Abstract
OBJECTIVE
Choroid plexus carcinoma (CPC) is a rare, primarily intraventricular neoplasm. Extent of resection correlates with improved outcomes but is limited due to tumor vascularity and size. Evidence on optimal surgical management and molecular drivers of recurrence remains limited. Here the authors characterize a case of multiply recurrent CPC treated with sequential endoscopic removals over 10 years and highlight its genomic properties.
OBSERVATIONS
Five years after standard treatment, a 16-year-old female presented with a distant intraventricular recurrence of CPC. Whole exome sequencing revealed NF1, PER1, and SLC12A2 mutations, FGFR3 gain, and no TP53 alterations. Repeat sequencing on recurrences 4 and 5 years later showed persistent NF1 and FGFR3 alterations. Methylation profiling was consistent with plexus tumor, subclass pediatric B. Short-term magnetic resonance imaging detected four total isolated recurrences, all treated with complete endoscopic resections at 5, 6.5, 9, and 10 years after initial diagnosis. Mean hospital stay for all recurrences was 1 day with no complications.
LESSONS
The authors describe a patient with four isolated recurrences of CPC over a decade, each treated with complete endoscopic removal, and identify unique molecular alterations that persisted without TP53 alterations. These outcomes support frequent neuroimaging to facilitate endoscopic surgical removal following early detection of CPC recurrence.
Keywords: choroid plexus carcinoma, endoscopic resection, recurrent, choroid plexus tumor, pediatric neurosurgery, whole exome sequencing
ABBREVIATIONS: 3D = three-dimensional, CPC = choroid plexus carcinoma, CPT = choroid plexus tumor, GTR = gross-total resection, MDM2 = mouse double minute 2, MPRAGE = T1-magnetization–prepared rapid gradient-echo, MRI = magnetic resonance imaging, VUS = variant of undetermined significance, WES = whole exome sequencing, WHO = World Health Organization
Choroid plexus tumors (CPTs) are rare, accounting for 3% of all brain tumors in children.1 Choroid plexus carcinomas (CPCs) differ from other classifications of CPT in that they frequently arise in the lateral ventricles, are invasive, and have a poor prognosis.2 Although there is no established standard of care given the paucity of controlled trials, the current goal of CPC management is successful gross-total resection (GTR) since it is the strongest predictor of overall survival.3–5 Unfortunately, approximately one-half of all GTRs fail because of the surgical challenges that arise when operating on these large, vascular tumors in patients whose median age is less than 2 years.6 In response to this, therapeutic schemes to facilitate safe and effective second-look surgery (i.e., neoadjuvant chemotherapy or arterial embolization) are advocated.5,7,8
Recurrence of CPC is common, but there are sparse reports of long-term outcomes for patients who have had recurrence, particularly in nonsyndromic cases.9,10 Likewise, there is limited knowledge regarding molecular drivers of tumor recurrence. Here we present a rare case of CPC with multiple local and distal recurrences over an approximate 10-year period, successfully managed with repeated endoscopic removals. Whole exome sequencing (WES) and targeted sequencing results reveal unique and persistent genetic alterations associated with this multiply recurrent tumor.
Illustrative Case
History and Examination
A 20-year-old female was referred to our institution for evaluation of CPC recurrence. She was initially diagnosed with CPC in the right atrium of the lateral ventricle at 11 years old after experiencing headaches, diplopia, and syncope leading to presentation to an outside emergency department. Magnetic resonance imaging (MRI) demonstrated compression of the right thalamus, significant mass effect, and concurrent hydrocephalus for which an external ventricular drain was placed emergently to relieve elevated intracranial pressure. Subtotal resection and a second-look GTR (resection 1) requiring right parietal craniotomy were performed over a period of 1 week and were followed by a 6-month regimen of adjuvant chemotherapy. Three years later, after increasingly worsening vision and diplopia, a CPC recurrence was found in the identical right thalamic location on surveillance MRI. This recurrence was treated with open resection 1 month later (resection 2).
