Abstract
BACKGROUND
Radiation-induced spinal cord cavernous malformations (RISCCMs) are a rare subset of central nervous system lesions and are more clinically aggressive than congenital cavernous malformations (CMs). The authors assessed the characteristics and outcomes of patients with RISCCM at a single institution and systematically reviewed the pertinent literature using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
OBSERVATIONS
Among the 146 spinal CMs at the authors’ institution, 3 RISCCMs were found. Symptom duration ranged from 0.1 to 8.5 months (mean [standard deviation], 3.2 [4.6] months), and latency ranged from 16 to 29 years (22.4 [9.6] years). All 3 RISCCMs were surgically treated with complete resection; 2 patients had stable outcomes, and 1 improved postoperatively. A review of 1240 articles revealed 20 patients with RISCCMs. Six of these patients were treated with resection, 13 were treated conservatively, and in 1 case, the treatment type was not stated. Five of the 6 patients treated surgically reported improvement postoperatively or at follow-up; 1 was stable, and none reported worsened outcomes.
LESSONS
RISCCMs are rare sequelae following radiation that inadvertently affect the spinal cord. Altogether, the frequency of stable and improved outcomes on follow-up suggests that resection could prevent further patient decline caused by symptoms of RISCCM. Therefore, surgical management should be considered primary therapy in patients presenting with RISCCMs.
Keywords: cavernoma, cavernous angioma, cavernous hemangioma, cavernous malformation, radiation, radiation therapy, radiation-induced
ABBREVIATIONS: CM = cavernous malformation, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RISCCM = radiation-induced spinal cord cavernous malformation, SCCM = spinal cord cavernous malformation, SD = standard deviation
A cavernous malformation (CM) is characterized by a nidus of abnormally dilated capillaries, which can occur in multiple bodily tissues. The overall prevalence of CMs is approximately 0.5% to 0.6% of the general population.1 CMs are often diagnosed after hemorrhagic symptoms occur. Intracerebral CMs exhibit a rupture rate of 0.7%–4.2%.2 Spinal CMs are relatively rare, comprising only 5% of all CMs and 5%–12% of all spinal vascular malformations.3–6 Within the spine, CMs can be extradural, intradural extramedullary, and intramedullary (spinal cord CMs [SCCMs]). Patients with SCCMs tend to present earlier in life than those with other CMs because of the higher propensity for symptoms with SCCMs. Approximately 10% of all SCCMs occur in the pediatric population.7
Conventional thinking holds that CMs arise from autosomal dominant patterns of inheritance in CCM1, CCM2, and CCM3 or by sporadic mutation.8 Radiation is increasingly recognized as a factor that contributes to cavernoma genesis. This phenomenon was first reported by Ciricillo et al.9 in 1994. Because of the delayed injury associated with radiation exposure, radiation-induced CMs exhibit a long and variable latency, ranging from 1 to 26 years.10 Although the mechanism behind this type of injury is not proven, it is thought to be due to hyalinization and fibrinoid necrosis of endothelial walls exposed to radiation.11 The changes to the vessel walls cause occlusion of blood flow and the characteristically dilated capillaries of CMs.
The literature shows that radiation-induced SCCMs (RISCCMs) are clinically distinct from traditional CMs. Cutsforth-Gregory et al.12 proposed that patients with CMs arising from radiation have a higher risk of hemorrhage and a younger age at presentation than those with naturally occurring CMs. Ducray et al.13 reported a high incidence of RISCCMs presenting as multiple lesions relative to naturally occurring CMs. Although treatment consensus guidelines for RISCCMs are lacking, their variable natural history and presentation suggest a course for RISCCMs potentially distinct from that for other CMs. This variable natural history could result in different outcomes and require distinct treatment. This study reports our single-center experience with RISCCM treatment and summarizes the literature in a systematic review.
Study Description
Methods
Institutional Patient Series
A prospectively maintained, institutional vascular malformation database was searched for all cases of SCCMs treated from April 29, 1986, to February 22, 2021. Patient presentation, operative notes, imaging, and follow-up were reviewed, with a special emphasis on a history of radiation exposure to the head, neck, cervical, thoracic, and lumbar regions. Demographic characteristics, radiological and intraoperative findings, and surgical outcomes were obtained and analyzed. A neurosurgeon determined whether radiation was the cause of the SCCM on the basis of the location and time course of previous radiation relative to the SCCM presentation.
