Abstract
Gout, a common form of inflammatory arthritis, is characterized by deposition of monosodium urate crystals in articular and periarticular tissues. Repeated flares of gout cause joint damage as well as significant health care utilization and decreased quality of life. Patients with CKD have a higher prevalence of gout. Treating Patients with CKD and gout is challenging because of the lack of quality data to guide management in this specific population. This often leads to suboptimal treatment of patients with gout and impaired renal function because concerns regarding the efficacy and safety of available gout therapies in this population often result in significant interphysician variability in treatment regimens and dosages. Acute gout flares are treated with various agents, including nonsteroidal anti-inflammatory drugs, colchicine, glucocorticoids, and—more recently—IL-1 inhibitors. These medications can also be used as prophylaxis if urate-lowering therapy (ULT) is initiated. While these drugs can be used in patients with gout and CKD, there are often factors that complicate treatment, such as the numerous medication interactions involving colchicine and the effect of glucocorticoids on common comorbidities, such as diabetes and hypertension. ULT is recommended to treat recurrent flares, tophaceous deposits, and patients with moderate-to-severe CKD with a serum urate goal of <6 mg/dl recommended to prevent flares. While many misconceptions exist around the risks of using urate-lowering agents in patients with CKD, there is some evidence that these medications can be used safely in Patients with renal impairment. Additional questions exist as to whether gout treatment is indicated for Patients on RRT. Furthermore, there are conflicting data on whether ULT can affect renal function and cardiovascular disease in patients. All of these factors contribute to the unique challenges physicians face when treating patients with gout and CKD.
Keywords: CKD, ESKD, hemodialysis, immunology, kidney disease, nephrotoxicity, peritoneal dialysis, rheumatology
Introduction
Gout is one of the most common forms of inflammatory arthritis in the world and is characterized by an inflammatory response to monosodium urate (MSU) deposition in articular and periarticular tissues. This often results in a flare presenting most commonly with debilitating pain and swelling of the affected joint. Gout occurs when the serum urate (SU) concentration exceeds urate solubility (>6.8 mg/dl), and MSU crystals deposit in and around the joints.1 The persistent burden of urate crystals can repeatedly trigger the nucleotide-binding domain, leucine-rich repeat containing (NLR) family pyrin domain containing 3 (NLRP3) inflammasome in macrophages and monocytes, leading to a proinflammatory response directed at the area of urate crystal deposition.2 When gouty arthritis becomes chronic, it can cause severe joint damage and lead to the development of visible MSU deposits known as tophi. This repeated inflammatory reaction and subsequent joint damage can lead to significant health care utilization and decreased quality of life in patients with gout.3,4 The global reported prevalence of gout ranges from 0.1% to 10%, including a prevalence between 3% and 4% in the United States, and this indicates a significant effect on both quality of life and the economic burden of health care systems worldwide.5,6
Gout and CKD
Patients with gout and CKD have increased difficulty in maintaining optimal SU concentrations. Because urate is largely excreted renally, serum levels increase when kidney function decreases; thus, hyperuricemia is more common in patients with stage 3–5 CKD.7 Mouse models indicate that hyperuricemia itself can affect kidney function by inducing afferent arteriolar fibrosis that decreases blood flow into the glomeruli.8 Predictably, studies also show a five-fold increase in the prevalence of gout in patients with an eGFR of ≤60 ml/min per 1.73 m2 when compared with patients with no kidney disease.7 Other studies report that in patients with gout, the prevalence of CKD stage ≥2 is over 70% while the prevalence of CKD stage ≥3 is around 24%.9,10 Urate crystals may contribute to inflammation and fibrosis in the kidney by depositing in the renal medulla and directly affecting the function of the kidney.11,12 To make matters more complex, there is also conflicting evidence about the effects of progressive CKD and gout; while some propose that persistent, low-grade inflammation in patients with CKD likely exacerbates flares in patients with gout and hyperuricemia, other studies have described that progression to ESKD is associated with a decrease in the frequency of gout flares.13,14
Treating patients with CKD and gout is challenging because of the lack of quality data to guide management in this specific population. Much of this stems from the exclusion of patients with CKD in clinical trials regarding gout management and the failure to stratify available trial results by renal function. An additional issue involves the lack of consistency in outcome measurements between studies.15 Without quality evidence, many physicians have concerns regarding the safety and efficacy of treating gout in patients with CKD.16 In clinical practice, there are significant differences in the treatment of gout patients with CKD among rheumatologists, nephrologists, and general practitioners.17 All of these factors can result in the suboptimal treatment of patients with gout.18
Approach to Treatment
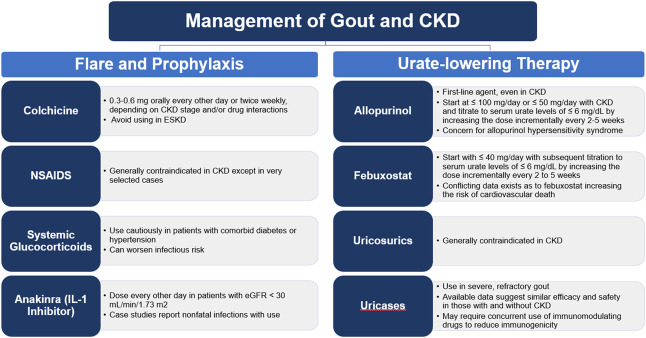
Treatment of gout is focused on two different methods of disease management. One involves treating the pain and inflammation surrounding an acute flare. Another aim of treatment involves lowering SU levels, thus preventing future flares. The most recent American College of Rheumatology (ACR) guidelines recommend this type of therapy in patients with tophaceous deposits, with radiographic evidence of bony damage, or with frequent gout flares (certainty of evidence (coe): high, moderate, high, respectively). It is also recommended in those patients who experience their first flare but may also have moderate-to-severe CKD (stage ≥3), SU concentration >9 mg/dl, or kidney stones (coe: very low).19 Urate levels can be lowered by various methods, including drugs that decrease uric acid production, increase uric acid secretion, or accelerate the breakdown of urate. This is known as urate-lowering therapy (ULT), and the ACR recommends a treat-to-target strategy of titrating ULT on the basis of serial urate levels (coe: moderate) to achieve a target SU level of < 6 mg/dl (coe: high). However, initiating ULT can precipitate a gout flare because of the rapid adjustment of SU levels. Preventing these flares often requires the use of short-term prophylaxis with one of the drugs that are also used in the treatment of acute flares. ACR guidelines strongly recommend the use of a prophylactic agent for 3–6 months in those patients initiating ULT (coe: moderate). A summary of available treatment options with specific aspects related to the treatment of patients with CKD or ESKD is included in Figure 1.
