Abstract
Amphotericin B colloidal dispersion (ABCD) is a new formulation of conventional amphotericin B designed to minimize drug distribution in the kidney and reduce nephrotoxicity. We studied the safety and efficacy of ABCD in 133 renally compromised patients with invasive fungal infections. Patients had either nephrotoxicity from amphotericin B or preexisting renal disease. Intravenous treatment with ABCD (4 mg/kg of body weight daily) was administered for up to 6 weeks. Evaluations included clinical response to treatment and adverse events, with emphasis on changes in serum creatinine levels. ABCD did not appear to have an adverse effect on renal function: mean serum creatinine level tended to decrease slightly with days on therapy, and increases were not dose related. Complete or partial response to treatment was reported for 50% of the 133 intent-to-treat patients and 67% of the 58 evaluable patients.
Amphotericin B has the broadest spectrum of activity of all available systemic antifungal agents. While the pharmacokinetics and pharmacodynamics of amphotericin B are not fully understood, its binding affinity for ergosterol is considered the primary reason for its antifungal efficacy, and its cholesterol-binding activity is believed to be the source of toxicity. Nephrotoxicity, which often requires discontinuation of therapy, has been reported in 60 to almost 90% of the patients who receive amphotericin B (6, 7, 15, 18). In addition, infusion-related toxicities, such as chills and fever, occur frequently.
The deleterious effects of amphotericin B on renal function may be dose related: end-stage renal disease requiring dialysis has been associated with total doses of ≥5 g. However, toxicity is not always dose dependent; even relatively modest doses may result in renal damage. For example, azotemia was reported in 26% of patients with cryptococcal meningitis treated with doses of only 0.3 mg/kg of body weight daily (25). Concern with the potential nephrotoxicity of amphotericin B has caused many clinicians to treat patients with suboptimal doses of the drug or to refrain from using it. Hence, newer formulations of amphotericin B that do not cause impairment in renal function are needed.
Amphotericin B colloidal dispersion (ABCD; amphotericin B cholesteryl sulfate complex for injection; AMPHOTEC [in the United States] or AMPHOCIL [outside the United States]; SEQUUS Pharmaceuticals, Menlo Park, Calif.) is a new formulation of amphotericin B, developed to reduce toxicity by minimizing the delivery of amphotericin B to host cells. ABCD is a high-affinity lipid complex consisting of amphotericin B and sodium cholesteryl sulfate in a near-1:1 ratio. ABCD has been shown to be significantly less toxic than amphotericin B in animals, without reduction in efficacy (11, 12). An early clinical trial reported that doses of 1.5 mg of ABCD per kg produced no more infusion-related toxicity than is typically produced by 0.75 mg of amphotericin B per kg administered to premedicated patients (23). Furthermore, studies both in vitro and in animal models indicate that ABCD is as effective as amphotericin B in treating the principal pathogenic fungi (1, 13, 17).
To reduce toxicity, amphotericin B has also been formulated in two other liposomal preparations, Abelcet and AmBisome. Abelcet is ribbonlike in structure and is a complex of amphotericin B and two phospholipids. It is approved for marketing in the United States, the United Kingdom, and several other European countries. AmBisome consists of unilamellar bilayer liposomes with amphotericin B intercalated within the membrane. It is approved for marketing in the United States and several European countries.
We present the results of a prospective study evaluating the safety and efficacy of ABCD in patients with renal impairment who were infected with invasive fungi.
MATERIALS AND METHODS
Patients.
This open-label study, conducted in 28 medical centers across the United States, included 133 hospitalized patients with invasive fungal infections and impaired renal function. An Investigational Review Board or Ethics Committee at each center approved this study, and written informed consent was obtained from the patient, a legal representative, or from the parent or legal guardian for minors. Patients were eligible for this study if they had nephrotoxicity due to amphotericin B therapy, as evidenced by an increase of ≥1.5 mg/dl in serum creatinine level or a doubling of serum creatinine level during amphotericin B therapy, or if they had an underlying renal insufficiency evidenced by a mean serum creatinine level of ≥2.0 mg/dl based on results from at least two samples before study entry. Patients were excluded from this study for any one of the following reasons: having a history of anaphylactoid or other serious allergic reactions to amphotericin B, having a life expectancy estimated to be less than 7 days, having a hemoglobin level of <7 g/dl, being a pregnant or lactating female, or being less than 2 years of age. Rigid documentation of systemic fungal infection was not a criterion for inclusion; fungal species or sites of infections were not predesignated. Where documentation was not available, patients could be treated empirically on the basis of clinical evidence or previously obtained mycologic data.
Study medication and administration.
