Abstract
The management of acute otitis media is complicated by the emergence of resistance to β-lactam and other antibiotics among common pathogens. We conducted a large, international study of infants and children with acute otitis media to identify pathogens and susceptibility patterns. During the winter of 1994 to 1995, middle ear fluid samples were collected from 917 patients with acute otitis media in Bulgaria, the Czech Republic, Hungary, Romania, Slovakia, Israel, and the United States. A single reference laboratory performed in vitro susceptibility testing. Pathogens were isolated from 62% of the patients. For Streptococcus pneumoniae (30% of the patients), untypeable Haemophilus influenzae (17%), and Moraxella catarrhalis (4%), there was significant variation among geographic regions (P < 0.001). The composite susceptibilities of these three organisms to amoxicillin ranged from 62% in the United States to 89% in Eastern and Central Europe; the corresponding susceptibilities to amoxicillin-clavulanate ranged from 90% in Israel to 95% in Eastern and Central Europe. β-Lactamase was produced by 31 and 100% of the isolates of H. influenzae and M. catarrhalis, respectively. More isolates of S. pneumoniae were susceptible to amoxicillin (90%) or amoxicillin-clavulanate (90%) than to penicillin (70%; P = 0.002). The prevalence of resistant S. pneumoniae was highest in patients less than 12 months of age. S. pneumoniae, H. influenzae, and M. catarrhalis remain the most important bacterial pathogens in patients with acute otitis media; however, their prevalence is variable and resistance patterns are changing.
Acute otitis media continues to be an important public health problem around the world. The most common bacterial pathogen, Streptococcus pneumoniae, has been implicated as part of the current antibiotic resistance crisis (33). The first cases of penicillin-resistant S. pneumoniae were reported in Australia and New Guinea in the early 1970s (21, 22). In less than a decade, highly resistant strains that exhibited resistance to multiple antibiotics were isolated from patients with invasive infections, as well as from carriers, in South Africa (3, 28). The prevalence of penicillin-resistant pneumococcal infections has escalated steadily. The increasing use of day-care facilities provides a vector for transmission of resistant pneumococci (10, 39). International spread of resistant isolates has been documented (30, 32, 43), so that penicillin-resistant pneumococcal infections have become a global problem (1, 2).
The optimal management of acute otitis media is widely debated for many reasons (6, 20, 38), beginning at the most basic level of medical management, because there is no consensus regarding diagnostic criteria (5). The selection of antibiotic therapy is usually empiric because of the difficulty of obtaining cultures. Although authorities usually agree that S. pneumoniae and untypeable Haemophilus influenzae are the most common bacterial pathogens (5), the prevalence of resistance is not as well documented. Eradication of pathogens from middle ear fluid and clinical outcome have been reported to be less favorable if pathogens with reduced antimicrobial susceptibility are present (12, 19).
To help answer these questions, we conducted a large, prospective study of bacterial isolates associated with acute otitis media. To minimize the risk of overlooking any isolates, especially penicillin-resistant S. pneumoniae, we evaluated a large number of patients in several countries. Strict diagnostic criteria for otitis media were used, including otoscopic findings of middle ear effusion or purulent otorrhea and local indicators of acute inflammation. This report summarizes the microbiological findings on pretherapy middle ear fluid specimens obtained from 917 patients with acute otitis media as part of a prospective, multinational clinical study.
(The clinical results reported here were published previously [23], and the preliminary microbiologic results were published as abstracts [26, 27].)
MATERIALS AND METHODS
Patients.
Infants and children up to 12 years of age who met strict criteria for acute otitis media with effusion (23) were eligible for enrollment. The lower age limits of eligibility were 3 months in the United States and Israel and, because of regulatory issues, 9 months in Eastern and Central Europe. Acute otitis media was diagnosed on the basis of otoscopic findings of either middle ear effusion or purulent otorrhea with a duration of less than 24 h.
Informed consent was obtained from parents or legal guardians. The protocol was approved by institutional review boards at participating institutions.
Microbiology.
Middle ear fluid samples were collected by tympanocentesis or myringotomy. If tympanostomy tubes were present or the tympanic membrane had ruptured, samples were collected on swabs. Initial isolation procedures were performed on blood and chocolate agar plates, by local microbiology laboratories, and all isolates recovered were forwarded to the reference laboratory of one of the investigators (M.R.J.) for confirmation of identification and in vitro susceptibility testing. Isolates received at the reference laboratory were identified by standard methods (34). Serotypes of penicillin-resistant S. pneumoniae isolates were determined by the Statens Seruminstitut, Copenhagen, Denmark, courtesy of Helle Bossen Konradsen.
Susceptibility testing.
MICs of penicillin, amoxicillin, amoxicillin-clavulanate, erythromycin, clindamycin, trimethoprim-sulfamethoxazole, tetracycline, and chloramphenicol were determined by the broth microdilution method of the National Committee for Clinical Laboratory Standards with cation-adjusted Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented with 5% lysed horse blood for S. pneumoniae (35). MICs of amoxicillin and amoxicillin-clavulanate were determined by using Haemophilus test medium for Haemophilus species and Moraxella (Branhamella) catarrhalis (35). β-Lactamase production was determined for Haemophilus species and M. catarrhalis by the chromogenic cephalosporin method by using nitrocefin as the substrate (34).
