Abstract
Objective:
Heated and humidified high flow nasal cannula (HFNC) is an increasingly used form of noninvasive respiratory support with the potential to generate significant tracheal pressure. The aim of this study was to quantify the pressure generated by HFNC within the trachea in anatomically correct, pediatric airway models.
Methods:
3D-printed upper airway models of a preterm neonate, term neonate, toddler, and small child were connected to a spontaneous breathing computerized lung model at age-appropriate ventilation settings. Two commercially available HFNC systems were applied to each airway model at increasing flows and the positive end-expiratory pressure (PEEP) was recorded at the level of the trachea.
Results:
Increasing HFNC flow produced a quadratically curved increase in tracheal pressure in closed-mouth models. The maximum flow tested in each model generated a tracheal pressure of 7 cm H2O in the preterm neonate, 10 cm H2O in the term neonate, 9 cm H2O in the toddler, and 24 cm H2O in the small child. Tracheal pressure decreased by at least 50% in open-mouth models.
Conclusions:
HFNC was found to demonstrate a predictable flow-pressure relationship that achieved sufficient distending pressure to consider treatment of pediatric obstructive sleep apnea and tracheomalacia in the closed-mouth models tested.
Keywords: Pediatric, OSA, HFNC, trachea, CPAP, tracheomalacia
1.1. Introduction
The first-line treatment for pediatric obstructive sleep apnea (OSA) is tonsillectomy and/or adenoidectomy when indicated.1 In other cases, continuous positive airway pressure (CPAP) is an available non-operative treatment modality for pediatric OSA.2 Effectiveness of CPAP in the pediatric population is limited by poor compliance.3 Many children, particularly those with craniofacial anomalies or patients requiring nasogastric tubes, are vulnerable to adverse effects of ill-fitting CPAP masks which can lead to skin irritation or breakdown, nasal trauma, and air leak around the mask rendering the therapy ineffective.4,5 Heated and humidified high-flow nasal cannula (HFNC) has greater patient comfort compared to CPAP and many studies show that HFNC is safe and well-tolerated in children.6–8 HFNC has been shown to treat OSA in CPAP-intolerant children by decreasing apnea-hypopnea index (AHI) and increasing oxygen saturation nadir.9–11 Ignatiuk et al. demonstrated that HFNC was able to treat OSA that persisted despite prior surgical management of adenotonsillar hypertrophy.10
HFNC also plays an important role in infants with respiratory failure, notably when weaning from non-invasive respiratory support such as CPAP.12,13 Of particular interest is the ability of HFNC to generate CPAP-like distending pressure and its theoretical application in the nonsurgical management of airway malacia. 7,8,14,15 In congenital airway malacia, both pediatric otolaryngologists and pulmonologists assist with diagnosis via nasopharyngolaryngoscopy and/or bronchoscopy.16 In the case of tracheomalacia, dynamic evaluation with tracheobronchoscopy and positive end expiratory pressure (PEEP) titration can help determine optimal to prevent collapse of the trachea.17 In fact, Vézina et al. demonstrated three cases of severe tracheomalacia successfully treated with home HFNC at 5 to 10 L/min on room air prior to complete weaning.18
The proposed mechanism of HFNC is multifactorial—Nasopharyngeal and oropharyngeal dead space is continuously flushed with oxygen while carbon dioxide is cleared and positive pressure is generated.7,14,19 Prior animal and in-vitro studies have shown that increasing flow leads to a higher positive pressure in the airway.8,19 In humans, there is debate on how much PEEP is provided by HFNC. Factors that influence PEEP include patient size, habitus, positioning, nasal prong sizing, flow delivered, and open versus closed mouth breathing.15,20,21 Ultimately, this flow-PEEP relationship has never been quantified at the level of the trachea in the various airway sizes encountered in the pediatric population.
The objective of this study was to quantify the effect of HFNC on airway pressure at the level of the trachea in anatomically correct, age-specific pediatric airways in a breathing lung model. We hypothesized that there is a flow-dependent effect on the tracheal pressure and that this effect changes based on the size of the airway.
2.1. Methods
A detailed description of the experimental setup, materials and methods is described in a previously published study.15 In the current study, tracheal pressure is the variable of interest, while the prior study focused on carbon dioxide clearance and intrapulmonary pressure generation.
