Abstract
Objective
To determine the impact of postoperative complications on long-term survival outcomes in patients with bladder cancer undergoing radical cystectomy.
Methods
This retrospective multi-institutional study included 766 bladder cancer patients who underwent radical cystectomy between 2011 and 2017. Patient characteristics, perioperative outcomes, all complications within 90 days after surgery and survival outcomes were collected. Each complication was graded based on the Clavien-Dindo system, and grouped using a standardized grouping method. The Comprehensive Complication Index, which incorporates all complications into a single formula weighted by their severity, was utilized. Overall survival and recurrence-free survival (local, distant or urothelial recurrences) were stratified by Comprehensive Complication Index (high: ≥26.2; low: <26.2). A multivariate model was utilized to identify independent prognostic factors.
Results
The incidence of any and major complications (≥Clavien-Dindo grade III) was 70 and 24%, respectively. In terms of Comprehensive Complication Index, 34% (261/766) of the patients had ≥26.2. Patients with Comprehensive Complication Index ≥ 26.2 had shorter overall survival (4-year, 59.5 vs. 69.8%, respectively, log-rank test, P = 0.0037) and recurrence free survival (51.9 vs. 60.1%, respectively, P = 0.0234), than those with Comprehensive Complication Index < 26.2. The Cox multivariate model identified the age, performance status, pT-stage, pN-stage and higher CCI (overall survival: HR = 1.35, P = 0.0174, recurrence-free survival: HR = 1.26, P = 0.0443) as independent predictors of both overall survivial and recurrence-free survival.
Conclusions
Postoperative complications assessed by Comprehensive Complication Index had adverse effects on long-term survival outcomes. Physicians should be aware that major postoperative complications can adversely affect long-term disease control.
Keywords: postoperative complication, radical cystectomy, bladder cancer, survival
Postoperative complications assessed by Comprehensive Complication Index had adverse effects on long-term survival after radical cystectomy. Physicians should be aware that major postoperative complications can deteriorate long-term outcomes in bladder cancer patients.
Introduction
Radical cystectomy (RC) in conjunction with regional lymph node dissection (LND) and urinary diversion is the mainstay of treatment for muscle-invasive or treatment-refractory non-muscle-invasive bladder cancers (BCs). However, it is well known that RC is correlated with significant perioperative morbidity (approximately 50–70%) (1–3). Recently, our group collected data on 90-day postoperative complications in patients treated by RC at Hokkaido University Hospital and our affiliated hospitals between 2011 and 2017 (recent cohort, n = 838), and compared the perioperative outcomes with those of our previous cohort (n = 919, 1997–2010). We observed that RC has remained correlated with significant postoperative morbidity [overall complications: 69% (580/838) in the recent cohort vs. 68% (629/919) in the previous cohort, and major complications (Clavien-Dindo, CD, grade ≥ III): 25% (211/838) in the recent cohort vs. 22% (201/919) in the previous cohort, respectively] over the past two decades (4).
Recent studies involving colorectal, gastric and esophagogastric cancer, or hepatocellular carcinoma resection have shown an adverse impact of postoperative morbidities on long-term survival outcomes (5–8), which remains unknown after RC. In the present study, we aimed to clarify the impact of postoperative complications on the long-term survival impact after RC.
Materials and methods
The Institutional Review Board approved this study (No. 017-0038). We reviewed the medical records of 838 patients with muscle invasive or treatment-refractory non-muscle-invasive BC who underwent RC at Hokkaido University Hospital and 19 affiliated institutions between 2011 and 2017. As mentioned before, in terms of the current landscape of postoperative complications after RC, we previously published a paper (4). In the present study, we updated the survival information. Excluding patients with distant metastasis (n = 29) at the time of RC, who died within 90 days after surgery (n = 38), in order to highlight the long-term survival effect, with the final pathology of lymphoma (n = 1), with advanced prostate cancer (n = 1), no follow-up data (n = 2) and treated for disease palliation (n = 1), 766 patients were included in the current survival analysis.
During the study period (2011–2017), the type of urinary diversion (i.e. ileal conduit, ileal neobladder or cutaneous ureterostomy) was performed based on the patients’ and surgeons’ decisions, and the region of LND was determined by each surgeon. Laparoscopic RC was performed mainly without robotic assistance, and urinary diversion was performed extracorporeally via a small incision (extracorporeal urinary diversion) in all patients. Perioperative systemic chemotherapy was also administered based on the patients’ and surgeons’ decisions.
Patient characteristics, perioperative outcomes and all complications within 90 days of surgery were reviewed. Tumor staging was performed according to the Union for International Cancer Control TNM classification 7th edition, and the tumor grade was assessed according to the 2004 WHO classification. Postoperative complications were also assessed. Each complication was graded according to the CD system (9) by co-authors at each hospital, and grouped according to a standardized grouping method, consisting of the 11 complication categories reported by Shabsigh et al. (1). If there was any contradiction between CD grading and the management for each complication, two authors (YS and TA) asked co-authors to review the medical charts, and corrected the misgrading. Furthermore, in order to examine the cumulative postoperative morbidity, the Comprehensive Complication Index (CCI), which incorporates all complications into a single formula weighted by their severity, was calculated for each patient by one author (YS), who was blinded to the final survival outcomes (10). CCI ranged from 0 (no complications) to 100 (death). According to previous studies, we utilized CCI of 26.2 as the cut-off point (equivalent to one grade IIIa complication by the CD system) (11,12).
In terms of postoperative follow-up, in general, computed tomography was performed every 6 months for the first two years. Subsequently, follow-up examinations were performed every 6–12 months. The primary outcome of the current study was the impact of any or major complications on long-term survival after RC. Regarding the definition of a major complication for the subsequent analyses, both the highest CD grade of ≥III in each patient and CCI ≥26.2 were utilized. The secondary outcome was the survival effect of each complication category. Any urine leak event was also evaluated separately, which may be associated with tumor dissemination if cancer cells remained within the urinary tract. Overall survival (OS) was defined as the interval between the date of surgery and that of death. Recurrence-free survival (RFS) was calculated from the date of surgery to disease recurrence or death from any cause. Local, distant and urothelial recurrences were included in the study.
