Abstract
Bis(isopropyloxymethylcarbonyl) 9-R-(2-phosphonomethoxypropyl)adenine [bis(POC)PMPA] has been identified as a novel prodrug of PMPA. The anti-human immunodeficiency virus activity of bis(POC)PMPA was >100-fold greater than that of PMPA in both an established T-cell line and primary peripheral blood lymphocytes. This improved efficacy was shown to be due to a rapid intracellular uptake of the prodrug resulting in an increased intracellular accumulation of PMPA diphosphate (PMPApp), the pharmacologically active metabolite. PMPApp levels in bis(POC)PMPA-treated cells exceeded by >1,000-fold the levels seen in cells treated with unmodified PMPA in both resting and activated peripheral blood lymphocytes. Significant differences in the intracellular catabolism of PMPA metabolites were noted between the resting and activated lymphocytes. The half-life for the disappearance of PMPApp, derived from either bis(POC)PMPA or PMPA, was 12 to 15 h in the activated lymphocytes and 33 to 50 h in the resting lymphocytes. This long persistence of PMPApp, particularly in resting lymphocytes, may be unique to the nucleoside phosphonate analogs and indicates that effective levels of the active metabolite can be achieved and maintained with relatively infrequent administration of the parent drug.
PMPA [9-R-(2-phosphonomethoxypropyl)adenine], a prototype agent of the class of acyclic nucleoside phosphonates, is a potent inhibitor of both hepadnaviruses and retroviruses, including the human immunodeficiency virus (HIV), the etiologic agent of AIDS (3–5, 11). Its antiviral activity is related to the preferential inhibition of the viral-specified DNA polymerases (3). PMPA possesses attributes which are desirable for an anti-HIV agent. It shows a lack of cross-resistance with most of the known clinically nucleoside-resistant HIV strains in vitro and remarkable efficacy in vivo in animal models of HIV infection (3, 14, 25, 26). Recently, it has been shown that PMPA is able to prevent the establishment of simian immunodeficiency virus (SIV) infection in macaques when treatment was started 48 h before or 24 h after virus inoculation (25). In addition to this prophylactic activity, PMPA shows efficacy against both chronic SIV infection in macaques and feline immune deficiency virus infection in cats (14, 26). Due to its efficacy and limited toxicity PMPA is currently undergoing phase I and II clinical trials for the treatment of HIV infection in AIDS patients. Preliminary results have shown that PMPA given intravenously appears safe and well tolerated and caused a 1.1 log reduction of HIV RNA levels after only eight doses (7).
Despite its demonstrated antiviral potency, PMPA has limited oral bioavailability in animals, presumably resulting from the presence of two negative charges on the phosphonyl group. This low oral bioavailability may require high dosages to maintain therapeutic drug levels; current clinical trials with PMPA employ daily infusions. Acyloxyalkyl esters of carboxylic acids, e.g., ampicillin and foscarnet, are known to increase the oral bioavailability of these compounds over that of the free parental drugs (8, 12, 21). In the case of 9-(2-phosphonomethoxyethyl)adenine (PMEA), the bis(pivaloyloxymethyl) [bis(POM)] group has been introduced (15, 19), and this prodrug (now known as adefovir dipivoxil) is currently in phase II and III clinical trials for the treatment of HIV and hepatitis B infection. However, pivaloyl-containing compounds generate pivalic acid during the release of the parent drug and can cause increased urinary carnitine loss (1, 13). We have sought to overcome this limitation of the pivaloyl esters by the development of a more suitable prodrug for PMPA. To this end, a novel series of alkyl methyl carbonate esters were synthesized (2). We describe here the cellular uptake, metabolism, and antiviral activities of one of the analogs, bis(isopropyloxymethylcarbonyl)PMPA [bis(POC)PMPA].
(Preliminary results of these studies have been presented previously [9].)
MATERIALS AND METHODS
Cells and viruses.
