Abstract
Esophagitis due to herpes simplex virus (HSV) infection is a rare entity in the immunocompetent population. It is usually seen in immunocompromised hosts, those with human immunodeficiency virus (HIV) infection, malignancies, and patients on immunosuppressive medications.
We present a case of a young immunocompetent man with anabolic steroid use who presented with esophagitis symptoms found to be from HSV infection. So far, the use of corticosteroids has been reported as a predisposing factor for HSV esophagitis in immunocompetent hosts in multiple case reports. However, our case suspects that transient immunosuppression with similar medication can cause HSV esophagitis in otherwise immunocompetent hosts.
Keywords: rare, anabolic steroid, immunocompetent, esophagitis, herpes simplex virus
Introduction
Esophagitis from the herpes simplex virus (HSV) is well-known and a potential complication in immunosuppressed populations. However, only a few cases have been reported in otherwise healthy and immunocompetent patients [1-4]. When seen in immunocompetent patients, HSV esophagitis is typically reported in males in their 30s [5]. Due to transient immunosuppression, immunocompetent patients may be susceptible to conditions typically seen in immunosuppressed patients. While anabolic steroids have been linked to immunosuppression [6], their association with HSV esophagitis has not been established. Herein, we present an interesting and rare case of HSV esophagitis in an immunocompetent host who reported intermittently using non-prescription anabolic steroids.
Case presentation
A 27-year-old male with no past medical history presented to the hospital complaining of burning pain extending from the throat to the epigastric region associated with nausea, bilious emesis with streaks of bright red blood, and odynophagia for five days. The patient mentioned experiencing generalized body aches but did not report any other symptoms, such as cough, diarrhea, constipation, chest pain, palpitation, back pain, headache, dizziness, or recent episodes of fever. He had been taking over-the-counter calcium carbonate regularly without significant relief. He did not have any previous esophagogastroduodenoscopy (EGD) or colonoscopy. He reported intermittent use of anabolic steroids (last use being two weeks before presentation) and occasionally smoking marijuana (last use being three weeks before presentation). He had been self-administering testosterone, which he procured from outside the country without a medical prescription. Based on his online search, he elected to inject himself with a dosage of up to 150 mg weekly. Computed tomography (CT) of the abdomen and pelvis with contrast was negative for any acute processes. The patient underwent EGD, which showed many cratered, punched-out esophageal ulcers with no active bleeding or stigmata of recent bleeding at 35-43 cm from the incisors (Figure 1).
Figure 1. Esophagogastroduodenoscopy (EGD) shows ulcerative esophagitis in the lower third of the esophagus.
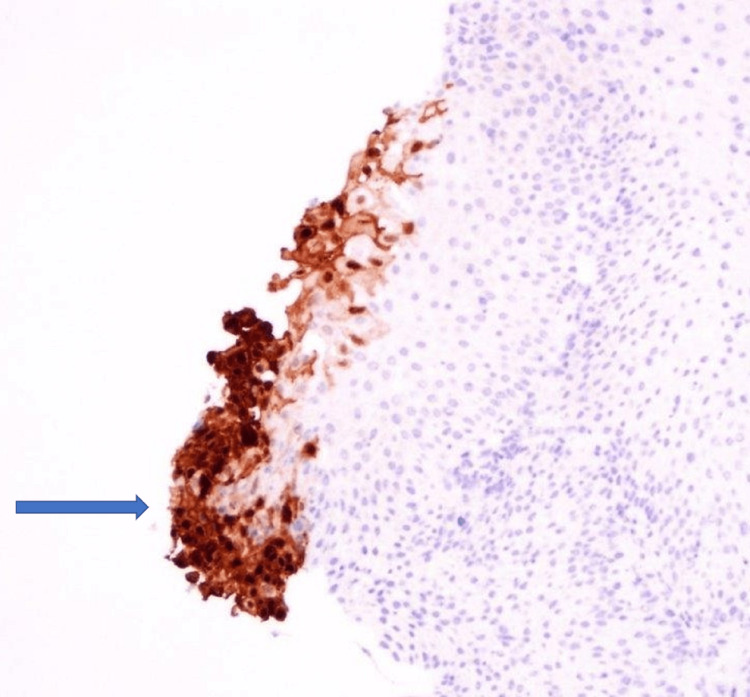
Biopsies were taken with cold forceps for histology and HSV and Epstein-Barr virus (EBV) staining. Herpetic esophagitis with ulcer was confirmed with immunostaining (Figures 2A, 2B, 3).