MICs were interpreted according to the National Committee for Clinical Laboratory Standards M100-S6 informational supplement (36). All β-lactamase-producing H. influenzae and M. catarrhalis isolates were interpreted as resistant to amoxicillin, regardless of the MICs.
Statistical design and analysis.
The target enrollment was set at 1,000 assessable patients to obtain 30 to 50 patients with acute otitis media due to S. pneumoniae isolates that were intermediately or fully resistant to penicillin. Data from Eastern and Central Europe were combined. Data analyses were performed with SAS Institute statistical software (41). Log-linear techniques were used to test for statistical independence of age (categorized), region of origin, and pathogen prevalence for each age group. Where the null hypotheses of independence were rejected, subsequent analyses were conducted at the appropriate level of detail for comparisons between age groups and regions. One-way analysis of variance or the Kruskal-Wallis test compared means across regions. Chi-square tests were used to test bivariate null hypotheses of independence. P values were adjusted for multiple comparisons as appropriate (16).
RESULTS
Patients.
Specimens of middle ear exudate were obtained from 917 assessable patients enrolled in the study during the winter of 1994 to 1995. Twenty-one investigators from Eastern and Central European countries enrolled 529 patients (58%). Four investigators from Israel enrolled 107 patients (12%). Ten centers in the United States, comprising six university-affiliated hospitals and four private practices, enrolled 281 patients (31%).
Mean ages (± the standard deviation) were 4.6 ± 2.9 years in Eastern and Central Europe, 1.4 ± 1.8 years in Israel, and 2.6 ± 2.3 years in the United States (P = 0.0001, between regions). Because of differences in the lower age limits of eligibility, younger patients were enrolled in the <12-month age group in the United States (mean, 7.8 ± 2.3 months; n = 63) and Israel (mean, 6.7 ± 2.7 months; n = 52) than in Eastern and Central Europe (9.2 ± 1.9 months; n = 25) (P = 0.0001, between regions).
Microbiology results.
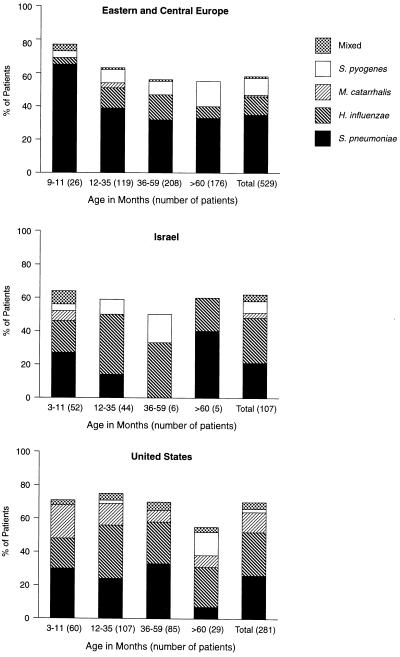
Specimens were obtained by tympanocentesis (80%) or from the discharge from ruptured tympanic membranes (18%) or tympanostomy tubes (2%) (Table 1). Pathogens were isolated from 569 patients (62%). The most frequently isolated pathogen was S. pneumoniae (30%), which was followed by untypeable H. influenzae (17%), S. pyogenes (7%), M. catarrhalis (4%), and mixtures of these pathogens (3%) (Fig. 1).
TABLE 1.
Culture results obtained by the specimen collection method
| Organism(s) | No. of isolates (% of patients)
|
|||
|---|---|---|---|---|
| Tympanocentesis | Ruptured tympanic membrane | Tympanostomy tube | All | |
| S. pneumoniae | 241 (33) | 34 (20) | 4 (22) | 279 (30) |
| H. influenzae | 140 (19) | 13 (8) | 3 (17) | 156 (17) |
| M. catarrhalis | 39 (5) | 1 (1) | 0 | 40 (4) |
| S. pyogenes | 37 (5) | 27 (16) | 2 (11) | 66 (7) |
| Mixed pathogensa | 23 (3) | 3 (2) | 2 (11) | 28 (3) |
| Contaminantb or no growth | 248 (34) | 91 (54) | 7 (39) | 346 (38) |
| Total | 728 (80) | 169 (18) | 18 (2) | 915 (100)c |
Mixed pathogens were S. pneumoniae and H. influenzae (n = 10), S. pneumoniae and M. catarrhalis (n = 7), S. pneumoniae and S. pyogenes (n = 4), H. influenzae and M. catarrhalis (n = 3), and H. influenzae and S. pyogenes (n = 4).
Staphylococcus aureus (n = 49) was interpreted as a contaminant.
The method of specimen collection from two patients, one with S. aureus and one with no growth, was missing.
FIG. 1.
Culture results of middle ear aspirates obtained from patients in Eastern and Central Europe, Israel, and the United States.
There were differences in the geographic distribution of potentially resistant pathogens (i.e., pathogens that have developed antimicrobial resistance, namely, S. pneumoniae, H. influenzae, and M. catarrhalis). Among the three geographic regions, significant differences (P < 0.001) were found for the following comparisons: any versus no pathogen, S. pneumoniae versus no S. pneumoniae, S. pneumoniae versus H. influenzae and M. catarrhalis, H. influenzae versus no H. influenzae, and H. influenzae versus S. pneumoniae and M. catarrhalis.