A test dose of 5 mg (modified for pediatric patients weighing <10 kg) was given on the first day; patients with an infusion-related toxicity of grade 2 or higher were premedicated with acetaminophen before the infusion was resumed. (The toxicity grade is based on the National Cancer Institute’s Common Toxicity Criteria. For example, a grade 2 toxicity involves fever of >38.0°C, chills requiring medication, or hypotension requiring fluid replacement.) ABCD was then administered once daily as a 4.0-mg/kg intravenous infusion, at a rate of 1 mg/kg/h. If this dosage was well tolerated for 2 weeks and renal function improved, an increase to 6.0 mg/kg daily was permitted. Ten patients received doses that ranged between 5.0 and 6.0 mg/kg. One patient received a dose of 6.20 mg/kg.
Patients were to receive ABCD until approximately 2 weeks after the disappearance of signs and symptoms of fungal infection, with a projected maximum of 6 weeks. Four patients with difficult-to-treat infections (three with Aspergillus pulmonary and sinus infection and one with Fusarium infection) were treated for 89, 110, 204, and 114 days, respectively. In the case of candidemia without evidence of invasive candidiasis, the treatment could be stopped 1 week after the disappearance of signs and symptoms, provided that neutropenia had resolved.
Monitoring.
The trial protocol required the following evaluations to be completed weekly: physical examination including neurologic assessment, complete blood cell count, blood chemistry and electrolyte analysis, and routine urinalysis.
Definition of infection.
Fungal infections were classified as definite or insufficient evidence. Classification was based on general clinical and mycologic criteria from the disease-specific guidelines of the Infectious Diseases Society of America and the Food and Drug Administration (19). For endemic mycoses, compatible clinical findings and isolation of the pathogen from any source were sufficient to classify the infection as definite. A positive cryptococcal antigen was considered evidence of definite infection, as was isolation of opportunistic fungi from biopsy specimens or from normally sterile body fluids or sites. Patients with probable infection, i.e., radiographic evidence of pulmonary infection and isolation of opportunistic fungi from one bronchopulmonary alveolar lavage specimen or two sputum specimens, were considered to have a definite infection. Additionally, any patient with at least one positive fungal blood culture was considered to have a definite fungal infection. Infections classified as insufficient evidence were either suspected or possible fungal infections.
Evaluations of efficacy and safety.
Therapeutic efficacy was determined by both clinical and mycologic responses. Clinical evaluation included signs and symptoms, radiographic changes, and histopathologic findings from biopsies or postmortem examinations. Mycologic evaluation was based on results of cultures, fungal antigen-antibody studies, and other microbiologic tests, such as nucleic acid probes. Response to treatment was rated as one of the following: complete response, no clinical or mycologic evidence of active infection and complete resolution of involved site at the end of therapy and at the 3-month follow-up; partial response, improvement in signs of disease activity but all criteria for complete response not being satisfied; failed, persistent clinical or laboratory evidence of fungal infection or continued treatment required during follow-up. Change in serum creatinine level was defined as the primary measure of renal safety; values before, during, and after therapy were compared within each patient. Additionally, adverse events were recorded along with their severity, relation to treatment, and outcome.
Statistical analysis.
Dosing information was summarized for all patients by fungal infection subgroup. Total dose, daily dose, and total days on treatment were statistically summarized by using mean, median, standard deviation (SD), and range. Because this was an open-label nonrandomized study, no formal statistical tests were performed on the efficacy analyses.
RESULTS
One hundred thirty-three patients (86 male, 47 female) were enrolled and received at least one dose of ABCD (Table 1). Their ages ranged from 21 months to 86 years. Sixty-eight patients (51%) were enrolled because of nephrotoxicity associated with previous treatment with amphotericin B. Forty-six patients (35%) were enrolled because of underlying renal insufficiency. The remaining 19 patients (14%) were enrolled because they were at significant risk of nephrotoxicity with amphotericin B as determined by their physicians. Sixteen of the 19 patients showed a marked trend toward nephrotoxicity while on amphotericin B therapy. Although these patients did not meet the exact criteria for enrollment due to nephrotoxicity, investigators did not want them to continue with amphotericin B therapy. One of the 19 patients was on renal dialysis.
TABLE 1.