Figure 1.
Specific aspects related to CKD and ESKD in the pharmacological treatment of patients with gout. NSAID, nonsteroidal anti-inflammatory drug.
Treatment of Flares and Prophylactic Agents in Gout and CKD
The 2020 ACR guidelines recommend nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and systemic glucocorticoids as options for the treatment of acute gout flares (coe: high).19 These medications can also be used as prophylactic agents to prevent flares in patients starting ULT. In certain situations, IL-1 inhibitors, such as anakinra and canakinumab, can be used for these indications as well. Unfortunately, in patients with both gout and CKD, there is a lack of consensus on proper dosing and monitoring in patients with acute flares and/or in need of prophylaxis.
NSAIDs
NSAIDs are largely contraindicated in advanced CKD because of the concern of nephrotoxicity. Given this known risk, the handful of case studies describing NSAID use in gout flare treatment largely highlighted the increased risk of AKI associated with using NSAIDs in this comorbid population.20 While prophylaxis dosing is often lower than the dosing used in acute flare treatment, the risk of kidney injury is still significant in patients with CKD. One exception may be patients with ESKD; while these patients certainly have high risk of side effects from NSAIDs, such as gastrointestinal bleeding, there are some physicians who suggest cautious, short-term use of NSAIDs in patients with no residual kidney function that would not affect BP or electrolyte dysfunction and would be safer than other alternatives that exist for pain control.21,22
Colchicine
Colchicine, an anti-inflammatory medication that blocks microtubule assembly and affects the function of the NLRP3 inflammasome, is another agent often used in patients with either acute gout flares or in need of prophylaxis when initiating ULT. While it is commonly used in clinical practice, there are little data regarding its safety and effectiveness in patients with gout and CKD. Colchicine is partially cleared by the kidney; thus, there can be increased toxicity in patients with CKD, including colchicine-induced rhabdomyolysis, neuromyopathy, or bone marrow suppression. Colchicine also has significant interactions with various drugs, such as statins, cyclosporine (historically part of renal transplant immunomodulation), and macrolide antibiotics, and this interaction is worsened when drug half-life is increased.23 Prior randomized clinical trials (RCTs) for colchicine in CKD did not report outcomes on the basis of renal function; thus, there is a lack of evidence in using colchicine in this population. Furthermore, case series and reports that did stratify results by eGFR used varied doses and dosing schedules. This led to highly variable results, as 12 of 19 patients in one review reported worsening kidney function while the other seven showed stable kidney function.20 Most of these case studies and reports that stratified safety events by renal function showed significant side effects or medication interactions; however, it is unclear whether these were directly caused by colchicine given the types of studies and heterogeneity between them. Ultimately, there was not enough evidence to make any conclusions on the safety or efficacy of colchicine in this patient population.20 One potential option that could be studied in the future involves low-dose colchicine because this has been shown to be similarly effective as high doses in patients without CKD.24 It has been our practice experience to dose colchicine for prophylaxis as 0.3–0.6 mg orally every other day or twice weekly, depending on how advanced a patient's CKD is and whether there is concurrent use of medications with colchicine interactions or added neuromuscular toxicity (such as statins).
Glucocorticoids
Systemic glucocorticoids are another option for both flare treatment and—less optimally but sometimes necessary—prophylaxis. Unfortunately, a review of the available data in steroid use in gout and CKD did not find enough evidence to make any conclusions of either the effectiveness or safety of steroids in this population because most of the studies were case reports. Interestingly, all of the available studies included patients with severe or refractory gout; thus, these findings may not be generalizable to the general population.20 Another concern that clinicians often face in patients with gout and CKD include the presence of other associated comorbidities, such as hypertension, diabetes, obesity, or infection risk, that may also preclude systemic glucocorticoid use in these patients.
IL-1 Inhibitors
Finally, IL-1 inhibitors are newer agents that have been used for gout flares and prophylaxis. Clinical trials of these drugs—anakinra, canakinumab, and rilonacept—excluded patients with advanced CKD (eGFR <30 ml/min per 1.73 m2), and only pooled results were reported as opposed to outcome data separated by CKD subgroups. In case reports and series of anakinra (n=7) and canakinumab (n=1) that did report outcomes stratified by renal function, only one case report of anakinra reported a decline in renal function.20,25 Furthermore, four of the studies reported nonfatal infections with anakinra. A case report evaluating canakinumab use in tophaceous gout showed good efficacy and no safety events or worsening in renal function.26 Importantly, the presence of CKD was not found to be a factor affecting response to anakinra in an analysis of hospitalized patients.27 While anakinra is becoming more widely available, it is not always appreciated that it is predominantly cleared by the kidneys and has a higher half-life in patients with CKD.28 Thus, it recommended that this medication is dosed every other day, rather than daily, in patients with eGFR <30 ml/min per 1.73 m2. In our experience, using IL-1 inhibitors for gout flare treatment and prophylaxis has been beneficial in difficult cases of patients with CKD or ESKD who lacked other treatment options.
