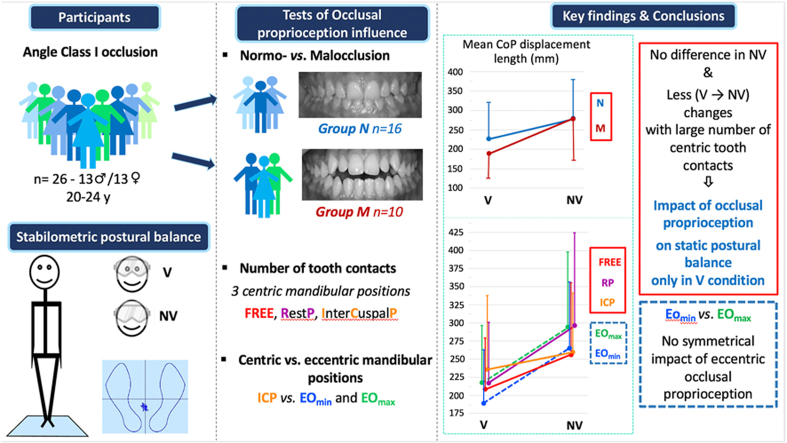
Abstract
Conflicting results on the effects of occlusal proprioceptive information on standing sway have been reported in the literature, partly due to the heterogeneity of the occlusal criterion studied and the experimental protocol used. In this study, occlusal functions, different mandibular positions and visual conditions were used to investigate the involvement of occlusal proprioception information in static postural balance. Postural adjustments of 26 healthy young adults, divided into Class I malocclusion and Class I normocclusion groups, were studied in upright position, in five mandibular positions (1 free, 2 centric and 2 eccentric), with and without vision. Due to different reported test durations, postural parameters were examined for the first and last halves of the 51.2 s acquisition time. A permutation ANOVA with 4 factors was used: group, mandibular position, vision, time window.
Mean length of CoP displacement was shorter with vision (ES = 0.30) and more affected by vision loss in the free than in the intercuspal mandibular position (ES = 0.76 vs. 0.39), which has more tooth contacts. The malocclusion group was more affected by vision loss (ES = 0.64). Unexpectedly, with vision, the mean length was smaller in one eccentric occlusion side compared to the other (ES = 0.51), but independent of the left or right side, and more affected by vision loss (ES = 1.04 vs. ES = 0.71). The first-time window of the acquisition time, i.e. 25.6 s, was sufficient to demonstrate the impact of dental occlusion, except for the sway area.
Comparison of the two visual conditions was informative. With vision, the weight of occlusal proprioception was not strictly related to occlusal characteristics (number of teeth in contact; centered or eccentric mandibular position), and it was asymmetrical. Without vision, the lack of difference between groups and mandibular positions suggested a sensory reweighting, probably to limit postural disturbance.
Keywords: Postural balance, Dental occlusion, Proprioception, Vision, Mandibular positions
Graphical abstract
1. Introduction
Postural stability in a standing position is only possible through a constant interaction of sensory information from different sensors, as evidenced by the observed disturbance when one of these sensors tends to dysfunction. In this interaction, the studies by Gangloff and Perrin [1] and Petrosini and Troiani [2] have demonstrated the influence of trigeminal afferents in postural control via vestibulospinal pathways and vestibular nuclei, respectively. However, as highlighted in the review by Sánchez et al. [3], the involvement of proprioceptive information from the masticatory apparatus remains controversial. Part of this controversy stems from its limited use in postural adjustments, making its involvement difficult to demonstrate. It may also be due to the type of the occlusal criterion used to select experimental participants and/or to methodological differences. In most of these postural studies, the occlusal criterion was defined according to either skeletal typology (maxillary-mandibular relationship) [4] or Angle classification (maxillary-mandibular tooth relationship) [1]. It has rarely been defined in terms of occlusal functions, namely occlusal stability in the intercuspal position (ICP), mandibular centering in the ICP, and anterior dental guidance of mandibular movements [5]. These occlusal functions, by regulating mandibular function through proprioception of the masticatory apparatus, could be the most involved in postural regulation. Furthemore, interocclusal devices or cotton rolls have been used to disturb occlusion in studies that have focused on the effect of dental occlusion on postural adjustment [6,7]. The disadvantage of these devices is that, by interfering with the teeth, gums, and jugular or labial mucosa, they impair not only dental occlusion but also many of the sensory afferents of the masticatory apparatus. Finally, postural assessment has been performed under different acquisition conditions that are likely to influence the postural parameters: the test duration (shorter [8,9], equal [4] or longer [10] than 30 s), the visual condition (with and without vision [6] or only in one of these two situations [11]) and the mandibular position adopted [4,11,12].
Among these mandibular positions, the rest position (RP) and the intercuspal position (ICP) are often used separately or together [3,13]. These two positions are opposite in terms of the amount of occlusal proprioceptive information from the mechanoreceptors of the periodontal ligaments: RP has no inter-occlusal contact, whereas ICP has maximum occlusal contact between the teeth. Conversely, eccentric occlusion (EO), which corresponds to the end of the lateral guidance pathway (either left or right), has been reported to play a major role in muscle activity and masticatory function along with the ICP [14,15], but has been less studied [16]. Similarly, while in stabilometric studies the mandibular position is generally freely chosen by the participants, this FREE position was rarely used in stabilometric studies focusing on the role of occlusion.
Manipulating the availability and/or amplitude of sensory information from one or more sensory system is one way to investigate the corresponding contribution to postural control, and the sensory reweighting that may result [17]. In the present stabilometric study, the effect of occlusal proprioception on postural adjustments was quantified in the standing position as a function of Class I occlusal characteristics (normocclusion vs. anterior open bite malocclusion) (Fig. 1), mandibular position (RP, FREE, ICP and EO) and visual condition (with or without). Due to the large variability in the test duration between studies, all parameters were compared between the first and last halves of the 51.2 s acquisition time recommended by the French Association of Posturology [18].
