysed |
Mitnik 1985.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Children were administered a first generation cephalosporin, nitrofurantoin or TMP‐SMX depending on the sensitivity of the organism cultured |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients analysed in group to which they were assigned |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
Principi 1990.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients analysed in group to which they were assigned |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
Sanchez 1990.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only, not enough detail provided |
| Selective reporting (reporting bias) | Unclear risk | Abstract only, not enough detail provided |
Shapiro 1981.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Source of funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patient and physician |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two children were excluded from analyses because the second urine culture was negative. |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
Stahl 1984.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Six girls were lost to follow‐up, in 3 girls the 2nd urine culture was negative and 1 girl had received antibiotics within the previous 2 weeks. One girl in the single‐dose group had an amoxicillin resistant organism and was switched to 10 days TMP‐SMX and then followed in the conventional therapy group. |
| Selective reporting (reporting bias) | Unclear risk | All planned outcomes were reported |
Wallen 1983.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At the 2‐4 day follow‐up, 6 girls were lost to follow‐up. By the 30‐40 day follow‐up, 10 girls were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
BUN ‐ blood urea nitrogen; CRP ‐ C‐reactive protein; ESR ‐ erythrocyte sedimentation rate; NS ‐ not stated; SCr ‐ serum creatinine; SMX ‐ sulfamethoxazole; TMP ‐ trimethoprim; UTI ‐ urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 1982 | Children with pre‐existing conditions, and who have symptoms of pyelonephritis are not reported separately from children with lower UTI |
| Anttila 1980 | Not RCT |
| Arap 1983 | Half of included children had fever and were not reported separately from those without |
| Arguedas 2009 | Children had complicated UTI |
| Arrieta 2001 | Included children had pyelonephritis |
| Asscher 1973 | Not an RCT; screening study only |
| Bahur 2003 | Included children had fever |
| Bailey 1977 | Almost half of the included children had known renal impairment |
| Baker 2001 | Included children were required to be febrile (i.e. systemic illness). |
| Bakkaloglu 1996 | Included children had pyelonephritis |
| Belet 2004 | Prophylaxis for preventing recurrence |
| Bose 1974 | More than half of the included children had pre‐existing renal abnormalities. |
| Bourillon 1994 | Included children had pyelonephritis |
| Caparelli 1983 | Some children had pyelonephritis; unclear how many |
| Carapetis 2001 | Most included children had systemic symptoms |
| Careddu 1987 | Study is conducted in symptomatic and asymptomatic children, but proportion of each is unknown. Also, 11/51 children had known renal abnormalities. |
| Carlsen 1985 | Prophylactic antibiotics |
| Chibante 1994 | Some children had complicated UTI and were not presented separately from those with lower UTI |
| Chong 2003 | Children had systemic symptoms |
| Chrapowicki 1975 | Included children had pyelonephritis |
| Clemente 1994 | Included children had fever |
| Dagan 1992 | Majority of included children had fever |
| De Garate 1988 | One third of included children had cystopyelitis or pyelonephritis and were not reported separately from those without |
| Ellerstein 1977 | Not enough information reported on symptoms to know whether children had lower UTI; 5/34 had reflux and 3/34 had abdominal pain, but other symptoms were not reported. |
| Elo 1975 | Two thirds of included children had renal abnormalities |
| Feldman 1975 | Some children had fever and were not reported separately from those without |
| Fischbach 1989 | Included children had signs of systemic illness (fever) |
| Francois 1995 | Included children had pyelonephritis |
| Francoise 1997 | Included children had pyelonephritis |
| Fuji 1987 | Some children had pyelonephritis and were not reported separately from children without. |
| Gaudreault 1992 | Comparison of short versus standard duration antibiotic for lower UTI ‐ included in Michael 2003 |
| Ginsburg 1982 | Approximately 1/3 of included children had fever and were not reported separately from those without |
| Gok 2001 | Approximately 1/3 of included children had pyelonephritis and over half had urinary tract abnormalities |
| Goldberg 1977 | Children with fever not reported separately from children without. |
| Gonzalez 1985 | 35% of included children had fever and were not reported separately from those without |
| Goos 2006 | Not a RCT, or quasi‐RCT |
| Goos 2007 | Not RCT |
| Goszczyk 2000 | Children received 3 months antibiotic treatment for preventing recurrence. |
| Gould 1975 | Unclear if participants were children. Included participants had prostatitis, acute cystitis, urethritis, and/or trigonitis but results were not reported separately. |
| Granados 1998 | Prophylactic antibiotics |
| Hansen 1981 | Approximately half of children presented with fever and were not reported separately from children without fever. |
| Hayashida 1970 | Some children had pyelonephritis and were not reported separately from those without |
| Helin 1981 | Comparison of short versus standard duration antibiotic for lower UTI ‐ included in Michael 2003 |