2.1.1. Pediatric Airway and Lung Models
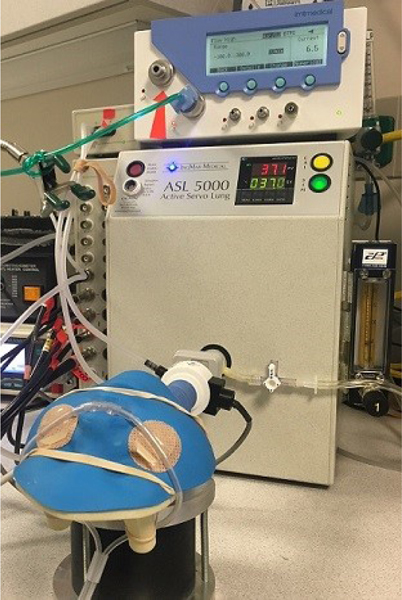
Airway models were printed using 3-dimensional rapid prototyping devices based on CT scans of the upper airways of a preterm neonate (28 weeks, 1 kg), term neonate (38 weeks, 4 kg), toddler (17 months, 10 kg), and a small child (5 years, 20 kg). Preterm neonate and small child models had established oral airway openings, so open-mouth and closed-mouth conditions were simulated in these models by oral occlusion using an airtight polymer. Term neonate and toddler did not have oral airway openings, so only closed-mouth conditions were simulated. Airway volumes were quantified with water to approximate previously published age-specific extrathoracic dead space values as show in Table 1 (adapted from Nielsen et al.).15,22 Models were attached to the ASL 5000 Test Lung (Ingmar Medical, Pittsburgh, Pennsylvania) in series with a pneumotachometer at the level of the trachea (Figure 1). Spontaneous breathing was simulated using age-specific normal values.23–25 The inspiratory-to-expiratory ratio was held constant at 1:3, and inspiratory effort was adjusted to maintain constant tidal volume (6 mL/kg) in each model at different HFNC flows.
Table 1:
Models, Respiratory Configuration, Nasopharyngeal volume, Flows
| Variable | Preterm Neonate (1 kg) | Term Neonate (4 kg) | Toddler (10 kg) | Small Child (20 kg) |
|---|---|---|---|---|
|
| ||||
| Tidal volume, mL | 6 | 24 | 60 | 120 |
| Respiratory Rate (breaths/min) | 50 | 30 | 25 | 20 |
| Airway volume, mL (above the tracheal sensor) | 1.5 | 7.7 | 10.2 | 16.3 |
| Flow settings tested, L/min | 2, 3, 4, 5, 6, 7, 8 | 2, 3, 4, 5, 6, 7, 8 | 2, 4, 6, 8, 10, 12, 14, 16, 18, 20 | 4, 8, 12, 16, 20, 24, 28, 32 |
Figure 1:
Experimental Setup. 3D-printed upper airway model with HFNC in place and attached to computerized breathing lung. Tracheal pressure sensor (white) in series with airway/lung model.
2.1.2. HFNC Systems and Nasal Prongs
Infant and Pediatric Optiflow Junior (Fisher & Paykel Healthcare, Auckland, New Zealand) and Precision Flow (Vapotherm, Exeter, New Hampshire) HFNC systems were used. Flows were confirmed with both systems using a calibrated, unidirectional gas flow analyzer (PF-350, ImtMedical, Buchs, Switzerland) before testing at each flow condition. Flow ranges were chosen based on flows from previously published age-specific studies.21,22 HFNC systems were preset to 37° C. HFNC sizing was based on manufacturer recommendations. The prongs were measured and secured in the models’ nares to achieve approximately 50% occlusion.
2.1.3. Experimental Procedures/Data Acquisition
PEEP data at the level of the trachea (Figure 1, white sensor) were collected in triplicate for each model and system. Continuous analog output of pressure data was processed using an analog-to-digital converter (ADInstruments, Colorado Springs, Colorado). Tracheal flow was measured using a calibrated heated pneumotachometer head (Hans Rudolph, Shawnee, Kansas) and acquired using a PowerLab Spirometer Pod (ADInstruments). All data were displayed and recorded digitally with LabChart 5.5.6 data analysis software (ADInstruments). Pressure and flow measurements were collected during spontaneous breathing for 1 min before and for 6 min after cannula placement to ensure a steady state had been achieved within the system. Immediately following cannula placement, ASL lung model muscle pressures were adjusted to maintain targeted, age-specific tidal volumes (Table 1).