Systematic Literature Review
A comprehensive search and review of the literature were conducted under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify all available cases of SCCMs that were the result of radiation therapy.14 The terms “cavernous malformation,” “cavernoma,” “cavernous hemangioma,” “cavernous angioma,” “radiation,” “spine,” “spinal cord,” and “intramedullary” were queried using the PubMed, Embase, Web of Science, and Cochrane databases. Two independent reviewers (S.W.K. and D.B.) analyzed articles and extracted data. Disagreements on inclusion were settled by a senior author (V.M.S.). Articles from January 1980 to December 2021 were included in the search. The title and abstract were reviewed first, and all studies describing cases of CMs that also mentioned radiation were considered. Articles that did not describe any involvement of radiation or that described CMs outside the spinal cord were excluded from further analysis. All articles were consolidated and reviewed.
Quantitative data are presented as counts, percentages, means (standard deviations [SDs]), and ranges.
Results
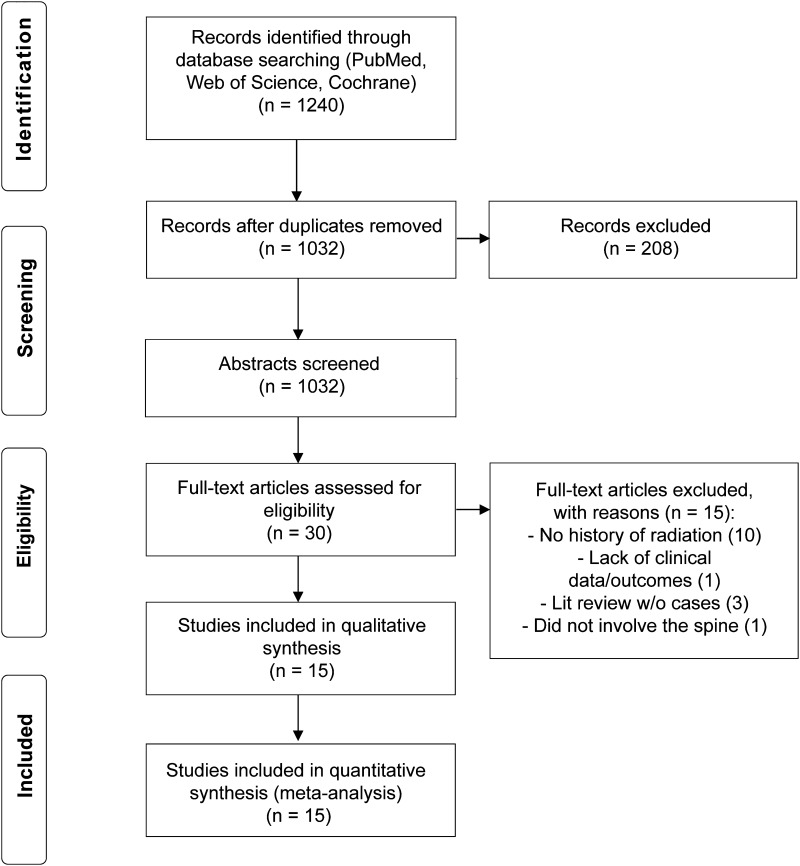
Three patients from our clinical cohort and 20 from the systematic literature review were included in the study. Figure 1 shows the number of RISCCM cases identified in the literature review.14 Tables 1–3 summarize the institutional and literature series, with the locations of the RISCCMs by spinal level and intra- or extramedullary location.13,15–28
FIG. 1.
PRISMA flow diagram.14
TABLE 1.
Patient characteristics and outcomes of institutional RISCCM cases
| Age (yrs), Sex | RISCCM Location | Dx Prompting Radiation | Latency (yrs) | Sxs | CM Size (cm)* | Sx Duration (mos) | Recurrent | Radiation Location | Outcome | FU (mos) |
|---|---|---|---|---|---|---|---|---|---|---|
| 43, F |
IM, C6 |
Hodgkin lymphoma |
29.2 |
Lt LE weakness, foot drop, bilat hand numbness/paresthesia |
0.5 |
8.5 |
No |
Mediastinum |
Improved |
8.5 |
| 50, F |
IM, T3 |
Hodgkin lymphoma |
NA |
Lt low-back pain, bilat hand numbness/paresthesia, facial twitching, blurry vision, balance/coordination problems |
2 |
1.0 |
No |
Mediastinum |
Stable |
25.2 |
| 35, M | IM, C1 | Rt facial rhabdomyosarcoma | 15.6 | Neck pain, rt numbness/paresthesia | 1.4 | 0.1 | No | Rt face (including mandible & sinusoidal cavities) | Stable | 0.8 |
Dx = diagnosis; FU = follow-up; IM = intramedullary; LE = lower extremity; NA = not available; Sx = symptom.
No patient had a family history of CM. All patients underwent resection without complications.
Largest diameter.
TABLE 3.