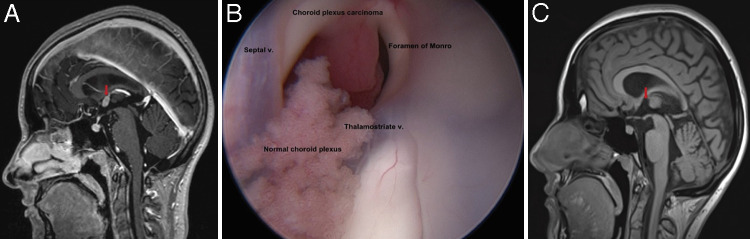
At the age of 16, 2 years after her first recurrence, the patient presented to our institution after surveillance MRI revealed a new 1.1 × 0.7 cm distal recurrence at the roof of the anterior third ventricle abutting the foramen of Monro without concurrent ventricular enlargement (Fig. 1A). Neurological examination was unremarkable including intact cranial nerve assessment and normal strength, tone, and deep tendon reflexes symmetrically. There was no disturbance of the hypothalamic-pituitary axis. This metastatic recurrence was deemed amenable to stereotactic navigation-guided endoscopic resection due to the intraventricular location and absence of drop metastases on cervical, thoracic, and lumbar spine MRI.
FIG. 1.
A: Preoperative sagittal postcontrast 3D T1-MPRAGE MRI demonstrating a new 1.1 × 0.7 cm distal recurrence at the roof of the anterior third ventricle abutting the foramen of Monro without concurrent ventricular enlargement. B: Anatomically labeled view into the foramen of Monro using a 30-degree angled endoscopic lens. The patient’s CPC is shown in contrast to normal choroid plexus, displaying its hypervascular and multilobulated nature as well as its attachment at the roof of the third ventricle. C: Postoperative postcontrast T1-weighted MRI revealed the absence of nodular enhancement, suggesting GTR without residual tumor. v. = vein.
Surgical Technique and Patient Outcome
CPC resection was performed under stereotactic guidance, utilizing endoscopic technique (resection 3, Video 1). The patient was positioned supine with 3-point fixation using a Mayfield head frame and a right coronal burr hole was created for entry. Utilizing a 6-mm obturator with a sheath, the endoscopic sheath (Lotta, Karl Storz) was passed utilizing navigational guidance into the frontal horn of the lateral ventricle. Inspection of the foramen of Monro with a 30-degree angled lens (Lotta, Karl Storz) revealed a hypervascular and multilobulated mass in the anterior third ventricle abutting the foramen of Monro (Fig. 1B). Bipolar cautery was used to create a plane of separation between the tumor and the adjoining subforniceal parenchyma. Any intraoperative venous hemorrhage encountered from either a subependymal vein or vascular pedicle was controlled with a combination of bipolar cautery, balloon tamponade, and constant irrigation. Once the tumor was sufficiently devascularized, suction aspiration was the primary means for tumor removal to achieve a GTR. Surgical pathology demonstrated neoplastic epithelial cells with nuclear pleomorphism, blurring of the papillary architectural pattern, focal necrosis, and mitotic activity, consistent with a diagnosis of recurrent CPC. The patient tolerated the procedure without complication and was discharged on postoperative day 1. Postoperative MRI revealed the absence of nodular enhancement suggesting GTR with no residual tumor (Fig. 1C).
VIDEO 1. Clip showing minimally invasive neuroendoscopic resection of patient’s third overall recurrence of CPC. A 30-degree angled lens offers a view into the foramen of Monro with anatomical labels provided. The patient’s CPC is shown in contrast to normal choroid plexus, displaying its hypervascular and multilobulated nature as well as its attachment at the roof of the third ventricle. A detailed description of the surgical technique for the procedure is described in the text. Click here to view.
Radiological Surveillance and Additional Intervention
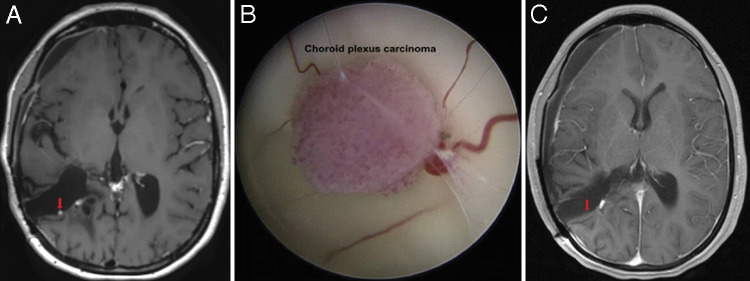
The patient remained asymptomatic for 2 years with regular 3-month follow-ups. However, surveillance imaging with postgadolinium T1-weighted MRI eventually revealed a 2.5-mm hyperintense, enhancing nodule located in the right temporoparietal resection tract concerning for a third local tumor recurrence (Fig. 2A). Repeat endoscopic GTR was achieved (resection 4) without complication or need for transfusion (Fig. 2B) and confirmed on postoperative T1 postcontrast MRI (Fig. 2C).
