Abstract
BACKGROUND
Gliosarcoma is a rare and highly malignant cancer of the central nervous system with the ability to metastasize. Secondary gliosarcoma, or the evolution of a spindle cell–predominant tumor after the diagnosis of a World Health Organization grade IV glioblastoma, has also been shown to metastasize. There is little information on metastatic secondary gliosarcoma.
OBSERVATIONS
The authors present a series of 7 patients with previously diagnosed glioblastoma presenting with recurrent tumor and associated metastases with repeat tissue diagnosis consistent with gliosarcoma. The authors describe the clinical, imaging, and pathological characteristics in addition to carrying out a systematic review on metastases in secondary gliosarcoma.
LESSONS
The present institutional series and the systematic review of the literature show that metastatic secondary gliosarcoma is a highly aggressive disease with a poor prognosis.
Keywords: secondary gliosarcoma, metastasis, malignant brain tumor, cancer
ABBREVIATIONS: BCNU = carmustine; CCNU = lomustine; CNS = central nervous system; CPT-11 = irinotecan; CT = computed tomography; EGFR = epidermal growth factor receptor; FISH = fluorescence in situ hybridization; GFAP = glial fibrillary acidic protein; IDH = isocitrate dehydrogenase; IHC = immunohistochemistry; KPS = Karnofsky Performance Status; MRI = magnetic resonance imaging; PCV = procarbazine, lomustine, and vincristine; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PTEN = phosphatase and tensin homolog; WHO = World Health Organization
Gliosarcoma is a rare malignant intrinsic brain tumor established as its own pathological entity.1 Gliosarcoma comprises <10% of all World Health Organization (WHO) grade IV glioblastomas and can be primary or secondary (arising from a previously diagnosed glioblastoma).2 Genomic studies between glioblastoma and gliosarcoma show overlapping (TERTp, CDKN2A/B, phosphatase and tensin homolog [PTEN]) and unique (BRAF, STAG2, SUZ12) genomic alterations.3 Despite these metabolic differences, the natural history of gliosarcoma is similar to that of glioblastoma,4–6 although it carries a greater propensity to metastasize outside the central nervous system.7–9 Glioblastomas have also been reported, although rarely, to metastasize. However, the literature supports spread through cerebrospinal fluid pathways as a more common route than hematogenous or direct spread. There are few studies that focus on metastasis in gliosarcoma and no studies exclusively studying metastatic secondary gliosarcoma, which, given the heterogeneity in high-grade glioma, may be a concrete clinical entity. In this study, we focus on a series of patients with gliosarcoma transforming from a pathologically proven glioblastoma presenting with metastases. We discuss the clinical characteristics, imaging findings, and pathological evaluation of a historical case series of patients, describe an illustrative case in detail, and carry out a systemic review of patients with secondary gliosarcoma and metastases.
Study Description
Patient Selection
Electronic medical records between 2000 and 2022 were reviewed to identify patients with pathologically diagnosed WHO grade IV glioblastoma with subsequent transformation to pathologically diagnosed gliosarcoma with new extraaxial metastases. All patients had at least 2 surgeries—on initial presentation and on recurrence with metastasis—for pathological diagnosis. Clinical, imaging, and pathological characteristics were obtained via retrospective chart review. At minimum, all patients had magnetic resonance imaging (MRI) studies with T1-weighted sequences with and without gadolinium contrast at a minimum of 2 time points: at initial presentation and upon recurrence. Surgical specimens underwent immunohistochemical staining, fluorescence in situ hybridization (FISH), and comprehensive genomic screening for mutational status (FoundationOne CDx, Foundation Medicine) when possible.