Patients with intra- and extramedullary RISCCM lesions
| Patient Source & Lesion Level | Location |
|
|---|---|---|
| IM | EM | |
| Institutional series |
4 |
0 |
| Cervical |
3 |
0 |
| Thoracic |
1 |
0 |
| Literature series |
8 |
12 |
| Cervical |
3 |
0 |
| Thoracic |
5 |
0 |
| Lumbar* |
0 |
1 |
| Cauda equina | 0 | 11 |
EM = extramedullary.
Data are presented as number of patients.
Spinal rootlets, L1–4.
Institutional Patient Series
Of the 2000 CMs identified in the institutional database, 146 were SCCMs, and 3 (2.1%) of these SCCMs were identified as RISCCMs (Table 1). The mean (SD) age of the 3 patients with RISCCM was 37.1 (7.5) years; 2 patients were women, and 1 patient was a man. Patients presented with numbness and paresthesia (n = 3), neck pain (n = 1), and weakness (n = 1). The duration of these symptoms ranged from 0.1 to 8.5 months (3.2 [4.6] months). None had a family history of CM. Two patients had a clear date for prior radiation; latency ranged from 16 to 29 years (22.4 [9.6] years). The mean RISCCM size was 1.5 (0.71) cm (range: 0.5–2.0 cm). RISCCMs occurred within the cervical (n = 2) and thoracic (n = 1) spine. Two patients received radiation to the mediastinum for Hodgkin lymphoma and 1 patient received radiation to the right face for rhabdomyosarcoma. All 3 RISCCMs were treated surgically with full resection. Based on Frankel scoring, 2 patients had stable outcomes and 1 had an improved outcome postoperatively. The follow-up ranged from 0.8 to 25.2 months (mean, 11.5 [12.5] months).
Systematic Literature Review
A total of 1240 articles were found in the literature. After review, 15 were used in the quantitative analysis (Fig. 1), revealing 20 cases of RISCCMs (Table 2). Patient ages ranged from 5 to 47 years; 18 patients were men and 2 were women. The most common presenting symptoms were lower-extremity weakness in 12 patients and back pain in 5 patients. Only 1 case of RISCCM was found incidentally. One patient reported a family history of CM. The RISCCMs occurred in the cervical (n = 3), thoracic (n = 5), and lumbar (cauda equina, n = 11) regions, and 1 RISCCM was within the spinal roots. Of the 14 cases with symptom duration reported, 9 were acute (≤1 month), 1 was subacute (1–3 months), and 4 were chronic (>3 months). The length of latency ranged from 5 to 47 years. The radiation dosage for the primary pathology was reported for 13 cases and ranged from 12 to 132.25 Gy. One case report did not include treatment type. Conservative treatment was reported for 13 patients. Of these 13 patients, 2 improved, 1 remained stable, and 6 worsened at the last follow-up; no outcome was reported for 4 patients treated conservatively. Six patients were treated with resection, with 5 of these cases reporting whether total (n = 4) or subtotal (n = 1) resection was achieved. Of the 6 patients undergoing resection, 5 improved postoperatively or at follow-up, and 1 was stable. Only 1 case report included a complication (postoperative hematoma). Follow-up length was reported in 14 cases and ranged from 1.5 to 144 months.
TABLE 2.
Patient characteristics and outcomes of literature review RISCCM cases
| Authors & Year | Age (yrs), Sex | RISCCM Location | Dx Prompting Radiation | Latency (yrs) | Sxs | CM Size > 1 cm | Sx Duration (mos) | Tx | EOR | Complications | Outcome | FU (mos) | Total Radiation Dose (Gy) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bowen et al., 199615 |
61, M |
CE |
Testicular seminoma |
11 |
Asymmetrical LE weakness |
No |
NA |
C |
|
None |
Worsened |
24 |
49.62 |
| 47, M |
CE |
Testicular teratoma |
10 |
Asymmetrical LE weakness, areflexia |
No |
NA |
C |
|
None |
Worsened |
84 |
44 |
|