Baseline demographic and disease characteristics—all patients
| Characteristic | Aspergillus | Candida | Othera | Total |
|---|---|---|---|---|
| Patients dosed (%) | 33 (25) | 67 (50) | 33 (25) | 133 |
| Male/female ratio | 20/13 | 40/27 | 26/7 | 86/47 |
| Age (yr) [median (range)] | 45 (6–77) | 46 (1–84) | 46 (16–86) | 46 (1–86) |
| Underlying disease or condition | ||||
| Hematologic malignancy | 15 | 12 | 12 | 39 (29.3%) |
| Solid tumor | 1 | 10 | 0 | 11 (8.3%) |
| Bone marrow transplant | 9 | 24 | 12 | 45 (33.8%) |
| Solid organ transplant | 4 | 4 | 1 | 9 (6.8%) |
| Otherb | 4 | 17 | 8 | 29 (21.8%) |
| Site of infection | ||||
| Lung | 26 | 9 | 14 | 49 (36.8%) |
| Skin | 2 | 0 | 2 | 4 (3.0%) |
| Blood and/or disseminated | 0 | 30 | 2 | 32 (24.1%) |
| Sinus | 1 | 0 | 2 | 3 (2.3%) |
| Otherc | 4 | 28 | 14 | 46 (34.6%) |
| Enrollment reason | ||||
| Amphotericin B toxicity | 20 | 27 | 21 | 68 (51.1%) |
| Renal insufficiency | 8 | 30 | 8 | 46 (34.6%) |
| Otherd | 5 | 10 | 4 | 19 (14.3%) |
| Baseline laboratory measurement | ||||
| ANCe (1,000/mm3) [mean (range)] | 6.49 (0–31.1) | 9.00 (0–38.8) | 9.75 (0–160) | 8.56 (0–160) |
| Serum creatinine (mg/dl) [mean (range)] | 2.28 (0.6–4.9) | 2.58 (0.8–7.2) | 2.63 (1.1–6.7) | 2.52 (0.6–7.2) |
| Total bilirubin (mg/dl) [mean (range)] | 2.84 (0.4–22.0) | 4.45 (0.2–43.0) | 4.56 (0.3–33.8) | 4.09 (0.3–33.8) |
Histoplasma/Coccidioides, Cryptococcus, other molds, and other yeasts.
Other underlying diseases or conditions included human immunodeficiency virus infection, AIDS, chronic obstructive pulmonary disease, surgery, coagulopathy, diabetes mellitus, chronic granulomatous disease, sarcoid tumor, Crohn’s disease, anemia, hypertension, immunosuppression, prosthesis, thrombocytopenia, or none.
Other sites of infection included bone, brain, chest wall, central nervous system, esophagus, gastrointestinal culture, Hickman catheter, hip joint fluid, liver, oropharynx, peritoneum, spleen, and urinary tract; also, one patient had >1 site of infection.
Patients did not meet entry criteria but were considered to be at risk of nephrotoxicity.
ANC, absolute neutrophil count.
Sixty-seven (50%) patients were infected with Candida, 33 (25%) were infected with Aspergillus, and 33 (25%) were infected with other fungal organisms. The most commonly observed underlying diseases or conditions were bone marrow transplantation (33.8%) and hematologic malignancy (29.3%). Fifty patients completed the entire study period. Reasons for early discontinuation included death in 44 patients, toxicity in 16, therapeutic failure in 10, and progression of underlying disease in 3. Ten patients discontinued for miscellaneous reasons unrelated to toxicity.
All 133 patients who received ABCD (intent-to-treat group) were assessed for both safety and efficacy, even if they had insufficient evidence of infection at baseline or had discontinued treatment within the first week of the study. An evaluable patient subgroup was selected by more stringent criteria for efficacy analysis, which required that patients (i) had a definite fungal infection at baseline, (ii) received ABCD for at least 7 of the first 10 days of the study, and (iii) took no concurrent systemic antifungal therapy during treatment with ABCD.
The baseline demographic characteristics of the 58 patients who qualified for inclusion in the evaluable patient group were similar to those of the intent-to-treat population, except that the evaluable group included a greater proportion of candidiasis patients: 30 (52%) were infected with Candida, 16 (28%) were infected with Aspergillus, and 12 (21%) had other fungal infections (three had cryptococcosis; four had other molds, one of whom had cryptococcosis as well; and six had Histoplasma capsulatum or Coccidioides immitis). Of the 75 patients excluded from the evaluable population, 45 had received less than 7 days of ABCD treatment within the first 10 days, 40 had insufficient evidence of a definite fungal infection at baseline, and 10 received additional, concurrent systemic antifungal medication. (Twenty patients were excluded for two reasons.)
Response to treatment.
Table 2 shows the response to treatment among the 58 evaluable patients, including a breakdown of response by underlying disease condition and by patients with a baseline absolute neutrophil count of <500/mm3. Of the 58 evaluable patients, 39 (67.2%) had either a complete or a partial response at end of treatment, with 19 (32.8%) having treatment failures. There were two reasons for treatment failure: death (14 patients) and persistent fungal infection (5 patients). The most common cause of death was fungal infection (seven patients). The highest cure rate was seen in patients with candidiasis: 17 of 30 (56.6%) had a complete response. When complete and partial responses were combined, recipients of bone marrow transplantation had the lowest response rate (4 of 15; 26.6%), compared to patients with solid tumors (6 of 6; 100%), solid organ transplants (5 of 6; 83.3%), and hematologic malignancy (14 of 17; 82.3%) and those with other underlying diseases (10 of 14; 71.4%).
