Abstract
BACKGROUND
Coil migration is a rare, but notable complication of endovascular treatment. Risk factors include communicating segment aneurysms, aneurysmal shape, and technical factors. Although cerebral blood flow obstruction caused by early coil migration requires urgent coil removal, delayed coil migration is often asymptomatic, making it difficult to determine a treatment strategy.
OBSERVATIONS
A 47-year-old woman was referred to the institute with acute-onset headache. She was diagnosed with subarachnoid hemorrhage due to rupture of the right internal carotid artery–posterior communicating artery aneurysm and underwent endovascular coil embolization. Following the procedure, the patient showed no obvious complications; however, 14 days later, images showed coil migration to the distal side, leading to surgical removal. Right frontotemporal craniotomy was performed, and the remaining coil was removed. The aneurysm was clipped again, and blood flow was confirmed. The patient was discharged 12 days after the craniotomy with transient oculomotor nerve palsy. At the 15-month follow-up, there was no aneurysm recurrence and the oculomotor nerve palsy showed improvement.
LESSONS
Retrieval of the migrated coil by craniotomy is an effective remedial measure; however, intraoperative complications are common. Early detection, established protocols, and prompt treatment decisions are important for preventing undesirable outcomes.
Keywords: delayed coil migration, surgical removal, coil embolization, intracranial aneurysm, intra-aneurysmal thrombi
ABBREVIATIONS: CT = computed tomography, DSA = digital subtraction angiography, ICA = internal carotid artery, ICG = indocyanine green, MCA = middle cerebral artery, PCoA = posterior communicating artery, 3D = three-dimensional
Coil embolization for intracranial aneurysms is one of the main treatment modalities in neurosurgery because of its safety and efficacy, low mortality rate, and better clinical outcomes than surgical clipping.1,2 Coil migration is a rare complication of endovascular embolization for cerebral aneurysms, with an estimated incidence of approximately 0.3%–6%.3–5 The detached material migrates along the parent vessel, eventually forming a thrombus that obstructs cerebral blood flow beyond the occlusion site, leading to cerebral ischemia or stroke if adequate restoration of blood flow is not achieved.6 Although cerebral blood flow obstruction caused by coil migration during endovascular treatment often requires urgent coil removal, delayed coil migration is often asymptomatic, making it difficult to determine a treatment strategy. Here, we report a case of delayed coil migration 2 weeks after endovascular coiling for a subarachnoid hemorrhage due to a ruptured aneurysm, which resulted in surgical removal of the migrated coil.
Illustrative Case
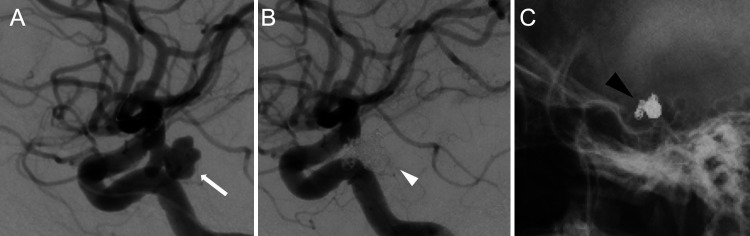
A 47-year-old woman was referred to our institute due to the acute onset of headache without any neurological deficit. Head computed tomography (CT) revealed a subarachnoid hemorrhage, and three-dimensional (3D) CT angiography showed an irregular aneurysm with multiple blebs in the right internal carotid artery–posterior communicating artery (ICA-PCoA) bifurcation, with a maximum diameter of 9.7 mm and a neck of 3.0 mm. A diagnosis of subarachnoid hemorrhage due to rupture of the right ICA-PCoA aneurysm was made, and endovascular coil embolization was performed (Fig. 1A and B). Under general anesthesia, a 7-Fr sheath and a 7-Fr Roadmaster 90 guiding catheter (Goodman) were placed in the right ICA, and 2 Excelsior SL-10 microcatheters (Striker) were placed in 2 compartments of the aneurysm. A Target XL 360 Soft 6 mm × 20 cm (Stryker) coil was used for framing, followed by the placement of GALAXY G3 XSFT 3 mm × 4 cm (Johnson & Johnson) and Target 360 Ultra 4 mm × 6 cm, 3 mm × 6 cm, and 2.5 mm × 4 cm (Stryker) coils into the aneurysm for double catheterization.
FIG. 1.
A: An irregular aneurysm with multiple blebs in the right ICA-PCoA bifurcation with a maximum diameter of 9.7 mm and a neck of 3.0 mm (white arrow). B: Endovascular coil embolization was performed without any intraoperative complications (white arrowhead). C: Skull radiographs obtained 1 and 7 days postsurgery showed no obvious coil migration (black arrowhead).