FIG. 2.
A: Preoperative axial postcontrast T1–sampling perfection with application optimized contrasts using different flip angle evolution (SPACE) MRI sequence showing focal punctate enhancement along the posterior wall of the patient’s prior right temporoparietal resection tract confirmed as local CPC recurrence. The third ventricle is notably free of disease. B: Endoscopic view of patient’s CPC recurrence in the previous right temporoparietal resection tract. The 2.5-mm tumor is multilobulated and hypervascular with surrounding vessels and attachment to parenchymal tissue at its base. C: Postoperative axial postcontrast T1-weighted MRI demonstrating absence of enhancement at the resection site, indicative of GTR. Vascular enhancement is noted adjacent to resected tumor site.
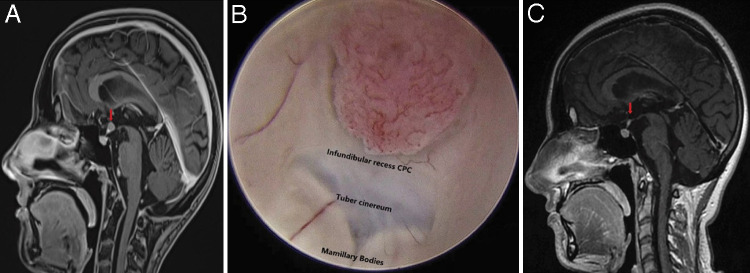
Two years from her third recurrence and now at the age of 20, surveillance imaging revealed a 0.7 × 0.5 cm enhancing nodular metastatic lesion located in the infundibular recess on sagittal postcontrast three-dimensional (3D) T1-magnetization–prepared rapid gradient-echo (MPRAGE) MRI sequence (Fig. 3A). A third endoscopic GTR was undertaken without complication or subsequent symptoms (resection 5; Fig. 3B and C). A fourth recurrence, measuring 0.4 × 0.4 cm, was found 5 months later along the posterior margin of the right parietal resection cavity on MRI (Fig. 4) and was treated endoscopically with similar results (resection 6).
FIG. 3.
A: Preoperative sagittal postgadolinium 3D T1-MPRAGE MRI demonstrating a 0.7 × 0.5 cm enhancing nodular focus along the infundibular recess of the third ventricle. B: Endoscopic view of patient’s disseminated CPC recurrence fungating into the third ventricle from the infundibular recess. C: Postoperative sagittal postgadolinium MRI exhibiting GTR of patient’s CPC. Pituitary stalk demonstrates mild enhancement with lack of adjacent tumoral enhancement suggesting absence of residual tumor.
FIG. 4.
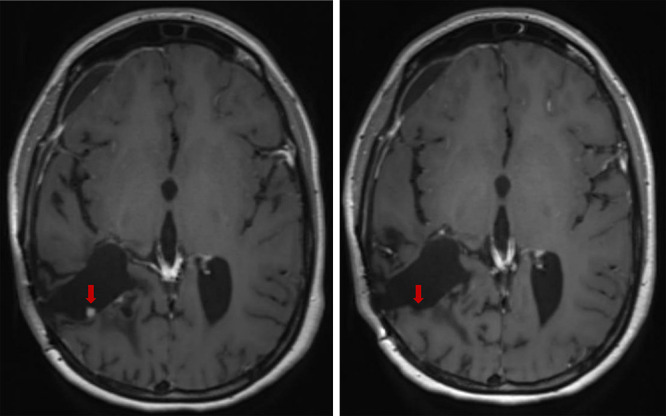
Left: Preoperative axial postcontrast T1-SPACE MRI demonstrating a 0.4 × 0.4 cm enhancing nodular focus along the posterior margin of the previous right parietal resection cavity. Right: Postoperative axial postgadolinium MRI exhibiting GTR of patient’s CPC 3 months after endoscopic intervention.