TABLE 2.
Response to treatment—evaluable patients
| Classification | Aspergillus | Candida | Othera | Total |
|---|---|---|---|---|
| Overall | ||||
| Total no. of patients (%) | 16 (28) | 30 (52) | 12 (20) | 58 |
| Complete response | 3 | 17 | 6 | 26 |
| Partial response | 7 | 3 | 3 | 13 |
| Failed | 6 | 10 | 3 | 19 |
| By underlying disease or condition (complete or partial response/no. of patients) | ||||
| Hematologic malignancy | 4/6 | 6/7 | 4/4 | 14/17 |
| Solid tumor | 1/1 | 5/5 | 0/0 | 6/6 |
| Bone marrow transplant | 0/3 | 4/9 | 0/3 | 4/15 |
| Solid organ transplant | 2/3 | 2/2 | 1/1 | 5/6 |
| Otherb | 3/3 | 3/7 | 4/4 | 10/14 |
| Total | 10/16 | 20/30 | 9/12 | 39/58 |
| By neutrophil count (ANCc < 500 at baseline) | ||||
| Total no. of evaluable patients | 4 | 5 | 1 | 10 |
| Complete response | 1 | 2 | 1 | 4 |
| Partial response | 1 | 0 | 0 | 1 |
| Failed | 2 | 3 | 0 | 5 |
Histoplasma/Coccidioides, Cryptococcus, other molds, and other yeasts.
Other underlying diseases or conditions included human immunodeficiency virus infection, AIDS, chronic obstructive pulmonary disease, surgery, coagulopathy, diabetes mellitus, chronic granulomatous disease, sarcoid tumor, Crohn’s disease, anemia, hypertension, immunosuppression, prosthesis, thrombocytopenia, or none.
ANC, absolute neutrophil count.
Forty-five of the 58 evaluable patients had received previous amphotericin B therapy. Twenty-nine of the 45 patients (64%) had a complete or partial response at end of treatment. A higher response rate was seen in the group of 13 evaluable patients who had not had previous amphotericin B therapy. In this group, 10 patients (10 of 13; 77%) had a complete or partial response at end of treatment. Thus, it did not appear that previous amphotericin B therapy was an important factor in determining the complete and partial response rates.
Posttreatment follow-up.
Patients were considered not assessable at follow-up if they were failures at the end of therapy, did not return, had been placed on an alternative systemic antifungal agent, or had died. Twenty (34.5%) of 58 evaluable patients who were assessed at follow-up examination had a positive response (complete or partial), with 15 (25.9%) cured and 5 (8.6%) improved compared to baseline. Median time to follow-up among evaluable patients was 90.5 days for Aspergillus-infected patients, 93.5 days for Candida-infected patients, and 98.0 days for those with other infections.
Mortality.
Seventy-four of the total 133 patients died (56%); 29 of these patients were in the evaluable group (29 of 58; 50%). Twenty-three of the total 133 patients (17%) died within 7 days of receiving the first dose of ABCD, which indicates these patients’ severity of illness. No deaths were attributed to ABCD therapy. Seventeen deaths were ascribed to fungal infection; all other deaths were due to either the underlying disease or complications involved in its treatment.
Safety.
The cumulative dose of ABCD ranged from 5 mg to 30.5 g (mean = 4.9 ± 4.9 [SD] g; median = 3.3 g). Daily doses ranged from 0.1 to 5.5 mg/kg (mean = 3.4 ± 0.98 [SD] mg/kg; median = 3.8 mg/kg), and mean total duration of treatment was 21 ± 25.7 (SD) days (median, 14 days; range, 1 to 207 days).
The most common adverse events judged as being possibly or probably related to ABCD (reported in ≥10% of patients) were chills (41%; 55 of 133), fever (18%; 24 of 133), and hypertension (10%; 13 of 133), with most of the events being mild or moderate in severity. In previous studies of ABCD, the incidence of hypertension has been less than 5%; this includes a double-blind randomized study involving 213 patients. The high incidence of patients with renal disease in this study may have contributed to the higher rate of hypertension.
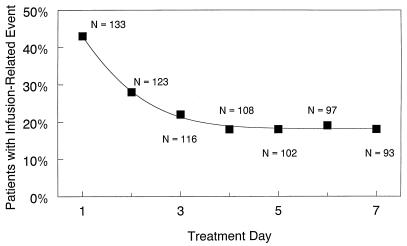
Infusion-related events occurred at least once in 74 patients (56%); for 15 patients, the events were categorized as severe. Although 57 patients (43%) experienced infusion-related toxicity on day 1, the proportion with infusion-related toxicities diminished steadily, with only 17 of 93 patients (18%) reporting these events by day 7 (Fig. 1).