The likelihood of isolating a potentially resistant pathogen decreased with increasing age. In Eastern and Central Europe, the prevalence of resistant pathogens fell from 73 to 40% as the patients’ ages rose from <12 to >60 months (P = 0.008) and from 57 to 44% as the ages rose from ≤35 to ≥36 months (P = 0.006). In the United States, the corresponding prevalence fell from 72 to 41% (P = 0.005) and from 72 to 62% (P = 0.03). There were not enough older patients in Israel to analyze this trend. The likelihood of isolating S. pneumoniae (P = 0.012) decreased in Eastern and Central Europe as the ages rose from ≤35 to ≥36 months, but not in the other regions.
Susceptibility testing.
In vitro susceptibility testing was performed on 454 potentially resistant pathogens that were received in viable condition by the reference laboratory. The composite susceptibility to amoxicillin for S. pneumoniae, H. influenzae, and M. catarrhalis was 76%, ranging from 62% in the United States to 89% in Eastern and Central Europe (Table 2). The corresponding susceptibility to amoxicillin-clavulanate was 94% overall, ranging from 90% in Israel to 95% in Eastern and Central Europe.
TABLE 2.
Susceptibility of isolates tested at reference laboratory,a including isolates from mixed infections
| Organism(s) | No. of susceptible isolatesb/total no. of isolates (% of isolates)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Amoxicillin
|
Amoxicillin-clavulanate
|
|||||||
| Europe | Israel | United States | Total | Europe | Israel | United States | Total | |
| S. pneumoniae | 154/166 (93) | 19/25 (76) | 70/80 (88) | 243/271 (90) | 155/166 (93) | 19/25 (76) | 70/80 (88) | 244/271 (90) |
| H. influenzae | 45/52 (87) | 23/31 (74) | 36/64 (56) | 102/147 (69) | 52/52 (100) | 31/31 (100) | 63/64 (98) | 146/147 (99) |
| M. catarrhalis | 0/5 (0) | 0/4 (0) | 0/27 (0) | 0/36 (0) | 5/5 (100) | 4/4 (100) | 27/27 (100) | 36/36 (100) |
| Total | 199/223 (89) | 42/60 (70) | 106/171 (62) | 345/454 (76) | 212/223 (95) | 54/60 (90) | 160/171 (94) | 426/454 (94) |
Isolates not received for testing were S. pneumoniae (n = 29), H. influenzae (n = 26), and M. catarrhalis (n = 14).
Susceptibility breakpoints: ≤0.5 μg/ml for S. pneumoniae and ≤4 μg/ml for H. influenzae and M. catarrhalis.
Of 147 isolates of H. influenzae, 31% produced β-lactamase, including 13% of 52 from Eastern and Central Europe, 26% of 31 from Israel, and 47% of 64 from the United States. Of 36 isolates of M. catarrhalis, all produced β-lactamase.
Of 271 isolates of S. pneumoniae, 30% were intermediately or fully resistant to penicillin, including 31% in Central and Eastern Europe, 52% in Israel, and 21% in the United States (P = 0.012). The prevalence of intermediately or fully penicillin-resistant S. pneumoniae isolates was variable among Central and Eastern European countries, ranging from 4% in the Czech Republic to 41% in Romania (Table 3).
TABLE 3.
Susceptibility of S. pneumoniae isolates from Eastern and Central Europe to penicillin
| Country (no. of isolates) | No. (%) of isolates
|
|||
|---|---|---|---|---|
| Susceptiblea | Intermediately resistant | Fully resistant | Intermediately or fully resistant | |
| Bulgaria (32) | 21 (66) | 7 (22) | 4 (13) | 11 (34) |
| Czech Republic (26) | 25 (96) | 1 (4) | 0 (0) | 1 (4) |
| Hungary (13) | 11 (85) | 2 (15) | 0 (0) | 2 (15) |
| Romania (82) | 48 (59) | 31 (38) | 3 (4) | 34 (41) |
| Slovakia (13) | 9 (69) | 3 (23) | 1 (8) | 4 (31) |
| All (166) | 114 (69) | 44 (27) | 8 (5) | 52 (31) |
Susceptible, ≤0.06 μg/ml; intermediate, 0.12 to 1 μg/ml; resistant, ≥2 μg/ml.
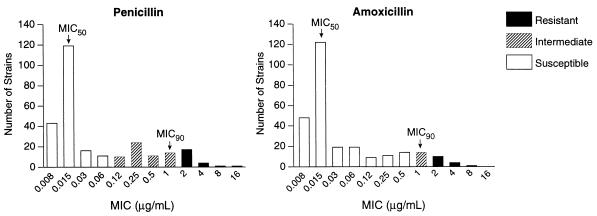
More isolates of S. pneumoniae were susceptible to amoxicillin (90%) or amoxicillin-clavulanate (90%) than to penicillin (70%; P = 0.002; Table 4). Figure 2 illustrates the distribution of MICs of penicillin and amoxicillin; MICs of amoxicillin-clavulanate were nearly identical to those for amoxicillin (data not shown). The three β-lactam antibiotics had identical MICs at which 50% of the isolates were inhibited (MIC50s (0.015 μg/ml) and MIC90s (1 μg/ml). However, while almost all fully and intermediately penicillin-susceptible strains were susceptible to amoxicillin and amoxicillin-clavulanate, penicillin-resistant strains were all intermediately or fully resistant to these agents (Table 5). Susceptibilities to non-β-lactam antimicrobials ranged from 59% for trimethoprim-sulfamethoxazole to 90% for chloramphenicol. When susceptibilities to other antimicrobials were stratified by penicillin susceptibility (Table 5), intermediately or fully penicillin-resistant isolates were more likely to be resistant to trimethoprim-sulfamethoxazole (82 versus 23%; P = 0.001) and erythromycin (39 versus 4%; P = 0.001) than were penicillin-susceptible isolates.
