Abstract
Polymyxin B (PMB) is a cyclic decapeptide antibiotic which also binds and neutralizes endotoxin. Unfortunately, PMB can be considerably nephrotoxic at clinically utilized doses, thereby limiting its utility as a therapeutic antiendotoxin reagent. We sought to change the pharmacokinetics and toxicity profile of PMB by covalently linking it to a human immunoglobulin G (IgG) carrier. Conjugates of PMB with IgG were prepared by EDAC [1-ethyl-3-(3-dimethylaminopropyl) carbodiimide]-mediated amide formation. Analysis by dot enzyme-linked immunosorbent assay with an anti-PMB monoclonal antibody showed that the purified conjugate contained bound PMB. The IgG-PMB conjugate reacted with lipid A and J5 lipopolysaccharide in Western blot assays in a manner comparable to that of whole antiserum with anti-lipid A reactivity; unconjugated IgG had no reactivity. The PMB bound in the conjugate retained its endotoxin-neutralizing activity compared to that of unbound PMB as evidenced by its dose-dependent inhibition of tumor necrosis factor release by endotoxin-stimulated human monocytes in vitro; unconjugated IgG had no activity. By this assay, the PMB-IgG conjugate was determined to have approximately 3.0 μg of bound functional PMB per 100 μg of total protein of conjugate (five molecules of PMB per IgG molecule). The PMB-IgG conjugate was also bactericidal against clinical strains of Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae relative to unconjugated IgG with MBCs of <4 μg of conjugate per ml for each of the tested strains. The conjugate appeared to be nontoxic at the highest doses deliverable and provided statistically significant protection from death to galactosamine-sensitized, lipopolysaccharide-challenged mice in a dose-dependent fashion when administered prophylactically 2 h before challenge. However, neither free PMB nor the PMB-IgG conjugate could protect mice challenged with endotoxin 2 h after administration. This suggests that these reagents can play a role in prophylaxis but not in therapy of sepsis. These experiments demonstrated that the PMB-IgG conjugate retains bound yet functional PMB as evidenced by its endotoxin-neutralizing activity both in vitro and in vivo. Further work is required to define the role that this or related conjugate compounds may play in the prophylaxis of endotoxin-mediated disease.
Septic shock remains a significant cause of morbidity and mortality despite the use of effective antibiotics and innovations in intensive care medicine (25). It has been estimated that there are approximately 300,000 cases per year in the United States (7). The mortality from sepsis exceeds 25% and in the presence of shock approaches 50% (14). Forty percent of the cases of sepsis are a consequence of infection with gram-negative bacteria (4). Bacterial lipopolysaccharide (LPS) or endotoxin precipitates the systemic inflammatory process, leading to sepsis and multiorgan failure by triggering the release of cytokines such as tumor necrosis factor (TNF) from macrophages (26). Of interest, endotoxemia has been demonstrated in up to 79% of septic patients and was detected in those with gram-positive bacterial and fungal infections as well (24).
Because of its role as a trigger of septic shock, endotoxin has been selected as a potential target for antisepsis strategies. Monoclonal antibodies (MAbs) against the active lipid A moiety of endotoxin have been developed and used therapeutically in clinical trials without significant success (23). Polymyxin B (PMB) is a cyclic decapeptide antibiotic which has been in clinical use for decades (28). It kills bacteria by disrupting cell membranes, presumably due to its ionic detergent action. Before clinically more effective drugs became available, PMB was used parenterally to treat serious Pseudomonas aeruginosa infections. More recently, its nephrotoxicity and modest efficacy following parenteral administration have relegated it to use primarily as a topical antibiotic (22).
PMB, in addition to its direct antimicrobial effects, binds stoichiometrically (1:1) to the lipid A moiety of bacterial LPS, and this binding results in the complete neutralization of endotoxin activity (20). Highly cationic PMB binds electrostatically to the anionic lipid A. PMB also utilizes hydrophobic binding between its acyl tail and the fatty acids of lipid A in this interaction. PMB was studied as an adjunct to effective antibiotics in an animal model of gram-negative bacterial sepsis in which it demonstrated protective efficacy independent of its antimicrobial activity (12). PMB has also been conjugated covalently to Sepharose and used in a plasmapheresis circuit to extract circulating endotoxin in septic animals. In one such experiment, use of a PMB column reduced mortality by 100% compared to that with a sham column (8). Unfortunately, the use of such a system may be too cumbersome for practical use in the clinical arena.
We sought to provide the endotoxin-neutralizing ability of PMB in a less toxic form with a longer half-life. We hypothesized that binding PMB to a carrier molecule would reduce rapid filtration through the renal glomeruli and thus prolong the PMB intravascular half-life. Since rapid filtration through the glomeruli and delivery to the renal tubules are the presumed mechanisms of PMB-induced nephrotoxicity, maintaining PMB in the intravascular space would also prevent nephrotoxicity (27). To this end, we had previously prepared a PMB-soluble starch conjugate by Schiff’s base chemistry (9). Other investigators have prepared a PMB-dextran conjugate by similar means (6, 16). In these conjugates, the covalently bound PMB retained its antiendotoxin abilities although the antimicrobial efficacy was significantly reduced (6). These conjugates were found to be almost completely nontoxic, and the PMB-dextran compound was efficacious in the galactosamine-sensitized mouse model of gram-negative bacterial sepsis (6). We hypothesized that covalent binding to a protein carrier could be another way of altering the toxicity and pharmacokinetics of PMB.