Urate-Lowering Therapy
As mentioned previously, patients with recurrent flares, tophaceous deposits, and moderate-to-severe CKD (stage ≥3) are recommended to be managed with ULT. Patients with moderate-to-severe CKD have a higher risk of developing severe gout and tophaceous deposits.29 ULT, when titrated to a goal SU level of <6 mg/dl, can reduce flares, dissolve tophi, and consequently improve quality of life.30 It has been a common practice among experienced rheumatologists to treat patients with severe tophaceous deposits to a stricter goal of <5 mg/dl, although this approach lacks supporting evidence. However, managing ULT in patients with CKD can be challenging for many physicians because there is concern of increased side effects in patients with abnormal kidney function. In addition, the need for additional prophylaxis to prevent further flares in this patient population complicates decision making for physicians. Unfortunately, RCTs of many of the ULTs used today excluded patients with severely impaired renal function; thus, there is a lack of data available in how to best treat patients with gout and CKD.31
Allopurinol
ACR guidelines strongly recommend the use of allopurinol, a xanthine oxidase inhibitor, as the preferred first-choice ULT, including in those with CKD (coe: moderate).19 Studies have shown that allopurinol can be used safely to reduce SU levels in patients with CKD.32,33 However, starting treatment with a low dose (≤100 mg/d) in patients with moderate-to-severe CKD is strongly recommended (coe: moderate) to prevent the development of allopurinol hypersensitivity syndrome.19,34 Oxypurinol, the active metabolite in allopurinol, is renally excreted; thus, levels are higher in patients with CKD.31 In clinical practice, the total dosage of allopurinol is often limited in patients with reduced creatinine in lieu of potential side effects; unfortunately, this practice often leads to undertreatment in patients with CKD. However, allopurinol can be safely up-titrated in these patients. The Veterans Affairs Comparative Effectiveness of Allopurinol and Febuxostat in Gout Management or VA STOP-GOUT study, which contained 351 participants with gout and stage 3 CKD, showed that following a treat-to-target strategy with initiation of allopurinol 100 mg and regularly increasing the dosage achieved a target SU level without increased toxicity or worsening kidney function in most participants.35 This is consistent with other studies that have shown that escalating allopurinol dosages to reach a target SU level can be performed safely, even in patients with creatinine clearance (CrCl) <30 ml/min.36 Population studies evaluating the pharmacodynamics of allopurinol have found that patients with larger weight and on diuretics, as can be seen in patients with CKD, may need higher allopurinol doses to reach goal SU levels.37 Ultimately, patients with gout and CKD may still require dosage titration to levels of 300 mg/d or above to reach the desired SU target.30
Febuxostat
Febuxostat is another xanthine oxidase inhibitor which may be used to treat gout in patients with CKD, and it has been shown to be effective and safe in patients with moderate-to-severe kidney disease.38 The ACR also strongly recommends using a reduced dose of febuxostat (<40 mg/d) with ongoing titration rather than starting with a higher dosage in these patients (coe: moderate).19 While some studies report that febuxostat is more effective than allopurinol in patients with mild-to-moderate CKD, others have reported that allopurinol is noninferior to febuxostat and either ULT, when titrated to a SU target, is effective.35,39 Of note, in patients with a history of kidney stones, both febuxostat and allopurinol are effective because both medications lower 24-hour uric acid secretion in urine.40
Regarding the cardiovascular risks of using febuxostat, the Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities or CARES trial revealed a higher risk of cardiovascular death and death from any cause in patients taking febuxostat compared with allopurinol.41 Unfortunately, these results were seen in secondary analyses that did not stratify patients on the basis of renal function. These results, however, led to the introduction of a black box warning for febuxostat. More recently, the Febuxostat versus Allopurinol Streamlined Trial or FAST looked at patients with gout and at least one additional cardiovascular risk factor.42 Unlike the CARES trial, the FAST showed that patients treated with febuxostat did not have an increased risk of cardiovascular death compared with patients on allopurinol and that patients with gout treated with febuxostat actually had fewer deaths when compared with the patients taking allopurinol. However, patients with advanced CKD were excluded from this trial.
Uricosurics
Another class of urate-lowering therapy (ULT) is uricosurics, drugs that prevent reabsorption of urate in the proximal tubule and promote renal clearance of urate.43 Examples of these medications include probenecid, benzbromarone, and lesinurad. Probenecid is generally avoided in patients with CrCl ≤50 ml/min because it is believed to be ineffective at that level of renal impairment.44,45 Thus, the ACR guidelines strongly recommend either allopurinol or febuxostat over probenecid for patients with moderate-to-severe CKD (stage ≥3) (coe: moderate).19 Although benzbromarone has shown to be effective in patients with CrCl ≤25 ml/min, it has been removed from many markets because of concerns for hepatotoxicity.46 Lesinurad, now withdrawn from both American and European markets after a business decision by the manufacturer, is also contraindicated with CrCl ≤45 ml/min.47
Uricases
Pegloticase, a mammalian recombinant uricase, is another medication used for ULT. Pegloticase works by metabolizing uric acid into allantoin, which is more soluble and readily excreted by the kidneys.48,49 One study, a post hoc subgroup analysis of two RCTs and their open-label extension phases, reported no overall difference in the efficacy or safety profile in patients on pegloticase with CKD compared with patients without CKD.50 At this moment, pegloticase is indicated for use in patients with severe gout refractory or intolerant to first-line ULTs, such as allopurinol or febuxostat.19 In addition, we have used pegloticase earlier when patients have severe and bulky tophaceous disease with profound disability, although this is not yet an approved indication. Immunogenicity, the need for frequent infusions, and an elevated cost are barriers to pegloticase use.
