Abstract
Proliferative myositis (PM) is a benign intramuscular tumor that might mimic a malignant one due to its unusual pseudosarcomatous inflammatory nature. In this report, we describe a patient who developed PM after vaccination with Sinopharm coronavirus disease (COVID-19) vaccine. A 73 years old man was admitted due to rapidly-growing painful mass in his left thigh from a few days ago, curtailing his walking. He received a recent COVID-19 vaccination (Sinopharm COVID-19 vaccine) about 5 days before the beginning of symptoms. No history of trauma was present. On physical exam, a round firm mass was found in lateral side of mid portion of left thigh within the muscle with tenderness on palpation. An oval-shaped well-defined intramuscular mass measured 15 × 41 mm was noted in vastus lateralis muscle in ultrasonography. Left thigh magnetic resonance imaging (MRI) showed a well-defined intramuscular mass with a definite margin of 19 × 39 mm. Finally, ultrasound (US)-guided core needle biopsy showed muscular tissue with a loose mass composed of plump fibroblasts and myofibroblasts and large ganglion-like cell with abundant amphophilic to basophilic cytoplasm, vesicular nuclei and prominent nucleoli. Pathology report showed a very rare case identified as proliferative myositis. It should be noted that we cannot make a direct link between these 2 events. PM is an extremely rare entity; however, its relation with COVID-19 vaccination might be a coincidence.
Keywords: COVID-19, Proliferative Myositis, Vaccination
Introduction
Coronavirus disease 2019 (COVID-19) is a condition caused by the SARS-CoV-2 coronavirus, which was initially discovered in Wuhan, China, in December 2019. Since that time, COVID-19 has spread globally, infecting more than 600 million individuals and killing more than 6 million people [1]. Several vaccines have been created and approved against the SARS-CoV-2. According to studies, COVID-19 vaccines are extremely safe and effective, capable of preventing severe COVID-19 symptoms, and can also lower hospitalization and fatality rates [2]. Nonetheless, several reports have been published regarding the possible adverse events after COVID-19 vaccination. One of the extremely rare adverse events was proliferative myositis (PM) and inflammatory myopathy.
PM is a benign intramuscular tumor that might mimic a malignant one due to its unusual pseudosarcomatous inflammatory nature. Kern initially described this entity in 1960 [3]. The median age of PM patients is 50 years old, and there is no gender predominance [4]. The shoulder, upper limb, trunk, head, and neck muscles are the most common sites for PM [4]. They appear as a single, solid, painful lump that quickly doubles in size [4]. The main differential diagnosis is sarcoma, which needs a distinct surgical strategy [5].In this report, we describe a patient who developed PM after vaccination with Sinopharm COVID-19 vaccine.
Case presentation
A 73-year-old man was admitted due to a rapidly-growing painful mass in his left thigh from a few days ago. As a result of the pain, he was barely able to walk properly. No other signs or symptoms were present. His past medical history was remarkable for chronic obstructive pulmonary disease (COPD), hypertension, and peptic ulcer disease (PUD). Drug history of the patient included theophylline, omeprazole, valsartan aspirin tablets, salmeterol and ipratropium bromide sprays. Further history taking revealed that he received a recent COVID-19 vaccination (Sinopharm COVID-19 vaccine) about 5 days before the beginning of symptoms. The vaccine had been injected intramuscularly into the left deltoid. No history of trauma was present. On physical exam, a round firm mass (measured approximately 4 cm) was found in lateral side of mid portion of left thigh within the muscle with tenderness on palpation. However, no skin changes or superficial signs were present. Vital signs were in normal limit, and other aspects of physical examination were unremarkable.
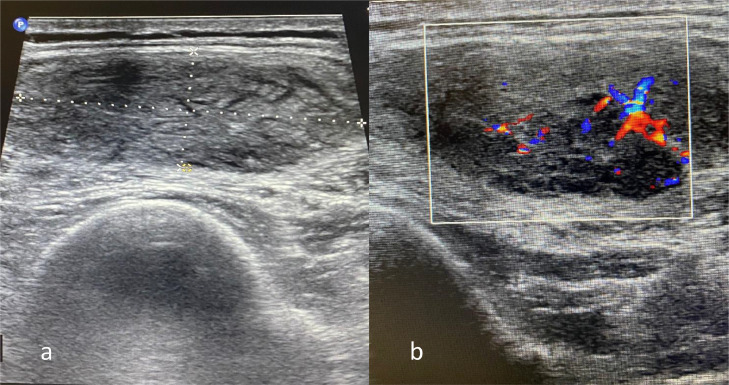
The mass was then examined with soft tissue ultrasonography (US) (Fig. 1). An oval-shaped well-defined intramuscular mass measuring 15*41 mm was noted in vastus lateralis muscle. The mass was hypoechoic with striated pattern. On color Doppler imaging (CDI), increased vascularity was noted within the lesion in comparison to surrounding normal muscle. No evidence of extra-fascial extension, bony invasion or regional lymphadenopathy was noted.
Fig. 1.
Ultrasonography of the lesion. (A) conventional ultrasonography and (B) color Doppler imaging.