Systematic Review
A systematic review was performed according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.10 Inclusion criteria were as follows: 1) clinical studies including case reports/case descriptions, 2) patients with gliosarcoma, and 3) extraaxial metastases of gliosarcoma. Exclusion criteria were as follows: 1) studies not reported in English and without English translation, 2) studies of primary (de novo) gliosarcoma, 3) studies without extraaxial metastases, 4) studies without a minimum 3-month clinical follow-up, and 5) studies without tissue diagnosis of both initial glioblastoma and subsequent gliosarcoma. Information sources included the MEDLINE PubMed database. The search strategy used was as follows: [gliosarcoma] AND [metastasis]. With this initial search, 75 articles were retrieved. For each of these 75 articles, the abstract was evaluated by a single independent reviewer using the inclusion and exclusion criteria. This was then filtered by another independent reviewer. Subsequently, a full-length review was performed on selected articles in parallel by independent reviewers. All full-length study evaluations were then filtered by another independent reviewer. Study variables obtained included the year of publication, the number of patients, and whether pathology or genomic data were provided. Variables of interest for comparison obtained included patient characteristics such as age and sex, initial surgery pathology, subsequent surgery pathology, location of metastasis, whether the metastasis was biopsy proven, adjuvant treatment(s), and overall survival from recurrence. Bias in the selection of studies was addressed by multiple independent reviewers, highlighting pathology results for primary surgery, secondary surgery, and metastases.
Case Series
We identified 7 patients with previously diagnosed glioblastoma presenting with tumor recurrence along with cranial and/or extracranial metastases, after which pathological diagnosis was deemed to be gliosarcoma. Clinical characteristics are described in Table 1. We also performed a systematic literature review to identify additional cases for comparison, as summarized in Table 1 and in the Discussion section. Within our case series, there were 3 (43%) women and 4 (57%) men. The mean age at initial diagnosis was 52.8 years (range 26–66 years). The initial location of the tumor was frontal in 2 cases (28.5%), parietal in 2 (28.5%), and temporal in 3 (42.9%). The initial pathological diagnosis was isocitrate dehydrogenase (IDH) wild-type glioblastoma in all 7 patients. Six patients (85.7%) had gross-total resection of the contrast-enhancing portion of the tumor. All patients had radiation therapy to the surgical bed. Adjuvant chemotherapy included temozolomide in 5 patients (71.4%), irinotecan (CPT-11) in 2 (28.5%), lomustine (CCNU) in 1 (14.3%), and procarbazine, lomustine, and vincristine (PCV) in 1 (14.3%). Four patients (57.1%) had bevacizumab therapy between transformation to gliosarcoma. The mean time to recurrence from pathological designation of glioblastoma to pathological designation of gliosarcoma with metastases was 11.7 months (range 5–22 months). The location of metastases was the dura in 4 patients (57.1%); skull in 4 (57.1%); and scalp, vertebral bodies, and lung in 1 patient each (14.3% each). All patients had surgery after recurrence and/or biopsies at all metastatic sites. All 7 patients had glial fibrillary acidic protein (GFAP)-negative spindle cell morphology on pathological review consistent with gliosarcoma. All specimens were positive for vimentin. One patient (14.3%) had epidermal growth factor receptor (EGFR) amplification, and 2 patients (28.5%) had PTEN deletions. No patients had IDH mutations. Overall survival after the second surgery was, on average, 4.14 months (range 1–12 months). These overall trends correlated well with the results of the systematic review, as summarized in the Discussion section.
TABLE 1.
