Abstract
Background
There are limited data about the association between body mass index (BMI), glycemic variability (GV), and life-related factors in healthy nondiabetic adults.
Methods
This cross-sectional study was carried out within our ethics committee-approved study called “Exploring the impact of nutrition advice on blood sugar and psychological status using continuous glucose monitoring (CGM) and wearable devices”. Prediabetes was defined by the HbA1c level of 5.7–6.4% and /or fasting glucose level of 100–125 mg/dL. Glucose levels and daily steps were measured for 40 participants using Free Style Libre and Fitbit Inspire 2 under normal conditions for 14 days. Dietary intakes and eating behaviors were assessed using a brief-type self-administered dietary history questionnaire and a modified questionnaire from the Obesity Guidelines.
Results
All indices of GV were higher in the prediabetes group than in the healthy group, but a significant difference was observed only in mean amplitude of glycemic excursions (MAGE). In the multivariate analysis, only the presence of prediabetes showed a significant association with the risk of higher than median MAGE (Odds, 6.786; 95% CI, 1.596–28.858; P = 0.010). Additionally, the underweight (BMI < 18.5) group had significantly higher value in standard deviation (23.7 ± 3.5 vs 19.8 ± 3.7 mg/dL, P = 0.038) and coefficient variability (22.6 ± 4.6 vs 18.4 ± 3.2%, P = 0.015), compared to the normal group. This GV can be partially attributed to irregularity of eating habits. On the contrary, the overweight (BMI ≥ 25) group had the longest time above the 140 or 180 mg/dL range, which may be due to eating style and taking fewer steps (6394 ± 2337 vs 9749 ± 2408 steps, P = 0.013).
Conclusions
Concurrent CGM with diet and activity monitoring could reduce postprandial hyperglycemia through assessment of diet and daily activity, especially in non- normal weight individuals.
Introduction
Obesity (body mass index (BMI) ≥25) has been consistently reported to be associated with the high risk of type2 diabetes mellitus (T2DM) [1, 2]. On the contrary, a large Japanese cohort study showed that underweight in adults aged 60–79 years may be associated with the risk of T2DM [3]. Jung et al reported that underweight, overweight (≥ 23 and ≺ 25), obese (≥ 25 and < 30), and severe obese (≥ 30) group had the higher hazard ratios for T2DM than normal group during follow up for 10 years [4]. Among Japanese women without parental DM history, combining “low” (< 25th percentile) BMI at age 18 years with current “middle” (25th to 74th percentile) or “high” (> 75th percentile) BMI had significantly high odds ratios (2.25 or 13.92) for adult-onset DM [5]. Furthermore, low BMI was associated with adverse coronary heart disease outcomes in Asian populations [6].
Continuous glucose monitoring (CGM), which can collect glucose data for several days in a non-invasive way, has been developed for diabetics to estimate and control their plasma glucose changes throughout the day. Prior studies have evaluated sensor glucose levels and glycemic variability (GV) in healthy individuals without diabetes [7–9]. Many self-reported non-diabetic participants have frequent glycemic excursions into the diabetic range: Fifteen % of healthy and 36% of prediabetic individuals had glucose levels above 200 mg/dl on CGM [10]. The quantity of carbohydrate has been shown to be a consistent predictor of postprandial blood glucose levels [11]. Moreover, not only type and amount of meals but also eating behaviors including eating frequency, skipping breakfast, snacking and eating speed, influence the onset of diabetes [12–14]. In a recent review, there are several reports on the association between sedentary time or exercise and glycemic excursions in patients with type 2 diabetes [15]. However, there are limited data combining CGM-measured glucose levels with diet and physical activity that could impact glycemia in individuals without diabetes [16].
We hypothesized that non-normal weight (underweight or overweight/obesity) people may be less active, and their eating behaviors such as skipping breakfast or eating fast may lead to greater GV, compared to normal weight people. The aim of this study is to examine the association between BMI, GV, and life-related factors such as diet, eating behaviors and daily activity in healthy non-diabetic individuals under normal conditions.
Materials and methods
Trial design
The present study was carried out within our ethics committee-approved study called “Exploring the impact of nutrition advice on blood sugar and psychological status using CGM and wearable devices: A feasibility study”. Briefly, this original research exploratory examined the relationship between blood glucose variability, activities of daily living obtained from wearable devices and psychological state obtained from questionnaires and ecological momentary assessment. Also, the extent to which intervention effects of advice on general diet and eating behavior influences these outcomes has been verified. Therefore, participants were monitored by CGM for two weeks before and after the dietary advice by our registered dietitian. However, in this cross-sectional study, we aimed to examine the association between BMI, GV, and life-related factors under normal conditions. In other words, this study is from the original clinical trial and the results presented from this cross-sectional trial are from the baseline prior to deploying the study intervention. The original research was approved by the institutional Review Board of Keio University Hospital (IRB No. 20211103) and has been registered in University Hospital Medical Information Network (UMIN) Clinical Trial Registry (UMIN000046858). Study participants signed a consent form for the study.
Participants
Healthy office workers without diabetes (previous diagnosis of diabetes or hemoglobin A1c (HbA1c) ≥ 6.5% or fasting plasma glucose ≥ 126 mg/dL), who had undergone a medical health check-up within 1 year were recruited via internal company communication in February 1, 2022 to February 28, 2022. A total of 40 individuals with a 1:1 male to female ratio were recruited for this research. All participants provided written informed consent before enrolling in the research. Prediabetes was defined by the HbA1c level of 5.7 to 6.4% and /or impaired fasting glucose level of 100 to 125 mg/dL [17] by the medical health check-up record. BMI was calculated as weight (kg) divided by the square of the body height (m). They were instructed not to change their usual diet and physical activity until receiving general dietary advice. Thus, the present study period was defined as the first 14 days during CGM monitoring. Data collection occurred between February and April 2022.
