Abstract
Few chemotherapeutic agents are available for the medical management of hydatid disease caused by the parasite Echinococcus granulosus. In order to test the potential of oxfendazole for the treatment of infection with this parasite, nine infected goats and four sheep were given oxfendazole twice weekly at a dose of 30 mg/kg of body weight for 4 weeks and monitored by ultrasound for an additional 4 weeks. Efficacy was finally evaluated by postmortem examination, including determination of protoscolex viability and cyst wall histology. In treated animals, protoscolices were dead or absent in 97% of cysts from oxfendazole-treated animals compared to 28% of cysts from untreated control animals. On postmortem examination, 53% of cysts from treated animals were found to be grossly degenerate. A sample of those cysts that appeared potentially viable all demonstrated evidence of severe damage to the cyst wall. By light microscopy, cysts showed severe disorganization of the adventitial layer with invasion of inflammatory cells and in some cases frank necrosis with no apparent adventitial layer. The follow-up period for assessment of the drug’s ability to cause complete degeneration and resorption of cysts was relatively short. This study, however, indicates that oxfendazole is at least as effective as and is easier to administer than albendazole for the treatment of hydatid disease.
Many tapeworms alternate their developmental cycle between intestinal stages in one host and tissue stages in another. Hydatid disease is the result of tissue invasion with the intermediate stage of a dog tapeworm, Echinococcus granulosus. The adult stage is a largely innocuous small tapeworm of dogs and other canids. The invasive intermediate stage (metacestode) takes the form of an enlarging cyst primarily in the liver and lungs of domestic and wild herd animals. These cysts may be found singly, in clusters, or in such numbers that they pack the peritoneal cavity. The principal sources of morbidity are pressure effects from cyst size (up to 48 liters), location in a sensitive organ (brain, reproductive tract, bone), or cyst rupture with subsequent anaphylaxis or dissemination of the infection (1). The disease can be found in any part of the world where slaughtering practices allow dogs to consume the organs of infected animals. The parasite can then complete its developmental cycle. There is also a sylvatic cycle for E. granulosus, in which transmission occurs between the wolf and wild ungulates in North America and Eurasia. Humans residing in sheep- and goatherding regions of the world can also develop these cysts. In parts of Kenya, China, and South America cysts are present in 4 to 10% of the population (4, 14, 21). Sporadic human cases occur in the western United States, Alaska, and Canada. An autopsy survey in Alaska indicated that 1% of natives had hydatid cysts (3). Until 1972 the prevalence of hydatid disease for sheep in Utah was 6 to 13%, but by 1984 it was less than 0.1% (2).
The structure of the hydatid cyst influences both the surgical and chemical approaches to therapy. The cyst is encased in a tough adventitial (fibrous) layer produced by the host. Within this capsule, the parasite produces a 50-mm-thick acellular complex of mucopolysaccharide and proteins that is lined by a single layer of germinal cells, the endocyst. These cells differentiate into immature parasites (protoscolices) that remain attached to the germinal layer or that settle in the cyst fluid. Protoscolices are capable of forming new cysts if spilled in the intermediate host following cyst rupture or during surgery. The cyst is filled with hypotonic fluid containing both host and parasite proteins. The cyst wall, therefore, must be capable of active transport and selective absorption and excretion (11). Drugs may have to penetrate all three layers of the cyst in order to kill the living parasite tissues.
For treatment of human hydatid disease, the best agent available is the benzimidazole albendazole. Most studies indicate that the efficacy of albendazole as measured by the disappearance of a cyst is generally less than 30% under ideal circumstances. Altogether, 60% of cysts show some response in the course of therapy, including shrinkage in size or detachment of cyst components from the wall. Albendazole must be taken daily for 4 to 6 weeks, and this course should be repeated an additional two or three times. The poor response of this infection to most chemotherapeutic agents has made hydatidosis primarily a surgical disease, and thus the role of chemotherapy is for prophylaxis against spillage during surgery, for the treatment of inoperable cases, or for use in areas without adequate surgical facilities. There is clearly a need for drugs that are more effective and easier to administer.