FIG. 1.
Rate of infusion-related toxicities by treatment day.
Sixteen patients discontinued prematurely because of toxicity or adverse events. Seven discontinuations were infusion related. Six were for renal toxicity or elevated serum creatinine levels. The three remaining discontinuations were for pulmonary edema, atrial fibrillation, and leg pain.
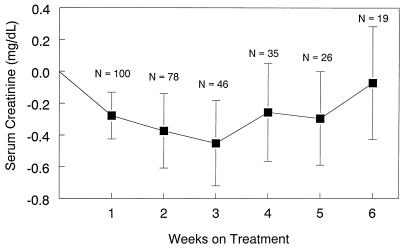
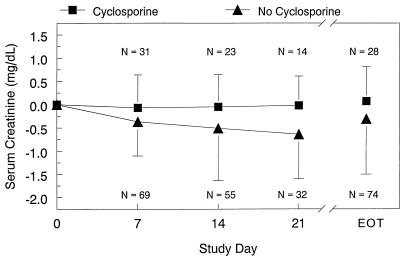
Although individual patients had increases in serum creatinine levels, there was no overall trend for increased creatinine levels, even in patients receiving concurrent cyclosporine. Mean serum creatinine levels decreased slightly from baseline. Figure 2 shows the overall change in serum creatinine levels, and Fig. 3 presents mean changes in serum creatinine levels among patients who were or were not treated concomitantly with cyclosporine. Table 3 presents median changes in creatinine clearance for all patients.
FIG. 2.
Mean change (± standard error) in serum creatinine levels over time.
FIG. 3.
Mean change (±SD) in serum creatinine level classified by cyclosporine group. EOT, end of treatment.
TABLE 3.
Median changes in creatinine clearance classified by fungal infection—all patients
| Measurement | Aspergillosis | Candidiasis | Other | Total |
|---|---|---|---|---|
| Baseline | ||||
| Mediana | 38.9 | 35.5 | 38.8 | 36.4 |
| Rangea | 14.7–111.5 | 7.2–76.0 | 17.3–96.6 | 7.2–111.5 |
| n | 28 | 60 | 21 | 109 |
| Change at day 7 | ||||
| Median | 7.2 | 6.5 | 0.0 | 5.6 |
| Range | −15.9–45.5 | −19.6–427.8 | −25.2–47.7 | −25.2–427.8 |
| n | 26 | 55 | 19 | 100 |
| Change at day 14 | ||||
| Median | 6.1 | 6.5 | 8.4 | 6.8 |
| Range | −27.9–43.2 | −16.7–72.8 | −35.2–47.7 | −35.2–72.8 |
| n | 19 | 45 | 14 | 78 |
| Change at day 21 | ||||
| Median | 13.6 | 5.2 | 6.8 | 5.5 |
| Range | −37.2–43.2 | −21.9–65.4 | −31.5–47.7 | −37.2–65.4 |
| n | 13 | 19 | 14 | 46 |
| Change at end of treatment | ||||
| Median | 6.7 | 6.0 | −3.1 | 5.4 |
| Range | −50.7–45.5 | −21.9–72.8 | −37.1–47.7 | −50.7–72.8 |
| n | 26 | 56 | 20 | 102 |
Values for medians and ranges are in milliliters per minute.
Baseline (defined as a window starting 2 days before and ending 3 days after the first dose of ABCD) and end-of-treatment serum potassium values were available for 102 patients; values at baseline and end of treatment were the same at a median of 3.9 meq/liter. Serum magnesium values were available for 79 patients; values at baseline and end of treatment were the same at a median of 1.6 meq/liter.
Baseline and end-of-treatment alkaline phosphatase values were available for 83 patients. Values changed from a median of 159 U/liter at baseline to 189 U/liter at the end of treatment. aspartate transaminase and bilirubin values were available for 62 and 84 patients, respectively. The median baseline and end-of-treatment values for aspartate transaminase were 35 and 37.5 U/liter, respectively; for total bilirubin, they were 1.9 and 2.3 mg/dl, respectively.
Similar laboratory values were found when renal function in patients with Candida was compared with that in the total group of patients. Serum creatinine values were identical in both groups at baseline (median, 2.3 mg/dl; 56 patients with candidiasis; 102 total patients) and end of treatment (median, 2.0 mg/dl). Serum potassium values were also identical in both groups at baseline (median, 3.9 meq/liter; 54 patients with candidiasis; 102 total patients) and end of treatment (median, 3.9 meq/liter). Blood urea nitrogen values were slightly different in the two groups. The median value at baseline for the 56 patients with candidiasis was 51.5 mg/dl, while for the 103 total patients, it was 50.0 mg/dl. The median value at end of treatment was 51.0 mg/dl for the patients with candidiasis and 50.0 mg/dl for the total group of patients.