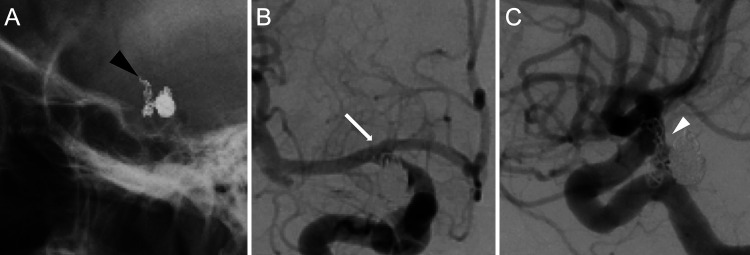
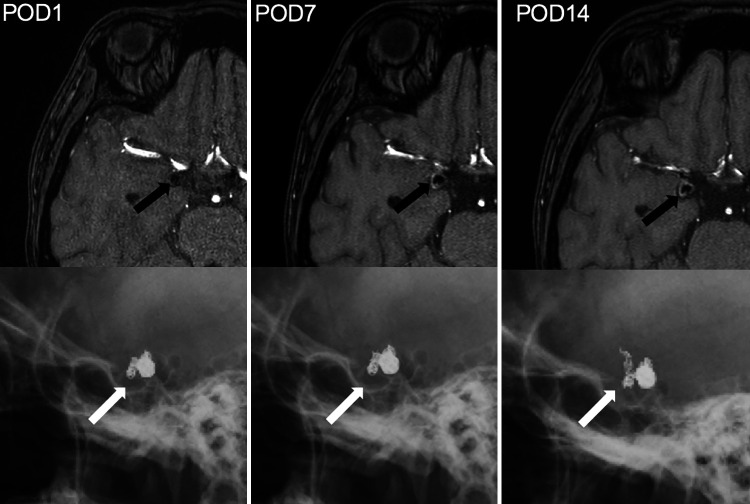
No obvious complications such as delayed cerebral vasospasm or coil compression were observed after coil embolization. Skull radiographs obtained 1 and 7 days postoperatively showed no obvious coil migration (Fig. 1C); however, images taken 14 days postsurgery showed migration of the coil to the distal side (Fig. 2A). Digital subtraction angiography (DSA) showed a 7.5-mm deviation of the coil loop from the obliterating side of the middle cerebral artery (MCA) (Fig. 2B). The previously embolized right ICA-PCoA bifurcation aneurysm was recanalized via coil migration (Fig. 2C). Magnetic resonance angiography showed increased signal intensity within the aneurysm (Fig. 3). Conservative treatment with antithrombotic drugs was performed because there was no neurological deficit. However, the large volume of the migrated coil was considered to carry a high risk of thrombosis; therefore, we decided to perform a craniotomy on day 14 of antithrombotic drug administration to remove the coil and clip the aneurysm.
FIG. 2.
A: Skull radiograph obtained 14 days postsurgery showed migration of the coil to the distal side (black arrowhead). B: DSA showed a 7.5-mm deviation of the coil loop to the obliterating side of the MCA (white arrow). C: A previously embolized right ICA-PCoA aneurysm was also recanalized by coil migration (white arrowhead).
FIG. 3.
Magnetic resonance angiography over time showed increased signal intensity within the aneurysm (black arrows) and the coil shown in corresponding skull radiographs (white arrows). A hematoma is present on the outside of the coil from postoperative day 1 to day 14.
Right frontotemporal craniotomy was performed, the transsylvian fissure was opened, the post–coil embolization aneurysm was identified at the ICA-PCoA bifurcation, and the migrated coil was visible through the right MCA M1 segment. A brown hematoma and strong adhesions were observed around the aneurysm. First, the ICA was temporarily trapped, the tip of the aneurysm was incised, the internal coil was removed, and the migrated coil in the MCA was unraveled and left behind, adhering to the vessel wall (Fig. 4A). After untrapping the ICA, indocyanine green (ICG) video angiography and Doppler ultrasound revealed poor blood flow from the ICA to M1, and thrombosis caused by the unraveled coil was suspected. An incision was made at the origin of M1, and the remaining coil was removed (Fig. 4B). The incision was sutured with 10–0 Ethilon, and although blood flow to the MCA was good, resumption of blood flow to the ICA was not achieved, suggesting thrombus formation. The ICA was trapped again, the clip was removed, and it was massaged after all coils in the aneurysm were removed, which showed good blood flow with backflow (Fig. 4C). After sufficient thrombus removal, the aneurysm was clipped again, and blood flow from the ICA to M1 was confirmed by ICG video angiography (Fig. 4D).