Novel approaches to the use of uricases incorporate the concurrent utilization of immunomodulating drugs (such as mycophenolate mofetil or methotrexate) to reduce immunogenicity to pegloticase and are rapidly becoming the treatment standard when using uricases.51–53 This has led to substantial improvements in sustained urate reduction in patients on pegloticase and is a viable option in many patients with CKD or after renal transplantation. Of note, in many patients with CKD, methotrexate is contraindicated or its dose needs to be substantially decreased.
Dialysis
Major questions exist concerning how to best treat patients with gout on RRT. Regarding acute treatment of gout flares in patients on RRT, glucocorticoids, preferably intra-articular, or IL-1 inhibitors are favored because colchicine and NSAIDs have many side effects in patients with limited renal function. While one study reported safety in using various doses of colchicine in a small cohort of hemodialysis (HD) patients, it is our opinion that it should be generally avoided in HD given the associated risks of toxicity and/or medication interactions in patients with renal impairment.54
For prophylaxis, systemic steroids are often used because of the side effects of other options, but this carries the known risks of chronic steroid use in this population. Approaches using very low doses of ULT with slower escalation and without prophylaxis have been tested with modest success and could be an option in patients with ESKD.55
Whether ULT needs to be continued in patients who become dialysis-dependent is another topic of interest. It has been reported that gout flares and tophi decrease after the initiation of HD.56 In fact, one study reported that new cases of gout diagnosed in patients on HD is quite rare (<3%).57 However, another study suggests that the overall prevalence of gout in this population is quite high, ranging from 13% in patients on HD to 21% in patients on peritoneal dialysis (PD).58 There are very little data on the use of ULT in patients on RRT, mostly limited to case studies and reports regarding allopurinol and febuxostat. For those who continue allopurinol during HD, it is best dosed after HD because oxypurinol is dialyzable.59
One study assessed HD patients with a history of hyperuricemia and/or gout to evaluate how SU levels fluctuated with sessions of thrice-weekly HD.60 Most of the patients in the kinetics study group had hyperuricemia before the initial HD session of the week (mean SU level of 7.1 [6.7–7.5] mg/dl). It showed that SU levels dropped by 80% immediately after one session of HD. While the SU level would increase in the day after the first session of HD, it still remained below hyperuricemic levels (6.8 mg/dl) until after the final session of the week, when it would slowly increase back to baseline levels after 2 days of no HD. Patients who were on ULT did not have any significant differences in their reduction in SU levels after HD compared with patients who were not on ULT. The effectiveness of dialyzing SU, the concern for adverse events from ULT in patients with significant CKD, and controversial data suggesting higher mortality in patients with very low SU levels argue against the need for ULT in this patient population.61 However, this study included patients who had been on HD for an average of 7 years and may not be generalizable to patients with gout newly starting HD or who may have a higher SU burden.
Another study analyzed the effects of PD on SU levels and showed that most patients undergoing PD have a normal SU level.62 This study included 20 patients, and only two patients remained hyperuricemic after a median of 20 months of PD. While patients on ULT in this study did have a statistically significantly lower mean urate level (4.2 mg/dl) when compared with patients who were not on ULT (5.6 mg/dl), both groups remained below the threshold of hyperuricemia. While no differences were seen when comparing automatic and manual forms of PD, there was a difference between the automated techniques showing that continuous cyclic PD was more effective at lowering SU levels than nocturnal intermittent PD.62 However, PD patients who are also prescribed diuretics and angiotensin-converting enzyme inhibitors to help with volume status and peritoneal membrane function may experience more episodes of gout because these drugs can cause hyperuricemia.58 We personally favor the continuation of ULT, in lower doses, if patients with ESKD on RRT are still having gout flares or if they have not achieved a SU level of <6 mg/dl. The need for ULT should be re-evaluated periodically after the initiation of RRT.
Lifestyle Modifications
While dietary changes may only result in small decreases in SU levels, certain foods or substances may trigger gout flares in susceptible patients19,63 Furthermore, obesity has been shown to increase the incidence of gout. Increasing body mass index over 5% was associated with more frequent gout flares while decreases in body mass index of at least 5% resulted in fewer flares.64 Other studies have reported that weight loss of more than 5 kg can reduce SU levels by 1.1 mg/dl in obese patients, and even larger decreases in SU levels can be seen in patients undergoing bariatric surgery.65,66 In addition to recommending weight loss (coe: very low), the ACR guidelines also recommend that patients with gout limit alcohol use, high purine foods, and high-fructose corn syrup (coe: low, low, and very low, respectively).19
Effects of ULT on Renal Function and Cardiovascular Disease
The role of ULT on the progression of CKD and cardiovascular health is a controversial topic. Mouse models have shown that increased levels of SU can lead to the activation of the renin-angiotensin-aldosterone system as well as oxidative stress and reduction of nitric oxide in the endothelium.67,68 This eventually leads to vascular changes in the kidney causing hypertension.69 These changes can also impair renal blood flow and hasten the development of CKD.70,71 One review of epidemiologic studies evaluating the link between SU levels and various conditions found that elevated urate concentrations were independently associated with hypertension, metabolic syndrome, type 2 diabetes mellitus (DM2), and incident CKD in patients with and without DM2.72 However, it is important to note that these studies are subject to confounders that may affect the association between SU levels and these diseases. Several small trials have shown that ULT can lessen the decline in eGFR in patients on allopurinol, around one-fourth of whom also had DM2, as well as in patients on febuxostat.73–75 Of note, recent literature suggests that using chlorthalidone, a thiazide-like diuretic, could be beneficial in patients with advanced CKD and hypertension.76 However, patients who received chlorthalidone had a higher incidence of hyperuricemia, and this is consistent with guidelines that recommend avoiding thiazide diuretics in patients with gout (coe: very low).19
However, other studies have argued against the role that ULTs may play in slowing the progression of CKD. One study using Mendelian randomization showed no relationship between SU levels and the risk of CKD.77,78 A recent study evaluated the effects of ULT in patients with long-standing DM type 1 with early-to-moderate CKD (stage 1–3) and found that, despite adequately controlling SU levels for 3 years with allopurinol, there was no difference in the progression of kidney disease between the allopurinol group and the placebo group.79 Another trial analyzed patients with stage 3 or 4 CKD, over 75% of whom were also hyperuricemic, who received either allopurinol or placebo. After 2 years of therapy, there was no difference in the rate of eGFR decline between the two groups, despite a reduction in SU levels of 35% in the allopurinol arm of the trial.32 These results are largely consistent with another trial looking at asymptomatic patients with hyperuricemia and stage 3 CKD that found that, when compared with placebo, febuxostat did not diminish the decrease in eGFR after 2 years of treatment.80 However, subgroup analysis of this study showed that patients in the febuxostat group who lacked proteinuria or who had a creatinine below the median level in the trial (<1.2±0.3 mg/dl) did show less progression of CKD when compared with the placebo arm. Prior studies have also described this association of hyperuricemia and progressive decline in eGFR in patients without proteinuria.81 While definitive evidence for using ULT in patients with asymptomatic hyperuricemia to prevent the progression of CKD is lacking, there may be a cause to explore the issue of treating those patients with earlier stage kidney disease without proteinuria.