Human immunoglobulin G (IgG) has a half-life of 21 days and possesses free carboxyl groups available for the formation of amide bonds with the free amines of PMB (19). The relatively long half-life of IgG makes it attractive as a potential prophylactic reagent for endotoxin-mediated sepsis. The synthesis, characterization, and preliminary studies of the efficacy of a PMB-human IgG conjugate in an animal model are described in this report.
(This work was presented in part at the American Federation of Clinical Research Meeting in Baltimore, Md., May 1992 [9], and at the 2nd International Endotoxin Society Meeting in Vienna, Austria, August 1992.)
MATERIALS AND METHODS
(i) Synthesis and purification of PMB-IgG conjugates.
The PMB-IgG conjugate was synthesized by combining 50 mg of pure human IgG (Sigma Biologics, St. Louis, Mo.) dissolved in 10 ml of 0.05 N NaCl solution with 25 mg of PMB (Sigma) in 5 ml of 0.05 N NaCl solution. The mixture was stirred continuously with a magnetic stirrer at 25°C with constant pH monitoring. The pH was continuously adjusted with 0.1 N HCl solution to maintain a pH of 5.5 ± 0.1. A total of 300 mg of EDAC [1-ethyl-3-(3-dimethylaminopropyl) carbodiimide] hydrochloride (Sigma) was added to this solution. Another 300 mg was added at 90 min. After one more hour of reaction time, the mixture was purified by dialysis against tissue-culture-grade phosphate-buffered saline (PBS; Hazleton Biologics, Lenexa, Kans.) at 5°C with 12,000- to 14,000-Da-molecular-mass-cutoff tubing (Spectrum, Houston, Tex.). Dialysis was performed for 3 days with daily changes of dialysis buffer. The final product was filter sterilized, aliquoted, and stored in sterile vials at 5°C. Chromatography of the product on a 1.6- by 90-cm column packed with Sephacryl S-300 gel (Pharmacia Fine Chemicals, Piscataway, N.J.) eluted with 0.01 M Tris–0.14 M NaCl–0.02% NaN3, pH 7.4, was performed with a flow rate of 20 ml/h. Serial 4.0-ml fractions, to a total of 40, were obtained. The void volume for the column as determined by Blue Dextran 2000 (Pharmacia) was at fraction 17. Optical densities of the fractions at 280 nm were determined. A PMB-sheep IgG conjugate was prepared as described above for comparison to the human conjugate; the sheep IgG was also purchased from Sigma. PMB can form close noncovalent associations with some proteins. A sham mixture of PMB and IgG was prepared by performing the reaction without EDAC. Some of this preparation was used directly in dot enzyme-linked immunosorbent assay (ELISA) as described below, and some was dialyzed for use in the TNF inhibition study. This was done to show that a covalent linkage was required for activity in the conjugate.
(ii) Immunologic characterization of the PMB-IgG conjugates.
To determine if PMB had been covalently incorporated into the conjugate, it was serially diluted twice and dot blotted onto nitrocellulose paper with controls with a dot blot apparatus at 25 μl/dot (Bio-Rad Laboratories, Richmond, Calif.). The starting concentrations for the dots were as follows: IgG, 250 μg/ml; PMB, 25 μg/ml; sham PMB-IgG noncovalent mixture, 25 μg of PMB and 250 μg of IgG; PMB-IgG conjugate, 10 μg/ml. The blots were blocked with 5% bovine serum albumin–casein blocker and then incubated with an anti-PMB MAb in ascites (clone 45, a murine IgM anti-PMB MAb which was the generous gift of Ben J. Appelmelk, Vrije Universiteit, Amsterdam, The Netherlands) at a 1/1,000 dilution in blocker for 4 h (2). After being washed with PBS, blots were incubated with alkaline phosphatase-labeled anti-mouse IgM (Jackson Laboratories, West Grove, Pa.) for 1 h and then washed and developed with Fast Red and Naphthol MX phosphate (Sigma) for 30 min.
Solid-phase binding of the PMB-IgG conjugate to lipid A was ascertained in a Western blot assay against purified Escherichia coli J5 LPS and purified E. coli lipid A (List Biologics, Campbell, Calif.). The J5 LPS is a rough LPS (Rc chemotype) which has lipid A accessible for reaction with anti-lipid A immunologic reagents (21). Briefly, 100 μg of purified J5 LPS or lipid A was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a 13% polyacrylamide gel with a preparative comb (Bio-Rad) (33). A silver stain (Bio-Rad) on part of the gel was performed to visualize LPS. The rest of the gel was transferred electrophoretically onto nitrocellulose, which was cut into strips. The strips were blocked with bovine serum albumin-casein and allowed to react with 20 μg of the PMB conjugates per ml overnight. A high-titered rabbit anti-J5 serum was used as a positive control at 1/100 (3). A prevaccination serum sample and a postvaccination human serum sample of a volunteer who had received a liposomal lipid A vaccine and was known to have had developed antilipid antibodies were also used as controls at 1/100 on the lipid A blots (1). The strips were then washed and allowed to react with the appropriate alkaline phosphatase-labeled secondary antibodies (Jackson) and developed as described above.