Statistical analysis
OS and RFS were estimated using the Kaplan–Meier method, and the log-rank test was used to compare survivals between the groups. Univariate and multivariate Cox models were utilized to determine independent survival predictors in the current cohort. The significant predictors in the univariate model were included in the multivariate model. The variables analyzed were sex, age (continuous), body mass index, average annual cystectomy volume (high: ≥10 per/year vs. moderate: 5 ≤ <10 per/year vs. low: <5 per/year), performance status (PS, 0 vs. 1 vs. 2 vs. 3), neoadjuvant chemotherapy (yes vs. no), adjuvant chemotherapy (yes vs. no), pathological T-stage (pT0-1 vs. pT2 vs. pT3-4), pathological N-stage (pN0 vs. pN+ vs. pNx), histology (pure urothelial carcinoma vs. urothelial carcinoma with variant histology), tumor grade (low vs. high), operative time (continuous), estimated blood loss (continuous), surgical approach (open vs. laparoscopic) and complications (none vs. minor/major, none/minor vs. major or CCI < 26.2 vs. CCI ≥ 26.2). All calculations were performed using JMP® Pro, version 16.0.0 (SAS Institute, Cary, NC, USA). Significance was set at p < 0.05.
Results
Table 1 shows a summary of the patient characteristics. The median patient age was 72 years (range, 34–93). Neoadjuvant chemotherapy was administered to 12% (95/766) of patients. In terms of urinary diversion, two-thirds of the patients (67%, 516/766) received an ileal conduit. Seventeen % (133/766) of patients had node metastasis on pathology.
Table 1.
Patients’ characteristics
| Characteristics | n = 766 |
|---|---|
| Sex | |
| Male | 567 (74%) |
| Female | 199 (26%) |
| Age at radical cystectomy (years), median (range) | 72 (34–93) |
| Body mass index (BMI) (kg/m 2), median (range) | 22.7 (13.5–34.5), n = 761 |
| Average annual cystectomy volume | |
| High (≥10 per year) | 257 (34%), 3 hospitals |
| Moderate (5 ≤ − < 10 per year) | 371 (48%), 8 hospitals |
| Low (<5 per year) | 138(18%), 9 hospitals |
| Performance status | |
| 0 | 661 (86%) |
| 1 | 82 (11%) |
| 2 | 14 (2.0%) |
| 3 | 2 (0.3%) |
| Unknown | 7 (0.9%) |
| No. neoadjuvant chemotherapy | 95 (12%) |
| No. adjuvant chemotherapy | 79 (10%) |
| No. form of urinary diversion | |
| Illeal conduit | 516 (67%) |
| Neobladder | 55 (7%) |
| Ureterocutaneostomy | 182 (24%) |
| Nephrostomy | 3 (0.4%) |
| Not performed | 10 (1.3%) |
| Type of neobladder | |
| Hautmann | 46 (84%) |
| Studer | 6 (11%) |
| Unknown | 3 (5%) |
| Pathological T-stage | |
| pT0 | 92 (12%) |
| pTa-is | 110 (14%) |
| pT1 | 107 (14%) |
| pT2 | 148 (19%) |
| pT3 | 239 (31%) |
| pT4 | 70 (9%) |
| Pathological N-stage | |
| pN+ | 133 (17%) |
| pN0 | 603 (79%) |
| pNx | 30 (4%) |
| Histology | |
| Pure urothelial carcinoma | 687 (90%) |
| Others | 79 (10%) |
| Grade | |
| high | 551 (72%) |
| low | 98 (13%) |
| unknown | 117 (15%) |
| Operative time (minutes), median (range) | 390 (75–820), n = 764 |
| Estimated blood loss (mL), median (range) | 1000 (0–9230), n = 760 |
| Surgical approach | |
| Open | 568 (74%) |
| Laparoscopic | 198 (26%) |
Complications
Table 2 summarizes the postoperative complications. The most common complications were infectious (39%, 300/766), followed by gastrointestinal (26%, 201/766), wound-related (18%, 136/766) and genitourinary (10%, 73/766) complications. Urine leakage was observed in 31 patients. Figure 1 shows the distribution of the highest CD grade complication per patient (a), and CCI (b). The incidences of any and major (≥CD grade III) complications were 70 (535/766) and 24% (187/766), respectively. In terms of CCI, 34% (261/766) of the patients had CCI ≥ 26.2.
Table 2.
Summary of postoperative complications
| Clavien-Dindo grade | |||||
|---|---|---|---|---|---|
| Category | No. of all patients (%) | ≤II | ≥III | Events | No. of patients |
| Gastrointestinal | 201 (26%) | 115 | 86 | Ileus | 174 |
| Bowel anastomosis leak/fistula | 15 | ||||
| Gastrointestinal ulcer/bleeding | 9 | ||||
| Enterocolitis | 14 | ||||
| Gastrointestinal perforation | 3 | ||||
| Infection | 300 (39%) | 259 | 41 | UTI | 218 |
| FUO | 27 | ||||
| Sepsis | 14 | ||||
| Other site infection | 69 | ||||
| Wound | 136 (18%) | 102 | 34 | SSI | 79 |
| Wound dehiscence | 65 | ||||
| Genitourinary | 73 (10%) | 29 | 44 | Hydronephrosis | 30 |
| Urine leak | 31 | ||||
| Renal failure | 13 | ||||
| Ileal conduit injury/necrosis | 3 | ||||
| Others | 2 | ||||
| Cardiac | 6 (0.8%) | 5 | 1 | Arryhythmia | 5 |
| Hypotension | 1 | ||||
| Pulmonary | 16 (2%) | 12 | 4 | Pneumonia | 14 |
| Respiratory distress | 1 | ||||
| Asthma attack | 1 | ||||
| Bleeding | 8 (1%) | 5 | 3 | Postoperative bleeding | 3 |
| Uretero-arterial fistula | 3 | ||||
| Hematoma (wound) | 1 | ||||
| Urinary tract bleeding | 1 | ||||
| Thromboembolic | 7 (0.9%) | 6 | 1 | Vascular thrombosis | 6 |
| Pulmonary embolism | 2 | ||||
| Neurological | 24 (3%) | 22 | 2 | Cerebrovascular event | 2 |
| Peripheral neuropathy | 2 | ||||
| Delirium/Agitation/Dementia | 17 | ||||
| Vertigo | 2 | ||||
| Insomnia | 1 | ||||
| Miscellaneous | 52 (7%) | 39 | 13 | Lymphocele | 7 |
| Dermatitis | 2 | ||||
| Liver dysfunction | 17 | ||||
| Electrolyte abnormality | 5 | ||||
| Compartment syndrome | 3 | ||||
| Drug eruption | 2 | ||||
| Loss of appetite | 2 | ||||
| Other rare complication | 15 | ||||
| Surgical | 12 (2%) | 2 | 10 | Rectal injury | 8 |
| Incisional hernia | 2 | ||||
| Intestinal injury | 1 | ||||
| Obturator nerve injury | 1 | ||||
| UTI = urinary tract infection | |||||
| FUO = fever of unknown origin | |||||
| SSI = surgical site infection | |||||
Figure 1.