| Maraire et al., 199916 |
22, M |
IM, T7 |
Infundibular germinoma |
5 |
Back pain, LE weakness, numbness, dysuria |
No |
0.5 |
R |
Total |
None |
Improved |
3 |
27 (15 fractions) |
| Narayan et al., 200317 |
17, M |
IM, T7–8 |
Medulloblastoma |
13 |
LE weakness & numbness, back pain |
No |
0.5 |
R |
Total |
None |
Improved |
1.5 |
27 |
| Hsia et al., 200318 |
40, M |
CE |
Hodgkin disease |
21 |
Lt foot drop, radiculopathy, back & leg pain |
Yes |
1 |
C |
|
None |
Worsened |
24 |
132.25 |
| 52, M |
CE |
Hodgkin disease |
17 |
Asymmetrical bilat weakness, wasting, hyporeflexia |
Yes |
6 |
C |
|
None |
Worsened |
24 |
93.63 |
|
| 50, F |
CE |
Hodgkin disease |
24 |
Asymmetrical bilat weakness, hyporeflexia |
Yes |
3 |
C |
|
None |
Worsened |
24 |
88 |
|
| Jabbour et al., 200419 |
33, M* |
CE |
Wilm tumor |
29 |
Incidental |
Yes |
NA |
R |
|
None |
Stable |
NA |
NA |
| Yoshino et al., 200520 |
16, F |
IM, T7, T9–10 |
Spinal astrocytoma |
8 |
LE weakness, hypoesthesia |
Yes |
Acute |
R |
Total |
None |
Improved |
24 |
40 |
| Labauge et al., 200621 |
78, M |
Spinal roots at L1–4 |
Hodgkin disease |
26 |
Asymmetrical lt LE weakness |
Yes |
NA |
NA |
|
NA |
NA |
NA |
45 (40 fractions) |
| Mathews et al., 200822 |
27, M |
IM, C2 |
ALL |
24 |
LE/UE weakness |
Yes |
Acute |
C |
|
None |
NA |
NA |
NA |
| Ducray et al., 200813 |
49, M |
CE |
Renal carcinoma |
20 |
Lt LE weakness, numbness, wasting, sexual dysfunction |
Yes |
48 |
C |
|
None |
Worsened |
144 |
NA |
| Ducray et al., 200813 |
52, M |
CE |
Hodgkin disease |
13 |
Rt foot drop |
Yes |
NA |
C |
|
None |
NA |
NA |
NA |
| Ducray et al., 200813 |
81, M |
CE |
Testicular seminoma |
47 |
Lower motor neuron syndrome, wasting, fasciculations |
No |
144 |
C |
|
None |
NA |
NA |
NA |
| Farid et al., 201423 |
68, M |
CE |
Testicular cancer |
NA |
Back pain, rt LE weakness |
Yes |
Acute |
C |
|
None |
NA |
NA |
NA |
| Won et al., 201524 |
63, M |
IM, T4–5 |
Lung cancer |
10 |
LE weakness, hypoesthesia |
No |
Acute |
C |
|
None |
Improved |
2 |
60 (30 fractions) |
| Drazin et al., 201725 |
76, M |
CE |
Abdominal sarcoma |
34 |
Gait disturbance, foot drop |
Yes |
6 |
C |
|
None |
Stable |
24 |
NA |
| Mikami et al., 201826 |
13, M |
IM, T1–3 |
ALL |
8 |
Chest pain, gait disturbance, & dysuria |
No |
Acute |
C |
|
None |
Improved |
7 |
12 (5 fractions) |
| Al-Jehani et al., 202027 |
41, M |
IM, C3–4 |
Mucoepidermoid cancer |
11 |
Leg paresthesia, weakness |
No |
Acute |
R |
Total |
Postoperative hematoma on MRI |
Improved |
3 |
66 |
| Oishi et al., 202028 | 37, M | IM, C7 | Medulloblastoma | 31 | Back pain, gait disturbance | No | NA | R | Subtotal | None | Improved | 24 | 14 (61 fractions) |
ALL = acute lymphocytic leukemia; C = conservative; CE = cauda equina; EOR = extent of resection; Hx = history; R = resection; Tx = treatment; UE = upper extremity.
Family history of CM noted.
Discussion
Observations
RISCCMs are a rare subset of central nervous system lesions and are more clinically aggressive than congenital CMs. In the single-institution case series, 3 patients with RISCCMs were identified, and their cases were reviewed. All 3 patients were treated surgically, resulting in complete resection with no postoperative complications. Our PRISMA-guided systematic literature review identified an additional 20 cases of RISCCM dating back to 1996. This review showed a diverse range of pathologies that required spinal radiation, established a diverse latency period between radiation and presentation, and showed the efficacy of surgical intervention to prevent the progression of symptoms. To the best of our knowledge, this study represents the most comprehensive and current review of RISCCMs to date.