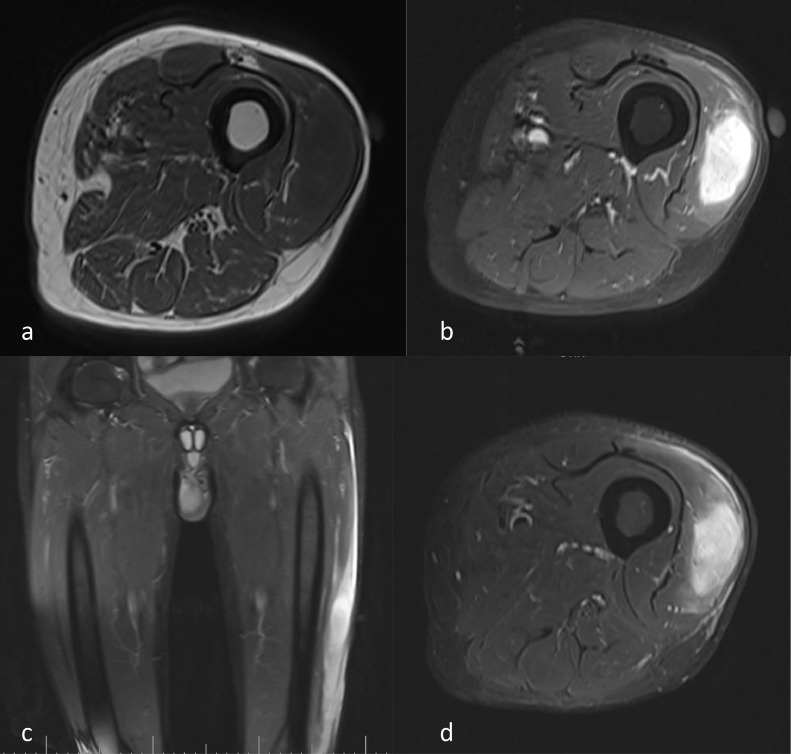
Subsequently, left thigh MRI with and without contrast showed a Well-defined intramuscular mass with a definite margin of 19*39 mm. Homogenous low-signal intensity on T1-weighted sequences and high-signal intensity on T2/proton density (PD) weighted sequences was seen in mid-portion of vastus lateralis muscle (Fig. 2). The mass had extensive surrounding vasogenic edema. After contrast injection, the mass showed intense homogenous enhancement. Based on imaging findings, neoplastic lesions such as sarcoma, nerve-sheath tumors, and metastasis were considered as differential diagnoses.
Fig. 2.
MRI of the lesion. (A) T1-weighted, (B) T1-weighted after injection of contrast, (C) short tau inversion recovery (STIR) in coronal view, and (D) STIR in axial view.
Finally, US-guided core needle biopsy was performed on the lesion. Sections showed muscular tissue with a loose mass composed of plump fibroblasts and myofibroblasts and large ganglion-like cell with abundant amphophilic to basophilic cytoplasm, vesicular nuclei and prominent nucleoli. Pathology report showed a very rare case identified as proliferative myositis. The patient was treated conservatively with a non-steroidal anti-inflammatory drug (NSAID), hot compress, and rest. The patient responded to the treatment and did not experience relapse.
Discussion
This study reported a rare case of PM after vaccination with Sinopharm COVID-19 vaccine. PM is considered a type of benign sarcomatous lesions that presents with rapidly growing intramuscular mass. The median age of affected patients is 50 years. The common locations of PM are the trunk and extremities [6]. The exact etiology of PM is unknown. Nonetheless, history of recent trauma, ischemia, vasculitis, and chromosomal abnormalities have been introduced as potential etiologies [7]. None of these etiologies were the cause of PM in our case. However, we cannot make a direct association between COVID-19 vaccination and the development of PM.
US is the first modality for initial investigation [7], which might show a “scaffolding” pattern on longitudinal views and “checkerboard” pattern on transverse views [8]. Previous studies have also characterized the appearance of PM as a well-defined, lobulated, expansive intramuscular lesion [8]. MRI findings of PM are a hypo- or similar signal compared to the muscle on T1-weighted sequences, with homogeneous enhancement [6], and a hyperintense soft-tissue mass on T2-weighted sequences [6].
In pathology examination, PM is generally characterized with 1) infiltration of muscle with large eosinophilic giant cells and 2) proliferative fibroblasts mainly targeting the interfascicular connective tissue. In contrast to other similar disorders like myositis ossificans or nodular fasciitis, the actual muscle is not involved and largely preserved in PM [7]. After the establishment of the diagnosis, the recommended treatment strategy is no specific treatment, because PM might disappear spontaneously. Excision of the lesion is generally for assessment a diagnosis or for cosmetic reasons [6].