Figure 2. Squamous epithelium shows herpes simplex virus (HSV) infection consisting of multinucleation, nuclear molding, and margination (A and B).
Figure 3. Immunostaining for the herpes simplex virus (HSV) highlights positive cells.
Typical cytomegalovirus (CMV) and periodic acid-Schiff (PAS) stains were negative. Serology testing was initially negative for HSV-1 immunoglobin M (IgM)/IgG, but two months later, IgG was positive. Hepatitis B, hepatitis C, chlamydia, gonorrhea, syphilis, HSV-2 IgM/IgG, and HIV were negative. Ceruloplasmin and alpha-1 antitrypsin (AAT) were within normal limits, and antinuclear antibodies (ANA) and anti-mitochondrial antibodies (AMA) were negative. The patient was treated with pantoprazole, sucralfate for eight weeks, and acyclovir for 10 days. At the three-month follow-up in the gastroenterology clinic, the patient reported a resolution of symptoms, including odynophagia.
Discussion
Herpes simplex virus esophagitis is frequently described in immunocompromised hosts and rarely in immunocompetent patients. It can occur as a primary infection or reactivation and is characterized by acute-onset systemic manifestations and extensive erosive-ulcerative involvement of the mid-distal esophagus [1]. The mean age of incidence is 35 years (with male predominance) [1,5]. Most cases of HSV esophagitis are due to HSV-1, but a few case reports of HSV-2 esophagitis have been described [5,7]. Patients usually have a prodromal systemic manifestation of fever, nausea, or vomiting before manifesting esophageal symptoms. The most common symptoms are odynophagia, dysphagia, heartburn, epigastric pain, or chest pain. Concurrent oropharyngeal lesions are uncommon in immunocompetent hosts, but immunocompromised hosts usually have co-existent herpes labialis or oropharyngeal ulcers [1,8,9]. In our case, an immunocompetent male presented with generalized body aches, epigastric pain, nausea, vomiting, and odynophagia with no fever before or during hospitalization.
The diagnosis of HSV esophagitis is usually based on endoscopic findings confirmed by histopathological examination. Endoscopic findings include friable mucosa with numerous well-circumscribed ulcers smaller than 2 cm, most commonly in the distal esophagus [1]. The margin of an ulcer, where viral cytopathic activity is most likely to be noticed, should be the location of biopsies. Multinucleated giant cells with ground-glass nuclei and eosinophilic inclusions are examples of histologic findings. Additionally helpful may be an immunohistochemical analysis for HSV glycoproteins [1,8]. Serology usually shows an acute infection pattern (positive IgM antibody and negative IgG antibody) for HSV type 1 (more frequently) or HSV type 2 infection, with possible seroconversion up to three to four weeks later [1]. In our case, the patient underwent EGD, showing characteristic ulcers in the distal esophagus. Histopathology, as well as immunostaining, were diagnostic of HSV. The patient’s serology was initially negative for HSV-1 IgM and IgG, but he later seroconverted. Testing for sexually transmitted infections, including HIV, was negative.
In immunocompetent patients, spontaneous remission typically happens within one to two weeks. However, some patients may respond more quickly to a brief course of oral acyclovir 200 mg five times per day or 400 mg three times per day for seven to 10 days [10]. To date, no trials have been done for using acyclovir in HSV esophagitis in immunocompetent patients, but prompt symptom resolution has been reported [11]. Our patient was treated with acyclovir along with proton pump inhibitors and sucralfate.
Multiple case reports have been published linking the use of corticosteroids with the development of HSV esophagitis, even in immunocompetent patients [12-14]. This may indicate that even in patients with intact immunity, transient immunosuppression can predispose them to HSV infection of the esophagus. However, no reports have been published about HSV esophagitis in those using anabolic steroids [6].