Areas for Future Direction
This review has summarized the available information regarding the efficacy and safety of acute gout treatment, prophylaxis, and ULT in patients with both gout and CKD. Table 1 presents a number of common mistakes and misconceptions we have encountered while taking care of gout patients with CKD/ESKD. Gout remains challenging to treat in this population because safety concerns are elevated in patients with CKD, especially regarding acute treatment and prophylaxis with NSAIDs, colchicine, and steroids. IL-1 inhibitors are an emerging therapy in this category but concerns also exist regarding their use in patients with CKD and associations with infections given the limited data available. ULT is another important category of gout treatment. While the bulk of data that exist regarding ULT pertains to the xanthine oxidase inhibitors allopurinol and febuxostat, there are still questions about using these medications in populations with impaired renal function, such as optimal dose-escalation strategies, risk of allopurinol hypersensitivity syndrome, and febuxostat-related cardiovascular events in this population. Uricosurics are largely contraindicated in patients with moderate-to-severe CKD, and little data exist as to the efficacy and safety of pegloticase in this population. Furthermore, additional evidence is needed as to whether ULT is required in patients on RRT and how to best treat gout flares in patients with ESKD.
Table 1.
Common missteps seen in gout patients with CKD and ESKD
| Common Missteps | Treatment/Solution |
|---|---|
| Gout flare and prophylaxis | |
| Colchicine dose is not adjusted, or medication interactions are not considered | Dose colchicine as 0.3–0.6 mg orally every other day or twice weekly, depending on how advanced a patient's CKD is and whether there is concurrent use of medications with colchicine interactions or added neuromuscular toxicity. Should be generally avoided in HD |
| Lack of prophylaxis use alongside ULT initiation | In patients starting ULT, adding a prophylactic agent is strongly recommended for 3–6 mo |
| ULT | |
| Initial allopurinol dose is too high | Start at ≤100 or ≤50 mg/d in patients with CKD and titrate to SU levels of ≤6 mg/dl by increasing the dose incrementally every 2–5 wk |
| Maximum allopurinol dose renally adjusted instead of adjusted to the SU target | Limiting max dose of allopurinol to a renally adjusted dosage often undertreats gout. Instead, regularly escalating allopurinol dosages to reach a target SU level (<6 mg/dl) can be performed safely, even in patients with CrCl <30 ml/min. Ultimately, patients with gout and CKD may require dosage titration to levels of 300 mg/d or above to reach the desired target |
| Assuming that febuxostat is more effective than allopurinol | Studies report that in patients with CKD, allopurinol is noninferior to febuxostat and either ULT, when titrated to a SU target, is effective |
| Stopping ULT while in a gout flare | ULT should be continued during a gout flare |
| ULT stopped during episodes of AKI | ULT should not be stopped during episodes of AKI |
HD, hemodialysis; ULT, urate-lowering therapy; SU, serum urate; CrCl, creatinine clearance.
As discussed, there are little evidence-based data on these treatments in patients with impaired renal function given the lack of patients with advanced CKD in many gout trials. In addition to including more patients with CKD, stratifying results by patients' renal function and implementing standardized outcome measures in reporting gout flares as well as SU levels would also improve the utility of clinical trials in this area and help practitioners better serve this patient population.
Disclosures
A. Gaffo reports the following: Employer: Birmingham VA Medical Center and University of Alabama at Birmingham; Consultancy: Atom and PK Med; and Patents or Royalties: UptoDate. The remaining author has nothing to disclose.
Funding
None.
Author Contributions
Supervision: Angelo Gaffo.
Writing – original draft: Vijay Kannuthurai.
Writing – review & editing: Angelo Gaffo, Vijay Kannuthurai.