TABLE 4.
Susceptibility of 271 isolates of S. pneumoniae to β-lactam antibiotics and other antimicrobials
| Antibiotic(s) | Breakpoint (μg/ml)
|
No. (%) of isolates
|
||||
|---|---|---|---|---|---|---|
| Susceptible | Intermediate resistance | Full resistance | Susceptible | Intermediately resistant | Fully resistant | |
| Penicillin | ≤0.06 | 0.12–1 | ≥2 | 189 (70) | 59 (22) | 23 (8) |
| Amoxicillin | ≤0.5 | 1 | ≥2 | 243 (90) | 13 (5) | 15 (6) |
| Amoxicillin-clavulanate | ≤0.5 | 1 | ≥2 | 244 (90) | 16 (6) | 11 (4) |
| Erythromycin | ≤0.25 | 0.5 | ≥1 | 231 (85) | 0 | 40 (15) |
| Clindamycin | ≤0.25 | 0.5 | ≥1 | 241 (89) | 0 | 30 (11) |
| Trimethoprim-sulfamethoxazole | ≤0.5 | 1–2 | ≥4 | 161 (59) | 0 | 110 (41) |
| Tetracycline | ≤2 | 4 | ≥8 | 210 (77) | 9 (3) | 52 (19) |
| Chloramphenicol | ≤4 | NAa | ≥8 | 245 (90) | NA | 26 (10) |
NA, not applicable.
FIG. 2.
MICs of penicillin and amoxicillin for 271 isolates of S. pneumoniae.
TABLE 5.
Susceptibility of 271 isolates of S. pneumoniae to various antimicrobials with respect to penicillin susceptibility
| Antibiotic(s) | No. (%) of isolatesa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Penicillin susceptibleb
|
Intermediately penicillin resistantc
|
Fully penicillin resistantd
|
|||||||
| S | I | R | S | I | R | S | I | R | |
| Amoxicillin | 189 (100) | 0 | 0 | 56 (95) | 3 (5) | 0 | 0 | 9 (39) | 14 (61) |
| Amoxicillin-clavulanate | 189 (100) | 0 | 0 | 57 (97) | 2 (3) | 0 | 0 | 13 (57) | 10 (43) |
| Erythromycin | 181 (96) | 0 | 8 (4) | 34 (58) | 0 | 25 (42) | 16 (70) | 0 | 7 (30) |
| Clindamycin | 186 (98) | 0 | 3 (2) | 36 (61) | 0 | 23 (39) | 19 (83) | 0 | 4 (17) |
| Trimethoprim-sulfamethoxazole | 145 (77) | 0 | 44 (23) | 14 (24) | 0 | 45 (76) | 1 (4) | 0 | 22 (96) |
| Tetracycline | 162 (86) | 7 (4) | 20 (11) | 37 (63) | 2 (3) | 20 (34) | 13 (57) | 0 | 10 (43) |
| Chloramphenicol | 177 (94) | NAe | 12 (6) | 52 (88) | NA | 7 (12) | 16 (70) | NA | 7 (30) |
S, susceptible; I, intermediately resistant; R, fully resistant.
n = 189.
n = 59.
n = 23.
NA, not applicable.
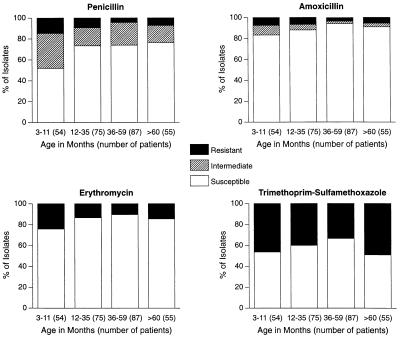
The prevalence of resistant S. pneumoniae was highest in the youngest age groups (Fig. 3). Values for intermediately or fully penicillin-resistant isolates were higher in the <12-month age group than in the ≥12-month age group in all regions (48 versus 26%; P = 0.001) and in the United States (47 versus 13%; P = 0.001); the corresponding differences were not statistically significant in Eastern and Central Europe (47 versus 30%) or in Israel (50 versus 13%). Amoxicillin-resistant S. pneumoniae was isolated from 10% of all patients, and its prevalence was highest in the <12-month age group in the United States (37% of 19 patients) and in the 12- to 23-month age group in Israel (60% of 5 patients) and Eastern and Central Europe (12% of 42 patients); however, these differences were not significant. Analogous findings were obtained for amoxicillin-clavulanate-resistant isolates of S. pneumoniae. Erythromycin and trimethoprim-sulfamethoxazole resistance was highest in the youngest and oldest children, but these differences were not significant.
FIG. 3.
Distribution of 271 isolates of S. pneumoniae by age group and antibiotic susceptibility.
