Abstract
BACKGROUND
Diffuse midline glioma (DMG), H3K27-altered, is a rare, highly malignant central nervous system neoplasm that arises in midline structures. They are more commonly encountered in children and are rarely encountered in adults, usually in the thalamus or spinal cord. The presence of the H3K27 mutation in the H3F3A gene automatically classifies a tumor as World Health Organization grade IV. These tumors carry a grim prognosis, with an overall median survival of less than 1 year.
OBSERVATIONS
The authors report the case of a 38-year-old male presenting with acute-onset urinary retention who was found to have an expansile, well-circumscribed mass involving the conus medullaris at the level of T12–L1. A T12–L1 laminectomy and tumor debulking were performed. Pathology revealed glial cells with astrocytic morphology among Rosenthal fibers, microvascular proliferation, and cellular atypia. The H3K27 mutation was confirmed.
LESSONS
DMG, H3K27-altered, is a rarely encountered entity that can present in numerous midline structures. If localized to the conus medullaris, it may present as acute-onset urinary retention in a previously asymptomatic patient. Further investigation is needed to characterize its molecular and clinical features in adults to improve the management of those presenting with these tumors.
Keywords: H3K27, diffuse midline glioma, conus medullaris, spinal cord tumor
ABBREVIATIONS: ADC = apparent diffusion coefficient, CNS = central nervous system, DMG = diffuse midline glioma, DWI = diffusion-weighted imaging, EMG = electromyography, MEP = motor-evoked potential, WHO = World Health Organization
Spinal cord tumors encompass approximately 15% of all central nervous system (CNS) neoplasms. They are commonly classified into 3 categories based on their depth within the spine: extradural (55%), intradural extramedullary (40%), and intramedullary (5%). Intramedullary tumors comprise astrocytoma (30%) and ependymoma (30%); the remaining 40% consist of miscellaneous, rarer neoplasms.1 Diffuse midline glioma (DMG), H3K27-altered, a subtype of pediatric-type diffuse high-grade gliomas, is a rare CNS neoplasm that is primarily found in children and less commonly in adults. It was previously classified as diffuse intrinsic pontine glioma, referring to high-grade gliomas that most commonly occur in the pons and primarily in children. In adolescents and adults, however, these tumors may arise in several midline structures, most commonly the thalamus and spinal cord.2
There are few reported cases of DMG, H3K27-altered, in adults, most of whom present with vague clinical symptoms. DMG, H3K27-altered, often mimics more common CNS neoplasms histopathologically and is thus frequently misdiagnosed on initial presentation. Most cases present with an astrocytic or ependymal morphology, although oligodendroglioma-resembling tumors have also been reported. They are usually highly vascularized and may possess other features, including central necrosis, multinucleation, pseudorosettes, and neuropil-like islands.2,3 DMGs are associated with uniformly dismal outcomes in pediatric patients, in whom median survival is estimated to be less than 1 year after diagnosis.4 Mortality in adults, however, is more variable.6
Illustrative Case
A 38-year-old male with no significant past medical history presented with acute, significant urinary retention for the previous 4 weeks, accompanied by left flank pain radiating to the left lower quadrant. During this time, he was experiencing both increasing urgency and difficulty with voiding. He reported improvement in pain whenever he was able to void more fully. He denied testicular pain or swelling, hematuria, and fever. Strength was 5/5 in all 4 limbs, with no motor drift. Sensation to light touch was decreased in the right lower extremity and perianal area.
Magnetic resonance imaging of the thoracic and lumbar spine was performed, which revealed a well-defined, expansile lesion with mild surrounding edema, involving the conus medullaris at the level of T12–L1 (Fig. 1). The lesion measured 2.1 × 1.8 × 4.64 cm in the transverse, anteroposterior, and cranial-caudal planes, respectively. Apparent diffusion coefficient (ADC) images revealed restricted diffusion in a hypointense mass at T12–L1 situated along the midline. The lesion was further delineated as an enhancing mass with surrounding edema and lacking central necrosis on T2-weighted and T1-weighted imaging. These radiological findings, along with the patient’s young age and no prior history of malignancy, raised the possibility of a spinal astrocytoma or ependymoma.
FIG. 1.