2.1.4. Data Analysis
Tracheal pressure values were measured at zero flow (for 1 minute) at end expiration with each respiratory cycle. Baseline (no flow) and response variable (with flow) of the tracheal pressures were determined by the arithmetic means of individual breath measurements acquired during the first minute preceding and fifth minute following cannula placement, respectively. In each model, the mean pressure value of the three data runs at gradually increased flows was plotted, and a best-fit trend line applied to elucidate the flow-tracheal pressure relationship. Data were analyzed with a simple linear regression model using mean tracheal pressure as the dependent variable and flow as the independent or input variable, including both a linear term (flow) and a quadratic term (flow2) as main input variables and allowing effect modification of the airway model and the HFNC system. All data were analyzed in R (v.3.5.1, R Foundation for Statistical Computing, Vienna, Austria).26 Regression analyses were done separately in each model and results are reported as trendline equation of the best-fit line, coefficient of determination (R2), and P-value. Separate regression analyses were done to determine tracheal pressure differences between open mouth versus closed mouth models (Preterm Neonate and Small Child).
3.1. Results
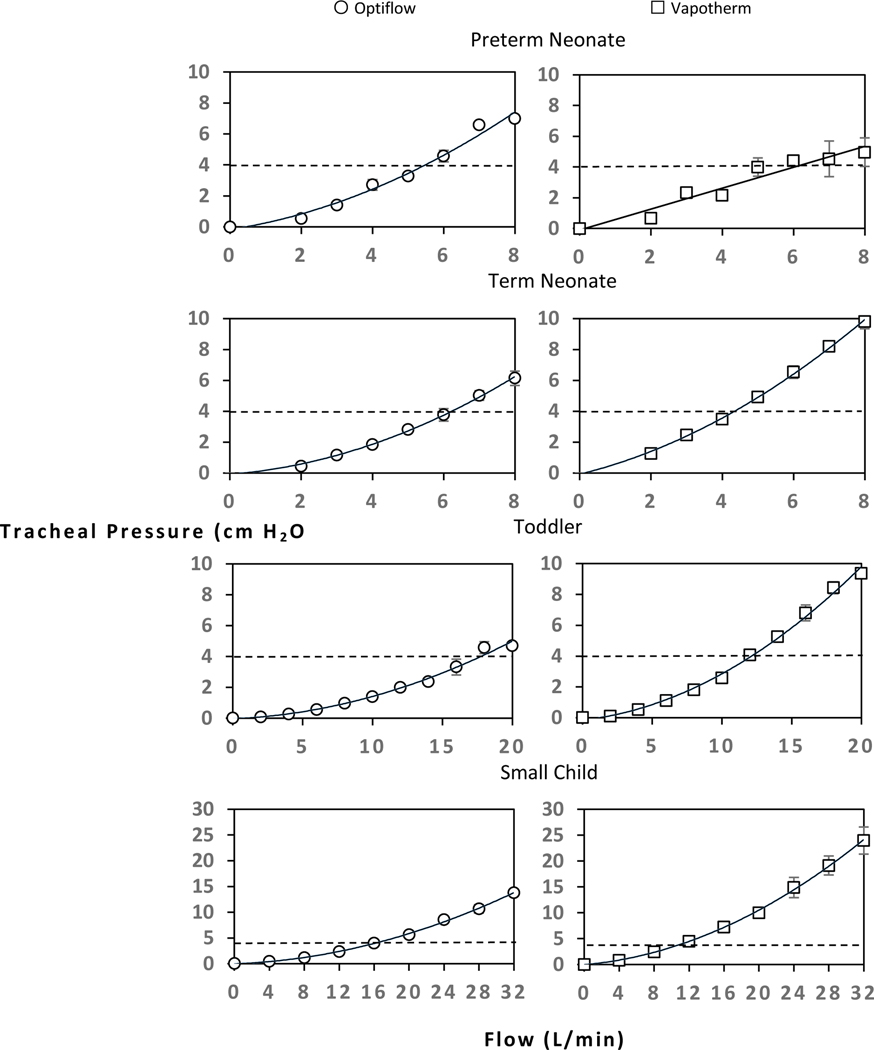
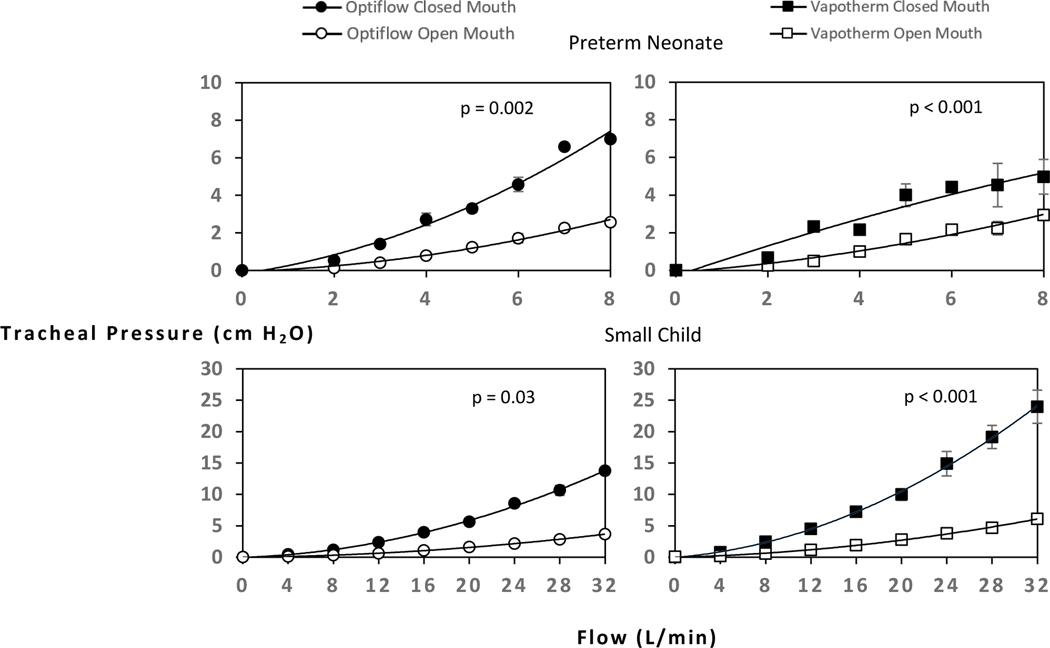