| Hoberman 1999 | Included children were required to have a temperature of > 38.3°C |
| Howard 1978 | Just under half of the included children had fever and approximately 65% had symptoms of malaise |
| Johnson 1993 | Comparison of short versus standard duration antibiotic for lower UTI ‐ included in Michael 2003 |
| Jojart 1991 | Comparison of short versus standard duration antibiotic for lower UTI ‐ included in Michael 2003 |
| Kenda 1995 | Not RCT |
| Khan 1987 | Not RCT |
| Kornberg 1994 | Comparison of short versus standard duration antibiotic for lower UTI ‐ included in Michael 2003 |
| Krepler 1976 | Included children had pyelonephritis |
| Lohr 1981 | Comparison of short versus standard duration antibiotic for lower UTI ‐ included in Michael 2003 |
| Lubitz 1984 | Symptoms not reported. 35% of included children had renal abnormalities |
| Madrigal 1988 | Comparison of short versus standard duration antibiotic for lower UTI ‐ included in Michael 2003 |
| Marild 2009 | Included children were required to have fever |
| McCracken 1981 | > 20% of children had fever, abdominal/flank pain and costovertebral tenderness indicating pyelonephritis. |
| Moe 1977 | Not all included children had bacteriologically proven UTI |
| Montini 2007 | Children had pyelonephritis |
| Nolan 1989 | Half of the included children had fever, loin pain and/or back pain |
| Noorbakhsh 2004 | Included children had pyelonephritis |
| Olbing 1971 | Some children had renal abnormalities; although the results refer to patients with and without abnormalities, no numbers are included so data cannot be extracted. |
| Palcoux 1986 | Half of included children had known renal abnormalities |
| Pitt 1982 | More than half of the included children had abdominal pain and/or fever |
| Pylkkanen 1981 | Compared 10‐day treatment with 42‐day treatment in children |
| Repetto 1984 | Children with fever were not analysed separately from children without fever |
| Rodriguez 1983 | Included children had fever |
| Ruberto 1984 | 52% of children had fever and were not reported separately from those without |
| Russo 1989 | Majority of included children had fever |
| Schach 1972 | Most children received concomitant surgical therapy |
| Sember 1985 | Some patients had fever and/or lumber pain and were not reported separately from patients without. |
| Stansfeld 1975 | Symptoms not reported. Approximately half of included children had reflux, but grade of reflux was not reported. |
| Stogmann 1983 | Most included children had fever |
| Sullivan 1980 | No symptoms of UTI reported. Bacteriological definition of UTI only. |
| Tambic 1992 | As per Michael 2003. Study was excluded because significantly more patients (32/59) with pyelonephritis were Included in the 7‐day group compared with 3‐day group (11/58) (Chi² = 15.65, df = 1; P < 0.001), which strongly suggested non‐random allocation. |
| Tapaneya 1999 | Included children were required to have fever |
| Tong 2005 | Some children had pyelonephritis but were not reported separately from those without |
| Toporovski 1988 | Included children presented with fever |
| Varese 1987 | One third of included children had known renal abnormalities and are not presented separately from those without |
| Vlatkovic 1972 | Included children had pyelonephritis |
| Vlatkovic 1974 | Included children had pyelonephritis |
| Weber 1982 | More than half of the included children had fever and were not reported separately from those without |
| Wientzen 1979 | Comparison of short versus standard duration antibiotic for lower UTI ‐ included in Michael 2003 |
| Zaki 1986 | Comparison of short versus standard duration antibiotic for lower UTI ‐ included in Michael 2003 |
randomised controlled trial‐ RCT; UTI ‐ urinary tract infection
Differences between protocol and review
Re‐defined the outcome of recurrence to include re‐infection; we used the definition recurrence (growth of original bacteria) and re‐infection (growth of new bacteria)
In some studies urine samples were collected using non‐invasive methods (clean‐catch, urine collection bag or pad) but if urine was unobtainable, several studies included the option of a supra‐pubic aspiration or catheter samples. We included studies that collected urine using supra‐pubic aspiration or catheters, as the difficulties in collecting urine from children, particularly infants can be problematic.
We initially defined recurrence as at least three episodes of cystitis/lower UTI; however in the included studies any recurrence was reported. We therefore included data on any recurrence.
Adverse effects were to be tabulated ‐ this was not performed.
Risk of bias assessment tool has replaced the quality assessment checklist.
Contributions of authors
Writing of protocol and review: AF, RM
Screening of titles and abstracts: AF, RM
Assessment for inclusion: AF, RM
Quality assessment: AF, RM
Data extraction: AF, RM
Data entry into RevMan: AF
Data analysis: AF, RM
Disagreement resolution: ML, KT
Declarations of interest
Anita Fitzgerald: Some of this work was undertaken when all authors were employed by, or were advisor's to, the National Collaborating Centre for Women’s and Children’s Health which received funding from NICE. The views expressed in this publication are those of the authors and not necessarily those of NICE.
Monica Lakhanpaul: I was the Clinical Director at the National Collaborating Centre for Women's Health and led the development of the NICE Urinary Tract Infection Guideline. I am no longer the Clincial Director but remain on the NCC‐WCH board and i am a NICE Fellow and member of the NHS evidence advisory team.