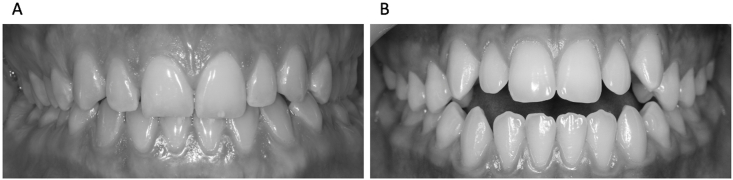
Fig. 1.
Two types of Angle Class I occlusion. Class I normocclusion (A) and anterior open bite malocclusion (B).
The first hypothesis was that static postural balance would be influenced by the amount of occlusal proprioceptive information, with more disturbances in the malocclusion group than in the normocclusion group, and in the mandibular positions with no (RP) or a reduced number (FREE) of occlusal contacts compared to the ICP position, especially without vision.
The second hypothesis was that eccentric mandibular occlusion (EO), regardless of its side, would result in less stability than ICP due to the reduced number of mechanoreceptors involved, but with similar effects in both normal and malocclusion groups.
2. Materials and methods
2.1. Participants
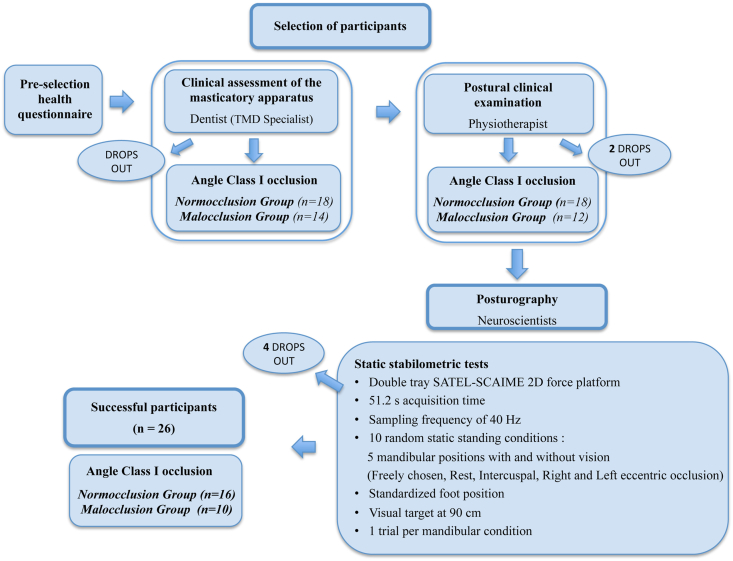
Prior to the experiment, the sample size was calculated using G*Power (Version 3.1.9.7) [19]. According to the experimental design and for a medium effect size (i.e. η2 = 0.06), 14 participants in each group were required to obtain a statistical power of 80%. As summarized in the flowchart (Fig. 2), participants, age-matched between 20 and 24 years, were selected based on a health questionnaire, followed by a clinical assessment of their masticatory apparatus by a dentist (Temporo-Mandibular Specialist) and a clinical postural examination by a physiotherapist (inclusion and exclusion criteria are detailed below). After a complete clinical examination, 30 healthy adults with a Class I Angle occlusion, defined as a normal sagittal intermaxillary position of both the first molar and the canine [20], were selected for the stabilometric study and completed all test sessions. However, after additional drops out (dental pain, discomfort, ankle sprain after recruitment, not previously reported by the participants, and poor recording quality), only the results of 26 of them were retained (Table 1; Supplementary Table 1 for individual values). The normocclusion group consisted of 16 participants (10 males and 6 females). The malocclusion group consisted of only 10 participants (3 males and 7 females). Despite a lower than expected number of participants, the statistical power obtained was greater than 65% (for a medium effect size), which is correct according to various studies in the field of biomechanics (statistical power <50%) [e.g. [21]].
Fig. 2.
Flowchart of Materials and Methods. Tmd, temporo-mandibular disorders.
Table 1.
Characteristics of participants (mean ± SD) per group.
| Whole group (n = 26) | Normocclusion group (n = 16) | Malocclusion group (n = 10) | |
|---|---|---|---|
| Gender | 13♂/13♀ | 10♂/6♀ | 3♂/7♀ |
| Age (yr.) | 22.1 ± 1.5 | 22.1 ± 1.3 | 22.1 ± 1.7 |
| Height (cm) | 171 ± 10 | 171 ± 12 | 170 ± 7 |
| Body mass (kg) | 61.7 ± 9.9 | 62.1 ± 9.9 | 61.0 ± 10.2 |
The clinical assessment of the masticatory apparatus included examination of the temporo-mandibular joint, muscles, and dental occlusion in accordance with international recommendations on diagnostic criteria for temporo-mandibular disorders for clinical and research applications [22] and the occlusal analysis reported by Okeson [20]. Dental occlusion was accurately analyzed using marker tapes (Bausch 8 μm) of three different colors and a Miller clamp. Inclusion criteria used for each group were as follows: The normocclusion group consisted of participants of Class I without malocclusion according to Casazza et al. [5], with anterior guidance (group or canine guidance in laterotrusion), stable intercuspal position (ICP) (equal distribution and simultaneity of occlusal contacts) and centered ICP (no difference between centric relation and ICP greater than 1 mm antero-posteriorly and 0.5 mm mediolaterally) (Fig. 1A). The malocclusion group included participants of Class I with anterior open bite of more than 4 anterior teeth (Fig. 1B). The following exclusion criteria were applied: Temporo-Mandibular Disorders, grinding, crown, 3 or more restored teeth, asymmetrical ICP due to the loss of 1 or 2 M, uncentered ICP, neuropathology, postural or visual disorders, vestibular syndrome, nicotine or alcohol use in the last 24 h, difficulty in achieving requested mandibular positions.
Clinical postural examination was designed to detect any major anatomical postural disorder (e.g., scoliosis) while standing in an erect position facing a grid-patterned wallpaper in a controlled standardized foot position.