References
- 1.Martillo MA, Nazzal L, Crittenden DB. The crystallization of monosodium urate. Curr Rheumatol Rep. 2014;16(2):400. doi: 10.1007/s11926-013-0400-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi: 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- 3.Flores NM, Nuevo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1–6. doi: 10.1080/13696998.2018.1532904 [DOI] [PubMed] [Google Scholar]
- 4.Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Ann Rheum Dis. 2007;67(9):1310–1316. doi: 10.1136/ard.2007.081604 [DOI] [PubMed] [Google Scholar]
- 5.Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–662. doi: 10.1038/nrrheum.2015.91 [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the national health and nutrition examination survey 2007-2008. Arthritis Rheum. 2011;63(10):3136–3141. doi: 10.1002/art.30520 [DOI] [PubMed] [Google Scholar]
- 7.Krishnan E. Reduced glomerular function and prevalence of gout: NHANES 2009-10. PLoS One. 2012;7(11):e50046. doi: 10.1371/journal.pone.0050046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzali M Kanellis J Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282(6):F991–F997. doi: 10.1152/ajprenal.00283.2001 [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med. 2012;125(7):679–687.e1. doi: 10.1016/j.amjmed.2011.09.033 [DOI] [PubMed] [Google Scholar]
- 10.Roughley MJ, Belcher J, Mallen CD, Roddy E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res Ther. 2015;17(1):90. doi: 10.1186/s13075-015-0610-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linnane JW, Burry AF, Emmerson BT. Urate deposits in the renal medulla. Nephron. 1981;29(5-6):216–222. doi: 10.1159/000182373 [DOI] [PubMed] [Google Scholar]
- 12.Stamp LK Farquhar H Pisaniello HL, et al. Management of gout in chronic kidney disease: a G-CAN Consensus Statement on the research priorities. Nat Rev Rheumatol. 2021;17(10):633–641. doi: 10.1038/s41584-021-00657-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mihai S Codrici E Popescu ID, et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. 2018;2018:1–16. doi: 10.1155/2018/2180373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohno I Ichida K Okabe H, et al. Frequency of gouty arthritis in patients with end-stage renal disease in Japan. Intern Med. 2005;44(7):706–709. doi: 10.2169/internalmedicine.44.706 [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, Tallon A, Taylor WJ, Gaffo A, Dalbeth N. How flare prevention outcomes are reported in gout studies: a systematic review and content analysis of randomized controlled trials. Semin Arthritis Rheum. 2020;50(2):303–313. doi: 10.1016/j.semarthrit.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 16.Fuldeore MJ, Riedel AA, Zarotsky V, Pandya BJ, Dabbous O, Krishnan E. Chronic kidney disease in gout in a managed care setting. BMC Nephrol. 2011;12(1):36. doi: 10.1186/1471-2369-12-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamp LK, Taylor W, Gaffo A. Variability in urate-lowering therapy prescribing: a gout, hyperuricemia and crystal-associated disease network (G-CAN) physician survey. J Rheumatol. 2021;48(1):152–153. doi: 10.3899/jrheum.200347 [DOI] [PubMed] [Google Scholar]
- 18.Jaffe DH Klein AB Benis A, et al. Incident gout and chronic kidney disease: healthcare utilization and survival. BMC Rheumatol. 2019;3(1):11. doi: 10.1186/s41927-019-0060-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FitzGerald JD Dalbeth N Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744–760. doi: 10.1002/acr.24180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pisaniello HL Fisher MC Farquhar H, et al. Efficacy and safety of gout flare prophylaxis and therapy use in people with chronic kidney disease: a Gout, Hyperuricemia and Crystal-Associated Disease Network (G-CAN)-initiated literature review. Arthritis Res Ther. 2021;23(1):130. doi: 10.1186/s13075-021-02416-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sriperumbuduri S, Hiremath S. The case for cautious consumption: NSAIDs in chronic kidney disease. Curr Opin Nephrol Hypertens. 2019;28(2):163–170. doi: 10.1097/mnh.0000000000000473 [DOI] [PubMed] [Google Scholar]
- 22.Tang KS, Shah AD. Nonsteroidal anti-inflammatory drugs in end-stage kidney disease: dangerous or underutilized? Expert Opin Pharmacother. 2021;22(6):769–777. doi: 10.1080/14656566.2020.1856369 [DOI] [PubMed] [Google Scholar]
- 23.Leung YY, Yao Hui LL, Kraus VB. Colchicine–update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45(3):341–350. doi: 10.1016/j.semarthrit.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060–1068. doi: 10.1002/art.27327 [DOI] [PubMed] [Google Scholar]
- 25.Direz G, Noël N, Guyot C, Toupance O, Salmon JH, Eschard JP. Efficacy but side effects of anakinra therapy for chronic refractory gout in a renal transplant recipient with preterminal chronic renal failure. Joint Bone Spine. 2012;79(6):631. doi: 10.1016/j.jbspin.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 26.Marotto D, De Santis A, Chessa D, Firinu D, Del Giacco S. A beacon in the dark: canakinumab. A new therapeutic perspective in chronic tophaceous gout. Rheumatol Ther. 2018;5(1):303–310. doi: 10.1007/s40744-018-0104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed HMA, Sun D, Gaffo A. Factors affecting response to anakinra in crystalline arthritis flares. J Clin Rheumatol. 2022;28(4):196–200. doi: 10.1097/rhu.0000000000001831 [DOI] [PubMed] [Google Scholar]
- 28.Yang BB, Baughman S, Sullivan JT. Pharmacokinetics of anakinra in subjects with different levels of renal function. Clin Pharmacol Ther. 2003;74(1):85–94. doi: 10.1016/s0009-9236(03)00094-8 [DOI] [PubMed] [Google Scholar]