Oxfendazole like albendazole is a benzimidazole used in veterinary medicine to control nematode infections. Oxfendazole and albendazole are similar in their antimicrobial spectra, but oxfendazole has a much longer half-life (15, 16). In addition, oxfendazole, unlike albendazole, is effective against the intestinal stage of E. granulosus as well as other cestodes in the gastrointestinal tract (7) and thus could be used to treat infection in dogs, the principal reservoir for human infection. Only one study has examined the effect of the drug on the tissue stage of tapeworm infections. Gonzales et al. showed that a single dose of 30 mg/kg of body weight of oxfendazole in pigs completely eliminated all tissue cysts of Taenia solium, an important human tapeworm (10). Though the hydatid cyst is much larger than and structurally different from the cyst of cysticercosis, this result prompted a trial of oxfendazole for the treatment of hydatid disease.
MATERIALS AND METHODS
Animals and treatment.
In order to obtain infected animals, goats and sheep were examined by ultrasound at an abattoir near the city of Nairobi. Animals found to have cysts that appeared typical of those of E. granulosus in the liver or abdomen were taken to Nairobi University of Veterinary Medicine. There they were quarantined for 1 week and dewormed with a single 130-mg dose of levamisole, a drug that does not affect tapeworms (12). The animals were then assigned serial numbers and randomized to the treatment or control group.
The treatment group received oxfendazole at 30 mg/kg twice weekly for 4 weeks. All animals were fed and watered ad libitum. Eight weeks after the start of treatment the animals were euthanized, and the lung, liver, and abdominal cavity were inspected. All visible cysts of the sheep parasite Taenia hydatigena were examined, and cysts of E. granulosus were dissected and aspirated, and a portion were fixed in formalin.
Ultrasound evaluation.
Goats were preferred for examination and the study, since sheep were difficult to shear down to the skin for an adequate ultrasound examination. Prior to treatment, views of the right lung, right lobe of the liver, and whole abdomen were obtained as described previously (18). The ultrasound examination was repeated 2, 4, and 8 weeks following the initial dose of oxfendazole. Cysts were measured at their greatest diameter and observed for typical signs of degeneration, i.e., decreased size, increased echogenicity, detachment of the endocyst, and collapse (6). The animals’ treatment status was unknown to the ultrasonographer. In order to estimate how closely the ultrasound appearance reflected actual size, an index of cyst size (maximum height times maximum width) was used for comparison.
Pathology, histology, and viability assays.
A standard postmortem examination was performed. Specifically, lungs, liver, abdominal cavity, kidneys, and spleen were visually inspected and dissected. All surface cysts were dissected intact, and the fluid was removed by needle aspiration. Portions of liver cysts not obviously degenerating were fixed in formalin and stained with hematoxylin and eosin. The viabilities of E. granulosus and T. hydatigena protoscolices were determined by eosin exclusion and observation for flame cell movement (22).
Data analysis.
Data were normalized by log transformation, and mean values and standard deviations were calculated and compared by Student’s t test for paired data. Qualitative variables were compared by Fisher’s exact test for chi-square values. Correlation coefficients comparing the size indices of ultrasound measurements with those of postmortem measurements were calculated with the computer program Cricket Graph (5). A P value of ≤0.05 was used to determine statistical significance.
RESULTS
Clinical response.
Approximately 200 goats and 20 sheep were scanned by ultrasound, and 12 goats and 5 sheep were entered into the study. The majority of the animals offered were in poor condition, since owners in this region were more likely to bring sickly animals for slaughter. All of the sheep and 30% of the goats had pneumonia and were treated with tetracycline at 20 mg/kg for 4 days. One goat died of pneumonia 2 weeks into the study, and autopsy revealed overwhelming pyogenic infection; this animal was not included in the analysis. One animal in the treatment group died 3 weeks after the final dose of oxfendazole, and two control animals (goat 3 and goat 6) died of pneumonia 2 weeks after the final dosing of the treatment group. Postmortem on these animals was performed within 4 h of their deaths, and the results are included in the study. No complications could be directly attributed to oxfendazole therapy. All animals gained weight, and no differences in weight gain were observed between the groups.
Ultrasound evaluation.