2.2. Ethics statement
This study was conducted in accordance with the Declaration of Helsinki. All procedures were reviewed and approved by the local Ethics Committee of Sud Méditerranée II (n° ID-RCB2012-AOO510-35). The participants provided their written informed consent to participate in this study.
2.3. Static stabilometric tests
All tests took place in the same laboratory environment and were performed by the same experimenters. The experimenter responsible for the postural assessment was unaware whether the participant was in the normocclusion or malocclusion group.
The stabilometric postural tests were performed under 10 random static standing conditions: 5 mandibular positions, with and without vision. Reliable stabilometric parameters have been reported when using a single trial of 25–40 s [23]. Due to the number of testing conditions and the total testing time, only one trial was performed for each condition. Each trial lasted 51.2 s, with a rest period of 30 s between trials. During each trial, participants stood as still as possible, barefoot, on a stabilometric platform. A removable wedge was used to ensure a given standardized foot position (heels 2 cm apart, opening angle of 30°). In all testing conditions, the participant wore a mask that laterally restricted the visual field. In the tests performed with vision, the participant was instructed to look straight ahead while standing 90 cm away from a visual target covered with randomly displaced printed geometric patterns to prevent exploratory saccades [24]. In the tests without vision, the mask was covered by a movable opaque visor while the participant was instructed to keep on looking straight ahead with both eyes opened. Five mandibular positions were tested: (1) the freely chosen position (FREE), without any instruction regarding tooth contact and tongue position, (2) the resting position (RP), in which the teeth are not in contact and tongue is not between the teeth [25], (3) the intercuspal position (ICP), in which the teeth make a maximum number of inter-occlusal contacts without clenching [26], (4 and 5) the eccentric lateral occlusion (EO) on edge-to-edge left and right obtained when the first pair of antagonist teeth make contact on the left or right side [8]. A phase of familiarization with these mandibular positions (except for FREE) preceded the acquisition phase.
All postural tests were performed on a double tray SATEL-SCAIME 2D-static force platform dimensioned: 2*(480 × 480 × 65 mm), equipped with 8 piezoelectric sensors. The force sensors are of SP4 “constant moment beams” type, with a precision of “C3 weighing class” and a sensitivity of 0.017% (2.0 mV/V ± 0.1). The acquisition time was 51.2 s at a sampling frequency of 40 Hz. The stabilometric platform was connected by USB port to a PC computer with a 24-bit analog-to-digital converter. The device was previously tested for reliability and validity [27].
2.4. Data analyses
Data were processed using MATLAB software (version 9.3-R2017b-Mathworks Inc., Novi, USA). The 4 variables commonly used to qualify postural balance were calculated from the force platform sensor data: length (in mm) and sway area (area of the 95% confidence ellipse in mm2) of the CoP displacement, mean position of the CoP on the anteroposterior and mediolateral axes (in mm relative to the center point (0,0) of the stabilometric platform). These dependent variables were calculated for each of the 10 tested conditions (5 mandibular positions, with and without vision) and for the first and last 25.6 s time periods. A 5th variable, the Romberg Quotient (100*area without vision/area with vision) was also computed [28]. The Romberg Quotient was used to determine individuals for whom visual information can be considered preponderant (>1) or not (≤1) in static postural control in standing position. For eccentric dental occlusion (EO), the sways area revealed significant inter-individual differences, with some participants being more stable on the left edge-to-edge side and others on the right side. We therefore distinguished the EO side for which the body sway area with vision was the smallest, called EOmin, from the other EOmax. The individually obtained EOmin and EOmax were retained for subsequent analysis of all other variables (e.g., displacement length) with and without vision.
2.5. Statistical analysis
Statistical analyses were performed with R software (v3.6.3, R Core Team, 2020, R Foundation for Statistical Computing, Vienna, Austria) and JASP (version 0.13.1). The Shapiro-Wilk test was used to check the normality of the data based on the residuals. As the data did not follow a normal distribution, a permutation ANOVA (lmPerm package for R, the R Core Team, 2020) was performed for the 5 dependent variables. The within-participant factors were vision (with vision, without), mandibular position (FREE, RP, ICP, EOmin, EOmax), and acquisition time (first and last 25.6 s). The between factor was the occlusion group (normocclusion, malocclusion). When a significant effect was found, planned contrasts were performed to assess factor-specific changes and to limit the number of pairwise comparisons. Indeed, it is not relevant to compare the effects of a given mandibular position in a condition with vision with those of another mandibular position without vision or the effects of FREE and RP with those of EOmin and EOmax. The alpha level was set at 0.05. Effect size (ES) was computed using Cohen's d coefficient [29]. It was assessed using the following thresholds: <0.2, 0.2 to <0.6, 0.6–1.2, and >1.2 for trivial, small, moderate and large effects, respectively [30]. Trivial effects (ES < 0.2) are not reported.
3. Results
3.1. Sway area
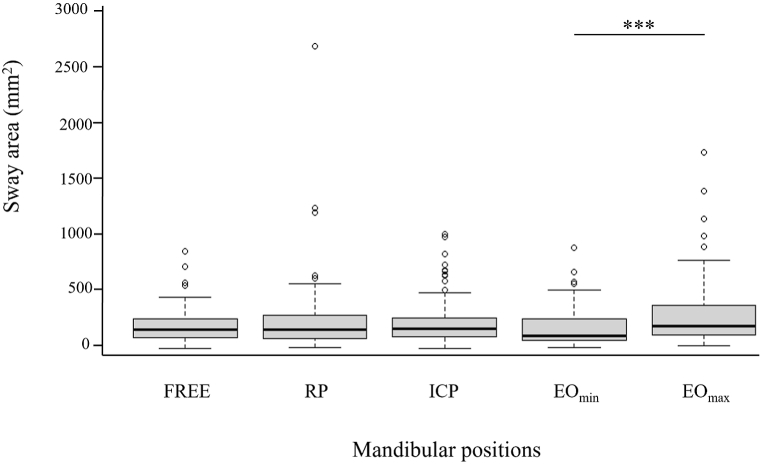
The analysis revealed significant effects of mandibular position (F(4,96) = 4.532, p < 0.001), acquisition time (F(1,24) = 6.061, p < 0.01) and vision (F(1,24) = 17.912, p < 0.001). As shown in Fig. 3, there was a difference between EOmin and EOmax resulting from the selection of these two eccentric positions. Independently of the visual condition, the sway area was also larger during the last 25.6 s compared to the first, and for without vision compared to the visual condition (Table 2).