There was an overall increase in tracheal PEEP as flow increased. All models except the preterm neonate (closed mouth) exhibited a quadratic curved relationship between flow and tracheal pressure, and the preterm neonate (closed mouth) exhibited a linear relationship (Figure 2 and Table 2). The toddler model using the Vapotherm device showed the greatest incremental rise in PEEP (Figure 2). The flow required to generate 6 cm H2O tracheal pressure increased with model size: preterm neonate, 7–8 L/min; term neonate, 6–8 L/min; toddler 16–20 L/min, small child, 12–20 L/min (Figure 2). The highest PEEP achieved in this study was approximately 24 cm H2O tracheal pressure in the largest model (small child, closed mouth) at the highest tested flow of 32 L/min using the Vapotherm device. As a useful visual reference, recommended CPAP initiation pressures in pediatric OSA (4 cm H2O) is plotted as a horizontal dashed line (Figure 2).27 Across all airway sizes, the same flow generated higher tracheal pressures in closed mouth models than in open mouth models (Figure 3).
Figure 2:
Closed mouth models’ tracheal pressures measured at baseline (spontaneous breathing without flow) and at increasing flows provided with two HFNC systems: Fisher and Paykel Optiflow and Vapotherm Precision Flow. Each data point represents the average of three runs at each flow, with error bars representing standard error between these runs. The data points are fitted with a quadratic, best fit trendline, except preterm neonate Vapotherm system, which has a linear trendline. The horizontal dashed line represents current guidelines for CPAP initiation pressure.