(iii) Human macrophage-induced TNF inhibition study.
Monocytes were purified from normal volunteers by leukapheresis, centrifugation on lymphocyte separation medium (Organon Teknika, Durham, N.C.), and counterflow centrifugation-elutriation (17, 29). Cells (2.5 × 105) were cultured in 24-well plates in 0.5 ml of RPMI 1640 medium with 10% heat-inactivated (56°C, 30 min) human AB serum (Sigma) and 10 ng of macrophage colony-stimulating factor per ml (kindly provided by Jay Stoudemire, Genetics Institute, Cambridge, Mass.). Cultures were fed with 0.2 ml of medium at 3 days. After 6 days in culture, medium was removed and replaced with 200 μl of 20% heat-inactivated serum in RPMI. To these wells was added 300 μl of RPMI containing dilutions of PMB-IgG, IgG, or PMB with or without 20 ng of LPS from E. coli O111:B4 (Sigma) per ml. Control wells received 300 μl of RPMI with or without LPS. Culture supernatant fluids were harvested 18 to 20 h later and frozen at −70°C until assayed for TNF content by ELISA kits according to the manufacturer’s instructions (Quantikine; R&D Systems, Minneapolis, Minn.). Data are expressed as picograms of TNF per milliliter in culture supernatant fluids. A similar experiment was conducted with LPS from E. coli O18 Bort, which had been prepared and purified from killed bacteria by the Westphal method (31).
(iv) Microtiter liquid-phase inhibitory-bactericidal assay.
Fresh clinical isolates of E. coli, P. aeruginosa, and Klebsiella pneumoniae were used to determine MICs and MBCs of the reagents by the microtiter method (21). Serial dilutions of PMB (1 mg/ml), PMB-IgG (2.5 mg/ml); and IgG (2.5 mg/ml) were performed in 96-well microtiter plates (Dynatech) with Mueller-Hinton broth medium (Becton Dickinson and Co., Cockeysville, Md.). Wells were inoculated with 500,000 CFU of organisms in 100 μl of broth. Growth was ascertained at 24 and 48 h by observation for turbidity in wells. At 48 h, 10 μl of the broth in the wells was subcultured and streaked onto Trypticase TSA II sheep blood agar plates (Becton Dickinson). Growth was checked at 24 and 48 h.
(v) Galactosamine-sensitized model of endotoxin-mediated sepsis.
The Galanos model of pure endotoxin-mediated sepsis was utilized to test the in vivo efficacy of the PMB-human IgG conjugate (13). Briefly, female 8-week-old BALB/c mice (Jackson Laboratory, Bar Harbor, Maine) were given either human IgG at 100 μg/kg of body weight or PMB-human IgG conjugate at doses of 3, 5, 10, 20, and 100 mg/kg intraperitoneally (i.p.) in a 1-ml total volume of sterile PBS. After 2 h, animals were challenged with 100 ng of E. coli O18 LPS (Bort strain) together with 20 mg of galactosamine (Sigma) in tissue culture-grade PBS. This challenge dose of LPS is a 100% lethal dose in this model. Mortality was assessed daily for 3 days. Survival to 72 h correlates with long-term survival in this model. A second experiment was done to test the therapeutic efficacy of the PMB-IgG conjugate versus free PMB. Briefly, three groups of mice at eight mice per group were challenged with the same dose of LPS and galactosamine as above but were treated 2 h after the challenge with IgG (100 mg/kg), PMB (5 mg/kg), and PMB-IgG conjugate (100 mg/kg). Differences in survival at the completion of each experiment were tested by Fisher’s exact method (32).
RESULTS
(i) Characterization of the PMB-IgG conjugate.
Chromatographic analysis of the PMB-IgG conjugate revealed a homogeneous product with a retention time (fraction 24) comparable to that of nonconjugated human IgG, suggesting that the apparent molecular size of IgG was not appreciably altered by the process of conjugation with PMB. There was no low-molecular-weight material consistent with unreacted PMB detectable in the product after the dialysis was completed.
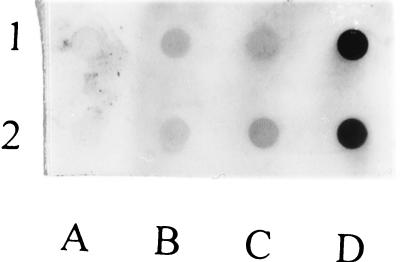
The anti-PMB MAb was found to react strongly with PMB-IgG well beyond the 10 and 5 μg/dot shown. In contrast, native IgG showed no reactivity at any dilution. Free PMB demonstrated weak reactivity, probably because free PMB does not absorb well enough to the nitrocellulose to allow reaction with the anti-PMB MAb. Similar binding was observed with the sham mixture, suggesting that the small amount of reactivity was due to the free PMB in the mixture (Fig. 1). Since free PMB did not bind well to the nitrocellulose and the sham mixture behaved similarly, it suggests that the observed reactivity must have been accounted for by PMB bound covalently to IgG and able to react immunologically with the anti-PMB MAb. The sheep PMB-IgG reacted similarly, as did the starch-PMB conjugate previously mentioned (data not shown).