Fig. 3.
Sway area as a function of mandibular position. Data are presented as median ± interquartile range. Extreme values are indicated by dots. ***p < 0.001 between mandibular positions.
Table 2.
Effect of the experimental conditions on postural parameters. Mean values (±SD) of postural parameters showing a significant experimental condition effect.
| Parameters | Vision condition | Mandibular position | Acquisition time |
|---|---|---|---|
| Sway area (mm2) Planned contrasts |
V: 204 ± 127 NV: 297 ± 205 ------------------------ V < NV *** ES = 0.56 |
EOmin: 200 ± 171 EOmax: 311 ± 287 --------------------- EOmin < EOmax*** ES = 0.55 |
First 25.6 s: 227 ± 132 Last 25.6 s: 274 ± 196 ------------------------ First < Last 25.6 s * ES = 0.28 |
| Romberg Quotient Planned contrasts |
NS | EOmin: 2.19 ± 1.36 EOmax: 1.63 ± 0.74 ICP: 1.58 ± 0.82 ---------------------- EOmin > EOmax* ES = 0.53 EOmin>ICP** ES = 0.56 |
NS |
| Mean Length (mm) Planned contrasts |
V: 210 ± 69 NV: 274 ± 87 ------------------------ V < NV*** ES = 0.82 |
NS | NS |
| Mean antero-posterior position (mm) Planned contrasts |
V: 4 ± 22 NV: 1 ± 22 ------------------------ V < NV*** ES = 0,21 |
NS | NS |
V: with vision, NV: without vision; ES: effect size; *p < 0.05, **p < 0.01, ***p < 0.001; NS: not significant.
3.2. Romberg Quotient
This analysis revealed a significant effect of mandibular position (F(4,96) = 2.148; p < 0.05). The Romberg quotient was greater in EOmin than in ICP and EOmax (Table 2). No difference was found between the 3 centered mandibular positions (FREE, RP and ICP).
3.3. Mean length of CoP displacement
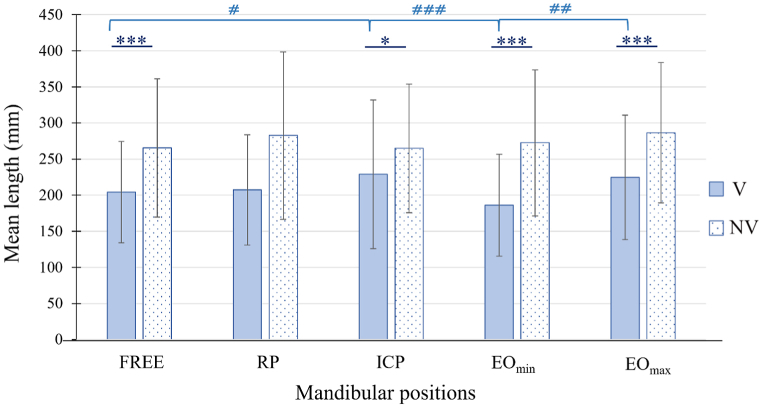
This analysis revealed a significant main effect of vision (F(1,24) = 50.368; p < 0.001). Independently of mandibular positions, the mean length of CoP displacement was longer (28% average increase) without vision than with vision (Table 2).
A significant interaction vision × mandibular position was found (F(4,96) = 3.185; p < 0.01). As shown by Fig. 4, the length of CoP displacement was less affected by the loss of vision in ICP than in all other mandibular positions (Table 3). This interaction vision × mandibular position resulted from the lack of difference between mandibular positions without vision, while significant differences were found with vision. Mean length of CoP displacement was longer in ICP than in FREE (p < 0.05; ES = 0.30) and was smaller in EOmin than in EOmax (p < 0.01; ES = 0.51) and ICP (p < 0.001; ES = 0.51).
Fig. 4.
Mean length of CoP displacement as a function of mandibular position and visual condition. Mean (±SD) length of CoP displacement with vision (V) and without vision (NV). *p < 0.05 and ***p < 0.001 between visual conditions. #p < 0.05, ##p < 0.01, ###p < 0.001 between mandibular positions in the condition with vision.
Table 3.
Absolute change in mean length of CoP displacement between visual conditions in each mandibular position.
| Mandibular Position |
Difference in Length of CoP displacement (mm) | Effect Size |
|---|---|---|
| FREE RP ICP EOmin EOmax |
61 ± 68 ** 75 ± 77 *** 36 ± 82 * 86 ± 74 *** 62 ± 64 *** |
0.76 0.83 0.39 1.04 0.71 |
*p < 0.05, **p < 0.01, ***p < 0.001.