Resistance patterns and serotypes were determined for 82 isolates of S. pneumoniae that were intermediately or fully resistant to penicillin (Table 6). The most common serotypes were 6A (24%) and 23F (21%). The most common pattern was resistance to penicillin and trimethoprim-sulfamethoxazole (38% of intermediately or fully resistant isolates), found in eight serotypes, followed by resistance to penicillin, trimethoprim-sulfamethoxazole, tetracycline, erythromycin, and clindamycin (22%), found predominantly in serotype 6A, and resistance to penicillin (13%), found in six serotypes. Thirty-nine isolates (48%) exhibited resistance to three or more antimicrobials.
TABLE 6.
Antibiotic resistance patterns and serotypes of 23 fully penicillin-resistant isolates and 59 intermediately penicillin-resistant isolates of S. pneumoniae
| Resistance patterna | No. of isolates
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6A | 6B | 9V | 14 | 19A | 19F | 23A | 23 F | Otherb | Total (%) | |
| P | 1 | 4 | 1 | 2 | 1 | 2 | 11 (13) | |||
| PE | 1 | 1 (1) | ||||||||
| PS | 2 | 1 | 6 | 4 | 2 | 2 | 11 | 3 | 31 (38) | |
| PECl | 1 | 1 (1) | ||||||||
| PTECl | 1 | 1 | 2 (2) | |||||||
| PEClS | 4 | 4 (5) | ||||||||
| PTEClS | 13 | 2 | 1 | 1 | 1 | 18 (22) | ||||
| PCES | 2 | 2 (2) | ||||||||
| PCTS | 2 | 5 | 1 | 8 (10) | ||||||
| PCTES | 2 | 2 (2) | ||||||||
| PCTEClS | 2 | 2 (2) | ||||||||
| Total | 20 | 4 | 10 | 13 | 3 | 7 | 2 | 17 | 6 | 82 (100) |
P, penicillin; E, erythromycin; S, trimethoprim-sulfamethoxazole; T, tetracycline; Cl, clindamycin; C, chloramphenicol.
Others belonged to serotype 5 (n = 1) or 15 (n = 2) or were untypeable (n = 3).
DISCUSSION
To our knowledge, this is the largest multinational, single-season microbiologic study of infants and children with acute otitis media. Bacterial pathogens were cultured from 62% of the patients who had acute otitis media as defined by strict diagnostic criteria. As expected, S. pneumoniae and H. influenzae predominated, but the prevalence, age distribution, and antibiotic susceptibility of these species varied among geographic regions. S. pneumoniae was more likely to be isolated from patients in Eastern and Central Europe (35% of patients) than from those in Israel (21%) or the United States (26%). In contrast, the prevalence of H. influenzae was higher in Israel (27%) and the United States (26%) than in Eastern and Central Europe (11%). Finally, the prevalence of M. catarrhalis was highest in the United States (12%), while the prevalence of S. pyogenes was highest in Eastern and Central Europe (10%).
While the identity of the organisms is not surprising, our findings, in agreement with those of other investigators, suggest that resistance patterns are changing. The proportions of fully or intermediately penicillin-resistant S. pneumoniae isolates are high, ranging from 30 to 50% in recent studies (7, 12, 15, 17, 19, 31, 40), each involving 61 to 155 isolates of S. pneumoniae from middle ear fluid. Intermediately penicillin-resistant isolates predominate in some regions, such as southern Israel, where Dagan et al. (15) reported that 38% of S. pneumoniae isolates were intermediately resistant and only 4% were fully resistant. In contrast, fully resistant isolates predominate in Paris, where Gehanno et al. (19) reported that 38% of isolates were fully resistant and only 12% were intermediately resistant. Numbers of intermediately or fully penicillin-resistant S. pneumoniae isolates from middle ear fluid were similar to (15, 24, 40) or higher than (8, 9, 17, 29, 44) those associated with invasive pneumococcal infections. Of 271 isolates of S. pneumoniae tested in our study, 30% were intermediately or fully resistant to penicillin and 8% were fully resistant. Interestingly, the prevalence of intermediately and fully penicillin-resistant S. pneumoniae isolates from Central and Eastern Europe in our study matched the prevalence of fully resistant nasopharyngeal isolates from children in the same countries (4). Finally, 48% of intermediately and fully penicillin-resistant isolates were resistant to multiple antimicrobial classes in our study.
Only 10% of the isolates of S. pneumoniae were resistant to amoxicillin or amoxicillin-clavulanate in our study, with no difference in activity between the latter two agents against this species. Doern et al. (17) reported that many isolates of penicillin-resistant S. pneumoniae were susceptible to amoxicillin or amoxicillin-clavulanate; amoxicillin and amoxicillin-clavulanate were more active against penicillin-resistant S. pneumoniae isolates than was cefuroxime and nearly as active as cefotaxime and ceftriaxone (17). Furthermore, Craig and Andes (11) evaluated the ability of currently approved dosage regimens, including β-lactam agents, macrolides, and trimethoprim-sulfamethoxazole, to provide concentrations above the MIC90 for at least 40% of the dosing interval. Amoxicillin-clavulanate and ceftriaxone were considered the best available antibiotics for covering the bacterial pathogens associated with otitis media, including intermediately and fully penicillin-resistant S. pneumoniae (11).
The most frequently isolated pneumococcal serotypes in our study were 6A (24%) and 23F (21%), while serotypes 9V, 14, and 19F collectively accounted for 37%. These serotypes also predominated in other studies of intermediately or fully penicillin-resistant S. pneumoniae isolated from middle ear fluid (7, 15, 40), other sites (8, 9, 17, 24, 29, 42, 44), and nasopharyngeal carriers (4, 13, 14).
