Abstract
BACKGROUND
Superimposed intracranial infection is an uncommon but clinically significant complication in patients with active coronavirus disease 2019 (COVID-19), particularly in those with predisposing immunocompromising conditions.
OBSERVATIONS
The authors describe a case of subdural empyema, secondary to extension from pansinusitis, in a 20-year-old otherwise healthy immunocompetent male who was recently diagnosed with COVID-19. Despite his critical condition at time of presentation, he made a full clinical recovery with aggressive multidisciplinary surgical management between neurosurgery and otolaryngology, despite negative cultures to guide directed antimicrobial therapy. Ultimately, use of molecular-based polymerase chain reaction testing diagnosed Aspergillus fumigatus as the offending pathogen after the patient had already recovered and was discharged from the hospital.
LESSONS
This case demonstrates the potential for significant superimposed intracranial infection even in young, healthy individuals, infected by COVID-19 and suggests an aggressive surgical approach to achieve source control, particularly in the absence of positive cultures to guide antimicrobial therapies, may lead to rapid clinical improvement.
Keywords: COVID-19, sinusitis, subdural empyema, immunocompetent
ABBREVIATIONS: COVID-19 = coronavirus disease 2019, MRI = magnetic resonance imaging, PCR = polymerase chain reaction
Sinonasal symptoms have become prevalent presenting symptom of coronavirus disease 2019 (COVID-19) during the ongoing pandemic.1 In rare instances, aggressive rhinosinusitis may develop, particularly in immunocompromised patients, and spread to nearby structures within orbit and anterior skull base.2–4 These cases are often in the setting of severe secondary fungal infection, especially mucormycosis,5 while rarely may also occur from secondary bacterial rhinosinusitis.6 To date, significant intracranial infectious complications related to COVID-19 have only been described in immunocompromised individuals.1–4,7,8 In this report, we describe presentation of pansinusitis, complicated by significantly large subdural empyemas, in an otherwise young, healthy male recently diagnosed with COVID-19 and the subsequent management of the intracranial and sinonasal complications.
Illustrative Case
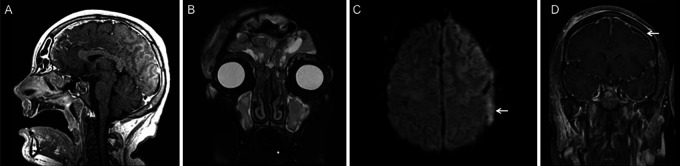
A 20-year-old healthy male, without a history of significant medical issues, developed mild respiratory symptoms and sinus congestion, for which he was started on empirical antibiotics. Further testing with reverse transcriptase-polymerase chain reaction (PCR) testing confirmed the diagnosis of COVID-19, for which he had not been previously vaccinated. Ten days later after diagnosis, he presented to the emergency department with progressive symptoms, albeit still relatively mild, and did not meet criteria for remdesivir or immunoglobulin therapy. He was subsequently discharged after symptomatic relief. The patient subsequently self-isolated in a hotel but was found 5 days later unresponsive and febrile, requiring emergent intubation. Magnetic resonance imaging (MRI) demonstrated pansinusitis (Fig. 1A, B), diffuse pachymeningeal and leptomeningeal enhancement, and a left frontoparietal diffusion-restricting and enhancing (Fig. 1C, D) subdural collection, concerning for empyema. He also exhibited findings of significant coagulopathy, which included a nonocclusive superior sagittal sinus thrombosis and thrombocytopenia, the latter of which improved with intravenous immunoglobulin administration. In addition, the patient developed new-onset generalized seizure, which was managed with antiepileptic medications.
FIG. 1.
Preoperative MRI at the time of presentation. Sagittal T1-weighted postcontrast (A) and coronal T2-weighted (B) MRI demonstrate significant enhancement and edema, involving the frontal, ethmoidal, maxillary, and sphenoid sinuses. Axial diffusion-weighted (C) and coronal T1-weighted postcontrast (D) MRI show a diffusion-restricting and enhancing left frontal subdural collection (arrows), consistent with subdural empyema.