Residual blood collected from rings after separation of platelets from the blood of healthy, HIV-negative donors was used as a source of lymphocytes. The peripheral blood mononuclear cells (PBMC) were separated over Ficoll-Hypaque gradient centrifugation. The cells were centrifuged at low speed to remove platelets, while contaminating erythrocytes were lysed by a brief exposure to hypotonic solution. H9 cells persistently infected with HIV type 1IIIB (H9/HIV-1IIIB) and MT-2 cells, a human T-cell lymphotropic-transformed CD4+ T-lymphocytic cell line, were obtained from the NIH AIDS Research and Reference Reagent Program. The virus 96-250, a primary isolate from a pediatric patient attending the St. Jude AIDS clinic, was isolated and propagated by PBMC coculture.
Compounds.
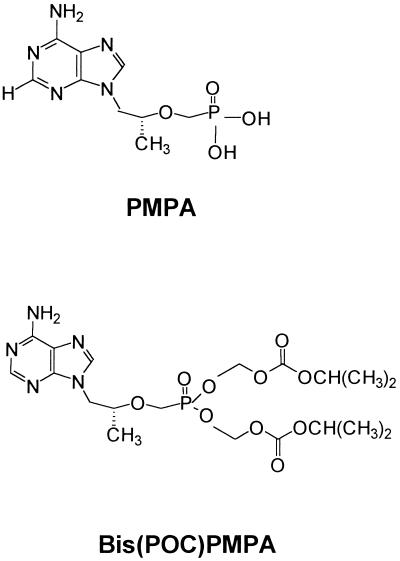
The structures of PMPA and bis(POC)PMPA are depicted in Fig. 1. The synthesis of the different phosphonates has been described elsewhere (2). The radiolabelled analogs [8-3H-adenine]bis(POC)PMPA ([3H]bis(POC)PMPA; specific activity, 28 Ci/mmol) and [2,8-3H-adenine]PMPA ([3H]PMPA; specific activity, 54 Ci/mmol) were obtained from Moravek Biochemicals (Brea, Calif.). The radiochemicals were verified by high-performance liquid chromatography (HPLC) before use and were estimated to be >95% pure.
FIG. 1.
Structures of PMPA and bis(POC)PMPA.
Antiretroviral assays.
The anti-HIV activities of PMPA and its prodrugs were determined in MT-2 cells and PBMC according to previously described procedures (23). Briefly, MT-2 cells or PBMC were infected with HIV-1 at a multiplicity of infection of 0.01, and the virus-infected cells were seeded at a concentration of 0.2 × 106 cells/ml in medium containing various concentrations of the drugs. After 5 days of incubation, the p24 antigen levels in the culture supernatants were determined by an in-house p24 antigen capture assay (22). A nonlinear curve-fitting program (Enzfitter; Elsevier-BioSoft, Evanston, Ill.) was used to calculate the drug concentrations yielding half-maximal inhibitions in p24 antigen production activity compared to that of the drug-free controls. Cell viability was monitored in replicate cultures by the XTT assay [2,3-bis-2-methoxy-4-nitro-5-sulfophenyl-5-(5-phenylamino)carbonyl-2H-tetrazolium hydroxide].
Metabolism of [3H]bis(POC)PMPA and [3H]PMPA.