Case series and systematic reviews
| Age(yrs) | Sex | PrimaryTumorLocation | Extent ofResection | AdjuvantTherapy | Time toTransformation(mos) | MetastasisLocation | Survival AfterTransformation(mos) | CaseSource |
|---|---|---|---|---|---|---|---|---|
| 52 |
F |
Temporal |
GTR |
PCV+RT |
8 |
Skull |
4 |
Institutional series |
| 62 |
M |
Temporal |
GTR |
CCNU+RT, CPT-11 |
14 |
Skull, dura |
2 |
Institutional series |
| 54 |
M |
Parietal |
STR |
TMZ+RT |
7 |
Dura |
7 |
Institutional series |
| 54 |
M |
Parietal |
GTR |
TMZ+RT, CPT-11, bevacizumab |
12 |
Lung |
2 |
Institutional series |
| 26 |
F |
Frontal |
GTR |
TMZ+RT, CCNU, bevacizumab |
5 |
Scalp, skull |
12 |
Institutional series |
| 56 |
F |
Frontal |
GTR |
TMZ+RT, bevacizumab |
22 |
Dura |
1 |
Institutional series |
| 66 |
M |
Temporal |
GTR |
TMZ+RT, bevacizumab |
14 |
Dura, skull, VB |
1 |
Institutional series |
| 47 |
M |
Temporal |
GTR |
BCNU+RT, TMZ, CPT-11 |
9 |
Lung, liver, spleen, scalp |
1.5 |
Beaumont et al., 20078 |
| 52 |
M |
Frontal |
GTR |
TMZ+RT |
5 |
Scalp, skull |
2 |
Bekar et al., 201011 |
| 57 |
F |
Temporal |
GTR |
TMZ+RT |
42 |
Scalp, zygoma, abdomen |
12 |
Dawar et al., 201312 |
| 37 |
M |
Temporal |
GTR |
TMZ+RT |
6 |
Skull, orbit, scalp, diaphragm |
2 |
Oberndorfer et al., 201313 |
| 63 |
M |
Frontal |
GTR |
RT |
12 |
Lung, bone, liver |
15 |
Choi et al., 201614 |
| 55 |
M |
Parietal |
GTR |
TMZ+RT |
6 |
Dura, skull, VB, lung |
2 |
Capion et al., 201915 |
| 69 |
M |
Frontal |
GTR |
TMZ+RT |
7 |
Liver |
1 |
Choi et al., 202016 |
| 53 | F | Temporal | GTR | TMZ+RT, bevacizumab | 15 | Spinal cord | 5 | Hsu et al., 202017 |
GTR = gross-total resection; RT = radiation therapy; STR = subtotal resection; TMZ = temozolomide; VB = vertebral body.
Illustrative Case
A 66-year-old male with a past medical history of hypertension and coronary artery disease initially presented with a 3-month history of headaches, nausea, and dizziness with recent falls. His history was negative for seizures. The initial neurological examination showed no focal neurological deficits. His Karnofsky Performance Status (KPS) was 90 on admission. MRI with and without gadolinium contrast was performed and showed a 3.2 × 4.8 × 4.2–cm heterogeneously enhancing, centrally necrotic mass in the right temporal pole with surrounding vasogenic edema and 5 mm of midline shift consistent with high-grade glioma (Fig. 1A). Computed tomography (CT) of the chest, abdomen, and pelvis was performed, which showed no evidence of other lesions. The patient was admitted and started on antiepileptic therapy (levetiracetam 1,000 mg twice daily) and steroids (dexamethasone 4 mg 3 times per day).
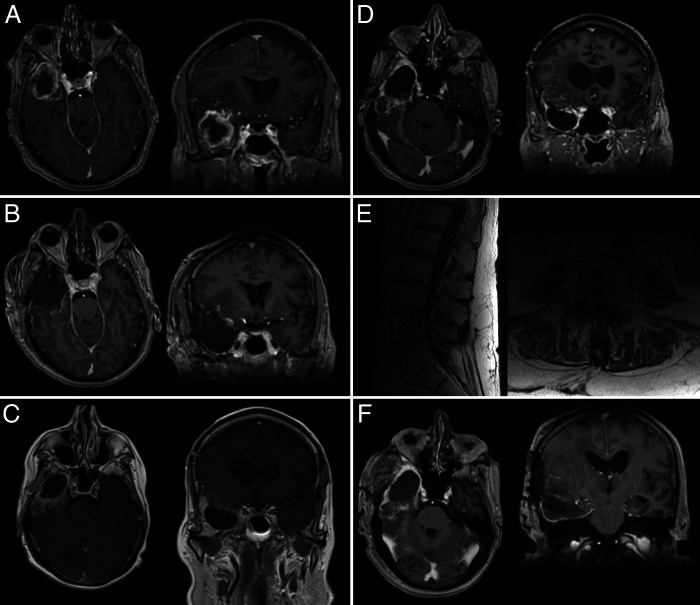
FIG. 1.
A: Brain MRI shows a right temporal lesion with necrosis and edema. B: Brain MRI obtained postoperatively shows gross-total resection of the lesion. C: Brain MRI shows recurrent lesion toward the posterior aspect of the resection cavity. D: Brain MRI obtained 2 weeks following admission shows extensive lesions. E: Lumbar spine MRI shows lesions near the conus and cauda equina and in the vertebral bodies. F: Brain MRI shows total resection of the recurrent temporal lesion.