Tumor Histopathology and Molecular Characterization
Resections 1 and 2
The pathology slides from the patient’s original resection were reviewed at three independent outside institutions, each rendering a diagnosis of choroid plexus carcinoma, World Health Organization (WHO) grade 3. Histological features included nuclear pleomorphism, five mitotic figures within 10 hpf, and a Ki-67 (MIB-1) labeling index of 20%. Staining for p53 was not consistent with an underlying TP53 mutation. Histological analysis of resection 2 revealed recurrent CPC with eight mitotic figures within 10 high-power field (hpf), and a Ki-67 (MIB-1) labeling index of 7.3%.
Resections 3 to 6
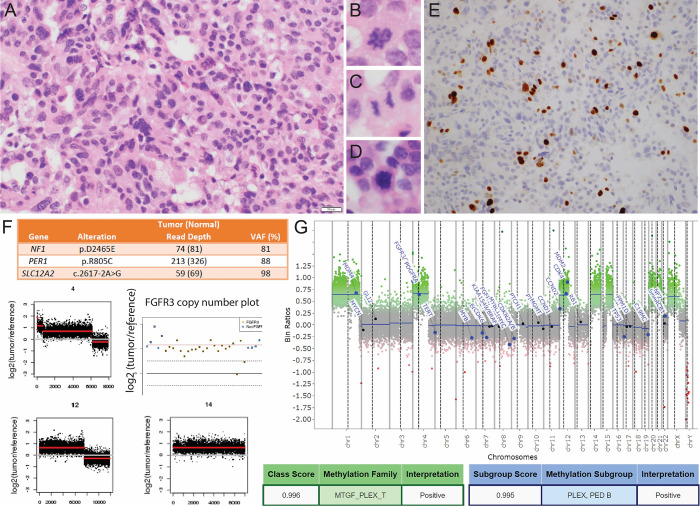
Histological findings of resection 3 included a papillary architectural pattern with focal sheet-like growth and nuclear pleomorphism (Fig. 5A), scattered mitotic figures (Fig. 5B–D), and a Ki-67 (MIB-1) labeling index of 10% (Fig. 5E), consistent with recurrent CPC. WES revealed two somatic missense variants of undetermined significance (VUSs) in the cancer-associated genes NF1 and PER1. In addition, 13 mutations were detected in other genes for which splice site or truncating mutations were detected: COL4A5 (nonsense mutation), CTPS2 (frameshift deletion), GALC (splice affecting variant), GLI3 (nonsense mutation), and SLC12A2 (splice affecting variant). Copy number gain of the FGFR3 locus was detected within the setting of broader chromosome 4 copy number changes and among multiple additional genome-wide copy number alterations highlighted in (Fig. 5F and G).
FIG. 5.
A: Hematoxylin and eosin (H&E) staining of resection 4 demonstrates nuclear pleomorphism and partial effacement of the papillary architecture. B–D: Several mitotic figures are identified within a limited amount of endoscopically recovered material. E: Immunohistochemistry shows an elevated Ki-67 (MIB-1) labeling index of 10%. F: Selected findings from WES include NF1, PER1, SLC12A2 mutations, FGFR3 gain, and additional chromosomal copy number alterations that are characteristic of CPC. G: Methylation array profiling on material from recurrence 6 showed similar copy number alterations as WES, and the profile was a high match for plexus tumor, subclass pediatric B.
Targeted sequencing performed on material from resection 5 (using the Oncomine version 2 platform) and resection 6 (using the TSO500 platform) showed persistence of the NF1 VUS and FGFR3 gain (other VUSs detected by WES were not covered by the more targeted panels). The TSO500 panel performed on resection 6 also detected several additional VUSs, none of which were detected by the WES assay performed on resection 3; these included BRAF p.P403T as well as ERCC1, GATA3, PRKDC, RANBP2, and STK11 alterations.