FIG. 1.
Immunologic reactivity of PMB-IgG with anti-PMB MAb (clone 45) by dot ELISA. Two dilutions of IgG (lane A) (250 and 125 μg/ml), PMB (lane B) (25 and 12.5 μg/ml), a noncovalent mixture of PMB and IgG (lane C) (25 μg of PMB–250 μg of IgG and 12.5 μg of PMB–125 μg of IgG per ml), and the PMB-IgG conjugate (lane D) (10 and 5 μg/ml, top and bottom, respectively) were prepared. Solutions were applied in 25-μl volumes, incubated, and then washed and allowed to react with a 1/1,000 dilution of ascites containing the anti-PMB MAb, clone 45. After development, marked reactivity consistent with the presence of bound PMB was noted only for the covalent PMB-IgG conjugate relative to the other reagents and the noncovalent mixture.
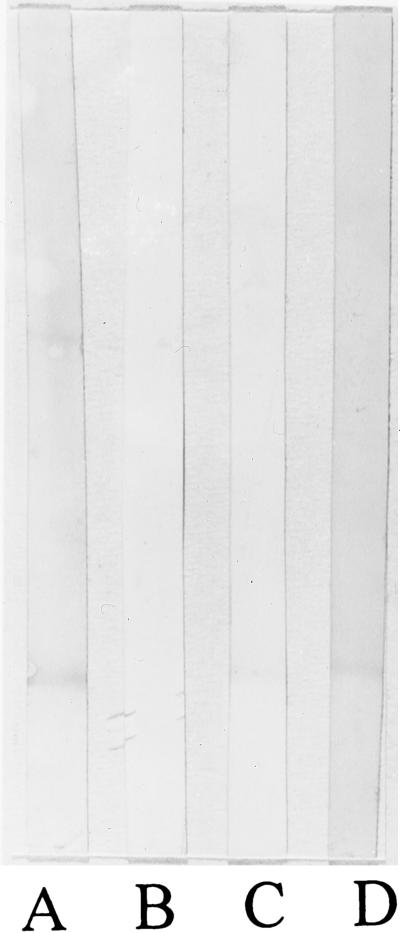
In the Western blot assay, both human PMB-IgG and sheep PMB-IgG reacted with the J5 LPS (Fig. 2). Unconjugated human IgG did not bind to the blots at all. Unconjugated sheep IgG behaved similarly (data not shown). The reactivity observed with the PMB-IgG conjugates was identical to that produced by reaction with the high-titered lapine anti-J5 antiserum with the blotted J5 LPS. The PMB-IgG conjugate also reacted against blotted lipid A like the anti-J5 serum and the post-lipid A vaccination serum. The prebleed human serum did not have measurable reactivity against lipid A (Fig. 3).
FIG. 2.
Immunologic reactivity of PMB-IgG types with E. coli J5 LPS by Western blot assay. LPS was electrophoresed on a 13% polyacrylamide gel and transferred to nitrocellulose for immunologic reaction with specific and control antibodies. Lane M, silver stain of molecular weight markers; lane A, silver stain of J5 LPS; lane B, human IgG (20 μg/ml); lane C, PMB-IgG human conjugate (20 μg/ml); lane D, PMB-IgG sheep conjugate (20 μg/ml); lane E, high-titered rabbit anti-J5 antiserum (1/100). The PMB-containing conjugates bound to the J5 LPS in a manner identical to that observed for the anti-J5 antiserum, whereas the parent IgG exhibited no visible binding.
FIG. 3.
Immunologic reactivity of PMB-IgG types with E. coli lipid A by Western blot assay. Lipid A was electrophoresed on a 13% polyacrylamide gel and transferred to nitrocellulose for immunologic reaction with specific and control antibodies. Lane A, high-titered anti-J5 antiserum (1/100); lane B, prebleed serum of human volunteer immunized with liposomal lipid A vaccine (1/100); lane C, postimmunization serum of human volunteer immunized with liposomal lipid A vaccine (1/100); lane D, PMB-IgG human conjugate (20 μg/ml). The human PMB-containing conjugate binds to the J5 LPS in a manner identical to that observed for the human postimmunization anti-lipid A and the anti-J5 antisera. The prebleed human serum had no measurable anti-lipid A binding.
(ii) In vitro and in vivo antiendotoxin properties of the PMB-IgG conjugate.