CGM procedures and measurement of glycemic variability
Glucose levels were assessed by using Freestyle Libre (Abbot, Tokyo, Japan), intermittent-scanning CGM. The sensor measures the glucose concentration in the interstitial fluid every 15 minutes for a period of 14 days. Sensor data was downloaded, processed, visualized and archived using a licensed software. Only those who covered more than 60% of CGM monitoring over 14 days were included in the final analysis.
As the currently adopted in the consensus on the use of CGM metrics [18, 19], the mean glucose level, standard deviation (SD), percent coefficient variability (CV), mean amplitude of glycemic excursions (MAGE), time above range (TAR), time in range (TIR), or time below range were calculated by means of software (Excel, Microsoft Office). We evaluated the GV as SD, CV and MAGE, and the definitions and interpretation of each index were described in previous report [20].
Evaluation of dietary history and behavior
For an assessment for daily food and nutrient intakes, a brief-type self-administered diet history questionnaire (BDHQ) consisting of 58-item food frequency questionnaires and 15-item diet history questionnaires [21, 22] was used. In addition, for the assessment for eating behavior, questionnaires from the Guideline for Obesity issued by the Japan Society for the Study of Obesity [23, 24] were excerpted and partially modified. It comprises 36-item detailed questions contained in the following 7 major categories: G1) recognition for weight and constitution, G2) external eating behavior, G3) emotional eating behavior, G4) sense of hunger, G5) eating style, G6) food preference, G7) regularity of eating habits. All items were rated on a scale of 1 (I don’t think so at all), 2 (I don’t think so), 3 (I think so a little), and 4 (I think), and the average score was calculated for each item. We used responses to three, two or seven questions in the G5, G6 or G7 categories, respectively, that were thought to affect blood glucose levels. Both questionnaires were examined at the end of the study.
Assessment of activity
Participants were instructed to wear a smartwatch-type activity tracer, Fitbit Inspire 2 (Fitbit Inc., Tokyo, Japan) [25], throughout the day, except when bathing. This activity monitor communicated with a smartphone application to provide feedback on the number of steps taken. The average step counts per day were calculated for each participant. For the final analysis, we used data of participants who covered at least 60% of their Fitbit wearing time, excluding sleep time, for 14 days.
Statistical analysis
All participants were included in the CGM analysis, but only one with normal weight was excluded from the analysis of daily activity due to lack of data by activity tracer. For continuous data, mean values were expressed with SD, and statistical differences between two groups as the reference of the normal weight group, were determined using the t-test or Mann-Whitney U., when the data was normally distributed or not, respectively. For categorical data, numbers were presented with percentage, and statistical differences were determined using the chi-square tests. To rule out multicollinearity, we ensured the absolute value of the correlation coefficient between the independent variables before regression analysis. Then, factors associated with the risk of higher than median MAGE (MAGE ≥52) by CGM were analyzed using logistic regression analysis. For sensitivity analyses, similar comparison of CGM metrics between two groups were performed by using the cutoff point of BMI of 23 kg/m2 in Asian-Pacific obesity guideline defined by the World Health Organization International Obesity Task Force for Asians (underweight ≺ 18.5, 18.5 ≤ normal weight ≺ 23, and overweight ≥ 23 kg/m2 [26]. All statistical analyses were performed using SPSS software version 24 (SPSS, Inc., Chicago, Ill). All p-values less than 0.05 were considered statistically significant.
Results
CGM metrics (GV and percentage of glucose sensor values) in healthy or prediabetes participants
Of the 40 healthy nondiabetic participants, 20 were female and 14 were prediabetic, with a mean age of 40.3 years. The average valid time of the Libre and Fitbit sensors was good at 92.2% and 88.0%, respectively, of the entire study period. In this study, all participants were included in the final analysis. Overall GV values and percentage of glucose sensor values are shown in Table 1. The prediabetes group was older than the healthy group, but there was no gender difference. All indices of GV were higher in the former group than in the latter group, but a significant difference was observed only in MAGE. Percentage of TAR (180) and TAR (140) were higher in the prediabetes group, compared to the healthy group, but there was no significant difference. Interestingly, 13 healthy participants (50%) had TAR (180) > 0, compared with 71% with prediabetes. We next investigated factors related to the risk of higher than median MAGE (MAGE ≥52) by CGM. In both Model 1, which included age, sex and the presence of prediabetes, and Model 2, which further adjusted for BMI, only the presence of prediabetes showed significant difference (Odds, 6.786; 95% CI, 1.596–28.858; P = 0.010) (Table 2).