Finally, methylation array profiling (on tissue from resection 5) showed several copy number alterations that aligned with those originally derived from the WES data, and additionally demonstrated a methylation class profile match with plexus tumor, subclass pediatric B using version 11 of the DKFZ CNS methylation classifier (Fig. 5G).11 The vast majority of tumors diagnosed as CPC in the pediatric setting belong to this methylation class and conversely 60% of tumors in this class are CPC. Thus, although the class is enriched for CPC relative to other CPT, it is neither completely sensitive nor specific for CPC relative to histological diagnosis.
Of note, WES and both targeted panels were negative for the detection of TP53 alterations. Moreover, we detected in this patient the presence of biallelic p.P72 in the TP53 gene as opposed to the p.R72 variant, the latter of which has been associated with TP53-wild-type CPC.12 Similarly, we did not detect the mouse double minute 2 (MDM2) single nucleotide polymorphism 309 that has also been associated with TP53-wild-type CPC.12
Discussion
Observations
CPC is considered a WHO grade 3 CPT, reflecting its risk for invasion and dissemination.13 Histopathology typically exhibits frequent mitotic figures, nuclear anaplasia, necrosis, and sheet-like growth. Proclivity for growth and invasion have been associated with factors such as age, anatomical location, epigenetic profile, and other molecular alterations including those involving TP53 and TERT.13–15 The tumor presented in this case lacked TP53 mutations or other polymorphisms that have been associated with reduced p53 activity including the TP53 p.R72 variant for the MDM2 oncoprotein. The presence of an FGFR3 gain is of potential interest given a study that identified a subtype of CPC with high expression of FGFR3, although the presence of FGFR3 overexpression or increased copy numbers of the gene has not demonstrated independent prognostic information to-date in CPTs.16
The tumor demonstrated persistence of an NF1 missense VUS at high variant allele frequency that is of potential interest given the gene’s known role in a spectrum of tumors including those involving the central nervous system; however, the biological relevance of this alteration is unknown and this gene was not found to be altered in a series of CPTs published by Thomas et al.15 in 2020. The remaining VUS mutations we detected were not demonstrated in this same published case series. Of potential interest was the presence of an ERRC2 alteration in one case of CPC in the series, and an ERCC1 alteration detected in resection 6 of the present case, a gene that is similarly involved in gene repair mechanisms. Finally, while it is noted that the gene SLC12A2 has been shown to be highly expressed in choroid plexus and plays a prominent role in regulating cerebrospinal fluid flow dynamics during postnatal murine development,17 whether or not the splice site alteration detected in this case (at high variant allele frequency) may have contributed to the oncogenic cascade of this tumor or merely represents a passenger mutation is not known.
At the genomic scale, this case demonstrated similar copy number alterations to those seen in the pediatric B subtype as demonstrated in Thomas et al.15 and that is also concordant with our methylation profiling findings. In particular, both WES and methylation profiling demonstrated persistent copy number gains across a 5-year time period in chromosomes 1, 4, 12, 14, 20, and 21, all of which commonly show gains in pediatric subtype B cases of CPT.
Surgical excision is the standard management strategy for CPC with adjuvant therapies provided at the discretion of the admitting institution. Wrede et al.,18 in their meta-analysis of over 800 cases of CPTs, determined that second-look surgery for recurrent or incompletely resected CPC improved 2-year overall survival by up to 40% compared with patients who did not undergo repeat excision. Therefore, second-look surgery should always be attempted, if feasible, in patients presenting with recurrence or isolated metastasis of CPC.
Over time, increased size, vascularity, mutagenicity, and dissemination of untreated CPC impact treatment strategy and may ultimately make GTR more difficult or impossible to achieve.15,18–20 Although several previous reports have detailed endoscopic resection for intraventricular choroid plexus papilloma,21–23 the role of upfront endoscopic resection of CPC has been limited historically, given the hemorrhagic nature and voluminous size of the tumor. Advantages of endoscopy for intraventricular tumor removal relate to the minimally invasive nature of the approach, including the potential for shorter length of stay, reduced morbidity, and control of blood loss, which is often a limiting factor in CPC resection.24 This property increases the feasibility of multiple resections with minimal impact on the patient relative to repeat craniotomy.