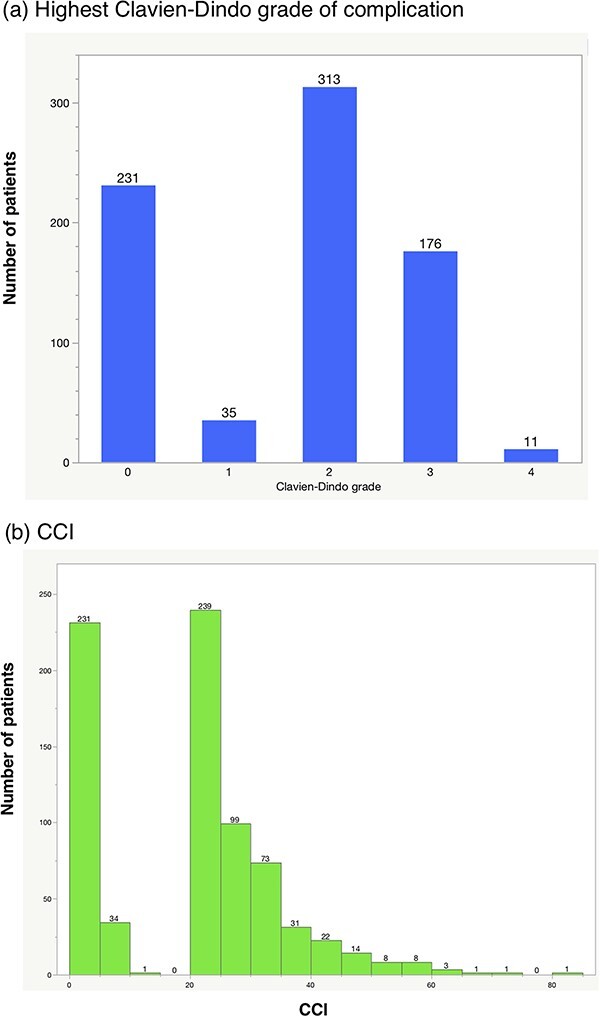
Distribution of the highest Clavien-Dindo (CD) grade of complication and Comprehensive Complication Index (CCI) in an independent patient population.
Survival analyses
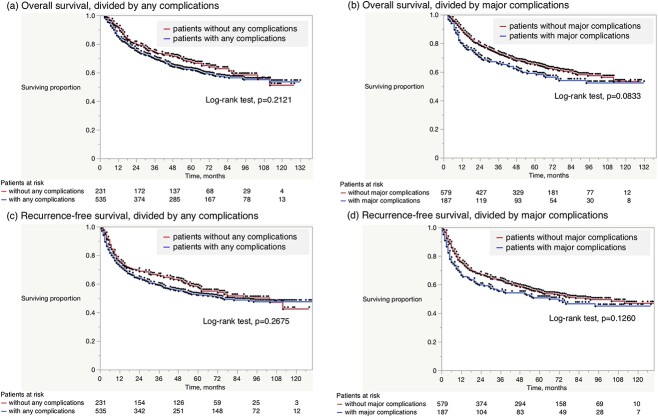
The median follow-up duration for the entire cohort was 53 months. Four-year OS and RFS rates were 66.4 and 57.4%, respectively. The effects of complications on OS and RFS are shown in Fig. 2. There was no significant difference in OS or RFS between patients with and without any complications (Fig. 2a and c). Major complications (≥CD grade III) were also not significantly correlated with poorer OS or RFS (Fig. 2b and d). In contrast, considering CCI, patients with CCI ≥ 26.2 had shorter OS (4-year, 59.5 vs. 69.8%, respectively, log-rank test, P = 0.0037, Fig. 3a) and RFS (51.9 vs. 60.1%, respectively, P = 0.0234, Fig. 3b), than those with CCI < 26.2.
Figure 2.
Kaplan–Meier estimates for overall and recurrence-free survival according to (a), (c) any complication, and (b), (d) major complications (≥CD grade III). There was no significant difference in overall survival (OS) or recurrence-free survival (RFS) between patients with and without any complications (a, c). Major complications were also not significantly correlated with poorer OS or RFS (b, d).
Figure 3.