Table 1. Summary of CGM metrics by presence of prediabetes in healthy non-diabetes participants (n = 40).
| Characteristics of research participants | Healthy (n = 26) | Prediabetes (n = 14) | P |
|---|---|---|---|
| Male (%) | 12 (46%) | 8 (57%) | 0.507 |
| Age, year (range) | 33.9 ± 9.2 (23∼56) | 52.3 ± 5.2 (45∼60) | 0.000 |
| BMI, kg/m2 (range) | 21.1 ± 2.8 (16.9∼28) | 21.5 ± 2.1 (18.5∼25.1) | 0.660 |
| FBS, mg/dL (range) | 84.2 ± 6.9 (67∼97) | 101.9 ± 9.2 (84∼117) | 0.000 |
| HbA1c, % (range) | 5.0 ± 0.2 (4.4∼5.5) | 5.6 ± 0.3 (5.0∼6.1) | 0.000 |
| Overall glucose distribution and variability | |||
| Mean, mg/dL | 104.1 ± 20.7 | 110.0 ± 8.7 | 0.316 |
| SD, mg/dL | 20.0 ± 4.1 | 22.1 ± 4.4 | 0.151 |
| CV, % | 18.2 ± 3.9 | 20.1 ± 3.7 | 0.163 |
| MAGE, mg/dL | 49.2 ± 9.4 | 56.0 ± 11.4 | 0.044 |
| Percentage of glucose sensor values | |||
| TAR (180), % | 0.9 ± 1.3 | 1.6 ± 1.5 | 0.086 |
| TAR (180) ≻ 0, % | 13 (50%) | 10 (71%) | 0.191 |
| TIR (70–180), % | 97.6 ± 2.8 | 97.8 ± 1.3 | 0.832 |
| TAR (140), % | 8.0 ± 7.1 | 10.6 ± 6.2 | 0.253 |
| TIR (70–140), % | 90.5 ± 7.2 | 88.6 ± 5.9 | 0.407 |
| Total energy, nutrients, food groups | |||
| Energy, kcal/d | 1482 ± 380 | 1814 ± 587 | 0.037 |
| Carbohydrate, % energy | 48.3 ± 6.9 | 46.9 ± 7.7 | 0.556 |
| Alcohol, % energy | 6.4 ± 7.0 | 5.0 ± 8.0 | 0.570 |
| Confections, g/d | 59.2 ± 45.7 | 62.5 ± 40.7 | 0.823 |
| Sugar sweetened beverages, g/d | 55.7 ± 73.8 | 97.1 ± 101.7 | 0.193 |
| Lifestyle behaviors | |||
| G5: Do you eat fast? | 2.9 ± 0.8 | 2.8 ± 01.0 | 0.645 |
| G6: Do you often eat snacks? | 2.2 ± 0.9 | 1.9 ± 0.9 | 0.313 |
| G6: Do you often drink? | 2.2 ± 0.9 | 1.9 ± 0.9 | 0.313 |
| G7: Do you often skip breakfast? | 2.1 ± 1.1 | 1.6 ± 1.2 | 0.237 |
| G7: Do you eat between meals during the day? | 2.6 ± 0.9 | 1.9 ± 0.8 | 0.023 |
| G7: Do you eat a late-night snack? | 2.0 ± 0.9 | 1.3 ± 0.5 | 0.011 |
| Dairy activity (n = 25, 14) | |||
| Average dairy step counts | 9172 ± 2056 | 9446 ± 3495 | 0.797 |
CGM, continuous glucose monitoring; SD, standard deviation; CV, coefficient variation; MAGE, mean amplitude of glycemic excursions; TAR, time above range; TIR, time in range. Prediabetes was defined by the HbA1c level of 5.7 to 6.4% and /or impaired fasting glucose level of 100 to 125 mg/dL.
Table 2. Multiple logistic regression analysis for the association between characteristics of the participants or lifestyle behaviors and the risk of higher than median MAGE (MAGE ≥52) by CGM.
| Odds | 95% CI | P | ||
|---|---|---|---|---|
| Model1 | sex | 0.501 | ||
| age | 0.293 | |||
| Prediabetes | 6.786 | 1.596–28.858 | 0.010 | |
| Model2 | sex | 0.501 | ||
| age | 0.293 | |||
| Prediabetes | 6.786 | 1.596–28.858 | 0.010 | |
| BMI | 0.874 |
MAGE, mean, amplitude of glycemic excursions; CGM, continuous glucose monitoring; Prediabetes was defined by the HbA1c level of 5.7 to 6.4% and /or impaired fasting glucose level of 100 to 125 mg/dL.
CGM metrics by BMI classification
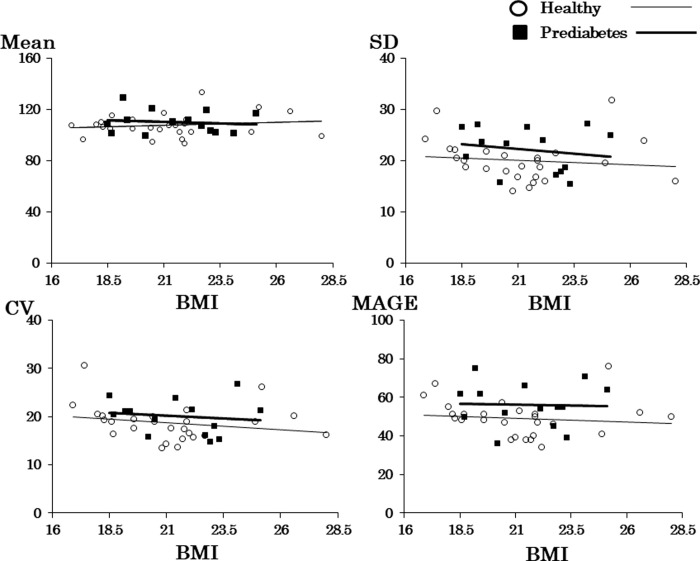
Fig 1 displays scatter plots for mean glucose concentration and each of the three GV indices, with closed squares and bold regression lines corresponding to prediabetes and open circles and thin regression lines to healthy participants. The bold lines for the prediabetes groups are always above the thin lines for the healthy group, except the upper left figure. Except for the upper left scatter plot of BMI and mean glucose concentration, the values for each GV index seem to be lowest around BMI of 21 kg/m2.