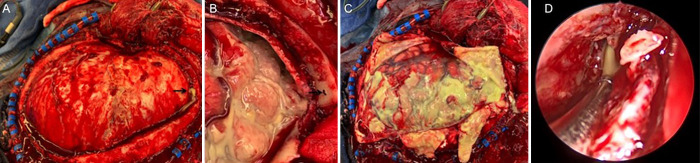
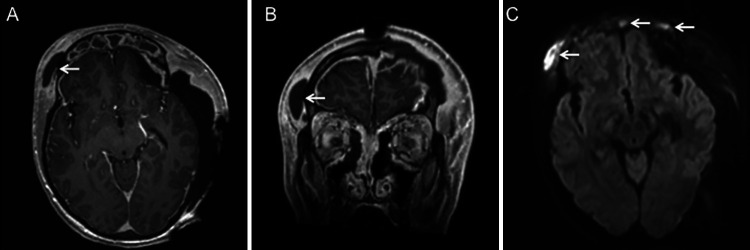
The patient underwent left decompressive hemicraniectomy for subdural empyema evacuation, which revealed pus above the dura (Fig. 2A), secondary to a bony defect in the frontal sinus (Fig. 2B), which was repaired. Gross subdural pus was found overlying the brain upon dural opening (Fig. 2C). Subsequently, he underwent bilateral complete endoscopic sinus surgery, which redemonstrated pus emanating from the frontal sinus (Fig. 2D). There was evidence of significant pansinus edema and purulence throughout consistent with acute rhinosinusitis. Despite these findings, cultures from the operating room did not yield growth of any bacterial or fungal organisms. He was maintained on empirical vancomycin, ceftriaxone, and metronidazole therapy throughout this time. Surveillance MRI performed 6 days after his initial hemicraniectomy demonstrated interval development of an enhancing, diffusion-restricting right frontal subgaleal collection with bony involvement, concerning for osteomyelitis (Fig. 3A-C). There was also complete opacification of the paranasal sinuses with areas of restricted diffusion in the frontal sinuses, concerning for persistent pansinusitis (Fig. 3C). The patient was subsequently taken for neurosurgical evacuation of the right-sided collection, removal of the infected bone, and mesh cranioplasty to replace the bony defect. The next day, he underwent endoscopic sinonasal surgery to debride further mucopurulence. The sinuses were noted to be open and draining without any communication into the intracranial space. Postoperatively, antimicrobial therapy was broadened to include high dose fluconazole and meropenem. However, final cultures were negative for growth of bacterial or fungal organisms, so he was tentatively prescribed a 6-week course of ceftriaxone and metronidazole. Two days after sinonasal surgery, the patient was successfully extubated, and he was following commands without any focal neurological deficits by the next day.
FIG. 2.
Intraoperative images. A gross image during craniectomy prior to dural opening demonstrates pus emanating from a defect in the frontal sinus (A, arrow), better visualized on close-up imaging after dural opening (B, arrow). There was significant pus overlying the brain surface upon dural opening (C). An intraoperative endoscopic view during sinus surgery (D) demonstrates pus draining from the opened right frontal sinus.
FIG. 3.
MRI prior to repeat surgical intervention. Axial (A) and coronal (B) T1-weighted postcontrast MRI demonstrate an enhancing right subgaleal collection (arrows) with corresponding diffusion-restriction in this area as well as within the paranasal sinuses (arrows, C).
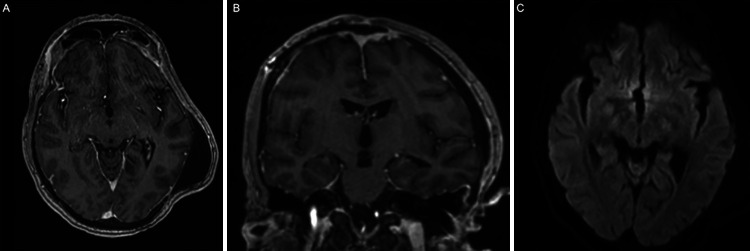
At time of discharge, 3 weeks after initial presentation, the patient had made a full neurological recovery. Shortly afterwards, PCR-based molecular testing of the brain abscess sample revealed a diagnosis of Aspergillus fumigatus, for which antibiotics were stopped, and he was switched to a 12-week course of voriconazole. At the 3-month follow-up, MRI demonstrated stability of a known pseudomeningocele with the absence of enhancement (Fig. 4A, B) or diffusion restriction (Fig. 4C) to suggest residual abscess. At the time of the last follow-up, 4 months after initial presentation, he had completed his antifungal therapy, was in neurologically intact condition, and had undergone a successful left cranioplasty procedure to address his existing bony skull defect.
FIG. 4.
Postoperative MRI at the 3-month follow-up. Axial (A) and coronal (B) T1-weighted postcontrast MRI demonstrate a stable pseudomeningocele with the absence of contrast enhancement or diffusion restriction (C) to suggest any possibility of residual abscess.