Edited (no change to conclusions)
References
References to studies included in this review
Ahmed 2001 {published data only}
- Ahmed M, Sloan JE, Clemente E. Clinical efficacy and safety of trimethoprim HC1 oral solution in the treatment of acute otitis media and urinary tract infection in children. Today's Therapeutic Trends 2001;19(2):63‐76. [EMBASE: 2001192856] [Google Scholar]
Avner 1983 {published data only}
- Avner ED, Ingelfinger JR, Herrin JT, Link DA, Marcus E, Tolkoff‐Rubin NE, et al. Single‐dose amoxicillin therapy of uncomplicated pediatric urinary tract infections. Journal of Pediatrics 1983;102(4):623‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CSG 1991 {published data only}
- Anonymous. Short‐term treatment of acute urinary tract infection in girls. Copenhagen Study Group of Urinary Tract Infections in Children. Scandinavian Journal of Infectious Diseases 1991;23(2):213‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fine 1985 {published data only}
- Fine JS, Jacobson MS. Single‐dose versus conventional therapy of urinary tract infections in female adolescents. Pediatrics 1985;75(5):916‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Grimwood 1988 {published data only}
- Grimwood K, Abbott GD, Fergusson DM. Single dose gentamicin treatment of urinary infections in children. New Zealand Medical Journal 1988;101(852):539‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Helin 1984 {published data only}
- Helin I. Three‐day therapy with cephalexin for lower urinary tract infections in children. Scandinavian Journal of Infectious Diseases 1984;16(3):305‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khan 1981 {published data only}
- Khan AJ, Kumar K, Evans HE. Three‐day antimicrobial therapy of urinary tract infection. Journal of Pediatrics 1981;99(6):992‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Komoroski 1999 {published data only}
- Komoroski EM, Lensing SY, Portilla MG, Krebel MS, Krebel SR, Kearns GL. Single‐dose intramuscular ceftriaxone for the treatment of uncomplicated cystitis in children and adolescents. Current Therapeutic Research ‐ Clinical & Experimental 1999;60(11):580‐54. [EMBASE: 1999426148] [Google Scholar]
Lidefelt 1991 {published data only}
- Lidefelt KJ, Bollgren I, Wiman A. Single dose treatment of cystitis in children. Acta Paediatrica Scandinavica 1991;80(6‐7):648‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Malaka‐Zafirui 1984 {published data only}
- Malaka‐Zafiriu K, Papadopoulos F, Avgoustidou‐Savopoulou P, Papachristos F. Comparison of cefadroxil and ampicillin in the treatment of urinary tract infections in children. Clinical Therapeutics 1984;6(2):178‐84. [MEDLINE: ] [PubMed] [Google Scholar]
Mitnik 1985 {published data only}
- Mitnik M, Gasc O, Gonzalez A. Short‐course treatment of urinary tract infections in children [Tratamiento abreviado de la infeccion del tracto urinario en ninos]. Pediatria 1985;28(3‐4):94‐6. [EMBASE: 1987126856] [Google Scholar]
Principi 1990 {published data only}
- Principi N, Corda R, Bassetti D, Varese LA, Peratoner L. Fosfomycin trometamol versus netilmicin in children's lower urinary tract infections. Chemotherapy 1990;36 Suppl 1:41‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sanchez 1990 {published data only}
- Sanchez UC, Balaguer A. Treatment for the urinary low tract infection. A study of 5 antibiotics. Anales Espanoles de Pediatria 1990;33(Suppl 41):85‐6. [Google Scholar]
Shapiro 1981 {published data only}
- Shapiro ED, Wald ER. Single‐dose amoxicillin treatment of urinary tract infections. Journal of Pediatrics 1981;99(6):989‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stahl 1984 {published data only}
- Stahl GE, Topf P, Fleisher GR, Norman ME, Rosenblum HW, Gruskin AB. Single‐dose treatment of uncomplicated urinary tract infections in children. Annals of Emergency Medicine 1984;13(9 Pt 1):705‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wallen 1983 {published data only}
- Wallen L, Zeller WP, Goessler M, Connor E, Yogev R. Single‐dose amikacin treatment of first childhood E. coli lower urinary tract infections. Journal of Pediatrics 1983;103(2):316‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adam 1982 {published data only}
- Adam D, Hager C, Dorn G, Bamberg P. A comparison of co‐trimazine once daily and co‐trimoxazole twice daily in treatment of urinary tract infections in children. Journal of Antimicrobial Chemotherapy 1982;10(5):453‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Anttila 1980 {published data only}
- Anttila R, Julkunen R, Seppanen J. Reduced sulfadiazine dose in the treatment of acute urinary tract infection in children. Annals of Clinical Research 1980, (25):25‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Arap 1983 {published data only}
- Arap S, Arap NW. Clinical study of efficacy and tolerance of trimethoprim plus sulfalene in children with acute urinary tract infections; comparison with co‐trimoxazole [Avaliacao clinica da eficacia e tolerabilidade de kelfiprim suspensao em criancas com infeccoes urinarias agudas. estudo comparativo com cortrimoxazol]. Revista Brasileira de Medicina 1983;40(3):91‐5. [EMBASE: 1983183026] [Google Scholar]
Arguedas 2009 {published data only}
- Arguedas A, Cespedes J, Botet FA, Blumer J, Yogev R, Gesser R, et al. Safety and tolerability of ertapenem versus ceftriaxone in a double‐blind study performed in children with complicated urinary tract infection, community‐acquired pneumonia or skin and soft‐tissue infection. International Journal of Antimicrobial Agents 2009;33(2):163‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arrieta 2001 {published data only}