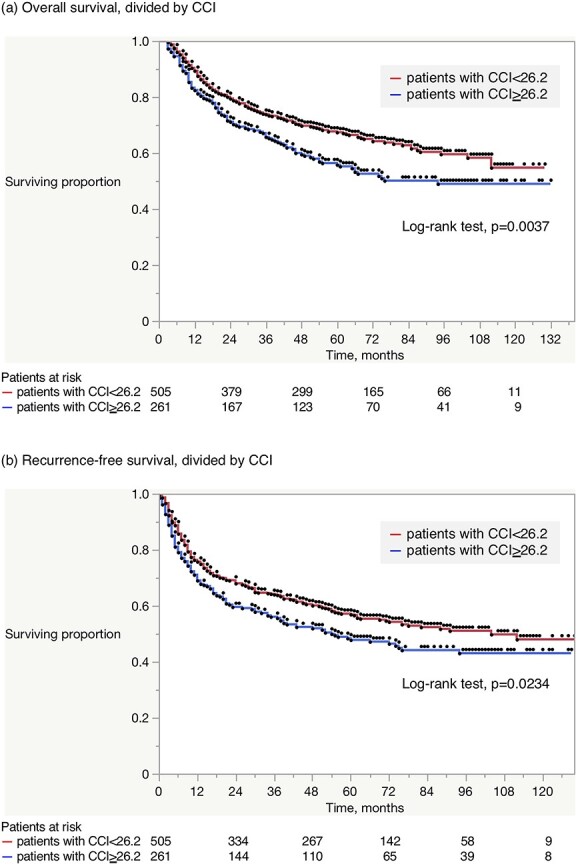
Kaplan–Meier estimates for (a) OS and (b) RFS according to CCI. Patients with CCI ≥ 26.2 had shorter OS (4 years, 59.5 vs. 69.8%, respectively, log-rank test, P = 0.0037) and RFS (51.9 vs. 60.1%, respectively, P = 0.0234), than those with CCI < 26.2.
Table 3 demonstrates the univariate and multivariate analyses of OS. Older age, higher PS, adjuvant chemotherapy, pT3-4, pN+, high grade and CCI ≥ 26.2 were significant adverse prognostic factors in univariate analyses. On multivariate analyses, age, PS, pT3-4, pN+ and CCI ≥ 26.2 remained independent predictors of OS. Table 4 presents the univariate and multivariate analyses data for RFS. Older age, higher PS, adjuvant chemotherapy, higher pathological T-stage, pN+ and CCI ≥ 26.2 were significant adverse prognostic factors in univariate analyses. In multivariate analyses, age, PS, pT3-4, pN+ and CCI ≥ 26.2 remained independent predictors of RFS.
Table 3.
Univariate and multivariate analyses of prognostic factors for overall survival (OS)
| OS | OS | |||
|---|---|---|---|---|
| Variables | Univariate analysis | p-value | Multivariate analysis | p-value |
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | |||
| Sex | ||||
| Male | 1 | |||
| Female | 0.78 (0.59–1.04) | 0.089 | ||
| Age, year | ||||
| Continuous | 1.05 (1.03–1.06) | <0.0001 | 1.03 (1.02–1.05) | <0.0001 |
| Body mass index (BMI) (kg/m 2), median (range) | 0.97 (0.93–1.00) | 0.062 | ||
| Average annual cystectomy volume | ||||
| High (>10 per year) | 1 | |||
| Moderate (5 < − < 10 per year) | 0.92 (0.71–1.19) | 0.52 | ||
| Low (<5 per year) | 0.95 (0.67–1.34) | 0.76 | ||
| Performance Status | ||||
| 0 | 1 | 1 | ||
| 1 | 1.70 (1.22–2.38) | 0.002 | 1.29 (0.91–1.82) | 0.16 |
| 2 | 4.03 (2.13–7.62) | <0.0001 | 3.36 (1.74–6.51) | 0.0003 |
| 3 | 3.31 (0.82–13.33) | 0.093 | 1.53 (0.36–6.51) | 0.57 |
| Unknown | 8.16 (3.56–18.69) | <0.0001 | 5.22 (2.19–12.44) | 0.0002 |
| Neoadjuvant chemotherapy | ||||
| No | 1 | |||
| Yes | 1.15 (0.81–1.62) | 0.44 | ||
| Adjuvant chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 1.72 (1.24–2.38) | 0.001 | 0.88 (0.60–1.29) | 0.52 |
| Unknown | 1.77 (0.44–7.13) | 0.42 | 0.85 (0.21–3.52) | 0.82 |
| Pathological T-stage | ||||
| pT0-1 | 1 | 1 | ||
| pT2 | 1.42 (0.97–2.08) | 0.069 | 1.19 (0.80–1.77) | 0.39 |
| pT3-4 | 4.03 (3.04–5.34) | <0.0001 | 2.96 (2.14–4.08) | <0.0001 |
| Pathological N-stage | ||||
| pN0 | 1 | 1 | ||
| pN+ | 3.09 (2.28–4.02) | <0.0001 | 2.08 (1.54–2.81) | <0.0001 |
| pNx | 1.16 (0.61–2.18) | 0.66 | 0.84 (0.42–1.67) | 0.62 |
| Histology | ||||
| Pure urothelial carcinoma | 1 | |||
| Others | 1.29 (0.90–1.86) | 0.17 | ||
| Grade | ||||
| Low | 1 | 1 | ||
| High | 1.54 (1.06–2.26) | 0.026 | 0.98 (0.65–1.47) | 0.93 |
| Unknown | 0.96 (0.59–1.57) | 0.87 | 0.89 (0.54–1.48) | 0.66 |
| Operative time (minutes), median (range) | 0.99 (0.99–1.00) | 0.18 | ||
| Estimated blood loss (mL), median (range) | 1.00 (0.99–1.00) | 0.19 | ||
| Surgical approach | ||||
| Open | 1 | |||
| Laparoscopic | 1.15 (0.88–1.49) | 0.3 | ||
| Complications | ||||
| None | 1 | |||
| Minor/major | 1.18 (0.91–1.53) | 0.21 | ||
| None/minor | 1 | |||
| Major | 1.26 (0.97–1.64) | 0.085 | ||
| CCI 26.2 low | 1 | 1 | ||
| high | 1.42 (1.12–1.80) | 0.004 | 1.35 (1.05–1.72) | 0.017 |
Table 4.