Discussion
Observations
Nasal inflammation, indicative of acute rhinosinusitis, may be present in up to one-half of patients experiencing headaches/facial pain and diagnosed with COVID-19.1 In the midst of the pandemic, there has been a surge of opportunistic fungal infections, particularly mucormycosis, in immunocompromised patients diagnosed with COVID-19.4 In severe cases, secondary fungal infection may spread into the orbit and/or erosion of the anterior skull base, including case reports of fungal brain abscesses in immunocompromised patients.2,3 Bacterial intracranial infection has also been reported in single case reports from the pediatric and adult population, predisposed to infectious spread secondary to diagnoses of multisystem inflammatory syndrome and chronic sinusitis, respectively.2,9 Whether complications are due to fungal or bacterial infection, cases required aggressive multidisciplinary surgical debridement to achieve source control but ultimately achieved good neurological outcomes.
Lessons
In contrast, our patient was an immunocompetent young healthy male with no prior medical history who suffered more severe subdural empyema burden, secondary to pansinusitis in the setting of COVID-19 diagnosis, and with a prolonged postoperative recovery. Despite negative bacterial and fungal cultures and stains, further PCR studies identified Aspergillus fumigatus as the likely causal organism, highlighting the utility of molecular-based testing. However, it is also important to recognize that we could not rule out an underlying bacterial or polymicrobial process, for which the cultures may have been negative because of the patient’s history of empirical antibiotic therapy.
Invasive aspergillosis is an uncommon but known severe complication of COVID-19 infection, particularly affecting the lungs.10 A recent meta-analysis of COVID-19–associated pulmonary aspergillosis reported a prevalence of 10% among patients with COVID-19 requiring intensive care unit-level care with a high mortality rate of 59.2%.11 Likewise, Aspergillus, alongside with the mucor species, has been reported as the major causative organisms in acute invasive fungal rhino-sinusitis after recent COVID-19 infection, particularly in immunocompromised patients.12,13 These patients have usually required surgical debridement in addition to antifungal therapy and typically present in a delayed fashion, 2 to 3 weeks after PCR-negative testing for COVID-19.14 In contrast, our patient’s clinical course was particularly more aggressive, manifesting with fulminant invasive sinusitis with intracranial involvement, despite immunocompetency, and in the setting of active COVID-19 infection diagnosed 2 weeks prior with progressive, albeit still mild, symptoms.
Subdural empyemas have a known association with sinusitis and most commonly present in pediatric and young adult males.15–17 In these patients, there is typically a bacterial etiology, most commonly from the Streptococcus and Staphylococcus species.18,19 Given our patient had received empirical antibiotic therapy prior to his acute neurological decompensation, it is plausible that an underlying bacterial sinusitis was the source of the subdural empyema, unrelated to his concurrent COVID-19 diagnosis. That said, given his ultimate PCR-based diagnosis of an Aspergillus infection and its strong association with COVID-19 related invasive sinusitis, we believe the pathophysiology of our patient’s subdural empyema diagnosis was linked to his concurrent COVID-19 infection.10–14
Despite multiple negative cultures from the blood, sputum, and operating room samples to guide antimicrobial therapy, the patient demonstrated rapid clinical improvement with aggressive surgical debridement, as evidenced by multiple neurosurgical and endoscopic endonasal procedures. By the time voriconazole was started after Aspergillus was diagnosed, the patient had already made full neurological recovery. In the absence of culture-guided antimicrobial treatment, it is rare to see such rapidity of clinical response in an immunocompetent host with source control alone. This case highlights that significant intracranial involvement can occur in patients with no significant predisposing risk factors who develop superimposed sinus infections from COVID-19. A high degree of clinical suspicion and an aggressive treatment approach may be indicated in these patients to prevent further intracranial sequela.
Acknowledgments
We acknowledge Dr. Albert Ko, MD, Professor of Medicine (Infectious Diseases), Public Health and Epidemiology at Yale School of Medicine, for his expert advice in the preparation of this manuscript.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Omay, Hong, Manes, Rimmer. Acquisition of data: Omay, Hong, Prust, Rimmer. Analysis and interpretation of data: Omay, Hong, Prust, Rimmer. Drafting the article: Hong. Critically revising the article: all authors. Reviewed submitted version of manuscript: Omay, Hong, Manes, Rimmer. Approved the final version of the manuscript on behalf of all authors: Omay. Statistical analysis: Omay. Administrative/technical/material support: Hong. Study supervision: Omay, Manes.