DISCUSSION
Our study demonstrates that ABCD can be safely administered to patients with invasive fungal infection who have preexisting renal insufficiency. Serum creatinine levels tended to decrease slightly over the course of the study, regardless of the total dose of ABCD or of concomitant administration of cyclosporine. The renal safety of ABCD was also demonstrated by the increase in creatinine clearance and by stable serum potassium values. ABCD was shown to be effective in many patients who had failed to respond to standard amphotericin B. However, any conclusions from this study are limited by the relatively small number of patients with uniform predisposing conditions and well-documented infections caused by a single species. In addition, the study did not include a control group, a problem with most studies evaluating patients with life-threatening fungal infections.
The response rates in the present study are consistent with those observed by others studying ABCD in the treatment of invasive fungal infection in this setting (5, 21). However, the scarcity of prospective randomized trials and the absence of standard criteria for diagnosis and evaluation of response make it more difficult to compare our data with data from studies of other antifungal agents.
Nevertheless, within these limitations, ABCD appeared to compare favorably with the historical response rates observed with amphotericin B. A recent comprehensive review of the published literature for the treatment of aspergillosis reported an overall response to amphotericin B of 34% among 314 patients with aspergillosis, compared to the 62% response rate for aspergillosis observed in the present study (8). The response rates observed in our study are also comparable to findings with the other lipid-associated formulations of amphotericin B, AmBisome and Abelcet (14, 22).
Our study is in agreement with studies reporting evidence of decreased nephrotoxicity with lipid formulations of amphotericin B (2, 4). Our data indicate that ABCD has decreased nephrotoxicity, as demonstrated by stabilization or improvement in renal function in patients with preexisting nephrotoxicity, including that induced by amphotericin B. This finding has also been noted among patients at higher risk of nephrotoxicity due to concurrent administration of cyclosporine.
Our data indicate that infusion-related adverse events occur with the administration of ABCD but tend to decrease over time. These can often be ameliorated by premedicating patients with acetaminophen, diphenhydramine, or hydrocortisone. A similar pattern also is known to occur with amphotericin B, as well as AmBisome and Abelcet (20, 24).
The results from this and other studies of ABCD suggest that ABCD would be particularly suitable in patients with invasive fungal infection and preexisting renal toxicity, including patients at higher risk due to concomitant nephrotoxic agents such as cyclosporine, tacrolimus, foscarnet, ganciclovir, and aminoglycoside antibacterials. Although ABCD therapy is more costly than therapy with amphotericin B, the renal system-sparing characteristics of ABCD may make it an appropriate choice in selected patient populations.
Therapy of severe fungal infections in immunocompromised patients is limited by the variable efficacy of the various antifungals and by irreversible tissue damage once invasive infection has been established. Hence, some investigators have recommended either the empiric administration of the maximum tolerated dose of amphotericin B or the prophylactic administration of this agent to high-risk patients (3, 10, 16). Since worsening renal function is usually the dose-limiting factor for amphotericin B, ABCD, because of its superior safety profile, could be useful in these settings. The results from this trial and other published reports with ABCD and other lipid formulations provide preliminary evidence on the value of such use (14, 19–22, 26). However, previous studies, including ours, enrolled only a limited number of patients with persistent, profound neutropenia. In these patients, response to amphotericin B is notoriously poor; however, early administration of high-dose antifungal therapy is advocated (3, 9).
Validation of the role of ABCD or other lipid formulations of amphotericin B in these situations will require controlled trials. Two prospective, blinded, randomized trials comparing ABCD to amphotericin B in high-risk and neutropenic patients with proven or suspected fungal infections have been completed. In one of these trials, a group of patients received ABCD concurrently with cyclosporine or tacrolimus. Results from these trials will provide more information about the usefulness of ABCD in high-risk and neutropenic patients.
In conclusion, amphotericin B formulated as ABCD was found to be effective and well tolerated in immunocompromised patients with invasive fungal infections. The major advantage of treatment with ABCD was reduced nephrotoxicity in patients with underlying renal impairment.