Fig 1. Scatter plots showing CGM metrics and BMI in the prediabetes group and the healthy group.
SD, standard deviation; CV, coefficient variation; MAGE, mean amplitude of glycemic excursions.
All 40 participants were grouped into underweight (n = 5), normal weight (n = 31) and overweight (n = 4) (Table 3). Compared to the normal weight group, the underweight group consisted only of women, and 13 and 1 prediabetic participants were included in the standard and overweight group, respectively. The underweight group had the lowest mean glucose concentration among the three groups, but had higher indices of GV (SD, CV and MAGE) than the normal weight group, especially significantly higher SD and CV. As for glucose sensor values, percentage of TAR (180) and TAR (140) were highest in the overweight group. Interestingly, all participants in the underweight group had TAR (180) > 0, compared to 48% in the normal weight group (P = 0.041). Compared to the normal weight group by the cutoff point of BMI of 23 kg/m2, the underweight group also had significantly higher SD and CV, and percentage of TAR (180) and TAR (140) were also highest in the overweight group (S1 Table).
Table 3. Summary of CGM metrics by BMI classification in healthy non-diabetes participants.
| BMI | Underweight | Normal weight | Overweight | P | ||
|---|---|---|---|---|---|---|
| (< 18.5) | (18.5 ≦ < 25) | (≥ 25) | ||||
| Characteristics of participants | Gr1 (n = 5) | Gr2 (n = 31) | Gr3 (n = 4) | Gr1 vs Gr2 | Gr1 vs Gr3 | Gr2 vs Gr3 |
| Male, % | 0 (0%) | 18 (58%) | 2 (50%) | 0.016 | 0.167 | 0.581 |
| Age, year | 35.4 ± 11.3 | 40.5 ± 12.4 | 45.3 ± 8.6 | 0.399 | 0.194 | 0.461 |
| BMI, kg/m2 | 17.8 ± 0.6 | 20.8 ±1.4 | 25.0 ± 1.6 | 0.000 | 0.000 | 0.000 |
| FBS, mg/dL | 86.6 ± 6.8 | 91.0 ± 12.5 | 89.8 ± 8.3 | 0.844 | 0.550 | 0.844 |
| HbA1c, % | 5.1 ± 0.3 | 5.3 ± 0.4 | 5.4 ± 0.3 | 0.297 | 0.110 | 0.441 |
| Prediabetes, (%) | 0 (0%) | 13 (42%) | 1 (25%) | 0.089 | 0.444 | 0.470 |
| Overall glucose distribution and variability | ||||||
| Mean, mg/dL | 105.8 ± 5.2 | 108.1 ± 9.0 | 114.0 ± 10.3 | 0.599 | 0.163 | 0.228 |
| SD, mg/dL | 23.7 ± 3.5 | 19.8 ± 3.7 | 24.2 ± 6.5 | 0.038 | 0.897 | 0.054 |
| CV, % | 22.6 ± 4.6 | 18.4 ± 3.2 | 21.0 ± 4.1 | 0.015 | 0.598 | 0.153 |
| MAGE, mg/dL | 56.6 ± 7.4 | 49.7 ± 10.2 | 60.5 ±12.0 | 0.154 | 0.567 | 0.057 |
| Percentage of glucose sensor values | ||||||
| TAR (180), % | 1.2 ± 0.4 | 0.9 ± 1.3 | 2.5 ± 2.5 | 0.649 | 0.287 | 0.046 |
| TAR (180) > 0, % | 5 (100%) | 15 (48%) | 3 (75%) | 0.041 | 0.444 | 0.323 |
| TIR (70–180), % | 95.0 ± 4.6 | 98.2 ± 1.5 | 97.3 ± 3.0 | 0.203 | 0.431 | 0.309 |
| TAR (140), % | 8.2 ± 1.1 | 8.2 ± 7.0 | 14.8 ± 7.9 | 0.994 | 0.197 | 0.092 |
| TIR (70–140), % | 87.6 ± 5.2 | 90.8 ± 6.7 | 85.3 ± 7.9 | 0.314 | 0.607 | 0.134 |
| Life style related factors | ||||||
| Energy (kcal/d) | 1406 ±180 | 1606 ± 409 | 1834 ±721 | 0.358 | 0.353 | 0.190 |
| < Estimated energy requirement (PAL I) | 4 (80%) | 28 (90%) | 3 (75%) | 0.466 | 0.556 | 0.687 |
| Nutrients (% energy) | ||||||
| Protein (% energy) | 14.8 ± 0.5 | 15.7 ± 2.3 | 17.8 ± 3.5 | 0.447 | 0.086 | 0.087 |
| Fat (% energy) | 28.3 ±1.8 | 29.4 ± 5.2 | 29.2 ± 2.0 | 0.358 | 0.505 | 0.915 |
| Carbohydrates (% energy) | 51.8 ± 4.2 | 47.2 ± 7.3 | 47.0 ± 8.6 | 0.183 | 0.305 | 0.955 |
| Alcohol (% energy) | 4.0 ± 5.1 | 6.4 ± 7.6 | 4.2 ± 8.4 | 0.496 | 0.970 | 0.583 |
| Food groups (g/d) | ||||||
| Rice (g/d) | 129.9 ± 64.1 | 216.3 ± 118.3 | 218.9 ± 125.2 | 0.036 | 0.257 | 0.969 |
| Bread (g/d) | 36.9 ± 21.2 | 32.2 ± 23.8 | 33.1 ± 32.3 | 0.679 | 0.838 | 0.942 |
| Noodles (g/d) | 93.1 ± 41.6 | 55.8 ± 37.8 | 86.1 ± 68.2 | 0.051 | 0.853 | 0.179 |