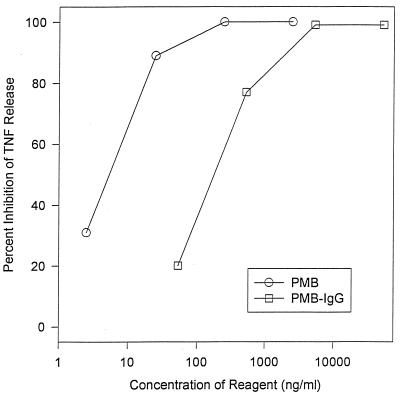
PMB, IgG, and PMB-IgG had negligible baseline endotoxin activity comparable to that of plain medium as measured by the induction of TNF from cultured human monocytes (data not shown). Both free PMB and PMB-IgG inhibited the release of TNF by E. coli O111 LPS in a dose-dependent fashion (Fig. 4). Unconjugated IgG had no intrinsic antiendotoxin activity, nor did the dialyzed sham PMB-IgG mixture (data not shown). With this graph, it was calculated that there was a 1.5-log difference in the potencies of PMB-IgG and free PMB by total protein weight. This suggests that there is approximately 3 μg of functional bound PMB per 100 μg of conjugate. With these calculations on a molar basis, there are on average, five molecules of functional PMB bound per molecule of IgG. Similar results were obtained with E. coli O18 LPS in this same assay (data not shown).
FIG. 4.
Comparison of free PMB versus PMB-IgG in TNF release inhibition assay. Cultured human monocytes release a fixed amount of TNF in response to incubation with 20 ng of pure E. coli O111:B4 LPS per ml. This experiment compares the abilities of varying concentrations (total protein) of either free PMB or the PMB-IgG conjugate to inhibit TNF release. Both reagents were found to inhibit the LPS-induced TNF release in a dose-dependent fashion. The free PMB was approximately 33 times more potent than the conjugate based on total protein weight. This suggests that there is approximately 3 μg of active PMB per 100 μg of PMB-IgG conjugate. Unconjugated IgG had no activity in this assay.
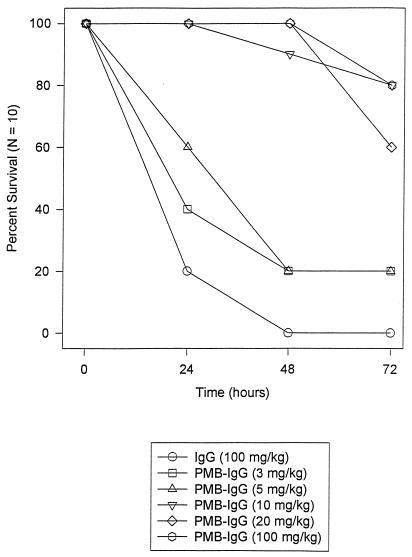
The PMB-IgG conjugate was found to convey dose-dependent protection in the galactosamine-sensitized mouse model of pure endotoxin lethal sepsis when used prophylactically (Fig. 5). Animals given 10, 20, or 100 mg of conjugate per kg were 60 to 80% protected from death relative to those given unconjugated IgG (0% protection). This protection was statistically significant (10 mg/kg, P = 0.0007; 20 mg/kg, P = 0.005; 100 mg/kg, P = 0.005 by two-tailed Fisher’s exact test). Low doses (3 or 5 mg/kg) of PMB-IgG afforded little protection (20%) (P = 0.47). In this model, 2.5 mg of PMB per kg given i.p. affords consistently high protection (>90%).
FIG. 5.
Ability of PMB-IgG to protect mice in a lethal galactosamine model of pure endotoxin-mediated sepsis. Groups of 10 animals each were challenged i.p. with 100 ng of E. coli O18 (Bort strain) and 20 mg of galactosamine per mouse. Two hours before challenge, animals were given IgG (100 mg/kg) or varying doses of the PMB-IgG conjugate i.p. Survival was tallied each day for 3 days. Survival at 3 days corresponds with long-term survival. The survival of animals receiving the three higher doses of conjugate was significantly improved compared to that of animals receiving IgG alone (P ≤ 0.005 by Fisher’s exact test). Outcome was not significantly improved in groups receiving the two lower doses of PMB-IgG conjugate.
There was 0% survival in all three groups (IgG, 100 mg/kg; PMB, 5 mg/kg; PMB-IgG conjugate, 100 mg/kg) given therapy 2 h after challenge, suggesting that PMB-based therapy is ineffective for established sepsis in this model.
(iii) Antibacterial properties of the PMB-IgG conjugate.
The unconjugated IgG possessed no intrinsic bacterial inhibitory or bactericidal activity. For both PMB and PMB-IgG, the 24-h MICs were identical to the 48-h readings. In this experiment, the MBCs determined by subculturing were the same as the MICs. PMB killed all three organisms at the highest dilution tested with a concentration of 250 ng/ml. The PMB-IgG conjugate killed P. aeruginosa and K. pneumoniae down to 3.4 μg/ml (total protein, 100 ng of PMB equivalent per ml). E. coli was killed at the highest dilution of the conjugate performed, which contained 0.9 μg/ml (total protein, 30 ng of PMB equivalent per ml).
DISCUSSION
We have demonstrated that PMB can be covalently bound to an IgG (human or sheep) carrier with the retention of its endotoxin-neutralizing activity. A noncovalent mixture of PMB and IgG did not have the same properties as the covalent conjugate. Through this linkage with PMB, the IgG carrier has essentially been given an artifical binding site for lipid A and can behave in some sense like an anti-lipid A antibody. This was demonstrated by the binding of PMB-IgG to lipid A and lipid A-containing J5 LPS in Western blot assays. The binding was identical to that observed with a vaccination-produced lapine anti-J5 antiserum in its reaction with both antigens (Fig. 2) and with a human anti-lipid A serum in its reaction with lipid A (Fig. 3).