Often-cited limitations of a purely endoscopic approach include restricted instrumentation for bimanual solid tumor dissection and constraints in achieving expedient hemostasis.22 The latter is of particular importance given the risk of intraoperative hemorrhage associated with this highly vascularized tumor. Intraoperative bleeding can disrupt the view through the endoscope, requiring regular irrigation and complicating extent of tumor removal.25 In our described approach and surgical video, hemostasis was successfully achieved with a combination of bipolar cautery, balloon tamponade, and constant irrigation. Overall, early detection of primary and recurrent CPC is key to limiting tumor vascularity and size to facilitate a purely endoscopic approach.22
Nonetheless, the above case demonstrates four separate GTRs achieved in the same patient utilizing endoscopic technique. This minimally invasive mode of surgical intervention was facilitated through utilization of close surveillance for tumor recurrence with frequent MRI and has contributed to a durable patient outcome over a decade of treatment.
Lessons
We have described the case of a patient with multiply recurrent, isolated, metastatic CPC over a 10-year period. This case highlights the role of early surveillance imaging, endoscopic surgery, and capability of a durable outcome in the face of multiple recurrences. Overall, this adds to the growing body of literature regarding metastatic CPC and outcomes of surgical management.26–30
The findings in this case raise the possibility that there may exist a subgroup of patients with histological evidence of CPC that cluster epigenetically with pediatric subgroup B but who lack evidence of TP53 mutation or polymorphisms associated with dysregulation of p53 in CPT. Further, this subgroup may have distinct outcomes with an individualized set of surgical management options. Increased analysis over broader series of patients with CPC will be necessary to determine this. Finally, whether the potential novel genes of interest in this case including NF1, PER1, and/or SLC12A2 contribute to tumor development in CPT would require functional studies or much larger series of cases than those published to-date.
We advocate for frequent surveillance with neuroimaging in these patients because this strategy can detect CPC recurrence early in the natural history of the tumor, prior to dissemination and substantial changes in size and vascularity, thus facilitating expedient second-look surgery while the tumor is still amenable to endoscopic resection. In conjunction with adolescent age of onset, this management strategy emphasizing early repeat endoscopic intervention generated a favorable and durable outcome for our patient.31,32
Given the high recurrence rate of this tumor,32,33 alternatives to open surgical techniques should continue to be explored to ensure safe and efficacious second-look surgery.34,35 As surgical and radiological management strategies for intraventricular CPC continue to be refined, we expect that minimally invasive endoscopic resection will come to occupy a larger role in the repertoire of the pediatric neurosurgeon.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Souweidane, Guadix. Acquisition of data: Souweidane, Guadix, Pisapia. Analysis and interpretation of data: Souweidane, Guadix, Pisapia. Drafting the article: all authors. Critically revising the article: all authors. Reviewed submitted version of manuscript: Souweidane, Guadix, Gundlach, Pisapia. Approved the final version of the manuscript on behalf of all authors: Souweidane. Administrative/technical/material support: Souweidane. Study supervision: Souweidane.
Supplemental Information
Videos
Video 1. https://vimeo.com/781781775.
Previous Presentations
Aspects of this work have been presented in poster form at the International Society for Pediatric Neuro-Oncology (ISPNO) Scientific Meeting in Hamburg, Germany from June 12–15, 2022 and published as an abstract supplement in Neuro-Oncology.