| Confections (g/d) | 73.4 ± 36.4 | 61.1 ± 46.2 | 38.0 ± 24.1 | 0.576 | 0.140 | 0.336 |
| Meat (g/d) | 64.7 ± 2.4 | 84.4 ± 36.9 | 82.0 ± 12.3 | 0.006 | 0.064 | 0.901 |
| Fish and shellfish (g/d) | 41.9 ± 15.1 | 63.5 ± 27.9 | 88.8 ± 48.3 | 0.103 | 0.146 | 0.127 |
| Vegetables (g/d) | 205.2 ± 39.3 | 212.3 ± 106.9 | 171.4 ± 37.3 | 0.885 | 0.231 | 0.457 |
| Fruits (g/d) | 85.2 ± 36.2 | 91.8 ± 90.0 | 90.5 ± 65.4 | 0.873 | 0.881 | 0.977 |
| Milk and milk products (g/d) | 87.6 ± 102.9 | 102.2 ± 75.6 | 122.3 ± 50.8 | 0.705 | 0.560 | 0.611 |
| Sugar sweetened beverages (g/d) | 13.3 ± 13.3 | 74.7 ± 85.7 | 106.5 ± 120.2 | 0.001 | 0.219 | 0.508 |
| Eating Behaviors G5: eating style | ||||||
| Do you eat fast? | 2.4 ± 0.5 | 2.9 ± 0.9 | 3.3 ± 1.0 | 0,240 | 0.136 | 0.479 |
| Do you eat without chewing too much? | 2.2 ± 0.4 | 2.9 ± 0.8 | 3.0 ± 0.8 | 0.066 | 0.101 | 0.754 |
| Do you have a lot of mouthfuls? | 2.0 ± 0.7 | 2.7 ± 0.8 | 3.0 ± 0.8 | 0.081 | 0.089 | 0.450 |
| Eating Behaviors G6: food preference | ||||||
| Do you often eat snacks? | 2.0 ± 0.7 | 2.0 ± 0.9 | 2.5 ± 1.3 | 1.000 | 0.480 | 0.305 |
| Do you often drink? | 2.0 ± 0.7 | 2.0 ± 0.9 | 2.5 ± 1.3 | 1.000 | 0.480 | 0.306 |
| Eating Behaviors G7: regularity of eating habits | ||||||
| Do you often skip meals during the day? | 2.6 ± 1.5 | 2.0 ± 1.0 | 2.0 ± 0.8 | 0.286 | 0.502 | 0.952 |
| Do you often skip breakfast? | 2.4 ± 1.5 | 1.9 ± 1.0 | 1.5 ± 1.0 | 0.360 | 0.343 | 0.471 |
| Do you have irregular mealtimes? | 2.8 ± 0.8 | 2.6 ± 0.8 | 2.5 ± 0.6 | 0.617 | 0.563 | 0.777 |
| Don’t you have time to eat slowly? | 3.2 ± 0.8 | 2.5 ± 0.7 | 2.5 ± 1.0 | 0.032 | 0.289 | 0.899 |
| Do you eat between meals during the day? | 3.2 ± 0.8 | 2.3 ± 0.9 | 1.8 ± 1.0 | 0.043 | 0.046 | 0.229 |
| Do you eat a late-night snack? | 2.2 ± 0.8 | 1.7 ± 0.9 | 1.3 ± 0.5 | 0.291 | 0.087 | 0.292 |
| Do you often drink canned juice, canned coffee, or energy drinks? | 1.6 ± 0.5 | 1.8 ± 0.9 | 1.5 ± 1.0 | 0.554 | 0.853 | 0.471 |
| Dairy Activity (n = 5, 30, 4) | ||||||
| Average dairy step counts | 8648 ± 2572 | 9749 ± 2408 | 6394 ± 2337 | 0.355 | 0.217 | 0.013 |
| Average daily step counts ≥ 10,000 | 2 (40%) | 14 (47%) | 0 (0%) | 0.585 | 0.278 | 0.104 |
CGM, continuous glucose monitoring; SD, standard deviation; CV, coefficient variation; MAGE, mean amplitude of glycemic excursions; TAR, time above range; TIR, time in range. Prediabetes was defined by the HbA1c level of 5.7 to 6.4% and /or impaired fasting glucose level of 100 to 125 mg/dL.
Comparison of diet history by BMI classification (Table 3)
Total energy intakes increased in the higher BMI group, but the ratio of each of the three macronutrients to total energy intake was approximately the same. Compared to the normal weight group as a reference, the overweight group had higher intake of some kind of foods such as sugar sweetened beverages, but they did not differ between the two groups. On the other hand, the underweight group showed lower intake of most kind of foods, except noodles and confections, than the reference group.
Comparison of eating behavior and daily activity by BMI classification (Table 3)
Each score in the G5 and G6 category increased in the higher BMI group. Compared to the normal weight group, the underweight group scored higher on most questions for the G7 category, especially lack of time to eat slowly and eating between meals during the day (3.2 ± 0.8 vs 2.5 ± 0.7, P = 0.032; 3.2 ± 0.8 vs 2.3 ± 0.9, P = 0.043). On the contrary, there was a significant difference in average daily step counts between the overweight group and the reference (6394 ± 2337 vs 9749 ± 2408 steps, P = 0.013).