Conclusions
We present a case of a young male with anabolic steroid use who complained of odynophagia. Upon conducting an endoscopy, we discovered ulcers in the lower esophagus caused by the herpes simplex virus. Given that herpes simplex virus esophagitis is usually seen in immunocompromised patients, common causes of immunosuppression, including HIV, were ruled out. Although there are no prior reports of this condition being linked to anabolic steroid use, we suspect that the patient's use of these steroids may have made him more vulnerable to the virus. While the causation cannot be established, it would be prudent for physicians to be mindful of anabolic steroid use as a potential cause of pathologies typically seen in immunocompromised patients. Additionally, it emphasizes the importance of reporting future cases of patients who use anabolic steroids and exhibit conditions typically found in immunosuppressed patients.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Herpes simplex virus esophagitis in the immunocompetent host: an overview. Ramanathan J, Rammouni M, Baran J Jr, Khatib R. https://pubmed.ncbi.nlm.nih.gov/11007213/ Am J Gastroenterol. 2000;95:2171–2176. doi: 10.1111/j.1572-0241.2000.02299.x. [DOI] [PubMed] [Google Scholar]
- 2.HSV esophagitis in an immunocompetent 17-year-old. Rajasekaran V, Rahaman V, Hou X, Steele RW. Glob Pediatr Health. 2021;8:2333794. doi: 10.1177/2333794X211052914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herpes simplex esophagitis in immunocompetent patients: a rare but easily managed condition. Ullah W, Abdullah HM, Amjad W, Hussain Q, Saeed J, Hurairah A. https://pubmed.ncbi.nlm.nih.gov/31465638/ S D Med. 2019;72:344–347. [PubMed] [Google Scholar]
- 4.Herpes simplex esophagitis in the immunocompetent host. Kato S, Yamamoto R, Yoshimitsu S, Shimazaki K, Ogawa S, Itoh K, Miura S. Dis Esophagus. 2005;18:340–344. doi: 10.1111/j.1442-2050.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 5.Herpes esophagitis in healthy adults and adolescents: report of 3 cases and review of the literature. Canalejo E, García Durán F, Cabello N, García Martínez J. Medicine (Baltimore) 2010;89:204–210. doi: 10.1097/MD.0b013e3181e949ed. [DOI] [PubMed] [Google Scholar]
- 6.Anabolic androgenic steroids effects on the immune system: A review. Marshall-Gradisnik S, Green R, Brenu EW, Weatherby RP. Cent Eur J Biol. 2009;4:19–33. [Google Scholar]
- 7.Herpes simplex virus-2 esophagitis in a young immunocompetent adult. Kadayakkara DK, Candelaria A, Kwak YE, Loeser C. Case Rep Gastrointest Med. 2016;2016:7603484. doi: 10.1155/2016/7603484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esophageal infections: risk factors, presentation, diagnosis, and treatment. Baehr PH, McDonald GB. Gastroenterology. 1994;106:509–532. doi: 10.1016/0016-5085(94)90613-0. [DOI] [PubMed] [Google Scholar]
- 9.Herpetic esophagitis in immunocompentent host: cases report. Diezma-Martín AM, Gigante-Miravalles E, Castro Limo JD, Quimbayo Arcila CA, Puche Paniagua JJ. BMC Infect Dis. 2020;20:605. doi: 10.1186/s12879-020-05328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etiology, diagnosis and treatment of infectious esophagitis. Rosołowski M, Kierzkiewicz M. Prz Gastroenterol. 2013;8:333–337. doi: 10.5114/pg.2013.39914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Infections of the esophagus: an update on risk factors, diagnosis, and management. Hoversten P, Kamboj AK, Katzka DA. Dis Esophagus. 2018;31 doi: 10.1093/dote/doy094. [DOI] [PubMed] [Google Scholar]
- 12.Herpes simplex virus esophagitis in the setting of acute use of corticosteroids for COPD exacerbation in an immunocompetent middle-aged woman. Haider MB, Basida B, Bapatla A, Ismail R, Hafeez W. Case Rep Gastroenterol. 2021;15:616–620. doi: 10.1159/000515413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A difficult case to swallow: herpes esophagitis after epidural steroid injection. Davis K, Prater A, Fluker SA, Klein R. Am J Ther. 2014;21:0–14. doi: 10.1097/MJT.0b013e318220500f. [DOI] [PubMed] [Google Scholar]
- 14.A case of herpes esophagitis after fluticasone propionate for eosinophilic esophagitis. Lindberg GM, Van Eldik R, Saboorian MH. Nat Clin Pract Gastroenterol Hepatol. 2008;5:527–530. doi: 10.1038/ncpgasthep1225. [DOI] [PubMed] [Google Scholar]