Untreated PBMC were used as resting lymphocytes (R-PMBC). PBMC cultured for 72 h in an activation medium (RPMI medium containing 20% fetal calf serum, 5 μg of phytohemagglutinin-P/ml, and 5% interleukin-2) were used as activated cells (phytohemagglutinin-activated PBMC [PHA-PBMC]). Resting or activated PBMC (106 cells per ml, 10-ml volume) were incubated in culture medium containing the indicated concentration of [3H]bis(POC)PMPA or [3H]PMPA. The activities of the intracellular metabolites PMPA diphosphate (PMPApp) and PMPA monophosphate (PMPAp) were analyzed as described previously (18, 23, 24). At different intervals, cells were centrifuged (1,000 × g for 10 min) and were washed with cold phosphate-buffered saline. The cell pellet was suspended in ∼1 ml of the incubation medium, layered onto 150 μl of Nyosil oil, and spun through the oil with an Eppendorf microcentrifuge. The supernatant was removed, and the cells were extracted with 200 μl of 70% ice-cold methanol–15 mM Tris buffer (pH 7.4). After standing on ice for 15 min, cell extracts were centrifuged with an Eppendorf centrifuge and analyzed by HPLC using a 250-mm Whatman Partisil-SAX anion exchange column. A linear gradient of 5 mM ammonium dihydrogen phosphate (pH 4.0) (buffer A) to 0.6 M ammonium dihydrogen phosphate buffer (pH 3.5) (buffer B) was used as follows (concentrations given for buffer B only): 0 to 30% buffer B for 15 min, 30 to 40% buffer B for 10 min, and 40 to 100% buffer B for 25 min, followed by 100% buffer B for 30 min. The column was run at a flow rate of 1.5 ml/min, fractions were collected at 40-s intervals, and the different fractions were assayed for radioactivity in a toluene-based scintillation fluid. A Whatman Partisil-5 reverse phase column was used to resolve bis(POC)PMPA from PMPA and its metabolites PMPAp and PMPApp; the column was eluted with a linear gradient of ammonium dihydrogen phosphate buffer (0.05 M), pH 6.0, to 60% acetonitrile. The identities of the metabolites were established by comparison to the elution profiles of authentic cold standards. The retention times for the different standards were as follows: PMPA, 14 min; PMPAp, 48 min; and PMPApp, 89 min. Cell numbers and cell volumes at each sampling interval were determined with a Coulter ZM 256-particle counter equipped with a Channelyzer (Coulter Electronics, Miami, Fla.). The mean single-cell volumes for resting and PHA-PBMC were 200 and 900 to 1,000 fl, respectively.
Intracellular retention of [3H]bis(POC)PMPA and [3H]PMPA in PBMC.
Ten-milliliter aliquots of R-PBMC and PHA-PBMC suspensions were incubated with 1 μM [3H]bis(POC)PMPA or 10 μM [3H]PMPA for 24 h. Cells were then washed and resuspended in drug-free medium, and aliquots were removed at different intervals over the next 48 h. Intracellular concentrations of PMPAp and PMPApp were determined as described in the previous section. PHA-PBMC were maintained in medium containing PHA and interleukin-2 throughout the study.
RESULTS
Anti-HIV activity of bis(POC)PMPA, bis(POM)PMPA, and PMPA.
The activities of the different phosphonates were evaluated against HIV-1IIIB and a clinical isolate, 96-250, in the lymphocyte cell line MT-2 and in PHA-stimulated PBMC. The EC50s (50% effective concentrations) of bis(POC)PMPA against HIV-1IIIB were 0.007 μM and 0.005 μM in MT-2 cells and PBMC, respectively (Table 1). These values are comparable to the EC50 of zidovudine, one of the most potent nucleoside inhibitors of HIV-1, and ∼100-fold lower than that for the unmodified PMPA. Interestingly, bis(POC)PMPA showed a more than threefold greater antiviral potency than a related prodrug, bis(POM)PMPA. PMPA and the prodrugs all showed greater antiviral effects against the primary clinical isolate 96-250 than against laboratory-adapted HIV-1IIIB.
TABLE 1.
Anti-HIV activity and cytotoxicity of PMPA compounds in MT-2 cells and PHA-PBMCa
| Compound | EC50 (μM)
|
IC50 (μM)b
|
|||
|---|---|---|---|---|---|
| MT-2 cells infected with HIV-1IIIb | PBMC infected with:
|
MT-2 cells | PBMC | ||
| HIV-1IIIb | 96-250 | ||||
| PMPA | 0.63 | 0.18 | 0.01 | 1,250 | 1,200 |
| Bis(POC)PMPA | 0.007 | 0.005 | 0.0004 | 22 | 29 |
| Bis(POM)PMPA | 0.05 | 0.05 | 0.007 | 7.5 | 8 |
MT-2 cells (2 × 103) and PHA-PBMC were exposed to 100 50% tissue culture infective dose of infectious HIV strains as indicated. The amount of p24 antigen produced was measured after 7 days. Average values from at least three different experiments are shown.