References
- 1. Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18(suppl 5):v1–v75. doi: 10.1093/neuonc/now207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun MZ, Oh MC, Ivan ME, et al. Current management of choroid plexus carcinomas. Neurosurg Rev. 2014;37(2):179–192. doi: 10.1007/s10143-013-0499-1. discussion 192. [DOI] [PubMed] [Google Scholar]
- 3. Sun MZ, Ivan ME, Clark AJ, et al. Gross total resection improves overall survival in children with choroid plexus carcinoma. J Neurooncol. 2014;116(1):179–185. doi: 10.1007/s11060-013-1281-5. [DOI] [PubMed] [Google Scholar]
- 4. Sun MZ, Ivan ME, Oh MC, et al. Effects of adjuvant chemotherapy and radiation on overall survival in children with choroid plexus carcinoma. J Neurooncol. 2014;120(2):353–360. doi: 10.1007/s11060-014-1559-2. [DOI] [PubMed] [Google Scholar]
- 5. Schneider C, Kamaly-Asl I, Ramaswamy V, et al. Neoadjuvant chemotherapy reduces blood loss during the resection of pediatric choroid plexus carcinomas. J Neurosurg Pediatr. 2015;16(2):126–133. doi: 10.3171/2014.12.PEDS14372. [DOI] [PubMed] [Google Scholar]
- 6. Pencalet P, Sainte-Rose C, Lellouch-Tubiana A, et al. Papillomas and carcinomas of the choroid plexus in children. J Neurosurg. 1998;88(3):521–528. doi: 10.3171/jns.1998.88.3.0521. [DOI] [PubMed] [Google Scholar]
- 7. Baro V, Gabrieli JD, Cester G, et al. Preoperative devascularization of choroid plexus tumors: specific issues about anatomy and embolization technique. Brain Sci. 2021;11(5):540. doi: 10.3390/brainsci11050540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Otten ML, Riina HA, Gobin YP, Souweidane MM. Preoperative embolization in the treatment of choroid plexus papilloma in an infant. Case report. J Neurosurg. 2006;104(6) suppl:419–421. doi: 10.3171/ped.2006.104.6.419. [DOI] [PubMed] [Google Scholar]
- 9. Hart S, Avery R, Barron J. Late recurrence of choroid plexus carcinoma. Childs Nerv Syst. 2020;36(8):1601–1606. doi: 10.1007/s00381-020-04663-x. [DOI] [PubMed] [Google Scholar]
- 10. McEvoy M, Robison N, Manley P, et al. Successful treatment of recurrent Li-Fraumeni Syndrome-related choroid plexus carcinoma. J Pediatr Hematol Oncol. 2017;39(8):e473–e475. doi: 10.1097/MPH.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. doi: 10.1038/nature26000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tabori U, Shlien A, Baskin B, et al. TP53 alterations determine clinical subgroups and survival of patients with choroid plexus tumors. J Clin Oncol. 2010;28(12):1995–2001. doi: 10.1200/JCO.2009.26.8169. [DOI] [PubMed] [Google Scholar]
- 13. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zaky W, Finlay JL. Pediatric choroid plexus carcinoma: Biologically and clinically in need of new perspectives. Pediatr Blood Cancer. 2018;65(7):e27031. doi: 10.1002/pbc.27031. [DOI] [PubMed] [Google Scholar]
- 15. Thomas C, Soschinski P, Zwaig M, et al. The genetic landscape of choroid plexus tumors in children and adults. Neuro Oncol. 2021;23(4):650–660. doi: 10.1093/neuonc/noaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Granberg KJ, Raina A, Lehtinen B, et al. Moderate-to-strong expression of FGFR3 and TP53 alterations in a subpopulation of choroid plexus tumors. Histol Histopathol. 2020;35(7):673–680. doi: 10.14670/HH-18-180. [DOI] [PubMed] [Google Scholar]
- 17. Xu H, Fame RM, Sadegh C, et al. Choroid plexus NKCC1 mediates cerebrospinal fluid clearance during mouse early postnatal development. Nat Commun. 2021;12(1):447. doi: 10.1038/s41467-020-20666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wrede B, Liu P, Ater J, Wolff JE. Second surgery and the prognosis of choroid plexus carcinoma--results of a meta-analysis of individual cases. Anticancer Res. 2005;25(6C):4429–4433. [PubMed] [Google Scholar]