References
- 1. Straburzyński M, Nowaczewska M, Budrewicz S, Waliszewska-Prosół M. COVID-19-related headache and sinonasal inflammation: a longitudinal study analysing the role of acute rhinosinusitis and ICHD-3 classification difficulties in SARS-CoV-2 infection. Cephalalgia. 2022;42(3):218–228. doi: 10.1177/03331024211040753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diwakar J, Samaddar A, Konar SK, et al. First report of COVID-19-associated rhino-orbito-cerebral mucormycosis in pediatric patients with type 1 diabetes mellitus. J Mycol Med. 2021;31(4):101203. doi: 10.1016/j.mycmed.2021.101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta V, Singh P, Sukriti K. Fungal brain abscess in a post COVID-19 patient. BMJ Case Rep. 2021;14(9):e246319. doi: 10.1136/bcr-2021-246319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mitra S, Janweja M, Sengupta A. Post-COVID-19 rhino-orbito-cerebral mucormycosis: a new addition to challenges in pandemic control. Eur Arch Otorhinolaryngol. 2022;279(5):2417–2422. doi: 10.1007/s00405-021-07010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meher R, Wadhwa V, Kumar V, et al. COVID associated mucormycosis: a preliminary study from a dedicated COVID Hospital in Delhi. Am J Otolaryngol. 2022;43(1):103220. doi: 10.1016/j.amjoto.2021.103220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Subramani M, Shankaran S. Bacterial sinusitis in the COVID-19 era: a reminder for antibiotic stewardship. Cureus. 2021;13(11):e19453. doi: 10.7759/cureus.19453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arteaga AA, Tran J, Frey H, Lewis AF. Rapidly progressive complicated acute bacterial sinusitis in the setting of severe pediatric SARS-CoV-2 infection. Ann Otol Rhinol Laryngol. 2022;131(10):1158–1163. doi: 10.1177/00034894211055337. [DOI] [PubMed] [Google Scholar]
- 8. Shahab A, Arora A, Chhina SS, Dhillon S, Nazir U. A unique triad of invasive sinusitis, brain abscess with focal cerebritis, and COVID-19. Am J Case Rep. 2021;22:e933177. doi: 10.12659/AJCR.933177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charlton M, Nair R, Gupta N. Subdural empyema in adult with recent SARS-CoV-2 positivity case report. Radiol Case Rep. 2021;16(12):3659–3661. doi: 10.1016/j.radcr.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White PL, Dhillon R, Cordey A, et al. A national strategy to diagnose coronavirus disease 2019-associated invasive fungal disease in the intensive care unit. Clin Infect Dis. 2021;73(7):e1634–e1644. doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kariyawasam RM, Dingle TC, Kula BE, Vandermeer B, Sligl WI, Schwartz IS. Defining COVID-19-associated pulmonary aspergillosis: systematic review and meta-analysis. Clin Microbiol Infect. 2022;28(7):920–927. doi: 10.1016/j.cmi.2022.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ismaiel WF, Abdelazim MH, Eldsoky I, et al. The impact of COVID-19 outbreak on the incidence of acute invasive fungal rhinosinusitis. Am J Otolaryngol. 2021;42(6):103080. doi: 10.1016/j.amjoto.2021.103080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. SeyedAlinaghi S, Karimi A, Barzegary A, et al. Mucormycosis infection in patients with COVID-19: a systematic review. Health Sci Rep. 2022;5(2):e529. doi: 10.1002/hsr2.529. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. El-Kholy NA, El-Fattah AMA, Khafagy YW. Invasive fungal sinusitis in post COVID-19 patients: a new clinical entity. Laryngoscope. 2021;131(12):2652–2658. doi: 10.1002/lary.29632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DelGaudio JM, Evans SH, Sobol SE, Parikh SL. Intracranial complications of sinusitis: what is the role of endoscopic sinus surgery in the acute setting. Am J Otolaryngol. 2010;31(1):25–28. doi: 10.1016/j.amjoto.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 16. Goldberg AN, Oroszlan G, Anderson TD. Complications of frontal sinusitis and their management. Otolaryngol Clin North Am. 2001;34(1):211–225. doi: 10.1016/s0030-6665(05)70307-8. [DOI] [PubMed] [Google Scholar]
- 17. Nicoli TK, Oinas M, Niemelä M, Mäkitie AA, Atula T. Intracranial suppurative complications of sinusitis. Scand J Surg. 2016;105(4):254–262. doi: 10.1177/1457496915622129. [DOI] [PubMed] [Google Scholar]
- 18. Felsenstein S, Williams B, Shingadia D, et al. Clinical and microbiologic features guiding treatment recommendations for brain abscesses in children. Pediatr Infect Dis J. 2013;32(2):129–135. doi: 10.1097/INF.0b013e3182748d6e. [DOI] [PubMed] [Google Scholar]
- 19. Patel AP, Masterson L, Deutsch CJ, Scoffings DJ, Fish BM. Management and outcomes in children with sinogenic intracranial abscesses. Int J Pediatr Otorhinolaryngol. 2015;79(6):868–873. doi: 10.1016/j.ijporl.2015.03.020. [DOI] [PubMed] [Google Scholar]