FIG. 4.
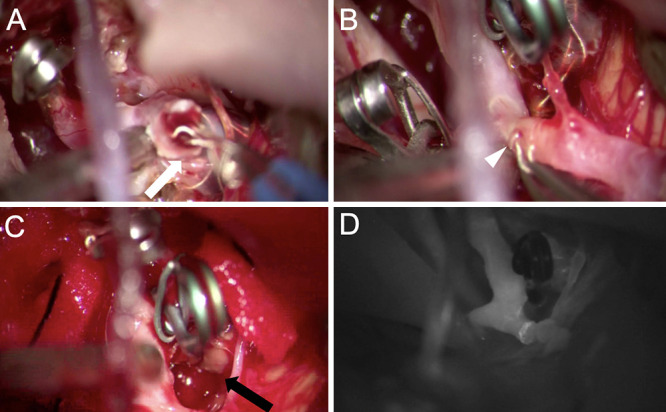
A: The tip of the aneurysm was incised, and the internal coil was removed (white arrow); the migrated coil in the MCA was unraveled in the process and left behind, adhering to the vessel wall. B: The MCA was temporarily trapped, arteriotomy was performed at the M1 origin, and the remaining coil was removed (white arrowhead). C: The ICA was trapped again, the clip was removed, and all of the coil in the aneurysm was removed (black arrow). D: With the thrombus sufficiently removed, the aneurysm was clipped again, and ICG test confirmed blood flow from the ICA to M1.
Postoperatively, the patient presented with transient oculomotor nerve palsy that improved over time. The patient was discharged 12 days after the craniotomy with no new neurological deficits. At the 15-month follow-up, there was no recurrence of the aneurysm and the oculomotor nerve palsy showed improvement.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
Coil migration is one of the most noted intraoperative and postoperative complications of endovascular treatment. If the coil deviates, it can cause a major stroke due to the obstruction of blood flow to the parent vessel, and appropriate treatment strategies are required.6 Risk factors for coil migration include communicating segment aneurysms, aneurysm shape (wide neck ≥ 4 mm, medium size ≤ 4 mm, aspect ratio ≤ 1.6), and technical factors such as the use of an undersized coil.5,6 In the case of intraoperative coil deviation, endovascular teams have been known to recognize coil dropout during coiling procedures and take urgent action.7 According to a literature review by Abdalkader et al.,5 coil migration can be classified into 2 groups, immediate and delayed, depending on when it occurs. Delayed coil migration is defined as coil deviation discovered after endovascular treatment.1,4–6 Its timing varies from a few hours to several months, depending on incidental discovery on follow-up imaging and the presentation of neurological symptoms due to coil occlusion.5,8–10 In the present case, delayed coil migration was observed, although none of the risks were present, as described above. We speculate that the coil, which was fixed intraoperatively within the aneurysm by the thrombus, lost stability and migrated out of the aneurysm when the fibrinolytic system became dominant. Colpan et al.11 reported that, when 34 ruptured aneurysms were examined by CT and magnetic resonance imaging, intra-aneurysmal thrombi were found in 9 cases (26.4%); 4 cases (11.7%) were detected by DSA. Coil embolization, with attention paid to the presence of intra-aneurysmal thrombi in the treatment of ruptured aneurysms, may reduce the risk of delayed coil migration.
To the best of our knowledge, there have been 10 reported cases in 4 studies of delayed coil migration where the coils were removed through craniotomy5,8–10 (Table 1). This includes our current case, bringing the total number of patients to 8, with 4 patients undergoing coil embolization for unruptured aneurysms and 4 for subarachnoid hemorrhage. The aneurysms were located in the ICA-PCoA and ICA–ophthalmic arteries in 2 patients each, in addition to 1 case each in the ICA–anterior choroidal artery, ICA–superior hypophyseal artery, ICA bifurcation, and MCA bifurcation. The length of the migrated coils ranged from 7.5 mm to 3 cm. The time from endovascular treatment to the detection of coil migration varied from 1 hour to a maximum of 3 months. The parent artery was occluded in half of the patients (4 cases) due to coil migration, whereas it was not occluded in the remaining 4 cases. Pre- and postoperative antithrombotic treatment was administered to 2 of the 8 patients. Of the 8 patients, 7 had complications after coil removal, with a good recovery outcome in 4, severe disability in 3, and death in 1 patient. This indicated that half of the patients had unfavorable outcomes.