Resistance to amoxicillin was common among nonpneumococcal organisms. Thirty-one percent of the isolates of H. influenzae produced β-lactamase, including 47% of those from the United States. Similarly, 51% of the H. influenzae isolates produced β-lactamase in a recent multicenter study of middle ear isolates from the United States (31). All isolates of M. catarrhalis produced β-lactamase in our study and, similarly, in a study of patients from rural Kentucky (7).
Young age appears to be an important risk factor for isolation of a bacterial pathogen from middle ear fluid and for antibiotic resistance. In our study, bacterial isolation was highest for the youngest patients and declined progressively with increasing age. In addition, the penicillin resistance of S. pneumoniae was higher in younger patients and occurred in nearly half of those in the <12-month age category. In contrast, isolates of S. pyogenes were found primarily in older children. Although we are not aware of previous reports of an association between age and the likelihood of isolation of a bacterial pathogen, others have described an association between young age and penicillin resistance of S. pneumoniae isolated from middle ear fluid (7, 40) or from other sites (8, 9). Hypoimmunogenic serogroups, mainly groups 6, 9, 14, 19, and 23, are most often carried by infants and young children (4, 14, 18, 25), whereas other serogroups are usually carried by older patients. Because infants and young children are more frequently treated with antibiotics, the corresponding serogroups are more likely to be exposed to antibiotics and therefore may be more likely to develop resistance than other serogroups.
Our microbiologic findings may have clinical implications because the selection of antibiotic therapy for intermediately or fully penicillin-resistant S. pneumoniae infections depends on patient factors, such as site of infection, and on antibiotic factors, such as route of administration. While serious pneumococcal infections such as meningitis require treatment with high-dose parenteral cefotaxime, ceftriaxone, vancomycin, or possibly a carbapenem (18, 25), the need for these antibiotics is not clear in the case of nonmeningeal infections. In a recent study of patients with pneumococcal pneumonia from Barcelona (37), parenteral penicillin therapy produced comparable outcomes and similarly low mortality, regardless of whether the infection was caused by penicillin-resistant or -susceptible isolates. The need for parenteral agents in patients with acute otitis media is especially doubtful because of the low morbidity, high frequency of spontaneous recovery, and frequent resolution of clinical signs and symptoms, despite the persistence of bacteria in middle ear fluid (5, 6, 18). However, Gehanno et al. (19) and Dagan et al. (12) reported an increased risk of clinical and microbiologic failure when oral β-lactam antibiotics with reduced activity against penicillin-resistant S. pneumoniae (i.e., cefaclor or cefuroxime axetil) were administered. On the other hand, the clinical response to amoxicillin-clavulanate therapy was independent of penicillin susceptibility in children with pneumococcal otitis media in the clinical analysis (23) of our study. In addition, our finding of a 23% improvement in the composite susceptibility of potentially resistant pathogens after addition of clavulanate to amoxicillin suggests that this combination may be appropriate for acute otitis media, particularly in the United States, where β-lactamase-producing organisms were most prevalent.
Our findings underscore the need for additional studies to assess the clinical relevance of our findings. In the meantime, practitioners should familiarize themselves with local microbiologic surveillance data. Our findings also underscore the need for vaccines to prevent colonization with otitis media pathogens, as has been achieved with H. influenzae type b conjugate vaccines in preventing bacteremia and meningitis due to this organism. While the current pneumococcal polysaccharide vaccines are poorly immunogenic in children under 3 years of age, conjugate pneumococcal vaccines, which should include serotypes associated with resistance, should greatly reduce the spread of these organisms (13) and the occurrence of disease in infants and young children. Development of vaccines against untypeable H. influenzae and M. catarrhalis could have similar efficacy against these species, but this approach has not been addressed.
ACKNOWLEDGMENTS
This work was supported by a grant from SmithKline Beecham Pharmaceuticals, Collegeville, Pa.
We thank the following investigators for enrolling their patients: Drs. Ashkenazi, Barzilai, Dagan, and Somekh of Israel; Drs. Fekete, Pataki, Reti, and Votisky of Hungary; Drs. Kovatchev, Manasieva, Pazardjikliev, Todorov, Ulevinov, and Wrantchewa of Bulgaria; Drs. Antolikova, Hacko, Hupkova, and Kisska of Slovakia; Drs. Kominek, Navratil, and Slapak of the Czech Republic; Drs. Balanescu, Florescu, Iorgu, and Mitrea of Romania; and Drs. Aronovitz, Blatter, Block, Blumer, Fiddes, Giebink, Harrison, Hoberman, Johnson, and Schwartz of the United States. We also thank Jim Poupard, SmithKline Beecham Pharmaceuticals, for study design; Pierre Geslin, Service de Microbiologie, Centre Hospitalier Intercommunal, Creteil, France, for coordinating transport of isolates from Europe and Israel; Saralee Bajaksouzian, Department of Pathology, Case Western Reserve University, Cleveland, Ohio, for technical assistance with identification and susceptibility testing of bacterial isolates; Sara M. Debanne and Grace W. Wu, Department of Epidemiology and Biostatistics, Case Western Reserve University, for statistical analysis; and Cindy W. Hamilton, Virginia Beach, Va., for editorial assistance.