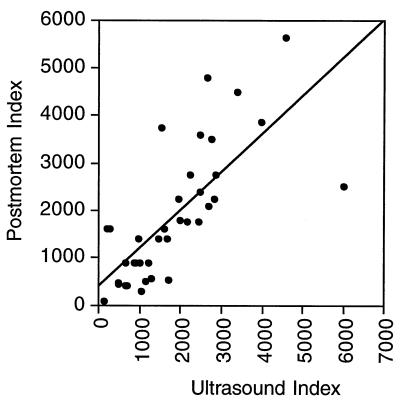
Ultrasound examination was confined to the right sides of the animals and the abdomens. The lungs were not specifically examined, since ultrasound has poor sensitivity for lung cysts due to the presence of air in this organ. Hair is not completely removed from animals by shaving; thus, the limit of resolution of ultrasound for cysts in animals is approximately 50 mm in any dimension (25) compared to 20 mm in humans. Ultrasound identified 92% (36 of 39) of cysts >50 by 50 mm in size that were located in the right lobe of the liver. There was a 10% false-positive rate due to two calcified nonviable cysts in a control animal and two cysts of T. hydatigena in infected animals. The difference in the size indices (maximum height times maximum width) of cysts found by ultrasound compared to postmortem indices was not statistically significant (1,797 ± 1,269 and 1,842 ± 1,387, respectively). Differences between the ultrasound and postmortem measurements ranged from a sevenfold underestimate to a threefold overestimate. The correlation coefficient of the two measurements was 0.736 (Fig. 1).
FIG. 1.
Comparison of ultrasound with postmortem cyst measurements. Ultrasound measurements were made 1 week prior to postmortem. The size index is the maximum width times the maximum length, with both length and width expressed in millimeters.
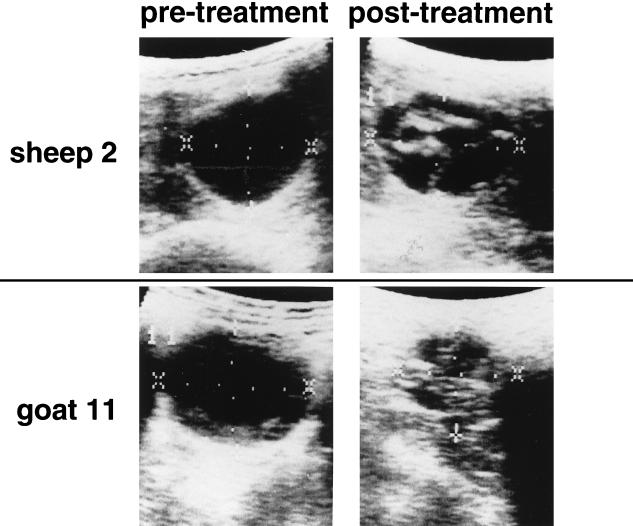
In animals receiving oxfendazole, 47% showed some change indicative of decreased cyst viability in only 2 months. Three cysts decreased in size, while the others showed increased echogenicity, mixed echogenicity, complete or partial detachment, or new calcification (Fig. 2). There were no changes in identifiable cysts of control animals.
FIG. 2.
Ultrasound changes in hydatid cysts in liver tissue before and after 4 weeks of oxfendazole therapy. Cyst sizes for sheep 2: pretreatment, 30 by 58 mm; posttreatment, 32 by 46 mm. Cyst sizes for goat 11: pretreatment, 38 by 49 mm; posttreatment, 34 by 37 mm. Collapsed endocyst is present in both cysts following treatment.
Protoscolex and cyst viabilities.
On postmortem a total of 93 E. granulosus cysts from 16 animals were identified. The five sheep harbored 52% of these (Table 1), consistent with the reported higher prevalence in this species due to their grazing closer to the ground than goats (13, 18). By dye exclusion and flame cell motility criteria, viable protoscolices were present in 72% (13 of 18) of cysts from control animals and 3% (2 of 75) of cysts from treated animals (P < 0.000001; two-tailed Fisher’s exact test). In treated animals, 35 cysts were found to be degenerate or calcified by visual inspection. The remaining 40 possessed normal-appearing membranes attached to the cyst wall. Only 2 of the 40 viable-appearing cysts from treated animals, however, contained living protoscolices (5%) compared to 13 of 14 (93%) apparently viable cysts in the control animals (Table 2). None of the six T. hydatigena cysts found in treated animals contained living organisms. Calcifications were found at postmortem in 16% of the animals receiving treatment and 22% of control animals.