- Arrieta AC, Bradley JS. Empiric use of cefepime in the treatment of serious urinary tract infections in children. Pediatric Infectious Disease Journal 2001;20(3):350‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Asscher 1973 {published data only}
- Asscher AW, McLachlan MS, Jones RV, Meller S, Sussman M, Harrison S, et al. Screening for asymptomatic urinary‐tract infection in schoolgirls. A two‐centre feasibility study. Lancet 1973;2(7819):1‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bahur 2003 {published data only}
- Bahar A, Karademir F, Unsur T, Ozyurt M, Gocmen I. Comparison of the efficacy and safety of isepamicin and amikacin in the treatment of urinary tract infection in children. Marmara Medical Journal 2003;16(2):107‐12. [EMBASE: 2004349632] [Google Scholar]
Bailey 1977 {published data only}
- Bailey RR, Abbott GD. Treatment of urinary‐tract infection with a single dose of amoxycillin. Nephron 1977;18(6):316‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baker 2001 {published data only}
- Baker PC, Nelson DS, Schunk JE. The addition of ceftriaxone to oral therapy does not improve outcome in febrile children with urinary tract infections. Archives of Pediatrics & Adolescent Medicine 2001;155(2):135‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bakkaloglu 1996 {published data only}
- Bakkaloglu A, Saatci U, Soylemezoglu O, Ozen S, Topaloglu R, Besbas N, et al. Comparison of ceftriaxone versus cefotaxime for childhood upper urinary tract infections. Journal of Chemotherapy 1996;8(1):59‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Belet 2004 {published data only}
- Belet N, Islek I, Belet U, Sunter AT, Kucukoduk S. Comparison of trimethoprim‐sulfamethoxazole, cephadroxil and cefprozil as prophylaxis for recurrent urinary tract infections in children. Journal of Chemotherapy 2004;16(1):77‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bose 1974 {published data only}
- Bose W, Karama A, Linzenmeier G, Olbing H, Wellmann P. Controlled trial of co‐trimoxazole in children with urinary‐tract infection. Bacteriological efficacy and haematological toxicity. Lancet 1974;2(7881):614‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bourillon 1994 {published data only}
- Bourillon A, Burgio GR, Steffens L, Kranz A, Noack M, Weippl G, et al. Cefetamet pivoxil in the treatment of acute urinary tract infections in children. Current Therapeutic Research ‐ Clinical & Experimental 1994;55(10):1161‐8. [EMBASE: 1994329580] [Google Scholar]
Caparelli 1983 {published data only}
- Caparelli AJ, Rios L, Reimondi R. Comparative study of the combination rifampicin‐trimetoprim vs cotrimoxazole in acute and chronic urinary infections in children [Estudio comparativo de la combinacion rifampicina + trimetoprim vs. cotrimoxazol en las infecciones agudas o cronicas del tracto urinario en ninos]. Investigacion Medica Internacional 1983;10(1):20‐9. [EMBASE: 1983143888] [Google Scholar]
Carapetis 2001 {published data only}
- Carapetis JR, Jaquiery AL, Buttery JP, Starr M, Cranswick NE, Kohn S, et al. Randomized, controlled trial comparing once daily and three times daily gentamicin in children with urinary tract infections. Pediatric Infectious Disease Journal 2001;20(3):240‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Careddu 1987 {published data only}
- Careddu P, Borzani M, Scotti L, Varotto F, Garlaschi L, Fontana P. Treatment of lower urinary tract infections in children: single dose fosfomycin trometamol versus pipemidic acid. Chemioterapia 1987;6(4):290‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Carlsen 1985 {published data only}
- Carlsen NL, Hesselbjerg U, Glenting P. Comparison of long‐term, low‐dose pivmecillinam and nitrofurantoin in the control of recurrent urinary tract infection in children. An open, randomized, cross‐over study. Journal of Antimicrobial Chemotherapy 1985;16(4):509‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chibante 1994 {published data only}
- Chibante A, Peixoto E, Lejeune R, Winter K, Kissling M. Clinical efficacy and safety of cefetamet pivoxil in toddlers. International Journal of Antimicrobial Agents 1994;4(3):203‐10. [EMBASE: 1994236751] [DOI] [PubMed] [Google Scholar]
Chong 2003 {published data only}
- Chong CY, Tan AS, Ng W, Tan‐Kendrick A, Balakrishnan A, Chao SM. Treatment of urinary tract infection with gentamicin once or three times daily. Acta Paediatrica 2003;92(3):291‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Chrapowicki 1975 {published data only}
- Chrapowicki T, Krzyzanowska‐Rogoziska T, Kurowska D. Treatment of acute and chronic urinary tract infections in children with an urinary chemotherapeutic agent [Die Behandlung von aktuen und chronischen Harnwegsinfekten bei Kindern mit eimen Harnweg‐Chemotherapeutikum]. Zeitschrift fur Allgemeinmedizin 1975;51(27):1215‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Clemente 1994 {published data only}
- Clemente E, Solli R, Mei V, Cera R, Caramia G, Carnelli V, et al. Therapeutic efficacy and safety of pidotimod in the treatment of urinary tract infections in children. Arzneimittel‐Forschung 1994;44(12A):1490‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Dagan 1992 {published data only}
- Dagan R, Einhorn M, Lang R, Pomeranz A, Wolach B, Miron D, et al. Once daily cefixime compared with twice daily trimethoprim/sulfamethoxazole for treatment of urinary tract infection in infants and children. Pediatric Infectious Disease Journal 1992;11(3):198‐203. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Garate 1988 {published data only}
- Gárate J, Pérez C, García‐Ibarra F, Díaz F, Aguilar L, Velasco J. Therapy of paediatric urinary tract infections with cefonicid. Acta Pediátrica Española 1988;46(3):183‐6. [EMBASE: 1988110858] [Google Scholar]