Shortly after admission, the patient was taken to the operating room for a right temporal craniotomy for resection of the tumor. The anterior temporal lobe and lesion anterior to the vein of Labbé were removed. Medially, the resection was carried to the tentorial incisura and the temporal horn of the lateral ventricle. Grossly, the tumor appeared as a high-grade glioma. Postoperatively, the patient was at his baseline neurological examination. Postoperative MRI with and without gadolinium contrast showed gross-total resection of the contrast-enhancing portion of the tumor and no postsurgical complication (Fig. 1B). The pathology was consistent with WHO grade IV IDH wild-type glioblastoma (see Pathological Findings section). The patient was discharged to home on postoperative day 3. The patient received postoperative concurrent temozolomide at 75 mg for 5 cycles and 60 Gy in 32 fractions. He tolerated this therapy with no issues or complications.
The patient was monitored with serial imaging every 3 months postoperatively. At approximately 12 months, the patient presented to the local emergency department with seizures and was found to have a 2.0 × 2.0 × 3.0–cm heterogeneously enhancing mass at the posterior aspect of the resection cavity (Fig. 1C). The patient was discharged on increased antiepileptic therapy and steroids. While awaiting new imaging and elective surgery for recurrent glioma, the patient presented with confusion and was admitted for further work-up. At this time, his KPS had declined to 70. New imaging showed stable recurrence at the posterior aspect of the resection, but now with 2 new subcentimeter dura-based lesions (right frontal and left frontal) involving the inner cortex of the skull. There was expansion of the right sphenoid wing and new enhancement in the right temporalis muscle (Fig. 1D). Repeat CT of the chest, abdomen, and pelvis was performed and was negative for any abnormality. MRI with and without contrast of the spinal axis was performed and showed enhancement of the cauda equina and large vertebral body lesions of L4 and L5 (Fig. 1E).
Given the presence of bony lesions, the L4 vertebral body lesion was biopsied via needle biopsy. Pathology showed atypical spindle cells. With these data, the case was discussed at the multidisciplinary tumor board. Given the unclear metastatic process with clear intracranial recurrence in a patient with functional independence prior to presentation with seizures, the decision was made to carry out repeat resection of the temporal tumor and biopsy of the right frontal dura-based lesion. The patient was taken to the operating room for this procedure. This lesion had invaded the skull, and this area was also biopsied. The intracranial mass was then resected with a complete temporal lobectomy together with a resection of involved mesial temporal structures (Fig. 1F). Postoperative imaging showed gross-total resection of the contrast-enhancing lesion and biopsy of the right frontal lesion.
The patient’s postoperative course was complicated by hydrocephalus. The patient was taken to the operating room 1 week after surgery for insertion of a left frontal ventriculoperitoneal shunt. The patient was discharged with palliative spinal radiation. The patient’s condition continued to decline, and eventually he was transferred to hospice, where he died 6 weeks after surgery. The family declined an autopsy.
Pathological Findings
Permanent sections obtained during the initial and subsequent resections and the vertebral body and frontal lesion biopsies were sent for multiple diagnostic studies, including immunohistochemistry (IHC), FISH, and genomic testing. Results are summarized in Table 2 and displayed in Fig. 2.
TABLE 2.