- 19. Mallick S, Benson R, Melgandi W, Rath GK. Effect of surgery, adjuvant therapy, and other prognostic factors on choroid plexus carcinoma: a systematic review and individual patient data analysis. Int J Radiat Oncol Biol Phys. 2017;99(5):1199–1206. doi: 10.1016/j.ijrobp.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 20. Jameel PZ, Varma A, Kumari P, Vagha K, Vagha J, Damke S. Choroid plexus carcinoma in an adolescent male: a case report. J Med Case Rep. 2021;15(1):184. doi: 10.1186/s13256-021-02801-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerard AW, Tailor J, Gradil C, Thakur B, Zebian B. Letter to the editor: endoscopic resection of intraventricular choroid plexus papillomas in infants. J Neurosurg Pediatr. 2017;19(1):122–125. doi: 10.3171/2016.5.PEDS16154. [DOI] [PubMed] [Google Scholar]
- 22. Santos MM, Souweidane MM. Purely endoscopic resection of a choroid plexus papilloma of the third ventricle: case report. J Neurosurg Pediatr. 2015;16(1):54–57. doi: 10.3171/2014.12.PEDS14287. [DOI] [PubMed] [Google Scholar]
- 23. Sufianov AA, Gaibov SS, Sufianov RA. Endoscopic monoportal removal of a choroid plexus papilloma in the posterior third ventricle in a child. J Neurosurg Pediatr. 2015;16(1):107–111. doi: 10.3171/2014.12.PEDS14306. [DOI] [PubMed] [Google Scholar]
- 24. Margetis K, Souweidane MM. Endoscopic treatment of intraventricular cystic tumors. World Neurosurg. 2013;79(2 Suppl):S19.e1–S19.e11. doi: 10.1016/j.wneu.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 25. Selvanathan SK, Kumar R, Goodden J, Tyagi A, Chumas P. Evolving instrumentation for endoscopic tumour removal of CNS tumours. Acta Neurochir (Wien) 2013;155(1):135–138. doi: 10.1007/s00701-012-1561-4. [DOI] [PubMed] [Google Scholar]
- 26. Bennedbaek O, Therkildsen MH. Choroid plexus carcinoma—report of a case with metastases within the central nervous system. Acta Oncol. 1990;29(2):241–243. doi: 10.3109/02841869009126551. [DOI] [PubMed] [Google Scholar]
- 27. Jo IY, Yeo SG, Oh HJ, Oh JS. Choroid plexus carcinoma with leptomeningeal spread in an adult: a case report and review of the literature. J Med Case Rep. 2021;15(1):286. doi: 10.1186/s13256-021-02887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ratha V, Kumar VRR. Transventricular migration of choroid plexus carcinoma causing an intraoperative conundrum: a case report with a review of the literature. Pediatr Neurosurg. 2019;54(5):341–346. doi: 10.1159/000500300. [DOI] [PubMed] [Google Scholar]
- 29. Donovan DJ, Prauner RD. Shunt-related abdominal metastases in a child with choroid plexus carcinoma: case report. Neurosurgery. 2005;56(2):E412. doi: 10.1227/01.neu.0000147982.80732.3d. [DOI] [PubMed] [Google Scholar]
- 30. Morshed RA, Lau D, Sun PP, Ostling LR. Spinal drop metastasis from a benign fourth ventricular choroid plexus papilloma in a pediatric patient: case report. J Neurosurg Pediatr. 2017;20(5):471–479. doi: 10.3171/2017.5.PEDS17130. [DOI] [PubMed] [Google Scholar]
- 31. Bettegowda C, Adogwa O, Mehta V, et al. Treatment of choroid plexus tumors: a 20-year single institutional experience. J Neurosurg Pediatr. 2012;10(5):398–405. doi: 10.3171/2012.8.PEDS12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hosmann A, Hinker F, Dorfer C, et al. Management of choroid plexus tumors-an institutional experience. Acta Neurochir (Wien) 2019;161(4):745–754. doi: 10.1007/s00701-019-03832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mosleh O, Tabori U, Bartels U, Huang A, Schechter T, Bouffet E. Successful treatment of a recurrent choroid plexus carcinoma with surgery followed by high-dose chemotherapy and stem cell rescue. Pediatr Hematol Oncol. 2013;30(5):386–391. doi: 10.3109/08880018.2012.756089. [DOI] [PubMed] [Google Scholar]
- 34. Souweidane MM, Luther N. Endoscopic resection of solid intraventricular brain tumors. J Neurosurg. 2006;105(2):271–278. doi: 10.3171/jns.2006.105.2.271. [DOI] [PubMed] [Google Scholar]
- 35. Qiao L, Souweidane MM. Purely endoscopic removal of intraventricular brain tumors: a consensus opinion and update. Minim Invasive Neurosurg. 2011;54(4):149–154. doi: 10.1055/s-0031-1284386. [DOI] [PubMed] [Google Scholar]