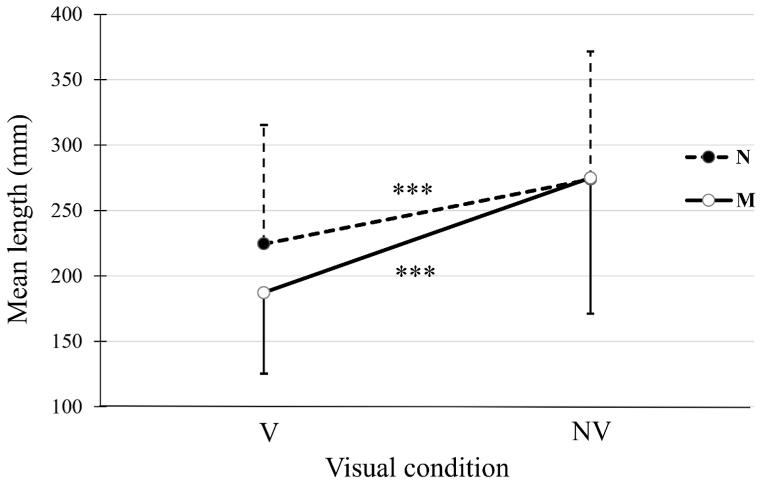
A significant interaction vision × group was found (F(1, 24) = 3.928; p < 0.001). The increase of CoP displacement length between the visual conditions was larger for the malocclusion group than for the normocclusion group (88 ± 50 mm, ES = 1.37 vs. 61 ± 47 mm, ES = 0.64; p < 0.001) (Fig. 5). However, no group effect was found in each visual condition.
Fig. 5.
Mean length of CoP displacement as a function of visual condition in each group. Mean length (±SD) of CoP displacement for the normocclusion (N) and malocclusion (M) groups. ***: p < 0.001 between the conditions with vision (V) and without vision (NV) for each group.
3.4. Mean CoP positions
Analyses of the mean CoP position revealed significant main effect and interactions along the antero-posterior, but not the medio-lateral axis. A significant effect of vision (F(1, 24) = 14.107; p < 0.001) was found for the antero-posterior axis: the no-vision condition was associated with a more forward mean CoP positioning than the vision condition. A vision × mandibular position interaction was found (F(4,96) = 2.053; p < 0.05), a more forward displacement being observed in FREE, ICP and EOmax with the vision loss (Table 4).
Table 4.
Absolute changes in mean CoP position on the antero-posterior axis (mm) between visual conditions.
|
Mandibular position |
Whole Group |
Normocclusion |
Malocclusion |
|||
|---|---|---|---|---|---|---|
| Mean ± SD | ES | Mean ± SD | ES | Mean ± SD | ES | |
| FREE | 6 ± 12 * | 0.26 | 10 ± 13 *** | 0.39 | NS | |
| RP | NS | 5 ± 9 * | 0.21 | NS | ||
| ICP | 6 ± 11 ** | 0.25 | 4 ± 12 | 9 ± 8 ** | 0.43 | |
| EOmin | NS | NS | NS | |||
| EOmax | 7 ± 9 *** | 0.31 | 7 ± 8 ** | 0.31 | 6 ± 11 * | 0.33 |
ES, effect size; *p < 0.05, **p < 0.01, ***p < 0.001; NS, not significant.
A triple interaction vision × mandibular position × group (F(4, 96)=2.199; p < 0.01) was also identified. The vision loss affected differently the two groups (Table 4). In the centric mandibular positions: the normocclusion group adopted a more forward positioning in FREE and RP, but not in ICP. For the malocclusion group, this more forward positioning was observed in ICP only. Regarding the eccentric mandibular positions, both groups showed a forward displacement with the vision loss in EOmax only. For the with-vision condition, the normocclusion group was positioned further forward in EOmin than in EOmax (p < 0.05; ES = 0.23) and the malocclusion group in EOmin than in ICP (p < 0.05; ES = 0.30).
4. Discussion
To our knowledge, this is the first study to compare various postural parameters in centric and eccentric occlusion conditions without the use of interocclusal devices or wax. Regardless of mandibular position and group, most of the stabilometric parameters studied showed significant differences between the two visual conditions, confirming the stabilizing role of visual information in postural control classically reported in the literature [26,31,32]. The loss of vision led to the adoption of a slightly more forward position, which could be explained by the loss of perception of visual verticality. In all experimental conditions, all postural parameters studied (except for the sway area) did not differ between the first and last halves of the total acquisition time. Thus, a test duration of 25.6 s should be sufficient to highlight some effects of occlusal proprioceptive information on postural control.
Our first hypothesis that static postural balance would be influenced by the amount of occlusal proprioceptive information, especially in the absence of vision, was partially confirmed. Differences between mandibular positions were found with vision rather than without vision, and interactions with the mandibular position or with the group were found between the two visual conditions.
For the sway area and the mean length of CoP displacement, regardless of the visual condition, no significant differences were found between the RP and ICP mandibular positions, or between the groups. This agrees with the absence of effect of mandibular position and/or occlusal characteristics on posturographic parameters, except for excessive “overbite” [e.g., [[31], [33]]]. In contrast, in asymptomatic participants and with vision, Sakaguchi et al. [8] reported a longer length in RP than in ICP, whereas Amaricai et al. [13] reported a smaller sway area in RP than in ICP. Other studies, such as Nowak et al. [28], focused only on the influence of severe malocclusion in ICP. They reported an increased instability (increased mean length and sway area) in case of malocclusion (Class II and III) compared to normocclusion (Class I), but no inter-group difference in RQ. The latter result is in line with our own observations. The conflicting results between RP and ICP could be attributed to the large variability in the mandibular position adopted in RP [34], which may have contributed to the large dispersion observed for the sway area in the current study. Regarding the effects of malocclusion on postural stabilization, additional factors such as occlusal criterion and malocclusion severity may explain the heterogeneity of results.
Interestingly, a shorter length was found in the freely chosen (FREE) mandibular position than in the ICP with vision, while a larger increase was found in FREE between the two visual conditions (with and without vision). FREE is expected to have fewer contacts and therefore less occlusal proprioceptive information compared to ICP. Based on the relative contribution of sensory information to postural control and its variation according to its availability and environmental conditions, known as “sensory reweighting” [35], the weight of visual information in postural balance should be greater in FREE than in ICP. Furthermore, when test conditions induce a change in the weight of one sensory information, that change is compensated for by changes in the weight of other sensory systems [36]. In this line, the current loss of vision had a greater effect in FREE, as shown by a greater increase in the mean length of CoP displacement than in ICP. This is also supported by the Vision × Group interaction found for the mean length parameter: With loss of vision, it increased more for the malocclusion group (with less relevant occlusal proprioceptive information) than for the normocclusion group.