Table 2:
Trendline and regression analysis of quadratic flow-PEEP relationship in each model
| Model | System | Trendline Equation | R2 | P-value |
|---|---|---|---|---|
|
| ||||
| Preterm Neonate, Closed Mouth | Optiflow Vapotherm | Ptrach = 0.07f2 + 0.35f − 0.17 Ptrach = 0.68f − 0.10* |
0.98 0.94 |
<0.001 <0.001* |
| Preterm Neonate, Open Mouth | Optiflow Vapotherm | Ptrach = 0.03f2 + 0.09f - 0.07 Ptrach = 0.02f2 + 0.19f − 0.10 |
0.99 0.97 |
<0.01 <0.001 |
| Term Neonate | Optiflow Vapotherm | Ptrach = 0.08f2 + 0.18f − 0.08 Ptrach = 0.09f2 + 0.57f − 0.07 |
0.99 0.99 |
<0.001 <0.001 |
| Toddler | Optiflow Vapotherm | Ptrach = 0.01f2 + 0.04f − 0.04 Ptrach = 0.02f2 + 0.10f − 0.16 |
0.99 0.99 |
<0.001 <0.001 |
| Small Child, Closed Mouth | Optiflow Vapotherm | Ptrach = 0.01f2 + 0.06f − 0.04 Ptrach = 0.02f2 + 0.15f − 0.03 |
0.99 0.99 | <0.001 <0.001 |
| Small Child, Open Mouth | Optiflow Vapotherm | Ptrach = 0.003f2 + 0.02f - 0.02 Ptrach = 0.005f2 + 0.05f − 0.02 |
0.99 0.99 | <0.001 <0.001 |
Linear trendline and linear regression analysis p-value for preterm neonate, closed mouth Vapotherm Ptrach = Tracheal pressure, cm H2O; f = Flow, L/min
Figure 3:
Open mouth vs closed mouth pressures differences in Preterm Neonate and Small Child models at increasing flows provided with two HFNC systems: Fisher and Paykel Optiflow and Vapotherm Precision Flow.
4.1. Discussion
This translational study was undertaken to better quantify tracheal pressure exerted by HFNC across a range of flows in anatomical pediatric airway models. To our knowledge, no prior studies have elucidated this flow-pressure relationship at this specific level of the airway. End-expiratory alveolar pressures with increasing flow have been measured by our group’s previous study (Nielsen et al.) and a recent study by Ejiofor et al. which both resulted in a reproducible flow-pressure relationship.15,28 As demonstrated in Figure 2, the major finding of this study is that in closed mouth models, HFNC can exert reproducible tracheal PEEP at levels that are commonly prescribed in CPAP therapy for pediatric OSA.27 Given this, the tracheal pressure generated by HFNC could theoretically be used as alternative treatment modality of congenital tracheomalacia. For pediatric OSA, the current guidelines for initiation and titration of CPAP are based on recommendations by the Positive Airway Pressure Titration Task Force of the American Academy of Sleep Medicine, which state that any patient less than 12 years old should be initiated on CPAP of 4 cm H20 with gradual CPAP increase of 1 cm H2O until all obstructive respiratory events are eliminated, with a maximum CPAP of 15 cm H2O.27 Other studies in infants with OSA have determined that CPAP levels of 4–6 cm H2O prevented obstruction and reversed sleep disturbances.29 The data presented in this study show that even at moderate flow HFNC can readily generate airway pressure within the recommended range of CPAP levels for treating pediatric OSA. This is demonstrated graphically as the horizontal dashed line on each plot in Figure 2.
4.1.1. Flow-PEEP relationship
An interesting finding in this study is the quadratic curved relationship between flow and trachea PEEP. This quadratic curved relationship in flow-PEEP is consistent with a Chinese study by Luo et al, which tested three HFNC devices not available in the United States.30 However, this contradicts previously reported data in flow-PEEP relationship in the adult population, with studies indicating that every 10 L/min of flow generates 1 cm H2O.31,32 In our data, the one linear relationship found in the preterm neonate closed mouth Vapotherm data we attribute to variation in cannula placement, which is discussed further in the study limitations. This quadratic flow-PEEP relationship is important to understand. If a provider assumes a linear flow-pressure relationship, it may seem that a term neonate (closed mouth breathing) using Vapotherm device getting flow of 6–8 L/min would generate a pressure of 4–6 cm H2O; however, based on our data the term neonate would be receiving 7–10 cm H2O and potentially a dangerous level of pressure.