TABLE 1.
Postmortem results from treated animals
| Animal | Total no. of cysts | Viable protoscolicesa | No. of cysts that wereb:
|
||
|---|---|---|---|---|---|
| Intact | Degenerate | Calcified | |||
| Goat 1 | 3 | 0 | 0 | 0 | 3 |
| Goat 2 | 5 | 0 | 1 | 3 | 1 |
| Goat 4c | 1 | 0 | 0 | 1 | 0 |
| Goat 5 | 5 | 0 | 0 | 3 | 2 |
| Goat 7 | 3 | 0 | 0 | 3 | 0 |
| Goat 8 | 9 | 0 | 8 | 0 | 1 |
| Goat 10 | 1 | 0 | 0 | 1 | 0 |
| Goat 11c | |||||
| Goat 14 | 3 | 0 | 1 | 1 | 1 |
| Sheep 2 | 4 | 0 | 3 | 1 | 0 |
| Sheep 4 | 22 | 1 | 14 | 5 | 3 |
| Sheep 5 | 5 | 0 | 3 | 2 | 0 |
| Sheep 6 | 14 | 1 | 10 | 4 | 0 |
| Total | 75 | 2 | 40 | 24 | 11 |
Number of cysts found with at least one viable protoscolex.
Intact, clear fluid with membranes attached to cyst wall; degenerate, turbid yellow fluid with collapsed membranes; calcified, chalky deposits in cyst wall.
Cysts of T. hydatigena were found, and all were dead as determined by dye exclusion and mobility assays. No cysts of E. granulosus were seen.
TABLE 2.
Postmortem results from control animals
| Animal | Total no. of cysts | Viable protoscolicesa | No. of cysts that wereb:
|
||
|---|---|---|---|---|---|
| Intact | Degenerate | Calcified | |||
| Goat 3 | 9 | 7 | 8 | 0 | 1 |
| Goat 6 | 3 | 3 | 3 | 0 | 0 |
| Goat 9 | 3 | 0 | 0 | 0 | 3 |
| Sheep 3 | 3 | 3 | 3 | 0 | 0 |
| Total | 18 | 13 | 14 | 0 | 4 |
Number of cysts found with at least one viable protoscolex.
Intact, clear fluid with membranes attached to cyst wall; degenerate, turbid yellow fluid with collapsed membranes; calcified, chalky deposits in cyst wall.
Cyst histopathology.
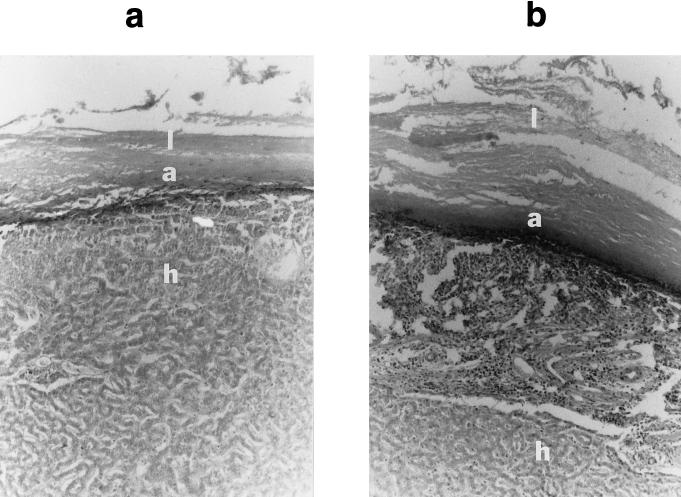
In the treatment group, 47% of cysts (35 of 75 cysts) identified on postmortem appeared grossly intact and potentially viable. To determine whether the viable-appearing cysts were in some way affected by oxfendazole treatment, sections from nine of them were examined histologically. All showed evidence of marked host cellular reaction consisting of infiltration of the adventitial layer with neutrophils, eosinophils, and plasma cells (Fig. 3). In addition to this inflammatory infiltrate, the new space between the liver tissue and cyst wall contained disorganized fibroblasts and mesenchymal cells. In the most necrotic areas, the laminate layer could not be collected together with adherent liver tissue and the adventitial layer appeared completely degenerate and was replaced by acute inflammatory cells (data not shown).