Ellerstein 1977 {published data only}
- Ellerstein NS, Sullivan TD, Baliah T, Neter E. Trimethoprim/sulfamethoxazole and ampicillin in the treatment of acute urinary tract infections in children: a double‐blind study. Pediatrics 1977;60(2):245‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Elo 1975 {published data only}
- Elo J, Ahava K. Cephalexin compared with ampicillin in urinary tract infections in children. Journal of Antimicrobial Chemotherapy 1975;1(3 Suppl):85‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feldman 1975 {published data only}
- Feldman W, Johnson DM, Newberry P, Weldon A, Naidoo S. Comparison of trimethoprim‐sulfamethoxazole with sulfamethoxazole in urinary tract infections of children. Canadian Medical Association Journal 1975;112(13 Spec No):19‐21. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Fischbach 1989 {published data only}
- Fischbach M, Simeoni U, Mengus L, Jehl F, Monteil H, Geisert J, et al. Urinary tract infections with tissue penetration in children: cefotaxime compared with amoxycillin/clavulanate. Journal of Antimicrobial Chemotherapy 1989;24 Suppl B:177‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Francois 1995 {published data only}
- Francois P, Croize J, Bost C, Wollschlager K. Comparative study of cefixime versus amoxicillin‐clavulanic acid combination in the oral treatment of urinary tract infections in children [Etude comparant le cefixime a l'association amoxicilline‐acide clavulanique dans le traitement par voie orale des infections urinaires de l'enfant]. Archives de Pediatrie 1995;2(2):136‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Francoise 1997 {published data only}
- Francois P, Bensman A, Begue P, Artaz M‐A, Coudeville L, Lebrun T, et al. Assessment of the efficacy and cost efficiency of two strategies in the treatment of acute pyelonephritis in children: Oral cefixime or parenteral ceftriaxone after an initial IV combination therapy [Evaluation de l'efficacite et du coat de deux strategies therapeutiques dans les pyelonephrites de l'enfant: Cefixime per os versus ceftriaxone parenterale en relais d'une bitherapie intraveineuse]. Medecine et Maladies Infectieuses 1997;27(Spec. Iss. June):667‐73. [EMBASE: 1997230388] [Google Scholar]
Fuji 1987 {published data only}
- Fujii R, Shinozaki T, Meguro H, Arimasu O, Izumi K, Osano M, et al. Comparative, controlled study on an ampicillin suppository (KS‐R 1) with an oral form of ampicillin in urinary tract infections. Japanese Journal of Antibiotics 1987;40(3):476‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Gaudreault 1992 {published data only}
- Gaudreault P, Beland M, Girodias JB, Thivierge RL. Single daily doses of trimethoprim/sulphadiazine for three or 10 days in urinary tract infections. Acta Paediatrica 1992;81(9):695‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ginsburg 1982 {published data only}
- Ginsburg CM, McCracken GH, Petruska M. Once‐daily cefadroxil versus twice‐daily cefaclor for treatment of acute urinary tract infections in children. Journal of Antimicrobial Chemotherapy 1982;10 Suppl B:53‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gok 2001 {published data only}
- Gok F, Duzova A, Baskin E, Ozen S, Besbas N, Bakkaloglu A. Comparative study of cefixime alone versus intramuscular ceftizoxime followed by cefixime in the treatment of urinary tract infections in children. Journal of Chemotherapy 2001;13(3):277‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goldberg 1977 {published data only}
- Goldberg IL, Weidman ML, Caplan AH, Williams TW, Stebbing N. Sulfacytine compared with sulfisoxazole in children with acute uncomplicated urinary tract infections. Current Therapeutic Research, Clinical & Experimental 1977;21(4):468‐72. [Google Scholar]
Gonzalez 1985 {published data only}
- Gonzalez E, Carranza C, Soto C, Romero P. Comparative study of the activity of trimethoprim‐sulfamethopyrazine and nitrofurantoin in urinary infections of children [Estudio comparativo sobre la actividad de trimetoprim‐sulfametopirazina y nitrofurantoina en infecciones urinarias del nino]. Revista Chilena de Pediatria 1985;56(5):341‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Goos 2006 {published data only}
- Goos KH, Albrecht U, Schneider B. Efficacy and safety profile of a herbal drug containing nasturtium herb and horseradish root in acute sinusitis, acute bronchitis and acute urinary tract infection in comparison with other treatments in the daily practice/results of a prospective cohort study [Wirksamkeit und Verträglichkeit eines pflanzlichen Arzneimittels mit Kapuzinerkressenkraut und Meerrettich bei akuter Sinusitis, akuter Bronchitis und akuter Blasenentzündung im Vergleich zu anderen Therapien unter den Bedingungen der täglichen Praxis]. Arzneimittel‐Forschung 2006;56(3):249‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goos 2007 {published data only}
- Goos KH, Albrecht U, Schneider B. On‐going investigations on efficacy and safety profile of a herbal drug containing nasturtium herb and horseradish root in acute sinusitis, acute bronchitis and acute urinary tract infection in children in comparison with other antibiotic treatments [Aktuelle Untersuchungen zur Wirksamkeit und Verträglichkeit eines pflanzlichen Arzneimittels mit Kapuzinerkressenkraut und Meerrettich bei akuter Sinusitis, akuter Bronchitis und akuter Blasenentzündung bei Kindern im Vergleich zu anderen Antibiotika]. Arzneimittel‐Forschung 2007;57(4):238‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goszczyk 2000 {published data only}