Detailed IHC, FISH, and genomic results
| Variable | Initial Resection | VB Biopsy | Repeat Resection + Frontal Biopsy |
|---|---|---|---|
| GFAP |
Positive |
Negative |
Negative |
| ATRX |
Positive |
— |
Positive |
| IDH1 |
Wild type |
— |
Wild type |
| Olig2 |
— |
Negative |
Negative |
| EMA |
— |
Negative |
— |
| S100 |
— |
Negative |
— |
| Desmin |
— |
Focally positive |
— |
| SMA |
— |
Focally positive |
— |
| Myogenin |
— |
Negative |
— |
| MyoD1 |
— |
Negative |
— |
| Vimentin |
— |
Positive |
— |
| CD56 |
— |
Patchy positive |
— |
| CD34 |
— |
Negative |
— |
| MPO |
— |
Negative |
— |
| P53 |
Rare positive nuclei |
— |
Rare positive nuclei |
| Ki67 |
Variable positivity |
Variable positivity |
Variable positivity |
| Proliferation index |
10–15% |
3–5% |
25–35% |
|
MGMT promoter |
Unmethylated |
— |
— |
|
EGFR amplification |
Negative |
— |
— |
| Chromosome 7 polysomy |
Negative |
— |
— |
| Monosomy 10 | Positive | — | — |
FoundationOne testing of the sample revealed the following genomic findings: NF1 (W267*, L190*, I1931fs*5), PTEN (splice site 209 + 5G>A), CBL (P417L), CDKN2A/B (loss), JAK1 (E188K)†, MITF (G344R)†, MLH1 (splice site 678-1G>A, P654L, S556N, G67E, splice site 1989G>A, splice site 381-1G>A)†, MLS2 (W5065*), NOTCH1 (A1650T)†, PMS2 (split site 903G>A)†, PTPN11 (E69K)†, SMARCA4 (S767F)†, TERT promoter (124C>T).
† Indicates subclonal mutations.
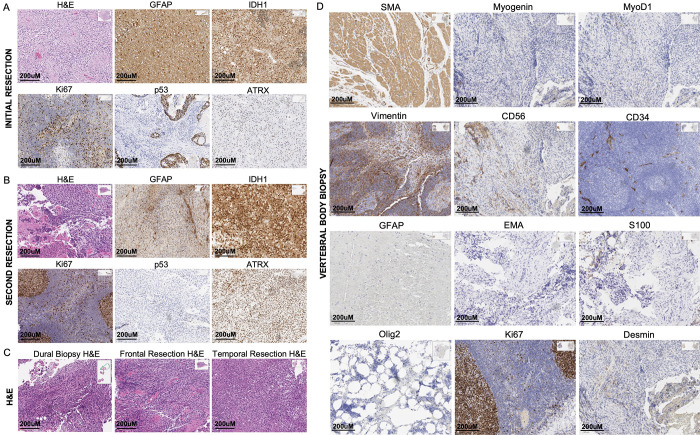
FIG. 2.
A: Pathologic stains from initial resection of the glioblastoma. B: Pathologic stains from resection of the recurrent lesion determined to be gliosarcoma. C: Hematoxylin and eosin (H&E) stains from various aspects of the recurrent lesion, including the frontal dural lesions, frontal part of the recurrent tumor, and temporal aspect of recurrent tumor. D: Various IHC stains performed on the vertebral biopsy sample obtained.
Sections from the first resection were classified as WHO grade IV IDH wild-type glioblastoma. Histologically, sections from the initial resection demonstrated hypercellular proliferation of pleomorphic glial cells with enlarged nuclei, atypical mitoses, palisading necrosis, and multifocal microvascular proliferation. IHC studies showed that the sample was GFAP positive, ATRX positive, and IDH1 wild-type. Additionally, these studies demonstrated rare positive nuclei for p53 and variable positivity for Ki-67 (proliferation index 10%–15%; Fig. 2A). Genomic studies showed that the sample was MGMT unmethylated. FISH studies showed that the sample was negative for EGFR gene amplification with no evidence of chromosome 7 polysomy and positive for monosomy 10 (loss of 1 copy of PTEN) in 70% of the nuclei examined.
Sections from the second resection of the temporal tumor were classified as being consistent with gliosarcoma, given a sarcomatous component that was additionally noted in the sample, new compared with prior. Histologically, these sections showed a high-grade spindle neoplasm with a predominantly mesenchymal appearance, 50% necrosis, numerous mitoses, and focal perivascular lymphocytic cuffing (Fig. 2B). IHC studies showed that the sample was GFAP negative, ATRX positive, Olig2 negative, and IDH1 wild-type. Studies also showed rare positive nuclei for p53 and variable positivity for Ki-67 (with a higher proliferation index compared with prior at 25%–35%). A frontal dural lesion biopsy was also obtained during this resection, pertinent for similar atypical spindle cells as noted in the frontal and temporal aspects of the resected sample (Fig. 2C).