REFERENCES
- 1.Appelbaum, P. C. 1994. Antibiotic-resistant pneumococci—facts and fiction. J. Chemother. 6(Suppl. 4):7–15. [PubMed]
- 2.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum P C, Bhamjee A, Scragg J N, Hallett A F, Bowen A J, Cooper R C. Streptococcus pneumoniae resistant to penicillin and chloramphenicol. Lancet. 1977;ii:995–997. doi: 10.1016/s0140-6736(77)92892-6. [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum P C, Gladkova C, Hryniewicz W, Kojouharov B, Kotulova D, Mihalcu F, Schindler J, Setchanova L, Semina N, Trupl J, Tyski S, Urbaskova P, Jacobs M R. Carriage of antibiotic-resistant Streptococcus pneumoniae by children in Eastern and Central Europe—a multicenter study with use of standardized methods. Clin Infect Dis. 1996;23:712–717. doi: 10.1093/clinids/23.4.712. [DOI] [PubMed] [Google Scholar]
- 5.Berman S. Otitis media in children. N Engl J Med. 1995;332:1560–1565. doi: 10.1056/NEJM199506083322307. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein J M. Otitis media with effusion: to treat or not to treat? J Respir Dis. 1995;16:88–99. [Google Scholar]
- 7.Block S L, Harrison C J, Hedrick J A, Tyler R D, Smith R A, Keegan E, Chartrand S A. Penicillin-resistant Streptococcus pneumoniae in acute otitis media: risk factors, susceptibility patterns and antimicrobial management. Pediatr Infect Dis J. 1995;14:751–759. doi: 10.1097/00006454-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Breiman R F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 9.Butler J C, Hofmann J, Cetron M S, Elliott J A, Facklam R R, Brieman R F The Pneumococcal Sentinel Surveillance Working Group. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention’s Pneumococcal Sentinel Surveillance System. J Infect Dis. 1996;174:986–993. doi: 10.1093/infdis/174.5.986. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control. Drug-resistant Streptococcus pneumoniae—Kentucky and Tennessee, 1993. Morbid Mortal Weekly Rep. 1994;43:23–25. , 31. [PubMed] [Google Scholar]
- 11.Craig W A, Andes D. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J. 1996;15:255–259. doi: 10.1097/00006454-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Dagan R, Abramson O, Leibovitz E, Lang R, Goshen S, Greenberg D, Yagupsky P, Leiberman A, Fliss D M. Impaired bacteriologic response to oral cephalosporins in acute otitis media caused by pneumococci with intermediate resistance to penicillin. Pediatr Infect Dis J. 1996;15:980–985. doi: 10.1097/00006454-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Dagan R, Melamed R, Muallem M, Piglansky L, Greenberg D, Abramson O, Mendelman P M, Bohidar N, Yagupsky P. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996;174:1271–1278. doi: 10.1093/infdis/174.6.1271. [DOI] [PubMed] [Google Scholar]
- 14.Dagan R, Melamed R, Muallem M, Piglansky L, Yagupsky P. Nasopharyngeal colonization in southern Israel with antibiotic-resistant pneumococci during the first 2 years of life: relation to serotypes likely to be included in pneumococcal conjugate vaccines. J Infect Dis. 1996;174:1352–1355. doi: 10.1093/infdis/174.6.1352. [DOI] [PubMed] [Google Scholar]
- 15.Dagan R, Yagupsky P, Goldbart A, Wasas A, Klugman K. Increasing prevalence of penicillin-resistant pneumococcal infections in children in southern Israel: implications for future immunization policies. Pediatr Infect Dis J. 1994;13:782–786. doi: 10.1097/00006454-199409000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Debanne S M, Rowland D Y. Proceedings of the Business and Economics Section. Washington, D.C: American Statistical Association; 1983. The conservative chi-square test for sparse data; pp. 705–708. [Google Scholar]
- 17.Doern G V, Bruggemann A, Holley H P, Jr, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedland I R, McCracken G H., Jr Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 19.Gehanno P, Lenoir G, Berche P. In vivo correlates for Streptococcus pneumoniae penicillin resistance in acute otitis media. Antimicrob Agents Chemother. 1995;39:271–272. doi: 10.1128/aac.39.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giebink G S. Otitis media update: pathogenesis and treatment. Ann Otol Rhinol Laryngol. 1992;101:21–23. doi: 10.1177/00034894921010s105. [DOI] [PubMed] [Google Scholar]
- 21.Hansman D, Devitt L, Miles H, Riley I. Pneumococci relatively insensitive to penicillin in Australia and New Guinea. Med J Aust. 1974;2:353–356. doi: 10.5694/j.1326-5377.1974.tb70836.x. [DOI] [PubMed] [Google Scholar]
- 22.Hansman D, Glasgow H, Sturt J, Devitt L, Douglas R. Increased resistance to penicillin of pneumococci isolated from man. N Engl J Med. 1971;284:175–177. doi: 10.1056/NEJM197101282840403. [DOI] [PubMed] [Google Scholar]