The IC50s for the uninfected cells were determined by XTT assay with various concentrations of each compound.
These compounds were also evaluated for their cytotoxicity to quiescent or proliferating PBMC as well as MT-2 cells. As shown in Table 1, bis(POC)PMPA inhibited the growth of MT-2 and PHA-PBMC with 50% inhibitory concentrations (IC50s) of 24 and 29 μM, respectively. These concentrations were well above the levels that provided the anti-HIV activities in these cells and gave selectivity indices (IC50/EC50 ratios) for bis(POC)PMPA of ∼3,000 and 6,000 in MT-2 cells and PBMC, respectively. These values were comparable to the selectivity index values of free PMPA, which were ∼2,000 and ∼7,000 in MT-2 cells and PBMC, respectively. Interestingly, bis(POM)PMPA was much more cytotoxic and showed lower selectivity than PMPA in these assays. None of these compounds, however, exerted any detectable toxicity against the quiescent PBMC up to concentrations well above 100 μM (as determined by dye exclusion).
Intracellular metabolism of [3H]bis(POC)PMPA and [3H]PMPA.
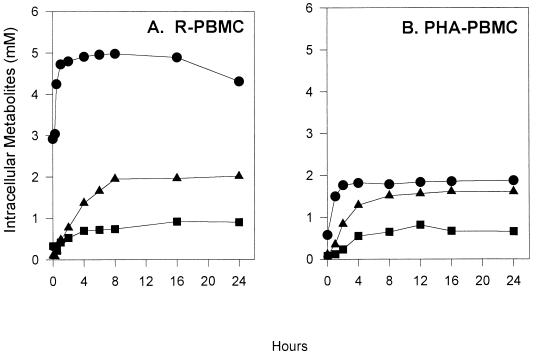
Other structurally related acyclic nucleoside analogs, e.g., PMEA and HPMPC, are internalized by endocytosis (6, 17). It is likely that PMPA is also internalized via endocytosis. PMPA is then phosphorylated by cellular nucleotide kinases to PMPApp, the putative active intracellular inhibitor of HIV reverse transcriptase. We investigated the metabolism of bis(POC)PMPA in R-PBMC and PHA-PBMC to establish the cellular pharmacokinetics of this compound. The time course of intracellular metabolism of [3H]bis(POC)PMPA in PBMC is depicted in Fig. 2. Both R-PBMC and PHA-PBMC showed a rapid accumulation of PMPA in the millimolar range within 1 h of incubation with 1 μM [3H]bis(POC)PMPA. Surprisingly, neither bis(POC)PMPA nor its monoester derivative were detected within the cell extracts at any of the time points examined (0.5 to 24 h), suggesting that bis(POC)PMPA is rapidly converted to PMPA within the cells. PMPAp and PMPApp accumulated steadily for 8 h, reaching a plateau at a total concentration of 2.8 and ∼2 mM in R-PBMC and PHA-PBMC, respectively. It is noteworthy that the accumulation of PMPA and its metabolites was dose dependent when PBMC were incubated with 100 nM to 10 μM of the drug (data not shown).
FIG. 2.
Intracellular metabolism of bis(POC)PMPA in PBMC. R-PBMC (A) and PHA-PBMC (B) were incubated with 1 μM [3H]bis(POC)PMPA. At different intervals, the concentrations of PMPA and its metabolites in cell extracts were analyzed by HPLC as described in Materials and Methods. •, [3H]PMPA; ▪, [3H]PMPAp; ▴, [3H]PMPApp.