To the best of our knowledge, SARS-CoV-2 vaccine-related myositis and myopathies have been reported in 3 cases. The first case was a localized myositis in a 56-year-old non-diabetic woman that had developed pain in left upper arm 8 days after a second dose of COVID-19 vaccine into her deltoid muscle [9]. Similar to our patient, she had no systemic signs and symptoms. she was managed with rest, cryotherapy, compression and NSAIDs. Her symptoms resolved after 6 weeks with no residual loss of function. The authors suggested that unknown mechanisms might have contributed to the inflammatory process in addition to minor muscle injury from injection [10]. However, in the other 2 reported cases, the myositis was systemic [11]. The patients had developed idiopathic inflammatory myopathy within 48 hours of receiving an mRNA vaccine (the BNT162b2 vaccine). These patients were treated successfully with corticosteroid and immunoglobulin therapy. Nonetheless, the authors stated that they could not find a direct link between COVID-19 vaccination and development of myositis. It was proposed that it is also possible that preexisting cancer or other causes might predispose the patients, and the COVID-19 vaccine provided the final blow [11].
The underlying mechanisms of drug-induced myopathy can be categorized into direct myotoxicity, immunologically related inflammation, indirect muscle damage. Direct myotoxicity, as in glucocorticoids, results from accumulation of drug in muscle tissues whereas indirect myotoxicity results from hyperthermia or hyperkinesis [12]. Immunologically related drug-induced myopathy has been seen in immune checkpoint inhibitors and interferon-alpha. However, the precise mechanisms are not elucidated [13]. Herein, the mechanisms of COVID-19 vaccine-related myositis in our case in unknown.
In conclusion, in this study, we reported a case of PM after vaccination with Sinopharm COVID-19 vaccine. It should be noted that we cannot make a direct link between these 2 events. PM is an extremely rare entity; however, its relation with COVID-19 vaccination might be a coincidence. Moreover, millions have been vaccinated with Sinopharm vaccine, and this is the first case of PM after Sinopharm vaccination. Thus, huge benefits of COVID-19 vaccination outweigh its potential harm.
Acknowledgments
Authors’ contributions
RE, AM, MSK, and KG conceived and designed the evaluation. RE, AM, MSK, and KG helped to collect clinical data, draft the manuscript and revised the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Patient consent
Informed consent for participation in the study and publication was obtained from the patient.
Footnotes
Competing Interests: We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Organisation WH . 2023. WHO coronavirus (COVID-19) dashboard.https://covid19.who.int Accessed Feb 1, 2023. [Google Scholar]
- 2.Tenforde MWOS, Self WH, Olson S, Talbot K, Lindsell C, Steingrub J, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years — United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674–679. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kern W. Proliferative myositis, a pseudosarcomatous reaction to injury: a report of seven cases. Arch Pathol. 1960;73:209–216. [PubMed] [Google Scholar]
- 4.Pagonidis K, Raissaki M, Gourtsoyiannis N. Proliferative myositis: value of imaging. J Comput Assist Tomogr. 2005;29(1):108–111. doi: 10.1097/01.rct.0000150142.14113.70. [DOI] [PubMed] [Google Scholar]
- 5.Enzinger FM, Dulcey F. Proliferative myositis. Report of thirty-three cases. Cancer. 1967;20(12):2213–2223. doi: 10.1002/1097-0142(196712)20:12<2213::aid-cncr2820201223>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Demir MK, Beser M, Akinci O. Case 118: proliferative myositis. Radiology. 2007;244(2):613–616. doi: 10.1148/radiol.2442041504. [DOI] [PubMed] [Google Scholar]
- 7.Shi J, Lewis M, Walsworth MK, Modaressi S, Masih S, Chow K. Proliferative myositis. Appl Radiol. 2018;47(1):43–45. [Google Scholar]
- 8.Wlachovska B, Abraham B, Deux J, Sibony M, Marsault C, Le Breton C. Proliferative myositis in a patient with AIDS. Skeletal Radiol. 2004;33(4):237–240. doi: 10.1007/s00256-003-0715-0. [DOI] [PubMed] [Google Scholar]
- 9.Theodorou DJ, Theodorou SJ, Axiotis A, Gianniki M, Tsifetaki N. COVID-19 vaccine-related myositis. QJM. 2021;114(6):424–425. doi: 10.1093/qjmed/hcab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bensalah A, Elbouardi N, Douida A, Haloua M, Alami B, Boubbou M, et al. Proliferative myositis: case report and review of the literature. Radiol Case Rep. 2021;16(7):1902–1906. doi: 10.1016/j.radcr.2021.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vutipongsatorn K, Isaacs A, Farah Z. Inflammatory myopathy occurring shortly after severe acute respiratory syndrome coronavirus 2 vaccination: two case reports. J Med Case Rep. 2022;16(1):57. doi: 10.1186/s13256-022-03266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun L, Trausch-Azar JS, Muglia LJ, Schwartz AL. Glucocorticoids differentially regulate degradation of MyoD and Id1 by N-terminal ubiquitination to promote muscle protein catabolism. Proc Natl Acad Sci U S A. 2008;105(9):3339–3344. doi: 10.1073/pnas.0800165105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunasso AMG, Aberer W, Massone C. New onset of dermatomyositis/polymyositis during anti-TNF-α therapies: a systematic literature review. Sci World J. 2014;2014 doi: 10.1155/2014/179180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.