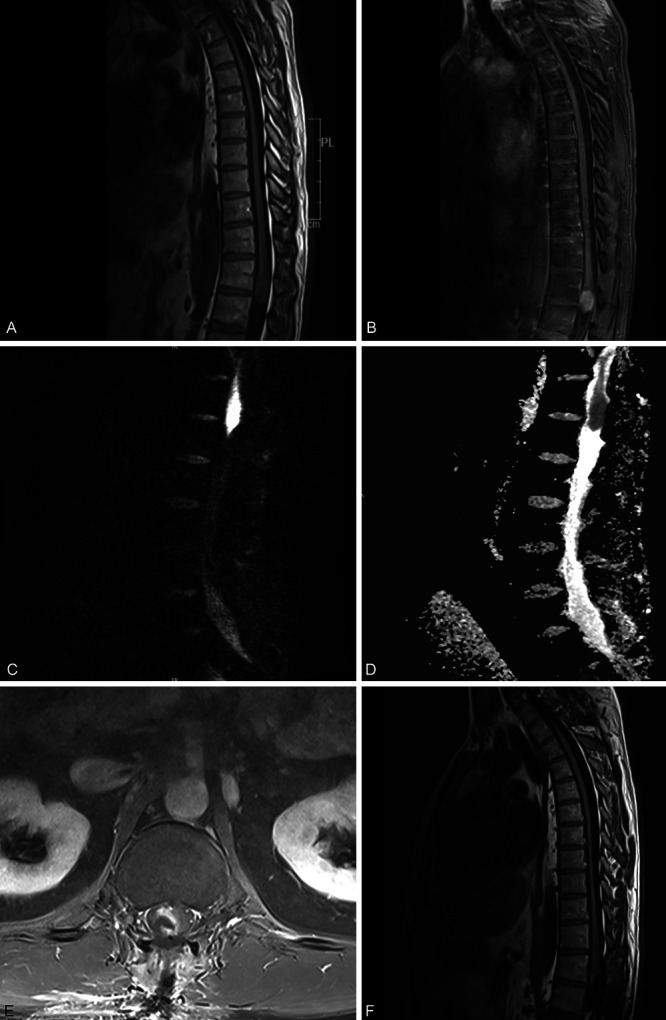
Sagittal magnetic resonance imaging (MRI) of the lumbar spine displaying the lesion at T12–L1: T1-weighted precontrast image (A), T1-weighted postcontrast image (B), DWI (C), and corresponding ADC image (D). Postoperative imaging was acquired 3 days following the resection: axial postcontrast volume interpolated breath-hold examination (VIBE) T1-weighted MRI (E) and sagittal postcontrast T1-weighted MRI (F).
The patient underwent T12–L1 laminectomy and intradural exploration for resection of the tumor. Upon opening the dura, the conus medullaris appeared swollen and discolored. Using a microscope, a subtotal tumor resection was performed with neuro-monitoring of motor-evoked potentials (MEPs) and electromyography (EMG). Results of MEP and EMG were stable and guided delineation of the margins of the tumor and healthy spinal tissue. The dura was closed in a watertight fashion, and the wound was closed in standard ashionn. The operation concluded, and the patient awoke, moving all 4 extremities voluntarily.
Postoperatively, the patient was alert and oriented and hemodynamically stable. He maintained full strength in all 4 extremities but was unable to contract his gluteus muscles. Additionally, he reported numbness and tingling around the anus and penis, as well as diminished sensation to his right lateral foot. He continued to have difficulty voiding and required reinsertion of a Foley catheter. The patient remained in the hospital for 12 days and was discharged to an acute rehabilitation center. He was examined 6 weeks later at a follow-up office visit. At the follow-up, there was slight improvement in his right foot paresthesia and he was awaiting another void trial. The patient was awaiting radiotherapy with adjuvant chemotherapy.
A sample of the tumor was collected during the operation and sent for histopathological analysis (Fig. 2). The sample contained an infiltrative neoplasm with glial cells, some spindled and arranged in a vaguely fascicular pattern, but most of the neoplasm lacked architectural patterning. Nuclei were pleomorphic with cellular atypia. Mitotic activity was found in some areas of the neoplasm. Rosenthal fibers were abundant. There was focal, mild, subtle (incipient) microvascular proliferation. Necrosis was not found. Additionally, the sample contained foci of perivascular lymphocytic infiltration composed of mostly B cells and few T cells and plasma cells, consistent with an inflammatory reaction. Molecular pathology later revealed the presence of several mutations: H3F3A, K27M; PPM1D, S421*; NF1, P185L; GLI3, V778I; and TET1, Q355H. The presence of the H3K27 mutation confirmed the diagnosis of DMG, H3K27-altered, CNS World Health Organization (WHO) grade 4 in this patient.
FIG. 2.