ACKNOWLEDGMENTS
We acknowledge Cathryn Evans of Chandos Communications and Melanie Rogers of SEQUUS Pharmaceuticals, Inc., for their help in preparing the manuscript. The following persons provided patients for the study: M. Abboud, Medical University of South Carolina, Charleston; E. Anaissie, M. D. Anderson Cancer Center, The University of Texas, Houston; S. Bertolone, University of Louisville, Children’s Hospital, Louisville, Ky.; N. Chao, Stanford University Hospital, Stanford, Calif.; K. De Santes, University of California, San Francisco; A. Deveikis, Long Beach Memorial Hospital, Long Beach, Calif.; G. Feleke, Nassau County Medical Center, East Meadow, N.Y.; D. Gervich, Mercy Medical Center, Des Moines, Iowa; A. Glatt, The Catholic Medical Center of Brooklyn and Queens, Jamaica, N.Y.; J. Graybill, Audie Murphy VA Hospital, San Antonio, Tex.; L. Kaplan, The University of Chicago Hospital, Chicago, Ill.; S. Kusne, Presbyterian University Hospital, Pittsburgh, Pa.; M. Martin, Oregon Health Sciences University, Portland; C. Miller, The Johns Hopkins University School of Medicine, Baltimore, Md.; V. Morrison, University of Minnesota, Minneapolis; M. Mustafa, Children’s Medical Center, Dallas, Tex.; G. Noskin, Northwestern Memorial Hospital, Chicago, Ill.; V. Pons, University of California, San Francisco; J. Pottage, Rush-Presbyterian-St. Luke’s Medical Center, Chicago, Ill.; C. Reis, University of California, San Francisco; K. Skahan, University of Cincinnati Medical Center, Cincinnati, Ohio; S. Spruance, University of Utah Health Science Center, Salt Lake City; D. Strike, Riverside Medical Center, Minneapolis, Minn.; W. Sutker, North Texas Infectious Diseases Consultants, Dallas; M. Trigg, University of Iowa Hospitals and Clinics, Iowa City; P. Weintrub, University of California, San Francisco; P. Wels, Millard Fillmore Hospital, Buffalo, N.Y.; and M. White, Memorial Sloan-Kettering Cancer Center, New York, N.Y.
The study was funded by SEQUUS Pharmaceuticals, Inc. (formerly Liposome Technology, Inc.).
REFERENCES
- 1.Allende M C, Lee J W, Francis P, Garrett K, Dollenberg H, Berenguer J, Lyman C A, Pizzo P A, Walsh T J. Dose-dependent antifungal activity and nephrotoxicity of amphotericin B colloidal dispersion in experimental pulmonary aspergillosis. Antimicrob Agents Chemother. 1994;38:518–522. doi: 10.1128/aac.38.3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anaissie E J, Gurwith M J. 19th International Chemotherapy Conference, Montreal, Canada, July 16–21. 1995. Safety and efficacy of amphotericin B colloidal dispersion (ABCD) [Google Scholar]
- 3.Anaissie E J, Pinczowski H. Invasive candidiasis during granulocytopenia. Recent Results Cancer Res. 1993;132:137–145. doi: 10.1007/978-3-642-84899-5_14. [DOI] [PubMed] [Google Scholar]
- 4.Anaissie E J, White M, Uzun O, Singer C, Bodey G P, Matzke D, Azarnia N, Lopez-Berestein G. Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Amphotericin B lipid complex (ABLC) versus amphotericin B (AMB) for treatment of hematogenous and invasive candidiasis: a prospective, randomized, multicenter trial, abstr. LM21; p. 330. [Google Scholar]
- 5.Bowden R A, Cays M, Gooley T, Mamelok R D, vanBurik J. Phase I study of amphotericin B colloidal dispersion for the treatment of invasive fungal infections after marrow transplant. J Infect Dis. 1996;173:1208–1215. doi: 10.1093/infdis/173.5.1208. [DOI] [PubMed] [Google Scholar]
- 6.Butler W T, Bennett J E, Alling D W, Wertlake P T, Utz J P, Hill G J., II Nephrotoxicity of amphotericin B: early and late effects in 81 patients. Ann Intern Med. 1964;61:175–187. doi: 10.7326/0003-4819-61-2-175. [DOI] [PubMed] [Google Scholar]
- 7.Clements J S, Jr, Peacock J E., Jr Amphotericin B revisited: reassessment of toxicity. Am J Med. 1990;88:22N–27N. [PubMed] [Google Scholar]
- 8.Denning D W. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:1–7. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 9.Denning D W, Stevens D A. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis. 1990;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 10.EORTC International Antimicrobial Therapy Cooperative Group. Empiric antifungal therapy in febrile granulocytopenic patients. Am J Med. 1989;86:668–672. doi: 10.1016/0002-9343(89)90441-5. [DOI] [PubMed] [Google Scholar]