- 23.Hoberman A, Paradise J L, Block S, Burch D J, Jacobs M R, Balanescu M I. Efficacy of amoxicillin/clavulanate potassium for acute otitis media: relation to Streptococcus pneumoniae susceptibility. Pediatr Infect Dis J. 1996;15:955–962. doi: 10.1097/00006454-199610000-00034. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann J, Cetron M S, Farley M M, Baughman W S, Facklam R R, Elliott J A, Deaver K A, Breiman R F. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N Engl J Med. 1995;333:481–486. doi: 10.1056/NEJM199508243330803. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs M R. Treatment and diagnosis of infections caused by drug-resistant Streptococcus pneumoniae. Clin Infect Dis. 1992;15:119–127. doi: 10.1093/clinids/15.1.119. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs M R, Bajaksouzian S, Burch D J, Poupard J, Appelbaum P C. Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Antimicrobial susceptibility of Streptococcus pneumoniae isolated from patients with acute otitis media in Eastern Europe, Israel and U.S., poster E29. [Google Scholar]
- 27.Jacobs M R, Burch D, Poupard J, Moonsammy G, Debanne S, Appelbaum P The International Otitis Media Study Group. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Microbiology of acute otitis media—results of an international study of 917 patients, abstr. K26; p. 254. [Google Scholar]
- 28.Jacobs M R, Koornhof H J, Robins-Browne R M, Stevenson C M, Vermaak Z A, Freiman I, Miller G B, Witcomb M A, Isaäcson M, Ward J I, Austrian R. Emergence of multiply resistant pneumococci. N Engl J Med. 1978;299:735–740. doi: 10.1056/NEJM197810052991402. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan S L The U.S. Pediatric Multicenter Pneumococcal Surveillance Group. One-year surveillance of systemic pneumococcal infections in children. Pediatr Res. 1995;37:179A. [Google Scholar]
- 30.Kristinsson K G, Hjalmarsdottir M A, Steingrimsson O. Increasing penicillin resistance in pneumococci in Iceland. Lancet. 1992;339:1606–1607. doi: 10.1016/0140-6736(92)91868-9. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 31.McLinn S, Williams D. Incidence of antibiotic-resistant Streptococcus pneumoniae and beta-lactamase-positive Haemophilus influenzae in clinical isolates from patients with otitis media. Pediatr Infect Dis J. 1996;15:S3–S9. doi: 10.1097/00006454-199609009-00001. [DOI] [PubMed] [Google Scholar]
- 32.Munoz R, Coffey T J, Daniels M, Dowson C G, Laible G, Casal J, Hakenbeck R, Jacobs M, Musser J M, Spratt B G, et al. Intercontinental spread of a multiresistant clone of serotype 23F Streptococcus pneumoniae. J Infect Dis. 1991;164:302–306. doi: 10.1093/infdis/164.2.302. [DOI] [PubMed] [Google Scholar]
- 33.Murray B E. Can antibiotic resistance be controlled? N Engl J Med. 1994;330:1229–1230. doi: 10.1056/NEJM199404283301710. [DOI] [PubMed] [Google Scholar]
- 34.Murray P R, Barron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. [Google Scholar]
- 35.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 36.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility tests, sixth informational supplement (M 100-S6). Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 37.Pallares R, Liñares J, Vadillo M, Cabellos C, Manresa F, Viladrich P F, Martin R, Gudiol F. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474–480. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 38.Paradise J L. Treatment guidelines for otitis media: the need for breadth and flexibility. Pediatr Infect Dis J. 1995;14:429–435. doi: 10.1097/00006454-199505001-00005. [DOI] [PubMed] [Google Scholar]
- 39.Reichler M R, Allphin A A, Breiman R F, Schreiber J R, Arnold J E, McDougal L K, Facklam R R, Boxerbaum B, May D, Walton R O, Jacobs M R. The spread of multiply resistant Streptococcus pneumoniae at a day care center in Ohio. J Infect Dis. 1992;166:1346–1353. doi: 10.1093/infdis/166.6.1346. [DOI] [PubMed] [Google Scholar]
- 40.Reichler M R, Rakovsky J, Sobotová A, Sláčiková M, Hlaváčová B, Hill B, Krajčíková L, Tarina P, Facklam R R, Breiman R F. Multiple antimicrobial resistance of pneumococci in children with otitis media, bacteremia, and meningitis in Slovakia. J Infect Dis. 1995;171:1491–1496. doi: 10.1093/infdis/171.6.1491. [DOI] [PubMed] [Google Scholar]
- 41.SAS Institute, Inc. SAS/STAT users’ guide, version 6. 4th ed. Cary, N.C: SAS Institute, Inc.; 1989. [Google Scholar]
- 42.Scott J A G, Hall A J, Dagan R, Dixon J M S, Eykyn S J, Fenoll A, Hortal M, Jetté L P, Jorgensen J H, Lamothe F, Latorre C, Macfarlane J T, Shlaes D M, Smart L E, Taunay A. Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7,000 episodes of invasive disease. Clin Infect Dis. 1996;22:973–981. doi: 10.1093/clinids/22.6.973. [DOI] [PubMed] [Google Scholar]
- 43.Stephenson J. Icelandic researchers are showing the way to bring down rates of antibiotic-resistant bacteria. JAMA. 1996;275:175. [PubMed] [Google Scholar]
- 44.Welby P L, Keller D S, Cromien J L, Tebas P, Storch G A. Resistance to penicillin and non-beta-lactam antibiotics of Streptococcus pneumoniae at a children’s hospital. Pediatr Infect Dis J. 1994;13:281–287. doi: 10.1097/00006454-199404000-00007. [DOI] [PubMed] [Google Scholar]