Regarding the change in mean CoP position along the antero-posterior axis with vision loss, only the normocclusion group moved forward in FREE and RP. The lack of significant shift in the malocclusion group could be attributed to the additional proprioceptive information provided by the lingual interposition in these two mandibular positions [37]. In ICP, only the malocclusion group moved forward, possibly because reduced anterior occlusal contact resulted in less proprioceptive information.
These overall results suggest that proprioceptive afferents of occlusal origin are involved in stabilizing the upright position under visual condition. Without vision, however, their influence was negligible, suggesting a sensory reweighting to limit postural disturbances, characterized by an increased contribution of other sensory inputs, especially vestibular, as hypothesized by Gangloff and Perrin [1].
Our second hypothesis regarding eccentric (EO) mandibular positions was partially supported: As expected, EO mandibular positions showed similar effects on postural adjustments in both groups. However, despite the limited number of tooth contacts, the stability was not lower than in ICP. Regardless of the group, in the visual condition, postural stability did not differ between ICP and EOmax, and it was even improved in EOmin (smaller sway area and mean length of CoP displacement). Interestingly, the loss of vision resulted in similar Romberg Quotient in ICP and EOmax, EOmin showing a larger one.
Based on sensory reweighting, if proprioceptive information of occlusal origin would have been the source of the greater postural stability in EOmin, removal of visual information should have had little effect. However, this was rather observed in EOmax. These results suggest a relatively greater influence of visual information in EOmin and occlusal proprioceptive information in EOmax. This asymmetry was observed in all participants and cannot be explained by the type of dental guidance or by an asymmetry in the masticatory cycle. Indeed, the participants with normocclusion had similar lateral occlusal guidance on both sides, whereas those with malocclusion should have been affected by a masticatory cycle asymmetry [38,39]. Furthermore, since suppression of vision resulted in both groups in an anterior shift in EOmax but not in EOmin, this asymmetry appears to be independent of occlusal characteristics. This could be related to a hemispheric predominance, as reported during mastication [40,41].
This study has limitations. Firstly, the diversity of protocols reported in the literature, particularly in terms of acquisition time, number of trials, standardization data, mandibular positions, and occlusal criteria, made it difficult to identify a protocol that would allow direct comparisons [4,42,43]. Secondly, although several studies [12,44,45] suggested the use of 3 repetitions to obtain reliable CoP parameters, the present protocol included only one trial for each test per session. Each participant completed 10 trials of 51.2 s each, with 30 s rest period in between. This choice was made to limit the decline in attention over the trials [46]. In the literature, stable and reliable stabilometric parameters have been reported for acquisition times of 25–40 s [23], whereas increased inter-individual variability has been found for repeated trials [47]. Thirdly, although a robust statistical analysis was used, it does not compensate for the imbalance and large inter-individual variability in the normocclusion and malocclusion groups. Finally, although some authors considered postural parameters to be gender dependent, others disagreed [48].
5. Conclusion
This study showed, with an acquisition time close to or equal to 30 s, that periodontal proprioceptive afferents had some influence on postural adjustments in the standing position. This effect was evident on the basis of the comparison of the postural parameters between the visual conditions (with and without vision). It showed a relatively greater role for visual information with fewer tooth contacts (freely chosen mandibular position) than with more (intercuspal mandibular position) and for participants with Class I anterior openbite malocclusion. It showed also an asymmetric weight of eccentric occlusal information. To assess the role of occlusal information in static postural balance, the rest mandibular position was not the most appropriate. Although these results are not clinically relevant, future studies of standing postural regulation should consider dental occlusion criteria and investigate the potential existence of a lateral dominance of occlusal proprioceptive information.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution statement
Anne GIRAUDEAU and Joëlle BARTHELEMY: conceived, designed and performed the experiments; analyzed and interpreted the data and wrote the paper. Caroline NICOL: analyzed and interpreted the data and wrote the paper. Robin MACCHI: analyzed and interpreted the data. Thelma COYLE analyzed and interpreted the data, contributed reagents, materials, analysis tools or data. Serge MESURE, Kelly BERDHA SETBON and Jean Daniel ORTHLIEB: performed the experiments.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Céline Boulanger, Mélanie Joinnet and Gaelle Cabon for their contribution to the recruitment of participants and the conduct of the experiment.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e20309.