Univariate and multivariate analyses of prognostic factors for recurrence-free survival (RFS)
| RFS | RFS | |||
|---|---|---|---|---|
| Variables | Univariate analysis | p-value | Multivariate analysis | p-value |
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | |||
| Sex | ||||
| Male | 1 | |||
| Female | 0.92 (0.72–1.17) | 0.47 | ||
| Age, year | ||||
| Continuous | 1.03 (1.02–1.04) | <0.0001 | 1.02 (1.00–1.03) | 0.023 |
| Body mass index (BMI) (kg/m 2), median (range) | 0.98 (0.95–1.01) | 0.17 | ||
| Average annual cystectomy volume | ||||
| High (≥10 per year) | 1 | |||
| Moderate (5 ≤ − < 10 per year) | 0.95 (0.75–1.20) | 0.67 | ||
| Low (<5 per year) | 1.17 (0.88–1.57) | 0.29 | ||
| Performance status | ||||
| 0 | 1 | 1 | ||
| 1 | 1.46 (1.07–1.98) | 0.017 | 1.13 (0.82–1.55) | 0.46 |
| 2 | 2.73 (1.49–4.99) | 0.001 | 2.11 (1.14–3.91) | 0.018 |
| 3 | 4.11 (1.02–16.57) | 0.047 | 1.97 (0.46–8.43) | 0.36 |
| Unknown | 4.84 (2.14–10.93) | 0.0001 | 3.00 (1.24–7.27) | 0.015 |
| Neoadjuvant chemotherapy | ||||
| No | 1 | |||
| Yes | 1.17 (0.86–1.59) | 0.31 | ||
| Adjuvant chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 1.94 (1.45–2.59) | <0.0001 | 0.96 (0.68–1.35) | 0.81 |
| Unknown | 1.56 (0.50–4.88) | 0.44 | 0.75 (0.23–2.39) | 0.62 |
| Pathological T-stage | ||||
| pT0-1 | 1 | 1 | ||
| pT2 | 1.51 (1.09–2.11) | 0.015 | 1.34 (0.96–1.88) | 0.088 |
| pT3-4 | 4.11 (3.20–5.27) | <0.0001 | 3.17 (2.42–4.16) | <0.0001 |
| Pathological N-stage | ||||
| pN0 | 1 | 1 | ||
| pN+ | 3.04 (2.41–3.85) | <0.0001 | 1.97 (1.50–2.59) | <0.0001 |
| pNx | 1.30 (0.76–2.22) | 0.34 | 1.02 (0.56–1.87) | 0.95 |
| Histology | ||||
| Pure urothelial carcinoma | 1 | |||
| Others | 1.31 (0.94–1.81) | 0.11 | ||
| Grade | ||||
| Low | 1 | |||
| High | 1.30 (0.94–1.78) | 0.11 | ||
| Unknown | 0.87 (0.57–1.32) | 0.51 | ||
| Operative time (minutes), median (range) | 0.99 (0.99–1.00) | 0.53 | ||
| Estimated blood loss (mL), median (range) | 1.00 (0.99–1.00) | 0.15 | ||
| Surgical approach | ||||
| Open | 1 | |||
| Laparoscopic | 1.11 (0.88–1.40) | 0.39 | ||
| Complications | ||||
| None | 1 | |||
| Minor/major | 1.14 (0.90–1.43) | 0.27 | ||
| None/minor | 1 | |||
| Major | 1.20 (0.95–1.52) | 0.13 | ||
| CCI 26.2 low | 1 | 1 | ||
| high | 1.28 (1.03–1.58) | 0.025 | 1.26 (1.01–1.57) | 0.044 |
Supplementary Tables 1 (OS) and 2 (RFS) summarize univariate analyses in terms of the survival impact of each category of complication according to the CD grading [(a) any grading, and (b) ≥ CD grade III]. After adjusting for other independent survival predictors including age, PS, pathological T-stage, and pathological N-stage, thromboembolic and neurological complications remained significant predictors of OS, both in any and major categorizations (Table 5). Regarding RFS, any thromboembolic or neurological complication, or major infectious complications remained significant predictors of RFS (Table 5). Urine leak was not associated with poorer OS (4-year survival estimates, without urine leak: 66.3% vs. with urine leak: 67.6%, P = 0.89) or RFS (without urine leak: 57.3% vs. with urine leak: 59.1%, P = 0.76).
Table 5.
Summary of hazard ratio of each complication category, after adjusting for age, PS, and pathological T-stage and N-stage
| OS | RFS | ||||
|---|---|---|---|---|---|
| Categories | Hazard ratio after adjusting (95% CI) | p-value | Categories | Hazard ratio after adjusting (95% CI) | p-value |
| Cardiac, any | Cardiac, any | ||||
| No | 1 | No | 1 | ||
| Yes | 1.70 (0.75–3.87) | 0.21 | Yes | 1.56 (0.69–3.54) | 0.29 |
| Thromboembolic, any | Thromboembolic, any | ||||
| No | 1 | No | 1 | ||
| Yes | 3.38 (1.50–7.64) | 0.003 | Yes | 2.64 (1.17–5.95) | 0.019 |
| Neurological, any | Neurological, any | ||||
| No | 1 | No | 1 | ||
| Yes | 1.71 (0.99–2.95) | 0.056 | Yes | 1.63 (1.01–2.64) | 0.018 |
| Infection, major | Infection, major | ||||
| No | 1 | No | 1 | ||
| Yes | 1.53 (0.97–2.40) | 0.068 | Yes | 1.56 (1.03–2.37) | 0.035 |
| Cardiac, major | Cardiac, major | ||||
| No | 1 | No | 1 | ||
| Yes | 2.10 (0.28–15.59) | 0.47 | Yes | 2.47 (0.34–18.22) | 0.37 |
| Thromboembolic, major | Thromboembolic, major | ||||
| No | 1 | No | 1 | ||
| Yes | 50.62 (6.17–415.2) | 0.0003 | Yes | 6.83 (0.93–50.37) | 0.059 |
| Neurological, major | Surgical, major | ||||
| No | 1 | No | 1 | ||
| Yes | 5.60 (1.38–22.76) | 0.016 | Yes | 1.92 (0.90–4.12) | 0.093 |
| Surgical, major | |||||
| No | 1 | ||||
| Yes | 1.81 (0.80–4.12) | 0.16 | |||
| RFS | RFS | ||||
| Categories | Univariate analysis | p-value | Categories | Univariate analysis | p-value |
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | ||||
| Gastrointestinal, any | Gastrointestinal, major (≥Clavien-Dindo grade III) | ||||
| No | 1 | No | 1 | ||
| Yes | 1.209 (0.963–1.517) | 0.1013 | Yes | 1.061 (0.767–1.468) | 0.7196 |
| Infection, any | Infection, major | ||||
| No | 1 | No | 1 | ||
| Yes | 1.089 (0.885–1.341) | 0.4208 | Yes | 1.727 (1.167–2.556) | 0.0063 |
| Wound, any | Wound, major | ||||
| No | 1 | No | 1 | ||
| Yes | 1.020 (0.784–1.327) | 0.8814 | Yes | 0.947 (0.582–1.541) | 0.8266 |
| Genitourinary, any | Genitourinary, major | ||||
| No | 1 | No | 1 | ||
| Yes | 1.084 (0.765–1.536) | 0.6505 | Yes | 1.339 (0.892–2.010) | 0.1588 |
| Cardiac, any | Cardiac, major | ||||
| No | 1 | No | 1 | ||
| Yes | 2.329 (1.039–5.221) | 0.0401 | Yes | 7.380 (1.029–52.91) | 0.0467 |
| Pulmonary, any | Pulmonary, major | ||||
| No | 1 | No | 1 | ||