Natural History
CMs of the brain and spinal cord are rare and are thought to arise congenitally.20,27 However, an increasing number of cases reporting the de novo formation in sporadic and familial cases presumes an acquired origin.17,19 Evidence supporting this theory is provided by several reports of the de novo formation of CMs in response to irradiation of the central nervous system, spinal cord, or even the whole body.16,17,20,24,27,29 The extremely rare occurrence and multifactorial genesis of these lesions leave their natural history to date unknown.20,27
Three hypotheses of the de novo formation of CMs are proposed. The first is that the CMs exist as radiographically occult lesions before radiation therapy.17,19,20,27 The radiation-induced transformation ensues from long-term sequelae associated with radiation therapy, including tissue proliferation, remodeling, and hemorrhaging.17,20,29
The second hypothesis of acquired CMs is based solely on radiation-induced cellular processes, such as vascular proliferation and dilation, hyalinization, fibrinoid necrosis, and the formation of telangiectasias.19,20 Narrowing of the vessels due to fibrosis and endothelial edema creates an ischemic environment resulting in the release of hypoxia-inducible factor 1, which leads to increased secretion of vascular endothelial growth factor and subsequent reactive neoangiogenesis.19 The key mediators of radiation-induced vascular alterations include vascular endothelial growth factor, basic fibroblast growth factor, and transforming growth factor α.17,27,29
The third hypothesis implicates the induction of somatic mutations through direct DNA damage caused by radiation therapy.17,19 Familial forms of CMs have been associated with genetic mutations on chromosomes 7q11–21, 7p13–15, and 3q25.2–27.3.17,30,31 Direct DNA damage to these loci may increase an individual’s susceptibility to CM development, especially in patients who already have a germline mutation and receive their second hit through irradiation.9,17,19,32,33
Although the distinct pathophysiological mechanisms of the de novo formation of CMs remain unclear, a clue exists indicating a pathophysiological difference between congenital and acquired CMs.27 Acquired CMs have a more aggressive natural history, and patients present with a higher risk of hemorrhage than those with congenital lesions.27 A previous study reported a higher association of RISCCM presenting as multiple CMs.13 Although our institutional case series did not include patients with multiple CMs, 11 of 20 RISCCM cases in our literature review reported multiple CMs at the initial diagnosis.
Primary or adjunct radiotherapy offers a suitable treatment option for various cranial neoplastic lesions, extracranial solid malignancies, and malignant hematological conditions, as demonstrated by the reported spectrum of diagnoses prompting radiation therapy in our literature review cases.27,29 Among these RISCCM cases, only a few developed after spinal-field irradiation. We reviewed 2 cases of medulloblastoma, 1 case of grade III astrocytoma, and 1 case of a suprasellar germinoma.16,17,20,28 All were treated with craniospinal axis irradiation, resulting in RISCCM.16,17,20,28 Additionally, extracranial solid malignancies and neoplastic hematological conditions described in the literature that prompted radiation included testicular cancer, Wilms tumor, lung cancer, Hodgkin disease, low-grade mucoepidermoid cancer of the parotid gland, renal carcinoma, testicular seminoma, testicular teratoma, and Philadelphia chromosome-positive acute lymphoblastic and acute lymphocytic leukemia.13,15,19,21–24,26,27 The radiation dose of craniospinal axis irradiation ranged from 12 to 132 Gy and was delivered in 5–40 fractions.15–18,20,21,24,26–28 Radiation-induced cranial CMs have been reported to develop after doses as low as 12–30 Gy.26 Thus, our finding is similar, as the lowest radiation dose was 12 Gy among our patients. Our review of institutional cases identified 3 patients who had received extraspinal radiation to the mediastinum and the right face due to Hodgkin lymphoma and rhabdomyosarcoma, respectively.
Patient Characteristics
A diagnosis of CM during infancy suggests a congenital origin.17 However, our findings suggest that RISCCMs occur between the third and fourth decade of life, with a mean patient age of 37.1 years in our case series. Data on the sex predominance of these lesions remain conflicting, and no cumulative analysis has yet been reported. In our case series of 3 patients, 1 was male. The literature review revealed that 18 of 20 cases occurred in men. Assuming similar patient characteristics between cranial and spinal radiation–induced CMs, these findings align with the literature review by Nimjee et al.,29 who found a 60% male predominance in cranial radiation–induced CMs.
RISCCMs can appear incidentally on imaging in patients without symptoms, or they can become symptomatic due to hemorrhaging and mass effect, resulting in pain, myelopathy, and sensorimotor deficits.17 In our literature review, extremity weakness was the most common symptom associated with RISCCMs (12 of 20 patients) and was associated with chest or back pain in almost half (6 of 12 patients) of the patients. In contrast, the 3 patients in our institutional case series predominantly presented with sensory deficits, including paresthesias and numbness, followed by motor deficits in 1 of 3 patients. These symptoms were accompanied by back pain in 1 patient and neck pain in 1 patient. The fact that none of our patients and only 1 in the literature had incidentally found lesions suggests that most patients with RISCCMs present with symptoms of myelopathy.
Furthermore, our data indicate that most RISCCMs are not linked to family history. Only 1 patient in our literature review had a family history of CMs, and none reported a family history of CMs in our case series. The etiology of a CM in the presence of family history and radiation exposure in the absence of genetic testing remains unclear. RISCCM latency ranged from 16 to 29 years in our case series and 5 to 47 years in the literature review cases.