PMB-IgG was able to inhibit the release of TNF from in vitro-cultured human monocytes which had been stimulated with purified LPS (endotoxin) (Fig. 4). The inhibition was clearly a function of concentration and was comparable in contour to that produced by free PMB alone. With this relationship, it was possible to calculate the amount of functional PMB bound per unit of protein mass of the conjugate. In the lot of conjugate used in these experiments, the mass ratio was approximately 33 to 1. In other words, each 100 μg of PMB-IgG contained 3 μg of functional PMB. This corresponds to approximately five molecules of active PMB per molecule of IgG. The liquid chromatography analysis suggested that the overall size of IgG was not altered appreciably by the conjugation process. This is also consistent with the determination of five molecules of PMB bound per molecule of IgG, since this degree of binding would increase the molecular weight of the complex by only 3% compared to that with IgG alone.
There may be some PMB bound to the IgG which is nonfunctional but apparently not enough to substantially alter its molecular weight. To determine exactly how much PMB is bound to the IgG would take more sophisticated methods such as quantitative gas chromatography with diaminobutyric acid as a standard. Diaminobutyric acid is an amino acid unique to PMB (28) and theoretically could be used to quantitate the total bound PMB, both functional and nonfunctional. We feel that the TNF release assay is the preferred test, however, since it quantitates functional bound PMB by bioassay. These data can be used in assessing the potency of the conjugate for use in dosing in animal models relative to unbound PMB. We have used the assay to compare lots of the PMB-IgG conjugate.
It may be possible to increase the amount of active PMB bound to the conjugate by alterations in the synthetic method such as altering the ratios of reactants or other reaction parameters. Further work needs to be performed to determine optimal parameters for maximum binding of active PMB to IgG. If the current lot were used in humans, however, approximately 20 μg of functional PMB equivalent per ml could be achieved by intravenous (i.v.) infusion of 4 g of PMB-IgG based on blood volume. This dose of IgG is comparable to the currently utilized clinical dose of i.v. immunoglobulin. This level of PMB is more than enough to inhibit TNF release by human monocytes. Although the in vivo experiments suggest that the conjugate can work at the level of the tissue macrophage, further studies using different animal models of sepsis will be required.
We were surprised to find that the PMB bound within the PMB-IgG conjugate retained significant bactericidal capabilities, comparable to those of the unbound PMB. The demonstrated MBCs of less than 4 μg of conjugate per ml (120 ng of PMB equivalent per ml) against the clinical strains of E. coli, P. aeruginosa, and K. pneumoniae are readily achievable on i.v. administration as discussed above. We thought that steric hindrance caused by binding to the IgG would substantially reduce its antimicrobial capability as occurred for the dextran-PMB (6) and starch-PMB (9) conjugates. The apparent retention of the antimicrobial effect of the PMB in the PMB-IgG conjugate in addition to its antiendotoxin activity is a potential therapeutic advantage. In addition to the direct microbicidal ability demonstrated here, the presence of PMB on the IgG may render it functional in the context of immune effector cells like polymorphonuclear leukocytes by virtue of the Fc region of the antibody. Opsonophagocytosis studies may elucidate such an additional effect.
Because of the relatively small amount of PMB bound to IgG in this conjugate, it was impossible to formally determine if the bound PMB was less toxic than the equivalent free PMB. The largest deliverable i.p. dose of conjugate in mice, containing 12 mg of PMB equivalent per kg, was found to be apparently nontoxic at 10 days by gross inspection. The largest deliverable dose of the conjugate i.v. was 1.2 mg/kg, which also appeared to be nontoxic. For comparison, 5 mg of free PMB per kg given i.v. is 100% lethal in mice (9a, 16, 22). Conjugates of PMB with carbohydrate carriers such as soluble starch or dextran have significantly more PMB bound to carrier on a weight basis; therefore, toxicity studies can and have been performed. For both the dextran and the starch PMB conjugates, no toxicity of the bound PMB was observed in doses exceeding 100 mg of PMB equivalent per kg (9a, 16). This suggests that the conjugation of PMB to a large carrier molecule does indeed reduce the toxicity substantially for the reasons discussed previously. The same effect would be predicted for the PMB-IgG but will require further study in other animal models in which larger amounts of reagent can be delivered. Formal testing for nonlethal toxicity, such as decrements in renal function or histopathologic damage, will be required before full conclusions on the toxicity of these conjugates can be rendered.
The IgG was chosen as a carrier because of its relatively long half-life of 21 days. This would make the PMB-IgG useful as a prophylactic agent if it retains the half-life of the parent carrier. To formally determine the biological half-life of the PMB-IgG would require the synthesis of an animal-specific (e.g., for rabbit), radiolabeled PMB-IgG for testing in that animal species (rabbit). This work remains to be performed. Half-life studies of a PMB-dextran 70 conjugate have been performed previously; the half-life was less than 5 h (6).