- 29.Dalbeth N, House ME, Horne A, Taylor WJ. Reduced creatinine clearance is associated with early development of subcutaneous tophi in people with gout. BMC Musculoskelet Disord. 2013;14(1):363. doi: 10.1186/1471-2474-14-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doherty M Jenkins W Richardson H, et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet. 2018;392(10156):1403–1412. doi: 10.1016/s0140-6736(18)32158-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farquhar H Vargas-Santos AB Pisaniello HL, et al. Efficacy and safety of urate-lowering therapy in people with kidney impairment: a GCAN-initiated literature review. Rheumatol Adv Pract. 2021;5(1):rkaa073. doi: 10.1093/rap/rkaa073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badve SV Pascoe EM Tiku A, et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med. 2020;382(26):2504–2513. doi: 10.1056/nejmoa1915833 [DOI] [PubMed] [Google Scholar]
- 33.Wei J Choi HK Neogi T, et al. Allopurinol initiation and all-cause mortality among patients with gout and concurrent chronic kidney disease: a population-based cohort study. Ann Intern Med. 2022;175(4):461–470. doi: 10.7326/m21-2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamp LK Taylor WJ Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64(8):2529–2536. doi: 10.1002/art.34488 [DOI] [PubMed] [Google Scholar]
- 35.O'Dell JR Brophy MT Pillinger MH, et al. Comparative effectiveness of allopurinol and febuxostat in gout management. NEJM Evid. 2022;1(3). doi: 10.1056/evidoa2100028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamp LK Chapman PT Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. doi: 10.1186/s13075-017-1491-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright DF, Duffull SB, Merriman TR, Dalbeth N, Barclay ML, Stamp LK. Predicting allopurinol response in patients with gout. Br J Clin Pharmacol. 2016;81(2):277–289. doi: 10.1111/bcp.12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saag KG, Whelton A, Becker MA, MacDonald P, Hunt B, Gunawardhana L. Impact of febuxostat on renal function in gout patients with moderate-to-severe renal impairment. Arthritis Rheumatol. 2016;68(8):2035–2043. doi: 10.1002/art.39654 [DOI] [PubMed] [Google Scholar]
- 39.Becker MA Schumacher HR Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63. doi: 10.1186/ar2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldfarb DS, MacDonald PA, Gunawardhana L, Chefo S, McLean L. Randomized controlled trial of febuxostat versus allopurinol or placebo in individuals with higher urinary uric acid excretion and calcium stones. Clin J Am Soc Nephrol. 2013;8(11):1960–1967. doi: 10.2215/CJN.01760213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White WB Saag KG Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;378(13):1200–1210. doi: 10.1056/nejmoa1710895 [DOI] [PubMed] [Google Scholar]
- 42.Mackenzie IS Ford I Nuki G, et al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet. 2020;396(10264):1745–1757. doi: 10.1016/s0140-6736(20)32234-0 [DOI] [PubMed] [Google Scholar]
- 43.Anzai N Ichida K Jutabha P, et al. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem. 2008;283(40):26834–26838. doi: 10.1074/jbc.c800156200 [DOI] [PubMed] [Google Scholar]
- 44.Perez-Ruiz F, Herrero-Beites AM, Atxotegi Saenz de Buruaga J. Chapter 12 - uricosuric therapy of hyperuricemia in gout. In: Terkeltaub R, ed. Gout & Other Crystal Arthropathies. W.B. Saunders; 2012:148–153. [Google Scholar]
- 45.Schlesinger N. In: Schlesinger N, Lipsky PE, eds. Chapter 15 - Current Pharmacological Treatments of Chronic Gout. Elsevier; 2019:169–177. [Google Scholar]
- 46.Wortmann RL. Recent advances in the management of gout and hyperuricemia. Curr Opin Rheumatol. 2005;17(3):319–324. doi: 10.1097/01.bor.0000162060.25895.a5 [DOI] [PubMed] [Google Scholar]
- 47.ZURAMPIC® (lesinurad) tablets [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207988lbl.pdf. 2015. [Google Scholar]
- 48.Guttmann A, Krasnokutsky S, Pillinger MH, Berhanu A. Pegloticase in gout treatment - safety issues, latest evidence and clinical considerations. Ther Adv Drug Saf. 2017;8(12):379–388. doi: 10.1177/2042098617727714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shannon JA, Cole SW. Pegloticase: a novel agent for treatment-refractory gout. Ann Pharmacother. 2012;46(3):368–376. doi: 10.1345/aph.1q593 [DOI] [PubMed] [Google Scholar]
- 50.Yood RA, Ottery FD, Irish W, Wolfson M. Effect of pegloticase on renal function in patients with chronic kidney disease: a post hoc subgroup analysis of 2 randomized, placebo-controlled, phase 3 clinical trials. BMC Res Notes. 2014;7(1):54. doi: 10.1186/1756-0500-7-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khanna PP Khanna D Cutter G, et al. Reducing immunogenicity of pegloticase with concomitant use of mycophenolate mofetil in patients with refractory gout: a phase II, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2021;73(8):1523–1532. doi: 10.1002/art.41731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albert JA, Hosey T, LaMoreaux B. Increased efficacy and tolerability of pegloticase in patients with uncontrolled gout Co-treated with methotrexate: a retrospective study. Rheumatol Ther. 2020;7(3):639–648. doi: 10.1007/s40744-020-00222-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bessen SY, Bessen MY, Yung CM. Recapture and improved outcome of pegloticase response with methotrexate-A report of two cases and review of the literature. Semin Arthritis Rheum. 2019;49(1):56–61. doi: 10.1016/j.semarthrit.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 54.Solak Y Atalay H Biyik Z, et al. Colchicine toxicity in end-stage renal disease patients: a case-control study. Am J Ther. 2014;21(6):e189–e195. doi: 10.1097/mjt.0b013e31825a364a [DOI] [PubMed] [Google Scholar]