Outcomes
The surgical outcomes of RISCCM treatment have only been reported individually in case reports, of which our series is the largest to date. We analyzed the postoperative outcomes of our single-center case series and compared them with outcomes reported in the literature. Of note, we present 3 surgically managed patients in our institutional series, whereas only one-third of the published reports were of cases managed with surgery. There are several advantages to the surgical management of CMs, including the potential for definitive treatment, reduction in bleeding risk, and improvement in symptoms. Correspondingly, a complication-free resection of these lesions is essential to achieve an unchanged or improved outcome and reveals the true benefit of resection with relief of mass effect,34 even for recurrent cases.35 None of our patients experienced intraoperative or immediate postoperative complications. This finding was also true for most cases reported in the literature review; even the 1 patient who developed a postoperative spinal hematoma had an improved outcome overall.27 Furthermore, the analysis of our institutional cases indicates that resection is associated with either a stable or an improved postoperative outcome. These findings align with those of our literature review, revealing an improved postoperative outcome in 5 of 6 patients after resection.
On the other hand, conservative management of CMs may be selected for asymptomatic lesions or when the risks and potential complications of surgery outweigh the potential benefits. Proof of this concept is exemplified by the findings of our literature review, in which the vast majority of lesions involving the cauda equina were managed conservatively, likely because of the high surgical risk associated with this delicate part of the spinal cord. Although conservative management evades the risks associated with resection, it does not improve symptoms and relies on close monitoring with neuroimaging. Among 13 patients treated conservatively, an improved outcome was reported in only 2 patients, and a worse outcome was reported in 6 patients.
In conclusion, conservative management remains a conflicting treatment strategy for these lesions, and surgical treatment of RISCCMs versus conservative management can affect the outcome. Ultimately, the decision of whether to surgically intervene or conservatively manage RISCCMs should be made on a case-by-case basis, taking into consideration the patient’s symptoms, the lesion size and location, the disease progression, and the risks and benefits of each approach.
Limitations
This study has limitations because of its retrospective character and corresponding data-collection bias. Additionally, the small sample size and single-institution background limit the generalizability of the findings and make it difficult to conduct cumulative analyses. The rarity of this disease subtype also contributes to a high degree of heterogeneity, most prominently in the follow-up duration, making it challenging to draw conclusions about the natural history and management of RISCCM.
Lessons
RISCCMs are rare sequelae following radiation that inadvertently affect the spinal cord. Knowledge of presenting symptoms can improve patient care in these rare circumstances. Altogether, the frequency of stable and improved outcomes on follow-up suggests that resection could prevent a further decline of symptoms in RISCCM. Therefore, surgical management should be considered the primary therapy in patients presenting with RISCCMs. Conservative management could be suitable for patients with minor symptoms, slow progression, advanced age, or increased risk factors for perioperative morbidity and mortality. The decision to operate should be made individually based on the patient’s symptoms and dynamics of progression, as with other CMs.
Acknowledgments
We thank the staff of Neuroscience Publications at Barrow Neurological Institute for assistance with manuscript preparation.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Lawton, Koester, Scherschinski, Srinivasan, Karahalios, Benner, Catapano. Acquisition of data: Scherschinski, Srinivasan, Benner. Analysis and interpretation of data: Lawton, Koester, Srinivasan, Benner. Drafting of the article: Lawton, Koester, Scherschinski, Srinivasan, Karahalios, Rumalla, Benner. Critically revising the article: Lawton, Koester, Scherschinski, Srinivasan, Karahalios, Catapano, Spetzler. Reviewed submitted version of the manuscript: Lawton, Koester, Scherschinski, Srinivasan, Karahalios, Catapano, Spetzler. Approved the final version of the manuscript on behalf of all authors: Lawton. Statistical analysis: Koester, Srinivasan. Administrative/technical/material support: Lawton, Srinivasan, Catapano. Study supervision: Lawton, Srinivasan.