Discussion
This is, to the best of our knowledge, the first study to investigate the association between BMI, GV, and life-related factors including diet, eating behaviors and daily activity, in healthy non-diabetic individuals under normal real-life conditions. Here, we demonstrated the impact of BMI on GV and the possibility of relationship between lifestyle factors and GV. Our results highlight that underweight (BMI < 18.5 kg/m2) group had the significantly higher value in SD and CV in comparison with normal weight group (18.5 ≤ BMI ≺ 25 kg/m2) as a reference, although the underweight group had the lowest mean value among the three groups. Results from BDHQ and the eating behavior questionnaire suggest that this GV could be partially caused by irregularity of eating habits including habitual eating between meals during the day. On the other hand, overweight group had highest percentage of TAR (180) and TAR (140) among the three groups, which may be due to eating style (i.e., eating fast) and taking fewer steps.
Insulin resistance and impaired insulin secretion are the two main components in the pathophysiology of T2DM. The predominant mechanism in lean diabetic patients was impaired insulin secretion, whereas that for obese subjects was insulin resistance [27]. Hyperglycemia is a causative factor for β-cell dysfunction before the onset of diabetes [28], and increased GV was related with decreased oral disposition index, a useful marker of islet β-cell function [29]. Thus, using CGM to assess the extent of postprandial hyperglycemia and GV in healthy individuals without diabetes is of significance for prediction and prevention of diabetes. All indices of GV and percentage of both TAR (180) and TAR (140) were higher in the prediabetes group than in the healthy group, but a significant difference was observed only in MAGE. Kishimoto et al. reported that the median CV, TAR (140) and TAR (180) were 18.3%, 10.4% and 0.6% in Japanese obese middle-aged men without diabetes [30]. Chakarova et al also found a significantly higher CV (20%) and significant increase of both SD and MAGE after adjustment for BMI, in the prediabetes group in comparison with the normal glucose tolerance group [31]. Our results were almost similar to those of these studies involving adults who were older and had higher BMIs than ours. Most interestingly, the underweight participants had the significantly higher values in SD and CV, compared to those with normal weight, in spite of lower mean glucose concentration. In Japan, it has been reported that young women tend to lose weight over the 25-year period [32]. According to Results of Year 2019 National Health and Nutrition Survey by Japanese Ministry of Health, Labor and Welfare [33], the percentage of people in their 20s with underweight has increased significantly in recent years, reaching the 20% level. Also, young underweight Japanese women had the higher prevalence of impaired glucose tolerance (IGT) than the normal weight women (13.3% vs 1.8%) and showed a lower insulinogenic index [34]. Together, these results suggest that the inability of β-cell function to compensate for decreased insulin sensitivity may contribute to the development of DM, especially in underweight women. In addition, the more muscle mass involved in glucose uptake, the better insulin resistance and the lower risk of prediabetes or overt diabetes [35]. Therefore, underweight women with lower average muscle mass may have severe insulin resistance in skeletal muscle. Thus, impaired insulin secretion and insulin resistance in skeletal muscle could be characteristic of underweight individuals with higher GV indices, resulting in postprandial hyperglycemia.
The mean caloric intake and daily activity in young women in Japan is very low and their daily steps count tends to be lower for these ten years [33]. Most participants (35 out of 40) including young women had the mean energy intakes lower than the estimated energy requirement for those with physical activity level I (low) in the “Dietary Reference Intakes for Japanese” by the Ministry of Health, Labor and Welfare [33]. No participants ate more than the recommended amount of carbohydrates per day (50–65% energy) [33]. On the other hand, as its recommendation regarding the amount of daily physical activity [36], it is considered ideal to secure "10,000 steps a day." The average step counts for this entire cohort was 9,264, similar to the underweight group (8,648), while the overweight group had a mean number of 6,394 steps, which was significantly lower. We therefore concluded that the higher GV in the underweight or overweight group was likely due to irregularity of eating habits or reduced daily physical activity. Regarding eating behaviors, the underweight eat more confections (73.4 g/d), but they were less aware of it (2.0 point). It was also found that the overweight tended to consume more sugar sweetened beverages (106.5g/d), but they didn’t pay much attention to it (1.5 point). In this study, we evaluated the factors associated with postprandial hyperglycemia in each of the three groups by BMI. Similarly, individual assessments are expected to identify factors that contribute to the individual’s postprandial hyperglycemia, and respective advice will help reduce glycemic excursions. Although lifestyle factors, including diet and physical activity, are often interrelated, interventions tend to focus on changing one health behavior rather than concurrently intervening on multiple behaviors. A meta-analysis of effects on glycemic control showed that lifestyle modifications based on physical or dietary intervention or both are associated with improvements in the 2-hour plasma glucose in IGT patients [37]. Much work is still needed to better understand how lifestyle factors may uniquely contribute to GV, and additional high-quality research on interventions designed to modify lifestyle behaviors is required to control blood glucose levels in healthy individuals.