4.1.2. Closed-Mouth versus Open-Mouth Breathing
A notable result in the current study is the difference of pressure generated in closed mouth and open mouth models. In the two models tested with open and closed mouth options, closed mouth pressures were significantly higher than open mouth pressures by at least 2-fold (Figure 3). This difference is consistent with prior studies. Parke et al. observed a 50–60% decrease in nasopharyngeal pressure with open mouth breathing.21,30 The non-occlusive design of HFNC enhances patient comfort, but comes at the expense of diminished PEEP with open mouth breathing.27 However, a literature review by Gonzales et al. describes enhanced oxygen delivery and improved washout of nasopharyngeal and oropharyngeal dead space with open mouth breathing.33 Hence, open versus closed mouth breathing with HFNC depends on the goals of therapy. When using HFNC for pressure-directed therapy in the case of physical airway obstruction, strategies to maintain a closed mouth would benefit the patient.
Overall, these data show that HFNC can exert a reproducible and therapeutic amount of tracheal pressure in pediatric airway models, which may allow clinicians to achieve a desired airway pressure in CPAP-intolerant patients, depending on the patient airway size and the HFNC system available to them. The potential to deliver a consistent pressure by adjusting flow, while also taking advantage of greater patient comfort and toleration, suggests that HFNC may be a useful alternative to CPAP to generate airway pressure and relieve obstruction in select patients.
4.1.3. Study Limitations
As translational research, our goal was to create a realistic model that can be extrapolated “from bench to bedside.” Nevertheless, our study has several limitations. We utilized tracheal pressure while most of the anatomic airway obstruction in pediatric OSA occurs in the nasopharynx, oropharynx, or hypopharynx.34 To obtain breath-by-breath pressure data in real-time while maintaining accurate airway anatomy, it was necessary to use an in-series pressure adaptor at the level of the trachea. In support of this study’s usage of tracheal pressure as a surrogate for upper airway pressure, Luo et al. measured airway pressure using HFNC and pressure catheter in the nasopharynx, supraglottis, and trachea with comparable pressure results.30 Another shortcoming is our 3D models are static, printed based on CT images of the upper airway and they lack the mucosal layer and the intrinsic upper-airway distensibility that may affect pressure and resistance in a real patient. This limitation also includes the physiologic changes seen in pediatric sleep apnea, including loss of airway tone, pharyngeal collapse, and glossoptosis, which may alter airway flow dynamics in real-time. Further, the rigid models are unable to replicate the inspiratory and expiratory movements of the larynx, so this area is not physiologically correct, but the models have accurate age-specific airway volume measurements.22 Despite these discrepancies, the tracheal pressure data obtained in our 3D models correlates well with HFNC-generated pressure data reported in infants by Hough et al.: In 11 infants with an average weight of 4.76 kg, they measured an end-expiratory esophageal pressure (used as a surrogate for tracheal pressure) of 6.9 ± 2.1 cm H2O at 8 L/min.31 This closely matches our term model (4 kg) data of 6.14 ± 0.5 cm H2O using the same system and flow (Figure 2). A final limitation of our study was the variation in the nasal cannula placement between runs, which increased variability between measurements. While attempts were made to ensure consistency of placement using unchanged position markers on the models, this variation in cannula placement represents real-world conditions in which the cannula position changes with patient positional changes which does alter laminar flow dynamics.
5.1. Conclusions
HFNC was found to demonstrate a predictable, quadratic flow-pressure relationship that achieved adequate airway pressures commonly used managed obstructive pathologies, such as pediatric OSA and congenital tracheobronchomalacia. We hope that these findings may serve as a starting point for clinicians to consider high flow treatment alternatives for pediatric obstructive airway pathologies, although further studies in human subjects are necessary to determine whether similar pressures are achievable at the bedside and the optimal flows for treatment of the upper airway.
Highlights.
Using HFNC, there is a reproducible flow-pressure relationship in the trachea
In closed mouth breathing, HFNC creates pressure that could treat airway obstruction
Using HFNC, there is an approximate 50% drop in pressure with open mouth breathing
Funding
This work was supported by the University of Washington Medical Student Research Training Program; University of Washington, Seattle, Washington, USA.