References
- 1. Koike T, Yanagimachi N, Ishiguro H, et al. High incidence of radiation-induced cavernous hemangioma in long-term survivors who underwent hematopoietic stem cell transplantation with radiation therapy during childhood or adolescence. Biol Blood Marrow Transplant. 2012;18(7):1090–1098. doi: 10.1016/j.bbmt.2011.12.582. [DOI] [PubMed] [Google Scholar]
- 2. Flemming KD, Link MJ, Christianson TJ, Brown RD., Jr Prospective hemorrhage risk of intracerebral cavernous malformations. Neurology. 2012;78(9):632–636. doi: 10.1212/WNL.0b013e318248de9b. [DOI] [PubMed] [Google Scholar]
- 3. Sandalcioglu IE, Wiedemayer H, Gasser T, Asgari S, Engelhorn T, Stolke D. Intramedullary spinal cord cavernous malformations: clinical features and risk of hemorrhage. Neurosurg Rev. 2003;26(4):253–256. doi: 10.1007/s10143-003-0260-2. [DOI] [PubMed] [Google Scholar]
- 4. Cosgrove GR, Bertrand G, Fontaine S, Robitaille Y, Melanson D. Cavernous angiomas of the spinal cord. J Neurosurg. 1988;68(1):31–36. doi: 10.3171/jns.1988.68.1.0031. [DOI] [PubMed] [Google Scholar]
- 5. Azad TD, Veeravagu A, Li A, Zhang M, Madhugiri V, Steinberg GK. Long-term effectiveness of gross-total resection for symptomatic spinal cord cavernous malformations. Neurosurgery. 2018;83(6):1201–1208. doi: 10.1093/neuros/nyx610. [DOI] [PubMed] [Google Scholar]
- 6. Requena I, Arias M, López-Ibor L, et al. Cavernomas of the central nervous system: clinical and neuroimaging manifestations in 47 patients. J Neurol Neurosurg Psychiatry. 1991;54(7):590–594. doi: 10.1136/jnnp.54.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deutsch H, Shrivistava R, Epstein F, Jallo GI. Pediatric intramedullary spinal cavernous malformations. Spine (Phila Pa 1976) 2001;26(18):E427–E431. doi: 10.1097/00007632-200109150-00023. [DOI] [PubMed] [Google Scholar]
- 8. Choquet H, Pawlikowska L, Lawton MT, Kim H. Genetics of cerebral cavernous malformations: current status and future prospects. J Neurosurg Sci. 2015;59(3):211–220. [PMC free article] [PubMed] [Google Scholar]
- 9. Ciricillo SF, Cogen PH, Edwards MS. Pediatric cryptic vascular malformations: presentation, diagnosis and treatment. Pediatr Neurosurg. 1994;20(2):137–147. doi: 10.1159/000120776. [DOI] [PubMed] [Google Scholar]
- 10. Heckl S, Aschoff A, Kunze S. Radiation-induced cavernous hemangiomas of the brain: a late effect predominantly in children. Cancer. 2002;94(12):3285–3291. doi: 10.1002/cncr.10596. [DOI] [PubMed] [Google Scholar]
- 11. Hassler O. Microangiographic studies on changes in the cerebral vessels after irradiation. II. Proton beam lesions in the rat. Acta Radiol Ther Phys Biol. 1966;4(5):394–400. doi: 10.3109/02841866609133159. [DOI] [PubMed] [Google Scholar]
- 12. Cutsforth-Gregory JK, Lanzino G, Link MJ, Brown RD, Jr, Flemming KD. Characterization of radiation-induced cavernous malformations and comparison with a nonradiation cavernous malformation cohort. J Neurosurg. 2015;122(5):1214–1222. doi: 10.3171/2015.1.JNS141452. [DOI] [PubMed] [Google Scholar]
- 13. Ducray F, Guillevin R, Psimaras D, et al. Postradiation lumbosacral radiculopathy with spinal root cavernomas mimicking carcinomatous meningitis. Neuro Oncol. 2008;10(6):1035–1039. doi: 10.1215/15228517-2008-069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bowen J, Gregory R, Squier M, Donaghy M. The post-irradiation lower motor neuron syndrome neuronopathy or radiculopathy? Brain. 1996;119(Pt 5):1429–1439. doi: 10.1093/brain/119.5.1429. [DOI] [PubMed] [Google Scholar]
- 16. Maraire JN, Abdulrauf SI, Berger S, Knisely J, Awad IA. De novo development of a cavernous malformation of the spinal cord following spinal axis radiation. Case report. J Neurosurg. 1999;90(2) suppl:234–238. doi: 10.3171/spi.1999.90.2.0234. [DOI] [PubMed] [Google Scholar]
- 17. Narayan P, Barrow DL. Intramedullary spinal cavernous malformation following spinal irradiation. Case report and review of the literature. J Neurosurg. 2003;98(1 Suppl):68–72. doi: 10.3171/spi.2003.98.1.0068. [DOI] [PubMed] [Google Scholar]
- 18. Hsia AW, Katz JS, Hancock SL, Peterson K. Post-irradiation polyradiculopathy mimics leptomeningeal tumor on MRI. Neurology. 2003;60(10):1694–1696. doi: 10.1212/01.wnl.0000063320.61458.d8. [DOI] [PubMed] [Google Scholar]