FIG. 3.
Histology of cysts from treated (a) and untreated animals (b). l, laminate layer; a, adventitial layer; h, liver tissue. Cyst lumen is at the top of the figure.
DISCUSSION
Oxfendazole and albendazole cannot be directly compared for efficacy in the treatment of hydatid disease based on the two existing studies with animals or on studies comparing humans and ruminants. The two drugs would have to be compared simultaneously by the same investigators or would have to be administered to humans. This preliminary trial of oxfendazole administered twice weekly, however, produced results similar to those of albendazole given once a day. Protoscolices in 97% of cysts from treated animals were killed. Further, gross or microscopic examination of a subsample of cysts (also capable of asexual reproduction and regeneration) showed that all were damaged. Oxfendazole given at a lower frequency than albendazole virtually eliminated all protoscolices in both lung and liver cysts. The cysts and protoscolices of a related parasite of sheep, T. hydatigena, also were killed in oxfendazole-treated animals. Though there were none of these parasites in control animals, the authors have never observed spontaneous degeneration of T. hydatigena, unlike E. granulosus. Oxfendazole, therefore, possesses significant activity against the intermediate stage of many species of tapeworms.
Interpretation of these results is somewhat complicated, however, by the variable presentation and course of natural hydatid disease. Although calcification is one way in which some cysts resolve spontaneously, death of cysts following chemotherapy may also produce calcification. Some spontaneously calcified cysts will, however, still have viable protoscolices. It is likely that some calcified cysts in both the treatment and control groups were already “dead.” If this were the case, the major effect on the results would be to underestimate the efficacy of treatment, since a larger percentage of cysts was calcified in the control group. Another consideration is that maturation and development of protoscolices is highly variable, and they may take years to emerge (8). In cysts with no evidence of protoscolices, these forms of the parasite may have been resorbed or were not present at the start of therapy. The histologic examination of a sample of cyst walls indicates that probably all suffered some damage. While the histologic findings may not carry the same weight as the evidence of nonviable protoscolices seen in other cysts, they are another indicator of drug efficacy. If only cysts with evidence of living or dead protoscolices were considered, 96% of protoscolices in the treatment group were dead (data not shown). In the control group, protoscolices were either absent or alive; none were dead. Parasite differences are not likely to confound the results, since the strain of E. granulosus found in sheep and goats in this region is the same as that infecting humans (23).
There are currently three treatment options for hydatid disease, surgery, ultrasound-guided aspiration, and chemotherapy (24). Each of these modalities has limitations depending on the specific case. Chemotherapy is the preferred treatment where cysts are inoperable, surgeons are not available, or the cysts are too numerous. Chemotherapy has also been used as an adjunct to surgery for prophylaxis against spillage of cyst contents. The most successful approved agents are the benzimidazoles mebendazole and albendazole. Both have been shown to be effective against hydatid disease in sheep (9, 20). For example, daily administration of albendazole for 6 weeks was sufficient to kill all protoscolices found in both lung and liver cysts of infected sheep (20).
In humans, albendazole appears to have the greatest efficacy of any agent; nevertheless, efficacy as measured by shrinkage or disappearance of cysts ranges only between 20 and 30% (1, 6, 24). The critical factors for success appear to be the ability of the drug to penetrate the complex cyst wall and the persistence of adequate levels of the active metabolite. The greater efficacy of albendazole compared to mebendazole or praziquantel is due to albendazole’s greater penetration and absorption (19). After a 10-mg/kg dose of albendazole, its active sulfide metabolite reaches a mean peak plasma concentration in sheep of 3.2 mg/ml 20 h after ingestion and disappears between 48 and 72 h (15). For oxfendazole, the parent drug is the active form, and a 10-mg/kg dose gives a peak of 0.76 mg/ml at 30 h and remains detectable for up to 7 days (16).