- Goszczyk A, Bochniewska V, Jung A. Clinical assessment of Uro‐Vaxom in the treatment and prophylaxis of recurrent urinary tract infection in children: preliminary results [Ocena kliniczna przydatnosci preparatu Uro‐Vaxom w leczeniu i profilaktyce nawracajacych zakzen ukladu moczowego u dzieci‐‐doniesienie wstepne]. Polski Merkuriusz Lekarski 2000;8(46):242‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Gould 1975 {published data only}
- Gould S. Urinary tract disorders. Clinical comparison of flavoxate and phenazopyridine. Urology 1975;5(5):612‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Granados 1998 {published data only}
- Granados EA. Which treatment should children with recurrent urinary infections, without anatomical anomalies, receive? [Que tratamiento deben de recibir los ninos con infecciones de orina recurrentes, sin anomalias anatomicas?]. Archivos Espanoles de Urologia 1998;51(4):354‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Hansen 1981 {published data only}
- Hansen PH, Kristensen KH, Lenler‐Eriksen HA, Pagh J, Ostergard JE. Pivmecillinam (Selexid) in acute cystitis. A comparative study of 3‐ and 7‐day treatments [Pivmecillinam (Selexid) til acute cystitis. En sammenlingning af 3 og 7 dages behandling]. Ugeskrift for Laeger 1981;143(11):670‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Hayashida 1970 {published data only}
- Hayashida K, Eto K, Shigematsu S. Clinical evaluation of rifampicin in urinary tract infections by double blind method. Japanese Journal of Antibiotics 1970;23(4):411‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Helin 1981 {published data only}
- Helin I. Short‐term treatment of lower urinary tract infections in children with trimethoprim/sulphadiazine. Infection 1981;9(5):249‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hoberman 1999 {published data only}
- Hoberman A, Wald ER, Hickey RW, Baskin M, Charron M, Majd M, et al. Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics 1999;104(1 Pt 1):79‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Howard 1978 {published data only}
- Howard JB, Howard JE Sr. Trimethoprim‐sulfamethoxazole vs sulfamethoxazole for acute urinary tract infections in children. American Journal of Diseases of Children 1978;132(11):1085‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 1993 {published data only}
- Johnson CE, Maslow JN, Fattlar DC, Adams KS, Arbeit RD. The role of bacterial adhesins in the outcome of childhood urinary tract infections. American Journal of Diseases of Children 1993;147(10):1090‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jojart 1991 {published data only}
- Jojart G. Comparison of 3‐day versus 14‐day treatment of lower urinary tract infection in children. International Urology & Nephrology 1991;23(2):129‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kenda 1995 {published data only}
- Kenda R, Kaplar‐Vucevac M, Jeler D, Kunstelj T, Truden E, Smole A. The treatment of bacterial infection of the urinary tract in infants and small children using Amoksiklav [Lijecenje bakterijskih infekcija mokracnih putova u dojencadi i male djece Amoksiklavom]. Paediatria Croatica 1995;39(3):155‐8. [EMBASE: 1996020626] [Google Scholar]
Khan 1987 {published data only}
- Khan AJ, Kumar K, Evans HE. Single‐dose gentamicin therapy of recurrent urinary tract infection in patients with normal urinary tracts. Journal of Pediatrics 1987;110(1):131‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kornberg 1994 {published data only}
- Kornberg AE, Sherin K, Veiga P, Mydlow PK, Collins JJ, Feld LG. Two‐day therapy with cefuroxime axetil is effective for urinary tract infections in children. American Journal of Nephrology 1994;14(3):169‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krepler 1976 {published data only}
- Krepler P, Steinbock H. Clinical testing of a combination of sulfametrol and trimethoprim (Lidaprim) in urinary tract infections of children [Klinische Prufung einer Kombination von Sulfametrol und Trimethoprim (Lidaprim) bei Harnwegsinfektionen von Kindern]. Wiener Medizinische Wochenschrift 1976;126(20‐22):317‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Lohr 1981 {published data only}
- Lohr JA, Hayden GF, Kesler RW, Gleason CH, Wood JB, Ford RF, et al. Three‐day therapy of lower urinary tract infections with nitrofurantoin macrocrystals: a randomized clinical trial. Journal of Pediatrics 1981;99(6):980‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lubitz 1984 {published data only}
- Lubitz L, Cullen J, Nolan T, Oberklaid F. A prospective study comparing single dose trimethoprim with a 7 day course of co‐trimoxazole in the treatment of urinary tract infection in children [abstract]. Australian Paediatric Journal 1984;20(3):236‐7. [Google Scholar]
Madrigal 1988 {published data only}
- Madrigal G, Odio CM, Mohs E, Guevara J, McCracken GH Jr. Single dose antibiotic therapy is not as effective as conventional regimens for management of acute urinary tract infections in children. Pediatric Infectious Disease Journal 1988;7(5):316‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marild 2009 {published data only}
- Marild S, Jodal U, Sandberg T. Ceftibuten versus trimethoprim‐sulfamethoxazole for oral treatment of febrile urinary tract infection in children. Pediatric Nephrology 2009;24(3):521‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McCracken 1981 {published data only}
- McCracken GH, Ginsburg CM, Namasonthi V, Petruska M. Evaluation of short‐term antibiotic therapy in children with uncomplicated urinary tract infections. Pediatrics 1981;67(6):796‐801. [MEDLINE: ] [PubMed] [Google Scholar]
Moe 1977 {published data only}