Some limitations of this study deserve comments. Since the original research was exploratory, no power or sample size was calculated during the study design. The small proportion of participants limited comparisons of GV and life-related factors according to BMI classification. Sex and /or age-based analyzes were also not possible. However, this feasibility study confirmed that data collection from CGM, wearable devices, and questionnaire is sufficiently achievable. Secondly, this cohort of healthy individuals recruiting through internal company communication might limit generalizability. Thirdly, the overweight group in this cohort had an average BMI of 26.2 kg/m2, close to that of the normal weight group in other studies. Fourthly, both dietary questionnaires were surveyed at the end of the study, which may have introduced recall bias, albeit for short-term memory within 14 days. Finally, we could not estimate insulin secretion and insulin sensitivity based on the OGTT. Regardless of these limitations, our results outline clear tendencies and show the necessity of future long-term research focused on the association between lower BMI and GV. The high data acquisition rate in this study seems to warrant the feasibility of future large-scale studies.
Conclusion
Significantly higher glycemic variability in SD and CV was observed for the underweight participants in comparison with the normal, which can be partly attributed to irregularity of eating habit (eating habit between meals) in free-living conditions. Future research is needed to determine which types of subjects with low BMI may have delayed insulin secretion and to identify the influence of low BMI on the development of IGT and/or T2DM. Concurrent CGM with diet and activity monitoring can yield health benefits to assess the impact of foods that individuals might consider healthy and to raise awareness of activity in daily living, i.e., enabling the practice of personalized medicine. These data also provide basic information for considering future treatments such as diet and exercise to predict or prevent the onset of diabetes.
Supporting information
(XLSX)
(DOCX)
Acknowledgments
We thank all participants who made the study possible and members of the Wellness Promotion Department at Mori Building Co., Ltd who helped recruit the participants.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors (KK, SK, TK) disclosed receipt of the following financial support for the research, authorship, and /or publication of this article: The study was funded by Mori Building Co., Ltd. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005; 366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1 [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM. Mechanism linking obesity to insulin resistance and type 2 diabetes. Nature. 2006; 444:840–846. doi: 10.1038/nature05482 [DOI] [PubMed] [Google Scholar]
- 3.Sairenchi T, Iso H, Irie F, Fukasawa N, Ota H, Muto T. Underweight as a predictor of diabetes in older adults: a large cohort study. Diabetes Care. 2008; 31:583–584. doi: 10.2337/dc07-1390 [DOI] [PubMed] [Google Scholar]
- 4.Jung JY, Park SK, Oh CM, Ryoo JH, Choi JM, Choi YJ. The risk of type 2 diabetes mellitus according to the categories of body mass index: the Korean Genome and Epidemiology Study (KoGES). Acta Diabetol. 2018; 55:479–484. doi: 10.1007/s00592-018-1112-4 [DOI] [PubMed] [Google Scholar]
- 5.Katanoda K, Noda M, Goto A, Mizunumz H, Lee JS, Hayashi K. Being underweight in adolescence is independently associated with adult-onset diabetes among woman: The Japan Nurses’ Health Study. J Diabetes Investig. 2019; 10:827–836. doi: 10.1111/jdi.12947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hioki H, Miura T, Motoki H, Kobayashi H, Kobayashi M, Nakajima H, et al. Lean body mass index prognostic value for cardiovascular events in patients with coronary artery disease. Heart Asia. 2015; 7:12–18. doi: 10.1136/heartasia-2015-010644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freckmann G, Hagenlocher S, Baumstark A, Jendrike N, Gillen RC, Rössner K, et al. Cintinuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J Diabetes Sci Technol. 2007; 1:695–703. doi: 10.1177/193229680700100513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group; Fox LA, Beck RW, Xing D. Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care. 2010; 33:1297–1299. doi: 10.2337/dc09-1971 [DOI] [PMC free article] [PubMed]
- 9.Akintola AA, Noordam R, Jansen SW, de Craen AJ, Ballieux BE, Cobbaert CM, et al. Accuracy of continuous glucose monitoring measurements in normo-glycemic individuals. PLoS ONE. 2015;10(10):e0139973. doi: 10.1371/journal.pone.0139973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zahedani AD, Torbaghan SS, Rahili S, Karlin K, Scilley D, Thakkar R, et al. Improvement in glucose regulation using a digital tracer and continuous glucose monitoring in healthy adults and those with type2 diabetes. Diabetes Ther. 2021;12:1871–1886. doi: 10.1007/s13300-021-01081-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gannon MC, Nuttall FQ, Westphal SA, Fang S, Ecran-Fang N. Acute metabolic response to high-carbohydrate, high- starch meals in subjects with type2 diabetes. Diabetes Care. 1998; 21:1619–1626. doi: 10.2337/diacare.21.10.1619 [DOI] [PubMed] [Google Scholar]
- 12.Van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary-patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med. 2002; 136:201–209. doi: 10.7326/0003-4819-136-3-200202050-00008 [DOI] [PubMed] [Google Scholar]