The PMB-IgG protected mice against lethal endotoxin challenge in a dose-dependent fashion when used prophylactically. The PMB-dextran was also found to be effective prophylactically in this model (6). The PMB-IgG conjugate may be proven to be of value as a prophylactic agent against sepsis in high-risk groups such as patients in intensive care units, those sustaining prolonged neutropenia, or those undergoing major abdominal surgery. Neither the conjugate nor the parent PMB was of any value in the treatment of sepsis in this model at 2 h postchallenge. This result is not unexpected. PMB or a conjugate containing it or a related endotoxin-neutralizing substance would have to neutralize the endotoxin before it gets a chance to interact with macrophages and initiate the cytokine cascade which results in sepsis. These experiments suggest that the PMB-IgG conjugate would be useful only if used prophylactically. Further study of the PMB-IgG conjugate as prophylaxis in other models of gram-negative bacterial sepsis, particularly those induced by live bacterial challenge, is warranted. This is especially important in view of the retained antibacterial activity of the conjugate.
The PMB-IgG conjugate described in this report was prepared by chemical means of bond formation. Recently, H. S. Warren and colleagues prepared an antiendotoxin peptide-IgG conjugate by chemical means as well (11). The antiendotoxin peptide CAP18(106-138), derived from human leukocytes, was chosen for conjugation. This conjugate, like PMB-IgG, inhibited endotoxin in vitro and protected mice challenged with endotoxin when administered prophylactically; it too retains the antimicrobial activity of the parent peptide. In addition to PMB and CAP18, there are other cationic peptides and low-molecular-weight proteins with PMB-like, antiendotoxin activity such as bactericidal-permeability-increasing protein (18), ceprocins (15), and Limulus-derived endotoxin-neutralizing protein (30) to be used as potential ligands on IgG. Fletcher and colleagues prepared a similar conjugate with a Limulus-derived protein (10). In addition to classical chemical means of coupling, a chimeric IgG (5) which expresses the active portion of these molecules as the active site of the antibody, hence creating an artifical binding site for lipid A, could be engineered. Expressing or coupling these small endotoxin-neutralizing peptides in IgG could also result in a family of nontoxic products with half-lives comparable to that of unengineered IgG.
We have demonstrated that PMB can be covalently linked to an IgG carrier with retention of its endotoxin-neutralizing and antibacterial activity. The conjugate conveys protection against lethal endotoxin challenge in mice when used prophylactically but not therapeutically. More studies are warranted to define the role that this or similar compounds containing antiendotoxin peptides coupled to IgG as a carrier may play in the prophylaxis of gram-negative bacterial sepsis.
REFERENCES
- 1.Alving C R. Liposomal vaccines: clinical status and immunological presentation for humoral and cellular immunity. Ann N Y Acad Sci. 1995;754:143–152. doi: 10.1111/j.1749-6632.1995.tb44447.x. [DOI] [PubMed] [Google Scholar]
- 2.Appelmelk B J, Su D, Marian A, Verweij-van Vught J J, Thijs B G, MacLaren D M. Polymyxin B-horseradish peroxidase conjugates as tools in endotoxin research. Anal Biochem. 1992;207:311–316. doi: 10.1016/0003-2697(92)90017-2. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharjee A K, Opal S M, Palardy J E, Drabick J J, Collins H, Taylor R, Cotton A, Cross A S. Affinity-purified Escherichia coli J5 lipopolysaccharide-specific IgG protects neutropenic rats against gram-negative bacterial sepsis. J Infect Dis. 1996;173:1157–1163. doi: 10.1093/infdis/170.3.622. [DOI] [PubMed] [Google Scholar]
- 4.Bone R C. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 5.Borrebaeck C A K, editor. Antibody engineering: a practical guide. W. H. New York, N.Y: Freeman and Co.; 1992. [Google Scholar]
- 6.Bucklin S E, Lake P, Logdberg L, Morrison D C. Therapeutic efficacy of a polymyxin B-dextran 70 conjugate in experimental model of endotoxemia. Antimicrob Agents Chemother. 1995;39:1462–1466. doi: 10.1128/aac.39.7.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. Increase in national hospital discharge survey rates for septicemia—United States: 1979–1987. Morbid Mortal Weekly Rep. 1990;39:31–34. [PubMed] [Google Scholar]
- 8.Cohen J, Aslam M, Pusey C D, Ryan C J. Protection from endotoxinemia: a rat model of plasmapheresis and specific absorption with polymyxin B. J Infect Dis. 1987;155:690–695. doi: 10.1093/infdis/155.4.690. [DOI] [PubMed] [Google Scholar]
- 9.Drabick J, Bhattacharjee A, Williams W, Siber G, Cross A S. Covalent polymyxin B-starch and polymyxin B-immunoglobulin G conjugates as novel anti-endotoxin reagents. Clin Res. 1992;40:287A. . (Abstract.) [Google Scholar]
- 9a.Drabick, J. J. 1993. Unpublished data.