Multiple assessments were performed on the vertebral body lesions, including IHC on tissues, flow cytometry, and fine-needle aspiration. Sections sent for permanent tissue analysis were assessed for a number of markers, as summarized in Table 2. Notably, samples were GFAP negative, Olig2 negative, and vimentin positive. They were focally positive for desmin and smooth muscle actin and showed variable positivity for Ki-67 (3%–5% proliferation index; Fig. 2D). Fine-needle aspiration showed disordered groups of atypical spindle cells. Flow cytometry showed no blasts, no monotypic B cells, and no T-cell aberrancies.
The tumor sample was sent to FoundationOne for a full diagnostic panel. The results showed microsatellite stability with a tumor mutational burden of 98 mutations/Mb. The genomic findings identified are summarized in Table 2. Notably, the patient did have mutations in MLH1, PTEN, NF1, TERT, and PMS2.
Discussion
Observations
In this descriptive study, we focus on the clinical situation of new extracranial lesions in a patient with pathologically diagnosed glioblastoma as the result of secondary transformation to gliosarcoma and subsequent gliosarcoma metastases. This is a rare scenario, with accordingly few cases reported in the literature.8,11–17 We identified a case series of 7 patients at our institution.
We additionally performed a PRISMA-based10 systematic review to identify patients with secondary gliosarcoma and metastasis. Each included patient was required to have a pathological diagnosis of glioblastoma followed by a pathological diagnosis of gliosarcoma with extraaxial metastasis. Of the 75 articles screened, 8 were selected after application of the inclusion and exclusion criteria (Fig. 3, Table 1).8,11–17 There were 3 (37.5%) female and 5 (62.5%) male patients. The mean age at diagnosis was 47 years (range 37–69 years). The primary glioblastoma location was frontal in 3 patients (37.5%), parietal in 1 (12.5%), and temporal in 4 (50%). All patients had gross-total resection of the initial tumor. The initial diagnosis was WHO grade IV glioblastoma in all cases. There were no cases of IDH mutation. Adjuvant radiation therapy was carried out in all patients, along with temozolomide in 7 (87.5%) and carmustine (BCNU), CPT-11, and bevacizumab in 1 patient each (12.5% each). The mean time to transformation (defined as pathologically diagnosed glioblastoma to pathologically diagnosed gliosarcoma) was 12.8 months (range 5–42 months). The most common locations of metastasis included the scalp in 4 patients (50%), skull in 4 (50%), lung in 3 (37.5%), liver in 3 (37.5%), and extracranial bony region in 3 (37.5%). The mean overall survival after transformation was 5.1 months (range 1–15 months). These results are overall similar to the trends identified in our case series of 7 patients.
FIG. 3.
PRISMA flow diagram. The PubMed database was searched for studies of gliosarcoma and metastases. Seventy-five studies met initial criteria and were reviewed for inclusion/exclusion criteria. This review identified 8 cases for inclusion in the analysis.
In an attempt to identify which patients with glioblastoma are more likely to experience subsequent transformation and metastases, we found no association with patient characteristics such as age or sex. In addition, patients with secondary gliosarcoma had primary glioblastoma tumors in the frontal, parietal, and temporal lobes, with no cases of occipital lobe glioblastoma converting to metastatic secondary glioblastoma, similar to previously reported data on both primary and secondary gliosarcoma.18 There were no cases of IDH-mutant astrocytoma19 converting to secondary gliosarcoma with metastasis, suggesting that this may be a process stemming from IDH wild-type glioblastoma alone. There was no specific adjuvant therapy that was associated with transformation. Vuong et al.,18 in a meta-analysis of primary versus secondary gliosarcoma, reported that secondary gliosarcoma was associated with higher rates of bevacizumab therapy. In our institutional series, 50% of patients were treated with bevacizumab therapy prior to transformation, and in the literature, only 12.5% were treated with bevacizumab prior to transformation. It is unclear if there is any association between bevacizumab therapy and the transformation from glioblastoma to gliosarcoma.