This research was considered exempt from IRB approval by Seattle Children’s Hospital.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mitchell RB, Archer SM, Ishman SL, et al. Clinical Practice Guideline: Tonsillectomy in Children (Update). Otolaryngol Neck Surg. 2019;160(1_suppl):S1–S42. doi: 10.1177/0194599818801757 [DOI] [PubMed] [Google Scholar]
- 2.Sullivan Colin E Berthon-Jones M Issa Faiq G, Eves L. REVERSAL OF OBSTRUCTIVE SLEEP APNOEA BY CONTINUOUS POSITIVE AIRWAY PRESSURE APPLIED THROUGH THE NARES. The Lancet. 1981;317(8225):862–865. doi: 10.1016/S0140-6736(81)92140-1 [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Rosen G, Ward SLD, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117(3):e442–451. doi: 10.1542/peds.2005-1634 [DOI] [PubMed] [Google Scholar]
- 4.Kushida CA, Halbower AC, Kryger MH, et al. Evaluation of a new pediatric positive airway pressure mask. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2014;10(9):979–984. doi: 10.5664/jcsm.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson D, Andersen C, O’Donnell CP, De Paoli AG, Manley BJ. High flow nasal cannula for respiratory support in preterm infants. In: The Cochrane Library. John Wiley & Sons, Ltd; 2016. doi: 10.1002/14651858.CD006405.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maggiore SM, Idone FA, Vaschetto R, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. 2014;190(3):282–288. doi: 10.1164/rccm.201402-0364OC [DOI] [PubMed] [Google Scholar]
- 7.Arora B, Mahajan P, Zidan MA, Sethuraman. Nasopharyngeal airway pressures in bronchiolitis patients treated with high-flow nasal cannula oxygen therapy. Pediatr Emerg Care. 2012;28(11):1179–1184. doi: 10.1097/PEC.0b013e318271a671 [DOI] [PubMed] [Google Scholar]
- 8.Kumar H, Spence CJT, Tawhai MH. Modeling the pharyngeal pressure during adult nasal high flow therapy. Respir Physiol Neurobiol. 2015;219:51–57. doi: 10.1016/j.resp.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 9.Hawkins S, Huston S, Campbell K, Halbower A. High-Flow, Heated, Humidified Air Via Nasal Cannula Treats CPAP-Intolerant Children With Obstructive Sleep Apnea. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2017;13(8):981–989. doi: 10.5664/jcsm.6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ignatiuk D, Schaer B, McGinley B. High flow nasal cannula treatment for obstructive sleep apnea in infants and young children. Pediatr Pulmonol. 2020;55(10):2791–2798. doi: 10.1002/ppul.25009 [DOI] [PubMed] [Google Scholar]
- 11.Joseph L, Goldberg S, Shitrit M, Picard E. High-Flow Nasal Cannula Therapy for Obstructive Sleep Apnea in Children. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2015;11(9):1007–1010. doi: 10.5664/jcsm.5014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosheh O, Edwards CT, Ramnarayan P. A nationwide survey on the use of heated humidified high flow oxygen therapy on the paediatric wards in the UK: current practice and research priorities. BMC Pediatr. 2020;20(1):109. doi: 10.1186/s12887-020-1998-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Delft B, Van Ginderdeuren F, Lefevere J, van Delft C, Cools F. Weaning strategies for the withdrawal of non-invasive respiratory support applying continuous positive airway pressure in preterm infants: a systematic review and meta-analysis. BMJ Paediatr Open. 2020;4(1):e000858. doi: 10.1136/bmjpo-2020-000858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated Humidified High-Flow Nasal Oxygen in Adults: Mechanisms of Action and Clinical Implications. Chest. 2015;148(1):253–261. doi: 10.1378/chest.14-2871 [DOI] [PubMed] [Google Scholar]
- 15.Nielsen KR, Ellington LE, Gray AJ, Stanberry LI, Smith LS, DiBlasi RM. Effect of High-Flow Nasal Cannula on Expiratory Pressure and Ventilation in Infant, Pediatric, and Adult Models. Respir Care. 2018;63(2):147–157. doi: 10.4187/respcare.05728 [DOI] [PubMed] [Google Scholar]