- 13.Mekary RA, Giovannucci E, Cahill E, Willett WC, van Dam RM, Hu FB. Eating pattern and type 2 diabetes risk in older women: breakfast consumption and eating frequency. Am J Clin Nutr. 2013; 98:436–443. doi: 10.3945/ajcn.112.057521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudo A, Asahi K, Satoh H, Iseki K, Moriyama T, Yamagata K, et al. Fast eating is a strong risk factor for new-onset diabetes among the Japanese general population. Scientific Reports. 2019; 9:8210. doi: 10.1038/s41598-019-44477-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparks JR, Kishman EE, Sarzynski MA, Davis JM, Grandjean PW, Durstine JL, et al. Glycemic variability: Importance, relationship with ohysical activity, and the influence of exercise. Sports Med Health Sci. 2021;11:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuBose SN, Li Z,, Sherr JL, Beck RW, Tamborlane WV, Shah VN. Effect of exercise and meals on continuous glucose monitor data in healthy individuals without diabetes. J Diabetes Sci Technol. 2021; 15:593–599. doi: 10.1177/1932296820905904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Standards of Medical care in diabetes-2010. Diabetes Care. 2010; 33:S11–S61. doi: 10.2337/dc10-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maahs DM, Buckingham BA, Castle JR, Cinar A, Damiano ER, Dassau E, et al. Outcome measures for artificial pancreas clinical trials: A consensus report. Diabetes Care. 2016; 39:1175–1179. doi: 10.2337/dc15-2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017; 40:1631–1640. doi: 10.2337/dc17-1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inaishi J, Saisho Y, Watanabe Y, Tsuchiya T, Sasaki H, Masaoka T, et al. Changes in glycemic variability, gastric emptying and vascular endothelial function after switching from twice-daily to once-weekly exenatide in patients with type 2 diabetes: a subpopulation analysis of the twin-exenatide study. BMC Endosc Disord. 2022; 22:20. doi: 10.1186/s12902-022-00932-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi S, Murakami K, Sasaki S, Okubo H, Hirota N, Notsu A, et al. Comparison of relativevalidity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16d dietary records in Japanese adults. Public Health Nutr. 2011; 14:1200–1211. doi: 10.1017/S1368980011000504 [DOI] [PubMed] [Google Scholar]
- 22.Omura Y, Murakami K, Matoba K, Nishimura R, Sasaki S. Effects of in individualized dietary advice compared with conventional dietary advice for adults with type 2 diabetes: A randomized controlled trial. Nutr, Metab & Cardiovasc Dis. 2022; 32:1035–1044. doi: 10.1016/j.numecd.2021.11.006 [DOI] [PubMed] [Google Scholar]
- 23.Inoue K, Maeda N, Kashine S, Fujishima Y, Kozawa J, Hiuge-Shimizu A, et al. Short-term effects of liraglutide onvisceral fat adiposity, appetite, and food preference: a pilot study of obese Japanese patients with type 2 diabetes. Cardiovasc Diabetol. 2011; 10:109. doi: 10.1186/1475-2840-10-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujishima Y, Maeda N, Inoue K, Kashine S, Nishizawa H, Hirata A, et al. Efficacy of liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, on body weight, eating behavior, and glycemic control, in Japanese obese type 2 diabetes. Cardiovasc Diabetol. 2012; 11:107. doi: 10.1186/1475-2840-11-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whelan ME, Denton F, Bourne CLA, Kingsnorth AP, Sherar LB, Orme MW, et al. A digital lifestyle behaviour change intervention for the prevention of type 2 diabetes: a qualitative study exploring intuitive engagement with real-time glucose and physical activity feedback. BMC Public Health. 2021; 21:130. doi: 10.1186/s12889-020-09740-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Expert Consultation (2004) Appropriate body-mass index for Asian population and its implications for policy and intervention strategies. Lancet. (2004) 363:157–163. doi: 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 27.Chan WB, Tong PC, Chow CC, So WY, Ng MC, Ma RC, et al. The association of body mass index, C-peptide and metabolic status in Chinese Type 2 diabetic patients. Diabet Med. 2004; 21:349–353. doi: 10.1111/j.1464-5491.2004.01158.x [DOI] [PubMed] [Google Scholar]
- 28.Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009; 32:Suppl2:S151-S156. doi: 10.2337/dc09-S301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen T, Xu F, Su JB, Wang XQ, Chen JF, Wu G, et al. Glycemic variability in relation to oral disposition index in the subjects with different stages of glucose tolerance. Diabetol Metab Syndr. 2013; 5:38. doi: 10.1186/1758-5996-5-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kishimoto I, Ohashi A. Hyperglycemia during continuous glucose monitoring in obese/overweight male individuals without diabetes. J Diabetes Sci Technol. 2021; 15:1198–1199. doi: 10.1177/19322968211018721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakarova N, Dimova R, Grozeva G, Tankova T. Assessment of glucose variability in subjects with prediabetes. Diabetes Res Clin Pract. 2019; 151:56–64. doi: 10.1016/j.diabres.2019.03.038 [DOI] [PubMed] [Google Scholar]
- 32.Takimoto H, Yoshiike N, Kaneda F, Yoshita K. Thinness among young Japanese women. Am J Public Health. 2004; 94:1592–1595. doi: 10.2105/ajph.94.9.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Health and Nutrition Survey | Health Japan 21 (nibiohn.go.jp)
- 34.Sato M, Tamura Y, Nakagata T, Someya Y, Kaga H, Yamasaki N, et al. Prevalence and features of impaired glucose tolerance in young underweight Japanese women. J Clin Endocrinol Metab. 2021; 106:e2053–e2062. doi: 10.1210/clinem/dgab052 [DOI] [PubMed] [Google Scholar]
- 35.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Finding from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011; 96:2898–2903. doi: 10.1210/jc.2011-0435 [DOI] [PubMed] [Google Scholar]
- 36.https://www.mhlw.go.jp/www1/topics/kenko21_11/b2.html (mhlw.go.jp)
- 37.Gong QH, Kang JF, Ying YY, Li H, Zhang XH, Wu YH, et al. Lifestyle Interventions for Adults with Impaired Glucose Tolerance: A Systematic Review and Meta-Analysis of the Effects on Glycemic Control. Intern Med. 2015;54:303–310. doi: 10.2169/internalmedicine.54.2745 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.