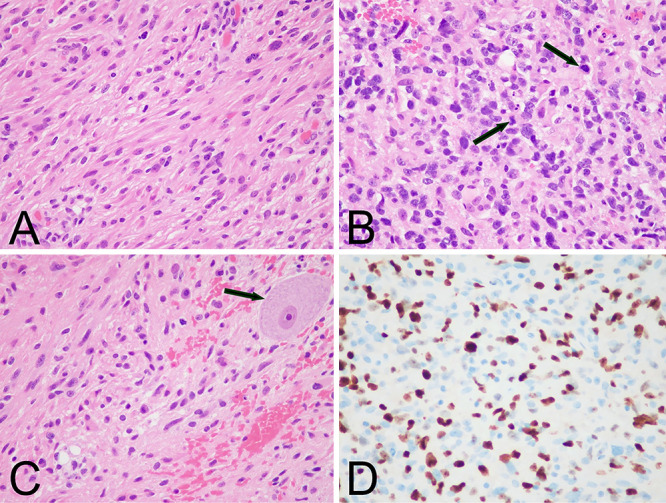
Microscopic pathology of the spinal cord neoplasm. A: Hematoxylin and eosin (H&E) stain showing a moderately cellular sample composed of cells with glial morphology. B: H&E stain of a sample with high cellularity and pleomorphism. Arrows indicate mitotic figures. C: H&E stain exhibiting neoplastic cells infiltrating normal spinal cord parenchyma. Nonneoplastic spinal cord anterior horn cell is indicated by an arrow. D: Ki-67 immunohistochemistry reveals labeling of approximately 30% of neoplastic cells in this area. Original magnification ×400 (A–D).
Discussion
Observations
We describe the case of a 38-year-old male presenting with acute urinary retention and an extensive intramedullary spinal cord tumor at T12–L1, later confirmed to be DMG, H3K27-altered. Currently, there have been fewer than 20 reported cases of spinal cord DMG, H3K27-altered, in adults and only 1 other case localized to the level of the conus medullaris.5–9
The radiographic features of DMG, H3K27-altered, are highly variable among the few reported cases. Nevertheless, identifying relevant features at presentation can improve prognostication and the choice of treatment. In our case, a well-defined, contrast-enhancing mass with surrounding edema was apparent. In contrast, most adult cases of DMG, H3K27-altered, have been reported to be partially enhancing or nonenhancing and do not usually present with edema.8,10 Diffusion-weighted imaging (DWI) is increasingly utilized in the diagnosis of CNS gliomas, as it can offer insight into cell density, and thus histological grade, noninvasively. Significant diffusion restriction in the volume of the lesion was present on ADC, likely indicating a high-grade, poorly differentiated neoplasm. In a healthy 38-year-old male with no past medical history or family history of malignancy, diffusion restriction on ADC suggests a tumor with significant malignant potential as opposed to a low-grade ependymoma or astrocytoma—pathologies more likely to be observed in our patient’s demographic. These findings can be better reconciled with more reported cases and improved imaging techniques over time.
The diagnosis of DMG, H3K27-altered, is confirmed with the presence of the H3.3, 3.1, or 3.2 K27 mutation in the H3F3A gene. The pathogenesis is the result of a total decrease in methylation of histone H3, leading to downstream epigenetic changes that augment the tumor’s malignant potential.11,12 Wild-type H3 variants also rarely present, possessing mutations in genes such as EGFR or TERT. Moreover, the majority of reported cases comprise tumors located in the brain; spinal cord tumors are less likely to harbor the H3K27 mutation in H3F3A and are more associated with mutations in NF1 and TERT.13 The presence of the H3K27 mutation has been associated with a fatal prognosis; compared with DMGs that harbor alternative mutations, neither the pathological features nor survival appear to be significantly different.14
As per the 2021 WHO guidelines, the presence of the H3K27 mutation indicates a high-grade, aggressive tumor, regardless of the apparent histopathological grade.15 However, retrospective studies have reported DMG, H3K27-altered, to be more common than previously thought, with up to 15% of adults with DMGs harboring the mutation. Ultimately, the epidemiology of DMG, H3K27-altered, is not well defined given the paucity of cases reported, along with the heterogeneity in tumor location, age, and patient-specific molecular pathology. Thus, the impact of tumor location in the prognosis of DMG, H3K27-altered, has yet to be conclusively investigated.11,12 The interactions between associated genes and tumor location are likely complex and multifactorial; our case is a unique addition to the sparse body of literature regarding this tumor.