- 10.Fletcher M A, Kloczewiak M, Loiselle P M, Amato S F, Black K M, Warren H S. TALF peptide-immunoglobulin conjugates that bind lipopolysaccharide. J Endotoxin Res. 1996;3:49–55. [Google Scholar]
- 11.Fletcher M A, Kloczewiak M A, Loiselle P M, Ogata M, Vermeulen M W, Zanzot E M, Warren H S. A novel peptide-IgG conjugate, CAP18(106–138)-IgG, that binds and neutralizes endotoxin and kills gram-negative bacteria. J Infect Dis. 1997;175:621–632. doi: 10.1093/infdis/175.3.621. [DOI] [PubMed] [Google Scholar]
- 12.Flynn P M, Shenep J L, Stokes D C, Fairclough D, Hildner W K. Polymyxin B moderates acidosis and hypotension in established, experimental gram-negative sepsis. J Infect Dis. 1987;156:706–712. doi: 10.1093/infdis/156.5.706. [DOI] [PubMed] [Google Scholar]
- 13.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;73:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goris R J A. Sepsis and multiple organ failure: the result of whole body inflammation. In: Faist E, Meakin T, Schildberg F W, editors. Host defense dysfunction in trauma, shock and sepsis. Berlin, Germany: Springer-Verlag; 1993. pp. 161–170. [Google Scholar]
- 15.Gough M, Hancock R E W, Kelly N M. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect Immun. 1996;64:4922–4927. doi: 10.1128/iai.64.12.4922-4927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handley, D. ,A. and P. Lake. January 1993. Polymyxin B conjugates. U. S. patent 5,177,059.
- 17.Hoover D L, Friedlander A M, Rogers L C, Yoon I K, Warren R L, Cross A S. Anthrax edema toxin differentially regulates lipopolysaccharide-induced monocyte production of tumor necrosis factor alpha and interleukin 6 by increasing intracellular cyclic AMP. Infect Immun. 1994;62:4432–4439. doi: 10.1128/iai.62.10.4432-4439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marra M, Wilde C G, Griffith J E, Snable J L, Scott R W. Bactericidal/permeability-increasing protein has endotoxin-neutralizing activity. J Immunol. 1990;144:662–666. [PubMed] [Google Scholar]
- 19.Morell A, Riesen W. Structure, function and catabolism of immunoglobulins. In: Nydegger U E, editor. Immunochemotherapy: a guide to immunoglobulin prophylaxis and therapy. London, United Kingdom: Academic Press; 1981. pp. 17–26. [Google Scholar]
- 20.Morrison D C, Jacobs D M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed., vol. 13, no. 25. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 22.Nord N M, Hoeprich P D. Polymyxin B and colistin. A critical comparison. N Engl J Med. 1964;270:1030–1035. doi: 10.1056/NEJM196405142702002. [DOI] [PubMed] [Google Scholar]
- 23.Opal S M. Clinical trials of novel therapeutic agents: why did they fail? In: Vincent J L, editor. Yearbook of intensive care and emergency medicine. Berlin, Germany: Springer-Verlag; 1995. pp. 425–436. [Google Scholar]
- 24.Opal S M, Palardy J E, Parejo N, Dubin P K, Pribble J, Stiles D, Vincent J-L, Fisher C J., Jr . Abstracts of 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Endotoxemia in patients with sepsis syndrome: therapeutic and prognostic implications, abstr. G88; p. 173. [Google Scholar]
- 25.Perl T M, Dvorak L, Hwant T, Wenzel R P. Long-term survival and function after suspected gram-negative sepsis. JAMA. 1995;274:338–345. [PubMed] [Google Scholar]
- 26.Raetz C R H, Ulevitch R J, Wright S D, Sibley C H, Ding A, Nathan C F. Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J. 1991;5:2652–2660. doi: 10.1096/fasebj.5.12.1916089. [DOI] [PubMed] [Google Scholar]
- 27.Seale T W, Rennert O M. Mechanisms of antibiotic-induced nephrotoxicity. Ann Clin Lab Sci. 1992;12:1–9. [PubMed] [Google Scholar]
- 28.Vaara M, Vaara T. Outer membrane permeability barrier disruption by polymyxin in polymyxin-susceptible and resistant Salmonella typhimurium. Antimicrob Agents Chemother. 1981;19:578–583. doi: 10.1128/aac.19.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahl L M, Katona I M, Wilder R L, Winter C C, Haraoui B, Scher I, Wahl S. Isolation of human mononuclear cell subsets by counter flow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte, T-lymphocyte, and monocyte-enriched fractions by flow cytometric analysis. Cell Immunol. 1984;85:373–378. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- 30.Warren H S, Glennon M L, Wainwright N, Amato S F, Black K M, Kirsch S J, Riveau G R, Whyte R I, Zapol W M, Novitsky T J. Binding and neutralization of endotoxin by Limulus antilipopolysaccharide factor. Infect Immun. 1992;60:2506–2513. doi: 10.1128/iai.60.6.2506-2513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 32.Woolson R F. Statistical methods for the analysis of biomedical data. New York, N.Y: John Wiley and Sons; 1987. pp. 215–221. [Google Scholar]
- 33.Ziegler E J, Douglas H, Sherman J E, Davis C E, Braude A I. Treatment of E. coli and Klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-Gal epimerase-deficient mutant. J Immunol. 1973;111:433–438. [PubMed] [Google Scholar]