Glioblastoma and gliosarcoma are differentiated by underlying mesenchymal differentiation and the presence of sarcomatous cells. This may be connected to the latter’s potentially greater propensity to metastasize through hematogenous and/or direct spread. These specific differences in the underlying biology between glioblastoma and gliosarcoma leading to diverging clinical behavior patterns is an important topic for further investigation. The time to transformation from initial diagnosis of glioblastoma to diagnosis of secondary gliosarcoma with metastasis was similar in our institutional study and in the literature, with an average of approximately 1 year. However, because imaging findings of glioblastoma and gliosarcoma are so similar, it is unclear when the transformation is truly occurring, because there is likely a latency period between conversion of the primary tumor to the development of extracranial metastases. Interestingly, metastasis with gliosarcoma was more often a direct extension (1) into the dura, skull, and scalp or (2) outside the central nervous system (CNS), such as the lung, liver, and bones, rather than (3) drop metastases into the spinal cord. This differs from the metastatic profile of most CNS tumors, which follows cerebrospinal fluid egress pathways.20 These data may suggest that gliosarcoma can simply spread by surgical contamination, through temporalis muscle contamination with a temporal craniotomy,12 or even at a distant site.11 Furthermore, the metastatic profile of gliosarcoma, being more similar to extracranial malignancies such as breast, lung, or colon cancer,21 suggests hematogenous spread in addition to spread through the neuroaxis.22
Lessons
Overall, we report the largest series of secondary gliosarcoma with metastases and systematically review the literature to characterize this rare entity. In patients diagnosed with glioblastoma, new extracranial lesions must put conversion of the tumor to gliosarcoma to the front of the differential diagnosis. Although the natural history of intracranial glioblastoma and gliosarcoma may be similar,4–6 after gliosarcoma metastasis, the prognosis becomes very grim.23 Further research into understanding the genomic alterations that may lead to transformation and subsequent metastasis is needed, along with specific therapies, separate from those for glioblastoma, for managing both intraaxial gliosarcoma and its extracranial metastases.
Acknowledgments
This work was supported by the Jonsson Cancer Center Foundation, University of California, Los Angeles (UCLA) and the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research, UCLA.
Disclosures
Dr. Liau reported being a member of the Board of Directors for ClearPoint Neuro, Inc. outside the submitted work.
Author Contributions
Conception and design: Patel, Baisiwala, Vivas, Bari. Acquisition of data: Patel, Baisiwala, Ko, Zubair, Vivas, Bari. Analysis and interpretation of data: Patel, Baisiwala, Ko, Zubair, Vivas, Everson, Bari. Drafting the article: Patel, Baisiwala, Ko, Li. Critically revising the article: Patel, Baisiwala, Ko, Zubair, Vivas, Everson, Bari. Reviewed submitted version of manuscript: Patel, Baisiwala, Ko, Zubair, Vivas, Everson, Liau. Approved the final version of the manuscript on behalf of all authors: Patel. Statistical analysis: Baisiwala. Administrative/technical/material support: Patel, Liau. Study supervision: Patel.
References
- 1. Lutterbach J, Guttenberger R, Pagenstecher A. Gliosarcoma: a clinical study. Radiother Oncol. 2001;61(1):57–64. doi: 10.1016/s0167-8140(01)00415-7. [DOI] [PubMed] [Google Scholar]
- 2. Cachia D, Kamiya-Matsuoka C, Mandel JJ, et al. Primary and secondary gliosarcomas: clinical, molecular and survival characteristics. J Neurooncol. 2015;125(2):401–410. doi: 10.1007/s11060-015-1930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zaki MM, Mashouf LA, Woodward E, et al. Genomic landscape of gliosarcoma: distinguishing features and targetable alterations. Sci Rep. 2021;11(1):18009. doi: 10.1038/s41598-021-97454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han SJ, Yang I, Ahn BJ, et al. Clinical characteristics and outcomes for a modern series of primary gliosarcoma patients. Cancer. 2010;116(5):1358–1366. doi: 10.1002/cncr.24857. [DOI] [PubMed] [Google Scholar]