- 55.Yamanaka H Tamaki S Ide Y, et al. Stepwise dose increase of febuxostat is comparable with colchicine prophylaxis for the prevention of gout flares during the initial phase of urate-lowering therapy: results from FORTUNE-1, a prospective, multicentre randomised study. Ann Rheum Dis. 2018;77(2):270–276. doi: 10.1136/annrheumdis-2017-211574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ifudu O, Tan CC, Dulin AL, Delano BG, Friedman EA. Gouty arthritis in end-stage renal disease: clinical course and rarity of new cases. Am J Kidney Dis. 1994;23(3):347–351. doi: 10.1016/s0272-6386(12)80995-4 [DOI] [PubMed] [Google Scholar]
- 57.Tan VS, Garg AX, McArthur E, Lam NN, Sood MM, Naylor KL. The 3-year incidence of gout in elderly patients with CKD. Clin J Am Soc Nephrol. 2017;12(4):577–584. doi: 10.2215/CJN.06790616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guedes M Zhao J LaMoreaux B, et al. Gout prevalence, practice patterns, and associations with outcomes in north American dialysis patients. Kidney360. 2023;4(1):54–62. doi: 10.34067/kid.0005392022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright DF Doogue MP Barclay ML, et al. A population pharmacokinetic model to predict oxypurinol exposure in patients on haemodialysis. Eur J Clin Pharmacol. 2017;73(1):71–78. doi: 10.1007/s00228-016-2133-y [DOI] [PubMed] [Google Scholar]
- 60.Arenas MD, Soriano R, Andrés M, Pascual E. Serum urate levels of hemodialyzed renal patients revisited. J Clin Rheumatol. 2021;27(8):e362–e366. doi: 10.1097/rhu.0000000000001438 [DOI] [PubMed] [Google Scholar]
- 61.Hu L Hu G Xu BP, et al. U-shaped association of serum uric acid with all-cause and cause-specific mortality in US adults: a cohort study. J Clin Endocrinol Metab. 2020;105(3):e597–e609. doi: 10.1210/clinem/dgz068 [DOI] [PubMed] [Google Scholar]
- 62.Diez-Lopez C, Perez-Contreras J, Andres M. Urate levels and clearance in renal patients under peritoneal dialysis. Nucleosides Nucleotides Nucleic Acids. 2021;40(7):720–731. doi: 10.1080/15257770.2021.1934482 [DOI] [PubMed] [Google Scholar]
- 63.Major TJ, Topless RK, Dalbeth N, Merriman TR. Evaluation of the diet wide contribution to serum urate levels: meta-analysis of population based cohorts. BMJ. 2018;363(8171):k3951. doi: 10.1136/bmj.k3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen UDT Zhang Y Louie-Gao Q, et al. Obesity paradox in recurrent attacks of gout in observational studies: clarification and remedy. Arthritis Care Res. 2017;69(4):561–566. doi: 10.1002/acr.22954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gibson T, Kilbourn K, Horner I, Simmonds HA. Mechanism and treatment of hypertriglyceridaemia in gout. Ann Rheum Dis. 1979;38(1):31–35. doi: 10.1136/ard.38.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dalbeth N Chen P White M, et al. Impact of bariatric surgery on serum urate targets in people with morbid obesity and diabetes: a prospective longitudinal study. Ann Rheum Dis. 2014;73(5):797–802. doi: 10.1136/annrheumdis-2013-203970 [DOI] [PubMed] [Google Scholar]
- 67.Mazzali M Hughes J Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. doi: 10.1161/hy1101.092839 [DOI] [PubMed] [Google Scholar]
- 68.Sanchez-Lozada LG Soto V Tapia E, et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol. 2008;295(4):F1134–F1141. doi: 10.1152/ajprenal.00104.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watanabe S Kang DH Feng L, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40(3):355–360. doi: 10.1161/01.hyp.0000028589.66335.aa [DOI] [PubMed] [Google Scholar]
- 70.Sanchez-Lozada LG Tapia E Santamaria J, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67(1):237–247. doi: 10.1111/j.1523-1755.2005.00074.x [DOI] [PubMed] [Google Scholar]
- 71.Nakagawa T Mazzali M Kang DH, et al. Hyperuricemia causes glomerular hypertrophy in the rat. Am J Nephrol. 2003;23(1):2–7. doi: 10.1159/000066303 [DOI] [PubMed] [Google Scholar]
- 72.Johnson RJ Bakris GL Borghi C, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the national kidney Foundation. Am J Kidney Dis. 2018;71(6):851–865. doi: 10.1053/j.ajkd.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goicoechea M Garcia de Vinuesa S Verdalles U, et al. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis. 2015;65(4):543–549. doi: 10.1053/j.ajkd.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 74.Siu YP, Leung KT, Tong MKH, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 75.Sircar D Chatterjee S Waikhom R, et al. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis. 2015;66(6):945–950. doi: 10.1053/j.ajkd.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 76.Agarwal R Sinha AD Cramer AE, et al. Chlorthalidone for hypertension in advanced chronic kidney disease. N Engl J Med. 2021;385(27):2507–2519. doi: 10.1056/nejmoa2110730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jordan DM Choi HK Verbanck M, et al. No causal effects of serum urate levels on the risk of chronic kidney disease: a Mendelian randomization study. PLoS Med. 2019;16(1):e1002725. doi: 10.1371/journal.pmed.1002725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hughes K, Flynn T, de Zoysa J, Dalbeth N, Merriman TR. Mendelian randomization analysis associates increased serum urate, due to genetic variation in uric acid transporters, with improved renal function. Kidney Int. 2014;85(2):344–351. doi: 10.1038/ki.2013.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doria A Galecki AT Spino C, et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med. 2020;382(26):2493–2503. doi: 10.1056/nejmoa1916624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kimura K Hosoya T Uchida S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis. 2018;72(6):798–810. doi: 10.1053/j.ajkd.2018.06.028 [DOI] [PubMed] [Google Scholar]
- 81.Tsai CW, Lin SY, Kuo CC, Huang CC. Serum uric acid and progression of kidney disease: a longitudinal analysis and mini-review. PLoS One. 2017;12(1):e0170393. doi: 10.1371/journal.pone.0170393 [DOI] [PMC free article] [PubMed] [Google Scholar]