- 16.Laryngomalacia, Tracheomalaciaand Bronchomalacia | Elsevier Enhanced Reader. doi: 10.1016/j.cppeds.2018.03.002 [DOI] [Google Scholar]
- 17.Reiterer F, Eber E, Zach MS, Müller W. Management of severe congenital tracheobronchomalacia by continuous positive airway pressure and tidal breathing flow-volume loop analysis. Pediatr Pulmonol. 1994;17(6):401–403. doi: 10.1002/ppul.1950170612 [DOI] [PubMed] [Google Scholar]
- 18.Vézina K, Laberge S, Nguyen TTD. Home high-flow nasal cannula as a treatment for severe tracheomalacia: A pediatric case report. Pediatr Pulmonol. 2017;52(8):E43–E45. doi: 10.1002/ppul.23688 [DOI] [PubMed] [Google Scholar]
- 19.Frizzola M, Miller TL, Rodriguez ME, et al. High-flow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatr Pulmonol. 2011;46(1):67–74. doi: 10.1002/ppul.21326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parke RL, Mcguinness SP. Pressures Delivered By Nasal High Flow Oxygen During All Phases of the Respiratory Cycle. Respir Care. 2013;58(10):1621–1624. doi: 10.4187/respcare.02358 [DOI] [PubMed] [Google Scholar]
- 21.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009;103(6):886–890. doi: 10.1093/bja/aep280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Numa AH, Newth CJ. Anatomic dead space in infants and children. J Appl Physiol Bethesda Md 1985. 1996;80(5):1485–1489. doi: 10.1152/jappl.1996.80.5.1485 [DOI] [PubMed] [Google Scholar]
- 23.Hall GL, Hantos Z, Peták F, et al. Airway and Respiratory Tissue Mechanics in Normal Infants. Am J Respir Crit Care Med. 2000;162(4):1397–1402. doi: 10.1164/ajrccm.162.4.9910028 [DOI] [PubMed] [Google Scholar]
- 24.Masters IB, Seidenberg J, Hudson I, Phelan PD, Olinsky A. Longitudinal study of lung mechanics in normal infants. Pediatr Pulmonol. 1987;3(1):3–7. doi: 10.1002/ppul.1950030104 [DOI] [PubMed] [Google Scholar]
- 25.Howlett G Lung Mechanics in Normal Infants and Infants with Congenital Heart Disease. Arch Dis Child. 1972;47(255):707–715. doi: 10.1136/adc.47.255.707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yumpu.com. R: A Language and Environment for Statistical Computing. yumpu.com. Accessed March 4, 2021. https://www.yumpu.com/en/document/read/6853895/r-a-language-and-environment-for-statistical-computing [Google Scholar]
- 27.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 28.Ejiofor BD, Carroll RW, Bortcosh W, Kacmarek RM. PEEP Generated by High-Flow Nasal Cannula in a Pediatric Model. Respir Care. 2019;64(10):1240–1249. doi: 10.4187/respcare.06470 [DOI] [PubMed] [Google Scholar]
- 29.Obstructive Sleep Apnea in Infants and Its Management With Nasal Continuous Positive Airway Pressure. Chest. 1999;116(1):10–16. doi: 10.1378/chest.116.1.10 [DOI] [PubMed] [Google Scholar]
- 30.Luo J-C, Lu M-S, Zhao Z-H, et al. Positive End-Expiratory Pressure Effect of 3 High-Flow Nasal Cannula Devices. Respir Care. 2017;62(7):888–895. doi: 10.4187/respcare.05337 [DOI] [PubMed] [Google Scholar]
- 31.Papazia L. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. :14. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura Masaji. High-Flow Nasal Cannula Oxygen Therapy in Adults: Physiological Benefits, Indication, Clinical Benefits, and Adverse Effects. Respir Care. 2016;61(4):529–541. doi: 10.4187/respcare.04577 [DOI] [PubMed] [Google Scholar]
- 33.Gonzales J, Collins K. A Narrative Summary of High Flow Nasal Cannula Therapy in the Adult Population. Published online 2017:9. [Google Scholar]
- 34.Dedhia RC, Rosen CA, Soose RJ. What is the role of the larynx in adult obstructive sleep apnea? The Laryngoscope. 2014;124(4):1029–1034. doi: 10.1002/lary.24494 [DOI] [PubMed] [Google Scholar]