TABLE 1.
Literature review of delayed coil migrations that were removed through craniotomy
| Authors & Year | Age (yrs) | Sex | Diagnosis | Max Diam (mm) | Neck (mm) | Aspect Ratio | Aneurysm Location | Length of Migrated Coil | Detection Time | Vessel Occlusion | Antithrombic Tx | Surgical Complication | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thronton et al., 200010 |
44 |
F |
SAH |
3 |
2 |
1.5 |
ICA-PCoA |
NA |
1 day |
Yes |
No |
Hemiparesis |
Severely disabled |
| 62 |
M |
Unruptured aneurysm |
4 |
2 |
2 |
ICA-SHA |
NA |
3 days |
No |
No |
None |
Good recovery |
|
| Wada et al., 20109 |
64 |
F |
SAH |
5.4 |
3.9 |
1.4 |
ICA-Acho |
NA |
24 days |
No |
No |
Leaving migrated part in M1 |
Good recovery |
| Motegi et al., 20128 |
59 |
M |
Unruptured aneurysm |
7 |
NA |
NA |
ICA bifurcation |
NA |
3 mos |
No |
Yes |
Proximal MCA had poor blood flow |
Good recovery |
| Abdalkader et al., 20195 |
NA |
NA |
SAH |
4.6 |
3.6 |
1.3 |
ICA-ophthalmic |
1 cm |
12 hrs |
Yes |
No |
Thromboembolic |
Death |
| NA |
NA |
Unruptured aneurysm |
3 |
3 |
1 |
ICA-ophthalmic |
3 cm × 2 |
1 hr |
Yes |
No |
Thromboembolic |
Severely disabled |
|
| NA |
NA |
Unruptured aneurysm |
3 |
3 |
1 |
MCA bifurcation |
3 cm |
2.5 hrs |
Yes |
No |
Thromboembolic |
Severely disabled |
|
| Present case | 47 | F | SAH | 9.7 | 3 | 3.2 | ICA-PCoA | 7.5 mm | 14 days | No | Yes | Oculomotor nerve palsy | Good recovery |
Acho = anterior choroidal artery; Diam = diameter; NA = not applicable or available; SAH = subarachnoid hemorrhage; SHA = superior hypophyseal artery; Tx = treatment.
Due to mixed reports in the literature, there are no standardized management or established guidelines for the retrieval of migrated coils; therefore, migrated coil retrieval is determined based on the patient’s clinical presentation and the importance of the occluded vessel and its collateral vessels. Particularly in cases of delayed coil migration, the patient may not be clinically symptomatic at the time of diagnosis, and endovascular retention may be chosen. Therefore, the decision regarding the retrieval method (endovascular or craniotomy) is left to the surgeon.4,5 There is no standard treatment strategy for antithrombotic therapy in patients with coil mass migration. In our literature review, 2 of the 8 patients were treated with antithrombotic therapy to prevent thrombus formation due to coil migration. Abdalkader et al.5 reported that medical management with antiplatelet and/or anticoagulant drugs should be considered if the migrated coil is distal and cannot be safely followed up, if there is no associated vessel occlusion, and if retrieval fails.
Romani et al.12 reported that coil removal after 1 month of coil embolization was a factor associated with a worse prognosis. The reasons for this based on the intraoperative findings are discussed below. Coils that are in contact with the vessel wall become strongly attached to the wall over time and are difficult to remove. On the other hand, the vessel wall itself undergoes degenerative changes and thinning, making it difficult to remove as it begins to adhere to the surrounding tissue.13
In the present case, the decision was made to remove the coil by craniotomy; however, owing to antithrombotic medication, there was a 14-day delay between the diagnosis of coil migration and retrieval. The decision to postpone the craniotomy for coil removal was made after careful consideration of the risks and benefits. At the time, the patient had no symptoms, and we believed that the risks associated with craniotomy were greater than the potential benefits. However, although the volume of the migrated coil was considered to be too large to have a high risk of thrombosis, we decided to remove the migrated coil. There was no thrombosis or neurological deficit, but the migrated coil’s volume was too large to be crimped onto the vessel wall with endovascular stenting. Therefore, direct surgery was chosen.
Consequently, the coil was unraveled intraoperatively because of adhesions caused by the intimal formation, resulting in temporary vessel occlusion. In such a situation, in the worst case, a deep anastomosis may be required to repair the vessel wall. Therefore, it is necessary to consider the period that has passed since coil migration as an indicator to perform a craniotomy.