| Yes | 1.327 (0.628–2.805) | 0.4581 | Yes | 0.9992 | |
| Bleeding, any | Bleeding, major | ||||
| No | 1 | No | 1 | ||
| Yes | 1.131 (0.422–3.031) | 0.806 | Yes | 0.9991 | |
| Thromboembolic, any | Thromboembolic, major | ||||
| No | 1 | No | 1 | ||
| Yes | 2.348 (1.047–5.265) | 0.0384 | Yes | 9.762 (1.358–70.19) | 0.0236 |
| Neurological, any | Neurological, major | ||||
| No | 1 | No | 1 | ||
| Yes | 2.028 (1.291–3.184) | 0.0021 | Yes | 3.108 (0.774–12.49) | 0.11 |
| Miscellaneous, any | Miscellaneous, major | ||||
| No | 1 | No | 1 | ||
| Yes | 1.318 (0.907–1.915) | 0.1473 | Yes | 0.856 (0.354–2.069) | 0.7295 |
| Surgical, any | Surgical, major | ||||
| No | 1 | No | 1 | ||
| Yes | 1.630 (0.771–3.443) | 0.2008 | Yes | 2.376 (1.124–5.021) | 0.0235 |
Discussion
This study investigated the prognostic impact of postoperative complications on long-term survival outcomes after RC for BC. When considering the most severe event in each patient, neither any nor CD grade ≥ III was associated with OS or RFS. Rather, when considering all events together with their respective severity with the use of CCI, which Slankamenac and colleagues created in order to assess cumulative postoperative morbidity (10), CCI was independently associated with both OS and RFS. Patients with CCI ≥ 26.2 had shorter OS (4-year, 59.5 vs. 69.8%, respectively, log-rank test, P = 0.0037) and RFS (51.9 vs. 60.1%, respectively, P = 0.0234), than those with CCI < 26.2. For the first time, we identified that postoperative complications assessed using CCI could impair long-term survival outcomes after RC. To date, the negative impact of postoperative complications on long-term survival outcomes beyond the postoperative periods has been reported in several cancers, such as colorectal, gastric and oesophagogastric cancer, or hepatocellular carcinoma (5–8). Taken together with these previous observations, postoperative complications could compromise long-term disease control after major cancer surgery.
To date, the CD Classification, a grading system based on the necessary treatment for proper management, has been widely utilized to report postoperative complications. CD Classification only accounts for the most severe complications in each patient, for example, patients with a single Grade 2 complication and those with multiple Grade 2 complications are categorized into the same category, ‘minor complication.’ However, infections requiring antibiotic treatment for a long time, or readmission for ileus, especially when multiple low-grade complications occur simultaneously in one patient, could significantly delay a patient’s full recovery to daily life. This is because, during the healing process, physicians may be reluctant to perform adjuvant chemotherapy, which could influence long-term disease control, as described below. In the present study, when using CCI to identify cumulative postoperative morbidity, the proportion of patients with major complications (CCI ≥ 26.2) increased to 34% (261/776) as compared with 24% (187/766) when considering only the highest-grade complications (≥CD grade III). Other researchers have also observed higher cumulative postoperative morbidity after RC than the CD Classification (13,14). Consistent with the present study, the prognostic impact of CCI has been reported for other malignancies (11,12,15). For example, Yamashita et al. observed that CCI ≥ 26.2 was independently associated with cancer-specific survival after resection of colorectal liver metastases (11).
Although the mechanism has not yet been fully clarified, one hypothesis is that the growth of residual cancer cells is fueled by inflammatory cytokines and growth factors that are stimulated by surgical stress (16–18). Another hypothesis is that an attenuated host immunological response during postoperative illness may promote tumorigenesis (19). In addition, the prolonged postoperative period required to recover from major complications could preclude adjuvant chemotherapy, which may result in a poorer long-term survival outcome. For example, Jin et al. observed that, in the US Gastric Cancer Collaborative (n = 824), patients with postoperative complications were less likely to undergo adjuvant chemotherapy (odds ratio = 0.5, P < 0.001), and such patients had a significantly increased hazard of death (HR-2.3, P < 0.001) (20). The delay in adjuvant chemotherapy (oral fluoropyrimidine derivative monotherapy) was also associated with shorter recurrence-free survival in gastric cancer patients (21). In the present cohort, after excluding patients without information on adjuvant chemotherapy (n = 7), among the pTanypN+ or pT3-4pNany patients (n = 335) who were considered to be candidates for adjuvant chemotherapy, those with CCI ≥ 26.2 were less likely to receive adjuvant chemotherapy than patients with CCI < 26.2 (25 vs. 15%, respectively, χ2 test, P = 0.0235, Supplementary Fig. 1a), which might result in poorer survival in patients with CCI ≥ 26.2. Furthermore, in patients who received NAC (n = 95), there was no significant difference in OS or RFS between patients with CCI ≥ 26.2 and those with CCI < 26.2 (4-year OS: 57.7 vs. 60.5%, log-rank test, P = 0.91, and 4-year RFS: 55.4 vs. 49.2%, log-rank test, P = 0.52, Supplementary Fig. 1b and c). Based on these observations, we believe that NAC could be a promising treatment option because it is not influenced by postoperative complications.