List of abbreviations
- CoP
Podal Center of Pressure
- ICP
Mandibular Intercuspal Position
- FREE
Freely chosen mandibular position
- EO
Eccentric lateral edge-to-edge occlusion
- EOmin
EO side for which the body sway area was smallest with vision
- EOmax
EO side for which the body sway area was greatest with vision
- RP
Mandibular Rest Position
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gangloff P., Perrin P.P. Unilateral trigeminal anaesthesia modifies postural control in human subjects. Neurosci. Lett. 2002;330(2):179–182. doi: 10.1016/S0304-3940(02)00779-6. [DOI] [PubMed] [Google Scholar]
- 2.Petrosini L., Troiani D. Vestibular compensation after hemilabyrinthectomy: effects of trigeminal neurotomy. Physiol. Behav. 1979;22(1):133–137. doi: 10.1016/0031-9384(79)90414-1. [DOI] [PubMed] [Google Scholar]
- 3.Sánchez S.J., Álvarez Herms J., Burtscher M. Dental occlusion and body balance: a question of environmental constraints? J. Oral Rehabil. 2019;46(4):388–397. doi: 10.1111/joor.12767. [DOI] [PubMed] [Google Scholar]
- 4.Arumugam P., Padmanabhan S., Chitharanjan A.B. The relationship of postural body stability and severity of malocclusion. APOS Trends Orthod. 2016;6:205–210. doi: 10.4103/2321-1407.186436. [DOI] [Google Scholar]
- 5.Casazza E., Ré J.P., Giraudeau A., Parfu A., Orthlieb J.D. Dental occlusion: proposal for a classification to guide occlusal analysis and optimize research protocols. J. Contemp. Dent. Pract. 2021;22(7):840–849. doi: 10.5005/jp-journals-10024-3113. [DOI] [PubMed] [Google Scholar]
- 6.Isaia B., Ravarotto M., Finotti P., Nogara M., Piran G., Gamberini J., Biz C., Masiero S., Frizziero A. Analysis of dental malocclusion and neuromotor control in young healthy subjects through new evaluation tools. J. Funct. Morphol. Kinesiol. 2019;4(1):5. doi: 10.3390/jfmk4010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabrera-Domínguez M.E., Domínguez-Reyes A., Pabón-Carrasco M., Pérez-Belloso A.J., Coheña-Jiménez M., Galán-González A.F. Dental malocclusion and its relation to the podal system. Frontiers in Pediatrics. 2021;9 doi: 10.3389/fped.2021.654229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi K., Mehta N.R., Abdallah E.F., Forgione A.G., Hirayama H., Kawasaki T., Yokoyama A. Examination of the relationship between mandibular position and body posture. Cranio. 2007;25(4):237–249. doi: 10.1179/crn.2007.037. 10.1179/crn.2007.037. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L., Xu L., Lu J., Cai B., Fan S. Static balance in participants with temporomandibular joint disc displacement without reduction versus healthy participants: a cross-sectional study. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2022;28 doi: 10.12659/MSM.934593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zurita-Hernandez J., Ayuso-Montero R., Cuartero-Balana M., Willaert E., Martinez-Gomis J. Relationship between unilateral posterior crossbite and human static body posture. Int. J. Environ. Res. Public Health, 2020. 2020;17(15):1–10. doi: 10.3390/ijerph17155303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohlendorf D., Fay V., Avaniadi I., Erbe C., Wanke E.M., Groneberg D.A. Association between constitution, axiography, analysis of dental casts, and postural control in women aged between 41 and 50 years. Clin. Oral Invest. 2021;25(5):2595–2607. doi: 10.1007/s00784-020-03571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scharnweber B., Adjami F., Schuster G., Kopp S., Natrup J., Erbe C., Ohlendorf D. Influence of dental occlusion on postural control and plantar pressure distribution. Cranio. 2017;35(6):358–366. doi: 10.1080/08869634.2016.1244971. [DOI] [PubMed] [Google Scholar]
- 13.Amaricai E., Onofrei R.R., Suciu O., Marcauteanu C., Stoica E.T., Negruțiu M.L., David V.L., Sinescu C. Do different dental conditions influence the static plantar pressure and stabilometry in young adults? PLoS One. 2020;15(2) doi: 10.1371/journal.pone.0228816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald J.W.C., Hannam A.G. Relationship between occlusal contacts and jaw-closing muscle activity during tooth clenching: Part I. J. Prosthet. Dent. 1984;52(5):718–729. doi: 10.1016/0022-3913(84)90149-5. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa T., Ogawa M., Koyano K. Different responses of masticatory movements after alteration of occlusal guidance related to individual movement pattern. J. Oral Rehabil. 2001;28(9):830–841. doi: 10.1046/j.1365-2842.2001.00672.x. [DOI] [PubMed] [Google Scholar]
- 16.Michalakis K.X., Kamalakidis S.N., Pissiotis A.L., Hirayama H. The effect of clenching and Occlusal instability on body weight distribution, Assessed by a Postural Platform. BioMed Res. Int. 2019 doi: 10.1155/2019/7342541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assländer L., Peterka R.J. Sensory reweighting dynamics in human postural control. J. Neurophysiol. (Bethesda) 2014;111(9):1852–1864. doi: 10.1152/jn.00669.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagey P.M., Weber B. Study of intra-subject random variations of stabilometric parameters. Med. Biol. Eng. Comput. 2010;48(8):833–835. doi: 10.1007/s11517-010-0656-4. [DOI] [PubMed] [Google Scholar]
- 19.Faul F., Erdfelder E., Lang A.G., Buchner A.G.* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 20.Okeson J.P. Management of Temporomandibular Disorders and Occlusion, Part I: Functional Anatomy. eighth ed. Elsevier Mosby; St Louis: 2019. pp. 47–100. ISBN 9780323676748. [Google Scholar]
- 21.Knudson D. Confidence crisis of results in biomechanics research. Sports BioMech. 2017;16(4):425–433. doi: 10.1080/14763141.2016.1246603. [DOI] [PubMed] [Google Scholar]