- 19. Jabbour P, Gault J, Murk SE, Awad IA. Multiple spinal cavernous malformations with atypical phenotype after prior irradiation: case report. Neurosurgery. 2004;55(6):1431. [PubMed] [Google Scholar]
- 20. Yoshino M, Morita A, Shibahara J, Kirino T. Radiation-induced spinal cord cavernous malformation. Case report. J Neurosurg. 2005;102(1) suppl:101–104. doi: 10.3171/ped.2005.102.1.0101. [DOI] [PubMed] [Google Scholar]
- 21. Labauge P, Lefloch A, Chapon F, et al. Postirradiation spinal root cavernoma. Eur Neurol. 2006;56(4):256–257. doi: 10.1159/000096676. [DOI] [PubMed] [Google Scholar]
- 22. Mathews MS, Peck WW, Brant-Zawadzki M. Brown-Séquard syndrome secondary to spontaneous bleed from postradiation cavernous angiomas. AJNR Am J Neuroradiol. 2008;29(10):1989–1990. doi: 10.3174/ajnr.A1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farid N, Zyroff J, Uchiyama CM, Thorson PK, Imbesi SG. Radiation-induced cavernous malformations of the cauda equina mimicking carcinomatous or infectious meningitis. A case report. J Neuroimaging. 2014;24(1):92–94. doi: 10.1111/j.1552-6569.2011.00606.x. [DOI] [PubMed] [Google Scholar]
- 24. Won YI, Kim CH, Chung CK, Yun TJ. Delayed diagnosis of probable radiation induced spinal cord vascular disorders. J Korean Neurosurg Soc. 2015;57(3):215–218. doi: 10.3340/jkns.2015.57.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drazin D, Kappel A, Withrow S, Perry T, Chu R, Phuphanich S. Post-irradiation lumbosacral radiculopathy associated with multiple cavernous malformations of the cauda equina: case report and review of the literature. Surg Neurol Int. 2017;8:26. doi: 10.4103/2152-7806.200574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mikami T, Kato I, Nozaki F, et al. Sudden spinal hemorrhage in a pediatric case with total body irradiation-induced cavernous hemangioma. Pediatr Blood Cancer. 2018;65(10):e27250. doi: 10.1002/pbc.27250. [DOI] [PubMed] [Google Scholar]
- 27. Al-Jehani H, Najjar A, Barnawi A, Shedid D. Radiation-induced cervical spinal cord cavernoma following head and neck radiotherapy: case report. J Neurol Surg Rep. 2020;81(3):e39–e41. doi: 10.1055/s-0040-1709714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oishi M, Fujisawa H, Tsuchiya K, Nakajima Y. Radiation-induced spinal cord cavernous malformations associated with medulloblastoma: case report and review of the literature. World Neurosurg. 2020;141:318–322. doi: 10.1016/j.wneu.2020.06.125. [DOI] [PubMed] [Google Scholar]
- 29. Nimjee SM, Powers CJ, Bulsara KR. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurg Focus. 2006;21(1):e4. doi: 10.3171/foc.2006.21.1.5. [DOI] [PubMed] [Google Scholar]
- 30. Marchuk DA, Gallione CJ, Morrison LA, et al. A locus for cerebral cavernous malformations maps to chromosome 7q in two families. Genomics. 1995;28(2):311–314. doi: 10.1006/geno.1995.1147. [DOI] [PubMed] [Google Scholar]
- 31. Dubovsky J, Zabramski JM, Kurth J, et al. A gene responsible for cavernous malformations of the brain maps to chromosome 7q. Hum Mol Genet. 1995;4(3):453–458. doi: 10.1093/hmg/4.3.453. [DOI] [PubMed] [Google Scholar]
- 32. Günel M, Awad IA, Finberg K, et al. Genetic heterogeneity of inherited cerebral cavernous malformation. Neurosurgery. 1996;38(6):1265–1271. doi: 10.1097/00006123-199606000-00059. [DOI] [PubMed] [Google Scholar]
- 33. Liquori CL, Berg MJ, Siegel AM, et al. Mutations in a gene encoding a novel protein containing a phosphotyrosine-binding domain cause type 2 cerebral cavernous malformations. Am J Hum Genet. 2003;73(6):1459–1464. doi: 10.1086/380314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Srinivasan VM, Karahalios K, Shlobin NA, et al. Residual and recurrent spinal cord cavernous malformations: outcomes and techniques to optimize resection and a systematic review of the literature. Oper Neurosurg (Hagerstown) 2023;24(1):44–54. doi: 10.1227/ons.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 35. Lu DC, Lawton MT. Clinical presentation and surgical management of intramedullary spinal cord cavernous malformations. Neurosurg Focus. 2010;29(3):E12. doi: 10.3171/2010.6.FOCUS10139. [DOI] [PubMed] [Google Scholar]