It is difficult to estimate the potential efficacy of treatment in humans based upon results obtained in animals. The criteria for success and parasite death are different, and ungulates achieve significantly higher concentrations of benzimidazoles than monogastric animals such as horses and humans (17). Following oxfendazole therapy there were ultrasound changes indicative of degeneration in 47% of cysts from treated animals after 1 month of therapy and a 1-month follow-up period. By contrast, changes in 60% of cysts in humans can be expected after multiple courses of daily therapy with a 6-month to 1-year follow-up period. Future investigations need to be extended for a longer period to definitively establish the effect of oxfendazole on the endocyst germinal layer and assess dose-response ratios. Ultrasound provides a simple and reliable way to use naturally infected animals to do such drug testing for hydatid disease.
In developing countries or any remote areas where pharmacies are scarce, those with hydatid disease often live long distances from health facilities. For them it is necessary to make repeated journeys or remain resident for months near a health facility to complete only one or two cycles of daily treatment. In addition, most control schemes involve treatment of infected dogs with praziquantel-laced baits, since albendazole is not effective against the adult tapeworm. Because of oxfendazole’s longer half-life, it may be possible to administer the drug weekly or even consider a shorter course of therapy. Oxfendazole also offers the possibility that the same drug might be used to control disease transmission by treatment of dogs, the animal reservoir for adult worms. Determination of its relative efficacy awaits a simultaneous comparison of oxfendazole therapy to albendazole or praziquantel therapy.
ACKNOWLEDGMENTS
This work was supported by NIH program project grant no. AI 33061.
We thank Syntex Animal Health Research and Development for their gift of the oxfendazole used in this study.
REFERENCES
- 1.Ammann R, Eckert J. Clinical diagnosis and treatment of echinococcosis in humans. In: Thompson R C A, Lymbery A J, editors. Echinococcosis and hydatid disease. Oxford, United Kingdom: CAB International; 1995. pp. 411–463. [Google Scholar]
- 2.Andersen F L, Crellin J R, Nichols C R, Schantz P M. Evaluation of a program to control hydatid disease in central Utah. Great Basin Nat. 1983;43:65–72. [Google Scholar]
- 3.Arthaud J B. Cause of death in 339 Alaskan natives as determined by autopsy. Arch Pathol. 1970;90:433–438. [PubMed] [Google Scholar]
- 4.Chi P, Fan Y, Zhang Z, Zhang Y, Hasyet M, Liu W, Jia W, Ye F, Chen Y, Ming R, Sung Y. The prevalence and distribution of hydatidosis in China. Xinjiang Agric Sci. 1989;3:35–38. [Google Scholar]
- 5.Computer Associates International. CA-Cricket Graph III, 1.5.1 ed. Islandia, N.Y: Computer Associates International, Inc.; 1993. [Google Scholar]
- 6.Davis A, Pawlowski Z S, Dixon H. Multicentre clinical trials of benzimidazolecarbamates in human echinococcosis. Bull W H O. 1986;64:383–388. [PMC free article] [PubMed] [Google Scholar]
- 7.Gemmell M A, Johnstone P D, Oudemans G. The effect of oxfendazole on Echinococcus granulosus and Taenia hydatigena infections in dogs. Res Vet Sci. 1979;26:389–390. [PubMed] [Google Scholar]
- 8.Gemmell M A, Lawson J R, Roberts M G. Population dynamics in echinococcosis and cysticercosis: biological parameters of Echinococcus granulosus in dogs and sheep. Parasitology. 1986;92:599–620. doi: 10.1017/s0031182000065483. [DOI] [PubMed] [Google Scholar]
- 9.Gemmell M A, Parmeta S N, Sutton R J, Khan N. Effect of mebendazole against Echinococcus granulosus and Taenia hydatigena cysts in naturally infected sheep and relevance to larval tapeworm infections in man. Z Parasitenkd. 1981;64:135–147. doi: 10.1007/BF00930490. [DOI] [PubMed] [Google Scholar]