- 11.Guo L S, Fielding R M, Lasic D D, Hamilton R L, Mufson D. Novel antifungal drug delivery: stable amphotericin B-cholesteryl sulfate discs. Int J Pharm. 1991;75:45–54. [Google Scholar]
- 12.Guo L S, Fielding R M, Mufson D. Pharmacokinetic study of a novel amphotericin B colloidal dispersion with improved therapeutic index. Ann N Y Acad Sci. 1991;618:586–588. [Google Scholar]
- 13.Hanson L H, Stevens D A. Comparison of antifungal activity of amphotericin B deoxycholate suspension with that of amphotericin B cholesteryl sulfate colloidal dispersion. Antimicrob Agents Chemother. 1992;36:486–488. doi: 10.1128/aac.36.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiemenz J W, Lister J, Anaissie E J, White M H, Dinubile M, Silber J, Horwith G, Lee L W. Emergency-use amphotericin B lipid complex (ABLC) in the treatment of patients with aspergillosis: historical control comparison with amphotericin B. Blood. 1995;86:3383. . (Abstract.) [Google Scholar]
- 15.Hoitsma A J, Wetzels J F, Koene R A. Drug-induced nephrotoxicity: aetiology, clinical features and management. Drug Saf. 1991;6:131–147. doi: 10.2165/00002018-199106020-00004. [DOI] [PubMed] [Google Scholar]
- 16.Holleran W M, Wilbur J R, DeGregorio M W. Empiric amphotericin B therapy in patients with acute leukemia. Rev Infect Dis. 1985;7:619–624. doi: 10.1093/clinids/7.5.619. [DOI] [PubMed] [Google Scholar]
- 17.Hostetler J S, Clemons K V, Hanson L H, Stevens D A. Efficacy and safety of amphotericin B colloidal dispersion compared with those of amphotericin B deoxycholate suspension for treatment of disseminated murine cryptococcosis. Antimicrob Agents Chemother. 1992;36:2656–2660. doi: 10.1128/aac.36.12.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janknegt R, de Marie S, Bakker-Woudenberg I A, Crommelin D J. Liposomal and lipid formulations of amphotericin B: clinical pharmacokinetics. Clin Pharmacokinet. 1992;23:279–291. doi: 10.2165/00003088-199223040-00004. [DOI] [PubMed] [Google Scholar]
- 19.Medoff, G., W. E. Dismukes, D. Pappagianis, R. Diamond, H. A. Gallis, and D. Drutz. 1992. Evaluation of new antifungal drugs for the treatment of systemic fungal infections. Clin. Infect. Dis. 15(Suppl. 1):S274–S281. [DOI] [PubMed]
- 20.Meunier, F., H. G. Prentice, and O. Ringden. 1991. Liposomal amphotericin B (AmBisome): safety data from a phase II/III clinical trial. J. Antimicrob. Chemother. 28(Suppl. B):83–91. [DOI] [PubMed]
- 21.Oppenheim B A, Herbrecht R, Kusne S. The safety and efficacy of amphotericin B colloidal dispersion in the treatment of invasive mycoses. Clin Infect Dis. 1995;21:1145–1153. doi: 10.1093/clinids/21.5.1145. [DOI] [PubMed] [Google Scholar]
- 22.Ringden, O., F. Meunier, J. Tollemar, P. Ricci, S. Tura, E. Kuse, M. A. Viviani, N. C. Gorin, J. Klastersky, P. Fenaux, H. G. Prentice, and G. Ksionski. 1991. Efficacy of amphotericin B encapsulated in liposomes (AmBisome) in the treatment of invasive fungal infections in immunocompromised patients. J. Antimicrob. Chemother. 28(Suppl. B):73–82. [DOI] [PubMed]
- 23.Sanders S W, Buchi K N, Goddard M S, Lang J K, Tolman K G. Single-dose pharmacokinetics and tolerance of a cholesteryl sulfate complex of amphotericin B administered to healthy volunteers. Antimicrob Agents Chemother. 1991;35:1029–1034. doi: 10.1128/aac.35.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharkey P K, Graybill J R, Johnson E S, Hausrath S G, Pollard R B, Kolokathis A, Mildvan D, Fan-Havard P, Eng R H, Patterson T F, Pottage J C, Jr, Simberkoff M S, Wolf J, Meyer R D, Gupta R, Lee L W, Gordon D S. Amphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1996;22:315–321. [PubMed] [Google Scholar]
- 25.Stamm A M, Diasio R B, Dismukes W E, Shadomy S, Cloud G A, Bowles C A, Karam G H, Espinel-Ingroff A. Toxicity of amphotericin B plus flucytosine in 194 patients with cryptococcal meningitis. Am J Med. 1987;83:236–242. doi: 10.1016/0002-9343(87)90691-7. [DOI] [PubMed] [Google Scholar]
- 26.White M H, Bowden R A, Sandler E, Graham M L, Noskin G, Wingard J R, Goldman M, McCabe A, Lin J S, Gurwith M, Miller C B. Amphotericin B colloidal dispersion (ABCD) vs. amphotericin B (AmB) in the empiric treatment of febrile neutropenic patients. Blood. 1996;88:1196. [Google Scholar]