- Moe OJ, Meberg A, Eng J. Ampicillin and pivampicillin in the treatment of urinary tract infection in children. Scandinavian Journal of Infectious Diseases 1977;9(1):31‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Montini 2007 {published data only}
- Montini G, Murer L, Gobber D, Comacchio S, Toffolo A, Dall'Amico R, et al. Oral vs initial intravenous antibiotic treatment of urinary tract infections in children: a multicentre study [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):816. [Google Scholar]
- Montini G, Toffolo A, Zucchetta P, Dall'Amico R, Gobber D, Calderan A, et al. Antibiotic treatment for pyelonephritis in children: multicentre randomised controlled non‐inferiority trial. BMJ 2007;335(7616):386. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nolan 1989 {published data only}
- Nolan T, Lubitz L, Oberklaid F. Single dose trimethoprim for urinary tract infection. Archives of Disease in Childhood 1989;64(4):581‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noorbakhsh 2004 {published data only}
- Noorbakhsh S, Lari AR, Masjedian F, Mostafavi H, Alaghehbandan R. Comparison of intravenous aminoglycoside therapy with switch therapy to cefixime in urinary tract infections. Saudi Medical Journal 2004;25(10):1513‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Olbing 1971 {published data only}
- Olbing H, Neussel H, Senge T, Hagel K, Linzenmeier G. Problems in the therapy of pseudomonas infections of the urinary tract. Alternating comparison of carbenicillin and gentamycin in children [Zur Problematik der Behandlung von Pseudomonas‐Infektionen der Harnwege. Alternierender Vergleich von Carbenicillin und Gentamycin bei Kindern]. Deutsche Medizinische Wochenschrift 1971;96(5):183‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palcoux 1986 {published data only}
- Palcoux JB, Raynaud EJ, Borderon JC, Dalous A, Geisert J, Pennaforte F, et al. Clinical trial of a clavulanic acid‐amoxicillin combination in urinary infections in children [Essai clinique de l'association acide clavulanique‐amoxicilline dans les infections urinaries de l'enfant]. Annales de Pediatrie 1986;33(8):761‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Pitt 1982 {published data only}
- Pitt WR, Dyer SA, McNee JL, Burke JR. Single dose trimethoprim‐sulphamethoxazole treatment of symptomatic urinary infection. Archives of Disease in Childhood 1982;57(3):229‐31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pylkkanen 1981 {published data only}
- Pylkkanen J, Vilska J, Koskimies O. The length of antimicrobial therapy in upper vs. lower urinary tract infection of childhood. Acta Paediatrica Scandinavica 1981;70(6):885‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Repetto 1984 {published data only}
- Repetto HA, MacLoughlin GJ. Single‐dose cefotaxime in the treatment of urinary tract infections in children: a randomized clinical trial. Journal of Antimicrobial Chemotherapy 1984;14 Suppl B:307‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rodriguez 1983 {published data only}
- Rodriguez W, Delucchi C, Bidegain MA, Rodriguez MS, Gleisner A, Figueroa S. Treatment of urinary tract infections in children with trimethoprim‐sulfamethoxypyridazine [Tratamiento de infecciones urinarias en ninas con trimetoprim‐sulfametopirazina]. Revista Chilena de Pediatria 1983;54(6):402‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Ruberto 1984 {published data only}
- Ruberto U, D'Eufemia P, Chiummariello R, Giardini O. Results of 3 days and 10 days treatment with nitrofurantoin or cotrimoxazole in urinary tract infections of childhood [Risultati della terapia per tre o dieci giorni con nitrofurantoina o cotrimoxazolo nelle infezioni urinarie del bambino]. Pediatria Medica e Chirurgica 1984;6(1):73‐5. [EMBASE: 1985042629] [PubMed] [Google Scholar]
Russo 1989 {published data only}
- Russo RM, Gururaj VJ, Laude TA, Rajkumar SV, Allen JE. The comparative efficacy of cephalexin and sulfisoxazole in acute urinary tract infection in children. Clinical Pediatrics 1989;16(1):83‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schach 1972 {published data only}
- Schach H, Scheidt J, Neussel H. Results of treatment with trimethoprim‐sulfamethoxazole in nonspecific urinary tract infections in pediatric urology [Behandlungsergebnisse mit der Kombination Trimethoprim‐Sulfamethoxazol bei unspezifischen chronischen Harnwegsinfekten in der Kinderurologie]. Monatsschrift fur Kinderheilkunde 1972;120(9):346‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Sember 1985 {published data only}
- Sember ME, Mosso F, Meregalli MA. Randomized bicentric study carried out with kelfiprim and cotrimoxazole in children with acute or chronic urinary infections [Estudio randomizado bicentrico realizado con kelfiprim y cotrimoxazol en ninos portadores de infecciones urinarias agudas o cronicas]. Prensa Medica Argentina 1985;72(9):301‐5. [EMBASE: 1986006721] [Google Scholar]
Stansfeld 1975 {published data only}
- Stansfeld JM. Duration of treatment for urinary tract infections in children. BMJ 1975;3(5975):65‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stogmann 1983 {published data only}
- Stogmann W, Fink M, Blumel P. Comparative study of Kelfiprim syrup in urinary tract and lower respiratory tract infections in children. Clinical Trials Journal 1984;21(3):147‐55. [EMBASE: 1984123036] [Google Scholar]
Sullivan 1980 {published data only}
- Sullivan TD, Ellerstein NS, Neter E. The effects of ampicillin and trimethoprim/sulfamethoxazole on the periurethral flora of children with urinary tract infection. Infection 1980;8 Suppl 3:S 339‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tambic 1992 {published data only}
- Tambic T, Oberiter V, Delmis J, Tambic A. Diagnostic value of a P‐fimbriation test in determining duration of therapy in children with urinary tract infections. Clinical Therapeutics 1992;14(5):667‐71. [MEDLINE: ] [PubMed] [Google Scholar]