- 10.Gonzales A E, Garcia H H, Gilman R H, Gavidia C M, Tsang V C W, Bernal T, Falcon N, Romero M, Lopez-Urbina M T. Effective, single-dose treatment of porcine cysticercosis with oxfendazole. Am J Trop Med Hyg. 1996;54:391–394. doi: 10.4269/ajtmh.1996.54.391. [DOI] [PubMed] [Google Scholar]
- 11.Hurd H. Echinococcus granulosus: a comparison of free amino acid concentration in hydatid fluid from primary and secondary cysts and host plasma. Parasitology. 1989;98:135–143. doi: 10.1017/s0031182000059771. [DOI] [PubMed] [Google Scholar]
- 12.Kates K C, Colglazier M L, Enzie F D, Lindahl I L, Samuelson G. Helminth control in grazing sheep: periodic treatment with levamisole, morantel, cambendazole and mebendazole. J Parasitol. 1974;60:989–995. [PubMed] [Google Scholar]
- 13.Liu F-J, Che X-H, Chang Q. Prevalence of hydatid cysts in livestock in the Xinjiang Uygur Autonomous Region, PRC. In: Anderson F L, Chai J-J, Liu F-J, editors. Compendium on cystic echinococcosis with special reference to the Xinjiang Uygur Autonomous Region, The People’s Republic of China. Provo, Utah: Brigham Young University Print Services; 1993. pp. 177–189. [Google Scholar]
- 14.Macpherson C N L, Zeyhle E, Romig T, Rees P H, Were J B O. Portable ultrasound scanner versus serology in screening for hydatid cysts in a nomadic population. Lancet. 1987;ii:259–262. doi: 10.1016/s0140-6736(87)90839-7. [DOI] [PubMed] [Google Scholar]
- 15.Marriner S E, Bogan J A. Pharmacokinetics of albendazole in sheep. Am J Vet Res. 1980;41:1126–1129. [PubMed] [Google Scholar]
- 16.Marriner S E, Bogan J A. Pharmacokinetics of oxfendazole in sheep. Am J Vet Res. 1981;42:1143–1145. [PubMed] [Google Scholar]
- 17.Marriner S E, Bogan J A. Plasma concentrations of fenbendazole and oxfendazole in the horse. Equine Vet J. 1985;17:58–61. doi: 10.1111/j.2042-3306.1985.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 18.Maxson A D, Wachira T M, Zeyhle E E, Fine A, Mwangi T W, Smith G. The use of ultrasound to study the prevalence of hydatid cysts in the right lung and liver of sheep and goats in Turkana. Int J Parasitol. 1996;26:1335–1338. doi: 10.1016/s0020-7519(96)00124-5. [DOI] [PubMed] [Google Scholar]
- 19.Morris D L, Chinnery J B, Georgiou G, Stamanakis G, Golematis B. Penetration of albendazole sulphoxide into hydatid cysts. Gut. 1987;28:75–80. doi: 10.1136/gut.28.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris D L, Clarkson M J, Stallbaumer M F, Pritchard J, Jones R S, Chinnery J B. Albendazole treatment of pulmonary hydatid cysts in naturally infected sheep: a study with relevance to the treatment of hydatid cysts in man. Thorax. 1985;40:453–458. doi: 10.1136/thx.40.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schantz P M, Chai J, Craig P S, Eckert J, Jenkins D J, Macpherson C N L, Thakur A. Epidemiology and control of hydatid disease. In: Thompson R C A, Lymbery A J, editors. Echinococcosis and hydatid disease. Oxford, United Kingdom: CAB International; 1995. pp. 233–331. [Google Scholar]
- 22.Smyth J D, Barrett N J. Procedures for testing the viability of human hydatid cysts following surgical removal especially after chemotherapy. Trans R Soc Trop Med Hyg. 1980;74:849–852. doi: 10.1016/0035-9203(80)90157-1. [DOI] [PubMed] [Google Scholar]
- 23.Wachira T M H, Bowles J, Zeyhle E, McManus D P. Molecular examination of the sympatry and distribution of sheep and camel strains of Echinococcus granulosus in Kenya. Am J Trop Med Hyg. 1993;48:473–479. doi: 10.4269/ajtmh.1993.48.473. [DOI] [PubMed] [Google Scholar]
- 24.WHO Informal Working Group. Guidelines for treatment of cystic and alveolar echinococcosis in humans. Bull W H O. 1996;74:231–242. [PMC free article] [PubMed] [Google Scholar]
- 25.Zeyhle, E. E. Personal communication.