Lessons
DMG, H3K27-altered, remains a rare condition with limited reported data regarding its epidemiology, clinical characteristics at presentation, effective treatment, and clinical management. We describe the clinical presentation, investigations, and postoperative follow-up of the second reported case of DMG, H3K27-altered, presenting at the level of the conus medullaris and the first case with diagnostic findings on DWI.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Saluja, Razzaq, Servider, Mushlin. Acquisition of data: Razzaq, Servider. Analysis and interpretation of data: Razzaq, Servider, Seidman, Mushlin. Drafting of the article: Saluja, Razzaq, Servider, Mushlin. Critically revising the article: all authors. Reviewed submitted version of the manuscript: all authors. Administrative/technical/material support: Seidman. Study supervision: Mushlin.
References
- 1. Khalid S, Kelly R, Carlton A, et al. Adult intradural intramedullary astrocytomas: a multicenter analysis. J Spine Surg. 2019;5(1):19–30. doi: 10.21037/jss.2018.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng L, Gong J, Yu T, et al. Diffuse midline gliomas with histone H3 K27M mutation in adults and children: a retrospective series of 164 cases. Am J Surg Pathol. 2022;46(6):863–871. doi: 10.1097/PAS.0000000000001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 4. Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32(4):520–537. e5. doi: 10.1016/j.ccell.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. López-Pérez CA, Franco-Mojica X, Villanueva-Gaona R, Díaz-Alba A, Rodríguez-Florido MA, Navarro VG. Adult diffuse midline gliomas H3 K27-altered: review of a redefined entity. J Neurooncol. 2022;158(3):369–378. doi: 10.1007/s11060-022-04024-5. [DOI] [PubMed] [Google Scholar]
- 6. Vuong HG, Ngo TNM, Le HT, Dunn IF. The prognostic significance of HIST1H3B/C and H3F3A K27M mutations in diffuse midline gliomas is influenced by patient age. J Neurooncol. 2022;158(3):405–412. doi: 10.1007/s11060-022-04027-2. [DOI] [PubMed] [Google Scholar]
- 7. Gu Q, Huang Y, Zhang H, Jiang B. Case report: five adult cases of H3K27-altered diffuse midline glioma in the spinal cord. Front Oncol. 2021;11:701113. doi: 10.3389/fonc.2021.701113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palpan Flores A, Rodríguez Domínguez V, Esteban Rodriguez I, Román de Aragón M, Zamarrón Pérez Á. H3K27M-mutant glioma in thoracic spinal cord and conus medullaris with pilocytic astrocytoma morphology: case report and review of the literature. Br J Neurosurg. doi: 10.1080/02688697.2021.1988054. Published online October 7, 2021. doi: 10.1080/02688697.2021.1988054. [DOI] [PubMed] [Google Scholar]
- 9. Peters K, Pratt D, Koschmann C, Leung D. Prolonged survival in a patient with a cervical spine H3K27M-mutant diffuse midline glioma. BMJ Case Rep. 2019;12(10):e231424. doi: 10.1136/bcr-2019-231424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yabuno S, Kawauchi S, Umakoshi M, et al. Spinal cord diffuse midline glioma, H3K27M-mutant effectively treated with bevacizumab: a report of two cases. NMC Case Rep J. 2021;8(1):505–511. doi: 10.2176/nmccrj.cr.2021-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schulte JD, Buerki RA, Lapointe S, et al. Clinical, radiologic, and genetic characteristics of histone H3 K27M-mutant diffuse midline gliomas in adults. Neuro-Oncol Adv. 2020;2(1):vdaa142. doi: 10.1093/noajnl/vdaa142. doi: 10.1093/noajnl/vdaa142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Argersinger DP, Rivas SR, Shah AH, Jackson S, Heiss JD. New developments in the pathogenesis, therapeutic targeting, and treatment of H3K27M-mutant diffuse midline glioma. Cancers (Basel) 2021;13(21):5280. doi: 10.3390/cancers13215280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vuong HG, Le HT, Jea A, McNall-Knapp R, Dunn IF. Risk stratification of H3 K27M-mutant diffuse midline gliomas based on anatomical locations: an integrated systematic review of individual participant data. J Neurosurg Pediatr. 2022;30(1):1–8. doi: 10.3171/2022.3.PEDS2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schreck KC, Ranjan S, Skorupan N, et al. Incidence and clinicopathologic features of H3 K27M mutations in adults with radiographically-determined midline gliomas. J Neurooncol. 2019;143(1):87–93. doi: 10.1007/s11060-019-03134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]