- 5. Han SJ, Yang I, Tihan T, Chang SM, Parsa AT. Secondary gliosarcoma: a review of clinical features and pathological diagnosis. J Neurosurg. 2010;112(1):26–32. doi: 10.3171/2009.3.JNS081081. [DOI] [PubMed] [Google Scholar]
- 6. Galanis E, Buckner JC, Dinapoli RP, et al. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: North Central Cancer Treatment Group results. J Neurosurg. 1998;89(3):425–430. doi: 10.3171/jns.1998.89.3.0425. [DOI] [PubMed] [Google Scholar]
- 7. Witwer BP, Salamat MS, Resnick DK. Gliosarcoma metastatic to the cervical spinal cord: case report and review of the literature. Surg Neurol. 2000;54(5):373–379. doi: 10.1016/s0090-3019(00)00315-3. [DOI] [PubMed] [Google Scholar]
- 8. Beaumont TL, Kupsky WJ, Barger GR, Sloan AE. Gliosarcoma with multiple extracranial metastases: case report and review of the literature. J Neurooncol. 2007;83(1):39–46. doi: 10.1007/s11060-006-9295-x. [DOI] [PubMed] [Google Scholar]
- 9. Cerame MA, Guthikonda M, Kohli CM. Extraneural metastases in gliosarcoma: a case report and review of the literature. Neurosurgery. 1985;17(3):413–418. doi: 10.1227/00006123-198509000-00003. [DOI] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bekar A, Kahveci R, Tolunay S, Kahraman A, Kuytu T. Metastatic gliosarcoma mass extension to a donor fascia lata graft harvest site by tumor cell contamination. World Neurosurg. 2010;73(6):719–721. doi: 10.1016/j.wneu.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 12. Dawar R, Fabiano AJ, Qiu J, Khushalani NI. Secondary gliosarcoma with extra-cranial metastases: a report and review of the literature. Clin Neurol Neurosurg. 2013;115(4):375–380. doi: 10.1016/j.clineuro.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 13. Oberndorfer S, Wöhrer A, Hainfellner JA, et al. Secondary gliosarcoma with massive invasion of meninges, skull base, and soft tissue, and systemic metastasis. Clin Neuropathol. 2013;32(6):522–524. doi: 10.5414/NP300643. [DOI] [PubMed] [Google Scholar]
- 14. Choi TM, Cheon YJ, Jung TY, Lee KH. A stable secondary gliosarcoma with extensive systemic metastases: a case report. Brain Tumor Res Treat. 2016;4(2):133–137. doi: 10.14791/btrt.2016.4.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Capion T, Hauerberg J, Broholm H, Muhic A. Multiple extracranial metastases from primary gliosarcoma in a patient with two previous different primary cancers. Case Rep Oncol Med. 2019;2019:7849616. doi: 10.1155/2019/7849616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi MG, Lee JH, Lee MS, Suh SJ, Lee YS, Kang DG. Primary gliosarcoma with extracranial metastasis. Brain Tumor Res Treat. 2020;8(1):53–56. doi: 10.14791/btrt.2020.8.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsu BH, Lee WH, Yang ST, Han CT, Tseng YY. Spinal metastasis of glioblastoma multiforme before gliosarcomatous transformation: a case report. BMC Neurol. 2020;20(1):178. doi: 10.1186/s12883-020-01768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vuong HG, Dunn IF. Primary versus secondary gliosarcoma: a systematic review and meta-analysis. J Neurooncol. 2022;159(1):195–200. doi: 10.1007/s11060-022-04057-w. [DOI] [PubMed] [Google Scholar]
- 19. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wright CH, Wright J, Onyewadume L, et al. Diagnosis, treatment, and survival in spinal dissemination of primary intracranial glioblastoma: systematic literature review. J Neurosurg Spine. 2019;31(5):723–732. doi: 10.3171/2019.5.SPINE19164. [DOI] [PubMed] [Google Scholar]
- 21. Wick MR. Metastases of malignant neoplasms: historical, biological, & clinical considerations. Semin Diagn Pathol. 2018;35(2):112–122. doi: 10.1053/j.semdp.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 22. Ramos R, Morais N, Silva AI, Almeida R. Gliosarcoma with neuroaxis metastases. BMJ Case Rep. 2015;2015:bcr2015212970. doi: 10.1136/bcr-2015-212970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feng SS, Li HB, Fan F, et al. Clinical characteristics and disease-specific prognostic nomogram for primary gliosarcoma: a SEER population-based analysis. Sci Rep. 2019;9(1):10744. doi: 10.1038/s41598-019-47211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]