Lessons
Coil migration following endovascular coiling of an intracranial aneurysm is rare. Coil embolization, with a focus on the presence of intra-aneurysmal thrombi in the treatment of ruptured aneurysms, may reduce the risk of delayed coil migration. Retrieval of the migrated coil by craniotomy is an effective remedial measure; however, intraoperative complications are common, so optimal timing of surgery should be considered.
Acknowledgments
We thank Editage Group for editing a draft of this manuscript.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Oda, Suzuki, Horio, Hirao, Takemoto, Morishita, Abe. Acquisition of data: Oda, Suzuki, Hirao, Takemoto. Analysis and interpretation of data: Oda, Suzuki, Hirao, Takemoto. Drafting of the article: Oda, Suzuki, Horio, Hirao, Takemoto, Morishita. Critically revising the article: Horio, Amamoto, Takemoto, Morishita, Abe. Reviewed submitted version of the manuscript: Horio. Approved the final version of the manuscript on behalf of all authors: Oda. Administrative/technical/material support: Kawano, Amamoto, Kobayashi. Study supervision: Horio, Kawano, Kobayashi, Morishita.
References
- 1. Molyneux AJ, Kerr RS, Yu LM, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 2. Eskey CJ, Meyers PM, Nguyen TN, et al. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association. Circulation. 2018;137(21):e661–e689. doi: 10.1161/CIR.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 3. Ding D, Liu KC. Management strategies for intraprocedural coil migration during endovascular treatment of intracranial aneurysms. J Neurointerv Surg. 2014;6(6):428–431. doi: 10.1136/neurintsurg-2013-010872. [DOI] [PubMed] [Google Scholar]
- 4. Turek G, Kochanowicz J, Lewszuk A, et al. Early surgical removal of migrated coil/stent after failed embolization of intracranial aneurysm. J Neurosurg. 2015;123(4):841–847. doi: 10.3171/2015.1.JNS132788. [DOI] [PubMed] [Google Scholar]
- 5. Abdalkader M, Piotin M, Chen M, et al. Coil migration during or after endovascular coiling of cerebral aneurysms. J Neurointerv Surg. 2020;12(5):505–511. doi: 10.1136/neurintsurg-2019-015278. [DOI] [PubMed] [Google Scholar]
- 6. Henkes H, Fischer S, Weber W, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery. 2004;54(2):268–280. doi: 10.1227/01.neu.0000103221.16671.f0. discussion 280–285. [DOI] [PubMed] [Google Scholar]
- 7. Muninthorn W, Kobkitsuksakul C, Boongird A. Emergency surgical removal of a migrated coil during embolization of a giant internal carotid artery aneurysm: illustrative case. J Neurosurg Case Lessons. 2022;4(9):CASE22287. doi: 10.3171/CASE22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Motegi H, Isobe M, Isu T, et al. A surgical case of delayed coil migration after balloon-assisted embolization of an intracranial broad-neck aneurysm: case report. Neurosurgery. 2010;67(2 Suppl):516–521. doi: 10.1227/NEU.0b013e3181f82588. [DOI] [PubMed] [Google Scholar]
- 9. Wada H, Tokumitsu N, Shirai W, Sako K, Kamada K. Ruptured aneurysm with delayed distal coil migration requiring surgical treatment. Case report. Neurol Med Chir (Tokyo) 2012;52(6):439–442. doi: 10.2176/nmc.52.439. [DOI] [PubMed] [Google Scholar]
- 10. Thornton J, Dovey Z, Alazzaz A, et al. Surgery following endovascular coiling of intracranial aneurysms. Surg Neurol. 2000;54(5):352–360. doi: 10.1016/s0090-3019(00)00337-2. [DOI] [PubMed] [Google Scholar]
- 11. Colpan ME, Sekerci Z, Hekimoglu B, Mogul DJ. Computer-assisted intraaneurysmal thrombus visualization. J Neuroimaging. 2006;16(1):59–68. doi: 10.1177/1051228405001528. [DOI] [PubMed] [Google Scholar]
- 12. Romani R, Lehto H, Laakso A, et al. Microsurgery for previously coiled aneurysms: experience with 81 patients. Neurosurgery. 2011;68(1):140–154. doi: 10.1227/NEU.0b013e3181fd860e. [DOI] [PubMed] [Google Scholar]
- 13. Deshaies EM. Extruded platinum coils from recurrent previously coiled intracranial aneurysms discovered at the time of microsurgical clip ligation. A report of three cases. Interv Neuroradiol. 2011;17(1):59–63. doi: 10.1177/159101991101700109. [DOI] [PMC free article] [PubMed] [Google Scholar]