Among postoperative complications, infectious complications were the most frequently reported as adverse prognostic factors in patients with hepatocellular, gastric, lung or colorectal cancers (8,22–24). As shown in Table 5, a major infectious complication (≥CD grade III) remained an independent adverse factor for RFS, but this was marginal (P = 0.0681) for OS. Although thromboembolic and neurological complications remained significant for OS and RFS, we could not draw a definitive conclusion because of the low number of events. A larger study is warranted to gain further insight into the survival impacts of each complication category.
In colorectal cancer, it was reported that long-term disease control was impaired by anastomotic leakage (25,26). It has been proposed that anastomotic leakage can lead to extraluminal dissemination of the remaining cancer cells. Based on this hypothesis, we compared survival curves between patients with and without urinary leakage. As described above, we did not observe significant differences in survival between patients with and without urine leak (4-year OS: without urine leak 66.3% vs. with urine leak 67.6%, P = 0.887 and 4-year RFS: without urine leak 57.3% vs. with urine leak 59.1%, P = 0.76).
Our study had several limitations. First, because of its retrospective design, some complications and comorbid conditions may not have been recorded. Second, variability among the participating hospitals regarding surgical techniques and postoperative management was another limitation. External validation is necessary to confirm the generalizability of the present findings. Nevertheless, this study is the first to demonstrate that postoperative morbidity can impair long-term disease control after RC, just as with other major cancer surgery. Physicians should be aware that major postoperative complications can impair long-term disease control.
Conclusions
Postoperative complications assessed by CCI independently had an adverse impact on long-term survival outcomes after RC. It is vital to mitigate postoperative complications, not only for early convalescence, but also for improving long-term disease control.
Supplementary Material
Acknowledgements
We thank Junji Ishizaki, Shuhei Ishikawa, Yuichiro Oishi, Chihiro Kamijyo, Keiji Sugishita, Tsuyoshi Furuno, Noboru Yamashita, Yuki Sugito, Tatsuya Hoshi, Oujiro Toukairin, Ataka Hanai, Kanta Hori, Kiyoto Nagamori, Madoka Higuchi, Mikio Konnno, Masaya Miyazaki, Ryo Kato, Ryo Kurosawa, Shogo Aizawa, Shigeru Harada, Shun Tsubouchi, Takuya Moriguchi, Takuto Morita, Tatsu Tanabe, Yurie Hirata, Yuki Muranishi, Takenori Ono and Gaku Tamaki for data collection.
Contributor Information
Takashige Abe, Department of Urology, Hokkaido University Graduate School of Medicine, Sapporo, Japan.
Shuhei Yamada, Department of Urology, Hokkaido University Graduate School of Medicine, Sapporo, Japan.
Hiroshi Kikuchi, Department of Urology, Hokkaido University Graduate School of Medicine, Sapporo, Japan; Department of Urology, Teine-Keijinkai Hospital, Sapporo, Japan.
Ataru Sazawa, Department of Urology, Obihiro Kousei Hospital, Obihiro, Japan.
Hidenori Katano, Department of Urology, Iwamizawa Municipal General Hospital, Iwamizawa, Japan.
Hidetaka Suzuki, Department of Urology, Hakodate Central Hospital, Hakodate, Japan.
Ichiro Takeuchi, Department of Urology, Tomakomai City Hospital, Tomakomai, Japan.
Keita Minami, Department of Urology, Sapporo City General Hospital, Sapporo, Japan.
Ken Morita, Department of Urology, Kushiro City General Hospital, Kushiro, Japan.
Kunihiko Tsuchiya, Department of Urology, KKR Sapporo Medical Center, Sapporo, Japan.
Norikata Takada, Department of Urology, Hokkaido Cancer Center, Sapporo, Japan.
Shintaro Maru, Department of Urology, Jinyukai Hospital, Sapporo, Japan.
Soshu Sato, Department of Urology, Ebetsu City Hospital, Ebetsu, Japan.
Takanori Yamashita, Department of Urology, Nayoro City General Hospital, Nayoro, Japan.
Tango Mochizuki, Department of Urology, Abashiri Kousei Hospital, Abashiri, Japan.
Tomoshige Akino, Department of Urology, KKR Tonan Hospital, Sapporo, Japan.
Yoshihiro Sasaki, Department of Urology, Kushiro Rosai Hospital, Kushiro, Japan.
Yuichiro Shinno, Department of Urology, Otaru General Hospital, Otaru, Japan.
Norihiro Murahashi, Department of Urology, Asahikawa Kousei Hospital, Asahikawa, Japan; Department of Urology, JCHO Sapporo Hokushin Hospital, Sapporo, Japan.
Takafumi Kawazu, Department of Urology, Hokkaido Urology Memorial Hospital, Sapporo, Japan.
Jun Furumido, Department of Urology, Hokkaido University Graduate School of Medicine, Sapporo, Japan; Department of Urology, Teine-Keijinkai Hospital, Sapporo, Japan.
Haruka Miyata, Department of Urology, Hokkaido University Graduate School of Medicine, Sapporo, Japan.
Ryuji Matsumoto, Department of Urology, Hokkaido University Graduate School of Medicine, Sapporo, Japan.
Takahiro Osawa, Department of Urology, Hokkaido University Graduate School of Medicine, Sapporo, Japan.
Sachiyo Murai, Department of Urology, Hokkaido University Graduate School of Medicine, Sapporo, Japan.