- 22.Schiffman E., Ohrbach R., Truelove E., Look J., Anderson G., Goulet J.P., List T., Svensson P., Gonzalez Y., Lobbezoo F., Michelotti A., Brooks S.L., Ceusters W., Drangsholt M., Ettlin D., Gaul C., Goldberg L.J., Haythornthwaite J.A., Hollender L., Jensen R., John M.T., De Laat A., de Leeuw R., Maixner W., van der Meulen M., Murray G.M., Nixdorf D.R., Palla S., Petersson A., Pionchon P., Smith B., Visscher C.M., Zakrzewska J., Dworkin S.F. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network and orofacial pain special interest group. J. Oral Facial Pain Headache. 2014;28(1):6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scoppa F., Capra R., Gallamini M., Shiffer R. Clinical stabilometry standardization: basic definitions–acquisition interval–sampling frequency. Gait Posture. 2013;37(2):290–292. doi: 10.1016/j.gaitpost.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Paya-Argoud M., Tardieu C., Cheynet F., Raskin A., Borel L. Impact of orthognathic surgery on the body posture. Gait Posture. 2019;67:25–30. doi: 10.1016/j.gaitpost.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz G., Uginčius P., Sebik O., Türker K.S. Tonic activity of the human temporalis muscle at mandibular rest position. Arch. Oral Biol. 2015;60(11):1645–1649. doi: 10.1016/j.archoralbio.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Perinetti G. Dental occlusion and body posture: no detectable correlation. Gait Posture. 2006;24(2):165–168. doi: 10.1016/j.gaitpost.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Rubio P.R., Bagur-Calafat C., López-de-Celis C., Bueno-Gracía E., Cabanas-Valdés R., Herrera-Pedroviejo E., Girabent-Farrés M. Validity and reliability of the satel 40 HZ stabilometric force platform for measuring quiet stance and dynamic standing balance in healthy subjects. Int. J. Environ. Res. Publ. Health. 2020;17(21):1–14. doi: 10.3390/ijerph17217733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowak M., Golec J., Wieczorek A., Golec P. Is there a correlation between dental occlusion, postural stability and selected gait parameters in adults? Int. J. Environ. Res. Publ. Health. 2023;20(2):1652. doi: 10.3390/ijerph20021652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J. second ed. Routledge; New York, USA: 1988. Statistical Power Analysis for the Behavioral Sciences; p. 567. [DOI] [Google Scholar]
- 30.Hopkins W., Marshall S., Batterham A., Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009;41(1):3–12. doi: 10.1249/MSS.0b013e31818cb278. [DOI] [PubMed] [Google Scholar]
- 31.Perinetti G. Temporomandibular disorders do not correlate with detectable alterations in body posture. J. Contemp. Dent. Pract. 2007;8(5):60–67. PMID: 16275097. [PubMed] [Google Scholar]
- 32.Baldini A., Nota A., Tripodi D., Longoni S., Cozza P. Evaluation of the correlation between dental occlusion and posture using a force platform. Clinics. 2013;68(1):45–49. doi: 10.6061/clinics/2013(01)oa07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perinetti G., Contardo L., Silvestrini-Biavati A., Perdoni L., Castaldo A. Dental malocclusion and body posture in young subjects: a multiple regression study. Clinics. 2010;65(7):689–695. doi: 10.1590/S1807-59322010000700007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michelotti A., Farella M., Vollaro S., Martina R. Mandibular rest position and electrical activity of the masticatory muscles. J. Prosthet. Dent. 1997;78(1):48–53. doi: 10.1016/S0022-3913(97)70087-8. [DOI] [PubMed] [Google Scholar]
- 35.Nashner L., Berthoz A. Visual contribution to rapid motor responses during postural control. Brain Res. 1978;150:403–407. doi: 10.1016/0006-8993(78)90291-3. [DOI] [PubMed] [Google Scholar]
- 36.Cenciarini M., Peterka R.J. Stimulus-dependent changes in the vestibular contribution to human postural control. J. Neurophysiol. 2006;95:2733–2750. doi: 10.1152/jn.00856.2004. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi H., Sueishi K. Malocclusion associated with abnormal posture. Bull. Tokyo Dent. Coll. 2003;44(2):43–54. doi: 10.2209/tdcpublication.44.43. [DOI] [PubMed] [Google Scholar]
- 38.Piancino M.G., Kyrkanides S. John Wiley & Sons; Ames, Iowa: 2016. Understanding Masticatory Function in Unilateral Crossbites; pp. 43–47. [DOI] [Google Scholar]
- 39.Slavicek G. The influence of occlusion on masticatory efficiency considering relevant influencing factors. Stoma Edu J. 2020;7(3):197–207. 10.25241/stomaeduj.2020.7(3).art.6. [Google Scholar]
- 40.Shinagawa H., Ono T., Ishiwata Y., Honda E., Sasaki T., Taira M., Iriki A., Kuroda T. Hemispheric dominance of tongue control depends on the chewing-side preference. J. Dent. Res. 2003;82(4):278–283. doi: 10.1177/154405910308200407. [DOI] [PubMed] [Google Scholar]
- 41.Nissan J., Gross M., Shifman A., Tzadok L., Assif D. Chewing side preference as a type of hemispheric laterality. J. Oral Rehabil. 2004;31(5):412–416. doi: 10.1111/j.1365-2842.2004.01256.x. [DOI] [PubMed] [Google Scholar]
- 42.Perinetti G., Primožič J., Manfredini D., Di Lenarda R., Contardo L. The diagnostic potential of static body-sway recording in orthodontics: a systematic review. Eur. J. Orthod. 2012;35(5):696–705. doi: 10.1093/ejo/cjs085. [DOI] [PubMed] [Google Scholar]
- 43.Michelotti A., Buonocore G., Farella M., Pellegrino G., Piergentili C., Altobelli S., Martina R. Postural stability and unilateral posterior crossbite: is there a relationship? Neurosci. Lett. 2006;392(1–2):140–144. doi: 10.1016/j.neulet.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Nota A., Tecco S., Cioffi C., Beraldi Padulo J., Baldini A. Occlusion time analysis in military pilots affected by bruxism. Sci. Rep. 2019;9(1):1–4. doi: 10.1038/s41598-018-38166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinsault N., Vuillerme N. Test–retest reliability of centre of foot pressure measures to assess postural control during unperturbed stance. Med. Eng. Phys. 2009;31(2):276–286. doi: 10.1016/j.medengphy.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Taylor M.R., Sutton E.E., Diestelkamp W.S., Bigelow K.E. Subtle differences during Posturography testing can influence postural sway results: the effects of talking, time before data acquisition, and visual fixation. J. Appl. Biomech. 2015;31(5):324–329. doi: 10.1123/jab.2014-0279. [DOI] [PubMed] [Google Scholar]
- 47.Pagnacco G., Carrick F.R., Wright C.H., Oggero E. Between-subjects differences of within-subject variability in repeated balance measures: consequences on the minimum detectable change. Gait Posture. 2015;41(1):136–140. doi: 10.1016/j.gaitpost.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 48.Maurer C., Holzgreve F., Erbe C., Wanke E.M., Kopp S., Groneberg D.A., et al. Influence of dental occlusion conditions on plantar pressure distribution during standing and walking–a gender perspective. Med. Eng. Phys. 2021;88:47–53. doi: 10.1016/j.medengphy.2020.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.