Tapaneya 1999 {published data only}
- Tapaneya‐Olarn C, Tapaneya‐Olarn W, Pitayamornwong V, Petchthong T, Tangnararatchakit K. Single daily dose of gentamicin in the treatment of pediatric urinary tract infection. Journal of the Medical Association of Thailand 1999;82 Suppl 1:S93‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Tong 2005 {published data only}
- Tong X‐S, Wang E‐Z, Feng L‐P. Clinical study of oral cefixime in the treatment of urinary tract infection in 35 children. Chinese Journal of Antibiotics 2005;30(5):311‐3. [EMBASE: 2005325330] [Google Scholar]
Toporovski 1988 {published data only}
- Toporovski J, Mimica I, Mimica L, Penon J, Cunha W, Umeda M. Treatment of urinary tract infections in children. Jornal de Pediatria 1988;64(9):371‐4. [EMBASE: 1989130628] [Google Scholar]
Varese 1987 {published data only}
- Varese LA. Trometamol salt of fosfomycin versus netilmicin: randomized multicenter study in children's lower urinary tract infections. European Urology 1987;13 Suppl 1:119‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vlatkovic 1972 {published data only}
- Vlatkovi G, Babi I. Treatment of urinary tract infection in the child using Ceporex (cephalexin) [Lijecenje infekcija mokracnog trakta djece Ceporexom (cefaleksin)]. Lijecnicki Vjesnik 1972;94(10):518‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Vlatkovic 1974 {published data only}
- Vlatkovi G, Babi I. Treatment of urinary tract infections in children by Urovalidin [Lijecenje infekcija mokracnog trakta djece urovalidinom]. Lijecnicki Vjesnik 1974;96(10):617‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Weber 1982 {published data only}
- Weber HP, Aberfeld U, Hildenbrand G, Knopfle G. Treatment of first urinary tract infection in children with cotrifamole and cotrimoxazole. A double‐blind study [Behandlung von ersten Harnwegsinfekten im Kindesalter mit Cotrifamol und Cotrimoxazol]. Deutsche Medizinische Wochenschrift 1982;107(24):937‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Wientzen 1979 {published data only}
- Wientzen RL, McCracken GH Jr, Petruska ML, Swinson SG, Kaijser B, Hanson LA. Localization and therapy of urinary tract infections of childhood. Pediatrics 1979;63(3):467‐74. [MEDLINE: ] [PubMed] [Google Scholar]
Zaki 1986 {published data only}
- Zaki M, Helin I. Nalidixic acid and trimethoprim/sulphamethoxazole as alternatives for short‐term treatment of urinary infections. Annals of Tropical Paediatrics 1986;6(3):205‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Coulthard 1997
- Coulthard MG, Lambert HJ, Keir MJ. Occurrence of renal scars in children after their first referral for urinary tract infection. BMJ 1997;315(7113):918‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzgerald 2012
- Fitzgerald A, Mori R, Lakhanpaul M. Interventions for covert bacteriuria in children. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD006943.pub2] [DOI] [PubMed] [Google Scholar]
Hellstrom 1991
- Hellstrom A, Hanson E, Hansson S, Hjalmas K, Jodal U. Association between urinary symptoms at 7 years old and previous urinary tract infection. Archives of Disease in Childhood 1991;66(2):232‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodson 2007
- Hodson EM, Willis NS, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003772.pub3] [DOI] [PubMed] [Google Scholar]
Keren 2002
- Keren R, Chan E. A meta‐analysis of randomized, controlled trials comparing short‐ and long‐course antibiotic therapy for urinary tract infections in children. Pediatrics 2002;109(5):E70‐0. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leibovici 1987
- Leibovici L, Alpert G, Laor A, Kalter‐Leibovici O, Danon YL. Urinary tract infections and sexual activity in young women. Archives of Internal Medicine 1987;147(2):345‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Messi 1988
- Messi G, Peratoner L, Paduano L, Marchi AG. Epidemiology of urinary tract infections and vesico‐ureteral reflux in children. Helvetica Paediatrica Acta 1988;43(5‐6):389‐96. [MEDLINE: ] [PubMed] [Google Scholar]
Michael 2003
- Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003966] [DOI] [PubMed] [Google Scholar]
Milo 2005
- Milo G, Katchman E, Paul M, Christiaens T, Baerheim A, Leibovici L. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004682.pub2] [DOI] [PubMed] [Google Scholar]
Stanley 2005
- Stanley R, Pagon Z, Bachur R. Hyperpyrexia among infants younger than 3 months. Pediatric Emergency Care 2005;21(5):291‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tran 2001
- Tran D, Muchant DG, Aronoff SC. Short‐course versus conventional length antimicrobial therapy for uncomplicated lower urinary tract infections in children: A meta‐analysis of 1279 patients. Journal of Pediatrics 2001;139(1):93‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vernon 1997
- Vernon SJ, Coulthard MG, Lambert HJ, Keir MJ, Matthews JN. New renal scarring in children who at age 3 and 4 years had had normal scans with dimercaptosuccinic acid: follow up study. BMJ 1997;315(7113):905‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Winberg 1974
- Winberg J, Andersen HJ, Bergstrom T, Jacobsson B, Larson H, Lincoln K. Epidemiology of symptomatic urinary tract infection in childhood. Acta Obstetricia et Gynecologica Scandinavica ‐ Supplement 1974, (252):1‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fitzgerald 2007
- Fitzgerald A, Lee CW, Mori R. Antibiotics for treating uncomplicated urinary tract infection in children. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006857] [DOI] [PMC free article] [PubMed] [Google Scholar]