Nobuo Shinohara, Department of Urology, Hokkaido University Graduate School of Medicine, Sapporo, Japan.
Funding
The study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Conflict of interest statement
The authors declare that they have NO affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Ethics statement
This study received Institutional Review Board Approval (No. 017-0038).
Author Contributions
T.A., S.Y. and H.K. designed the research. T.A., S.Y., H.K., A.S., H.K., H.S., I.T., K.M., K.M., K.T., N.T., S.M., S.S., T.Y., T.M., T.A., Y.S., Y.S., N.M., T.K., J.F., H.M., R.M. and S.M. collected the data. T.A. and S.Y. analyzed the data or performed statistical analysis. T.O. and N.S. supervised the project. T.A. wrote the paper. All authors read and approved the final manuscript.
References
- 1. Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009;55:164–76. [DOI] [PubMed] [Google Scholar]
- 2. Takada N, Abe T, Shinohara N, et al. Peri-operative morbidity and mortality related to radical cystectomy: a multi-institutional retrospective study in Japan. BJU Int 2012;110:E756–64. [DOI] [PubMed] [Google Scholar]
- 3. Novara G, De Marco V, Aragona M, et al. Complications and mortality after radical cystectomy for bladder transitional cell cancer. J Urol 2009;182:914–21. [DOI] [PubMed] [Google Scholar]
- 4. Yamada S, Abe T, Sazawa A, et al. Comparative study of postoperative complications after radical cystectomy during the past two decades in Japan: radical cystectomy remains associated with significant postoperative morbidities. Urol Oncol 2022;40:11.e17–25. [DOI] [PubMed] [Google Scholar]
- 5. Saunders JH, Yanni F, Dorrington MS, et al. Impact of postoperative complications on disease recurrence and long-term survival following oesophagogastric cancer resection. Br J Surg 2020;107:103–12. [DOI] [PubMed] [Google Scholar]
- 6. Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg 2015;261:497–505. [DOI] [PubMed] [Google Scholar]
- 7. Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 2011;253:890–9. [DOI] [PubMed] [Google Scholar]
- 8. Yang T, Liu K, Liu CF, et al. Impact of postoperative infective complications on long-term survival after liver resection for hepatocellular carcinoma. Br J Surg 2019;106:1228–36. [DOI] [PubMed] [Google Scholar]
- 9. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1–7. [DOI] [PubMed] [Google Scholar]
- 11. Yamashita S, Sheth RA, Niekamp AS, et al. Comprehensive Complication Index predicts cancer-specific survival after resection of colorectal metastases independent of RAS mutational status. Ann Surg 2017;266:1045–54. [DOI] [PubMed] [Google Scholar]
- 12. Tu RH, Lin JX, Li P, et al. Comprehensive Complication Index predicts cancer-specific survival of patients with postoperative complications after curative resection of gastric cancer. Gastroenterol Res Pract 2018;2018:4396018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haas M, Huber T, Pickl C, et al. The comprehensive complication index is associated with a significant increase in complication severity between 30 and 90 days after radical cystectomy for bladder cancer. Eur J Surg Oncol 2021;47:1163–71. [DOI] [PubMed] [Google Scholar]
- 14. Vetterlein MW, Klemm J, Gild P, et al. Improving estimates of perioperative morbidity after radical cystectomy using the European Association of Urology quality criteria for standardized reporting and introducing the Comprehensive Complication Index. Eur Urol 2020;77:55–65. [DOI] [PubMed] [Google Scholar]
- 15. Ortiz-Lopez D, Marchena-Gomez J, Nogues-Ramia E, et al. Utility of a new prognostic score based on the Comprehensive Complication Index (CCI(R)) in patients operated on for colorectal cancer (S-CRC-PC score). Surg Oncol 2022;42:101780. [DOI] [PubMed] [Google Scholar]
- 16. Bohle B, Pera M, Pascual M, et al. Postoperative intra-abdominal infection increases angiogenesis and tumor recurrence after surgical excision of colon cancer in mice. Surgery 2010;147:120–6. [DOI] [PubMed] [Google Scholar]
- 17. Abramovitch R, Marikovsky M, Meir G, Neeman M. Stimulation of tumour growth by wound-derived growth factors. Br J Cancer 1999;79:1392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- 19. Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg 2011;253:798–810. [DOI] [PubMed] [Google Scholar]
- 20. Jin LX, Sanford DE, Squires MH III, et al. Interaction of postoperative morbidity and receipt of adjuvant therapy on long-term survival after resection for gastric adenocarcinoma: results from the U.S. Gastric Cancer Collaborative. Ann Surg Oncol 2016;23:2398–408. [DOI] [PubMed] [Google Scholar]
- 21. Nakanishi K, Kanda M, Ito S, et al. Delay in initiation of postoperative adjuvant chemotherapy with S-1 monotherapy and prognosis for gastric cancer patients: analysis of a multi-institutional dataset. Gastric Cancer 2019;22:1215–25. [DOI] [PubMed] [Google Scholar]
- 22. Andalib A, Ramana-Kumar AV, Bartlett G, Franco EL, Ferri LE. Influence of postoperative infectious complications on long-term survival of lung cancer patients: a population-based cohort study. J Thorac Oncol 2013;8:554–61. [DOI] [PubMed] [Google Scholar]
- 23. Han WH, Oh YJ, Eom BW, Yoon HM, Kim YW, Ryu KW. Prognostic impact of infectious complications after curative gastric cancer surgery. Eur J Surg Oncol 2020;46:1233–8. [DOI] [PubMed] [Google Scholar]
- 24. Farid SG, Aldouri A, Morris-Stiff G, et al. Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg 2010;251:91–100. [DOI] [PubMed] [Google Scholar]
- 25. Bell SW, Walker KG, Rickard MJ, et al. Anastomotic leakage after curative anterior resection results in a higher prevalence of local recurrence. Br J Surg 2003;90:1261–6. [DOI] [PubMed] [Google Scholar]
- 26. Law WL, Choi HK, Lee YM, Ho JW, Seto CL. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg 2007;11:8–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.