In parallel experiments with 10 μM [3H]PMPA, the cellular uptake and intracellular metabolism of PMPA was quite slow in both R-PBMC and PHA-PBMC. The accumulation of PMPA, and its biologically active metabolites, was barely detectable during the first 6 h of incubation. Unlike cells treated with 1 μM bis(POC)PMPA, which accumulated PMPA metabolites in the millimolar range, the intracellular concentrations of PMPA, PMPAp, and PMPApp were only ∼1.7, 0.4, and 1.2 μM in R-PBMC, and 1.0, 0.2, and 0.4 μM in PHA-PBMC, respectively, after 24 h of incubation with 10 μM PMPA (Table 2).
TABLE 2.
PMPA nucleoside levels in PBMC incubated with 10 μM [3H]PMPAa
| Cell type | Drug | Incubation time (h) | Intracellular concn (μM) of:
|
||
|---|---|---|---|---|---|
| PMPA | PMPAp | PMPApp | |||
| R-PBMC | PMPA | 6 | 1.0 | 0.1 | 0.35 |
| 12 | 1.5 | 0.15 | 0.50 | ||
| 24 | 1.7 | 0.45 | 1.24 | ||
| Bis(POC)PMPA | 6 | 4,960 | 720 | 1,660 | |
| 12 | 4,885 | 915 | 1,960 | ||
| 24 | 4,305 | 900 | 2,010 | ||
| PHA-PBMC | PMPA | 6 | 0.4 | 0.11 | 0.14 |
| 12 | 0.7 | 0.10 | 0.19 | ||
| 24 | 0.9 | 0.21 | 0.40 | ||
| Bis(POC)PMPA | 8 | 1,789 | 645 | 1,511 | |
| 12 | 1,837 | 816 | 1,562 | ||
| 24 | 1,875 | 657 | 1,608 | ||
R- and PHA-PBMC (107 cells) were incubated with 10 μM [3H]PMPA for the times indicated. Cells were then washed with cold buffer and extracted with 70% ice-cold methanol. Nucleoside analogs were quantified by HPLC as described in Materials and Methods. Data are the means of two separate experiments. Variability in these experiments was less than 15% of the values obtained.
Intracellular decay of PMPA metabolites in PHA-PBMC and R-PBMC.
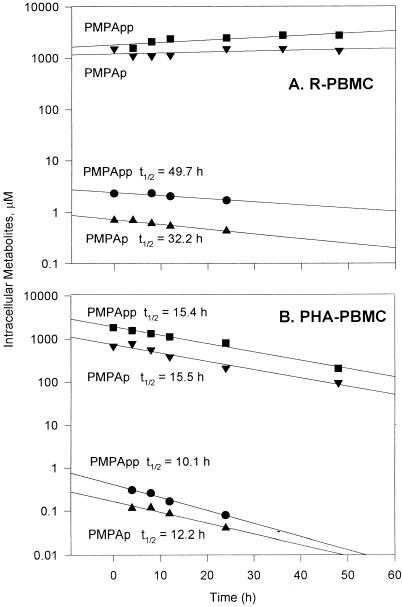
An important characteristic of any drug is the amount of time that the active drug metabolite persists in the cells. To investigate the intracellular decay of PMPA metabolites, PBMC were loaded with 1 μM [3H]bis(POC)PMPA or 10 μM [3H]PMPA for 24 h, and the intracellular levels of the drug metabolites were determined at different intervals after drug removal. As shown in Fig. 3, the intracellular clearance of PMPA metabolites was linear in PBMC. PMPAp and PMPApp decayed with half-lives (t1/2s) of 15 and 11 h, respectively, in PHA-PBMC preloaded with either bis(POC)PMPA or PMPA. These values are similar to results previously obtained in human T-lymphocytic cell lines (19). In marked contrast, the intracellular t1/2s of PMPAp and PMPApp were much longer (33 and 49 h, respectively) in R-PBMC preloaded with PMPA. In bis(POC)PMPA-treated R-PBMC, there was very little clearance, and t1/2s could not be estimated. The high cellular concentration of PMPA that accumulates from bis(POC)PMPA during the loading period is likely to be a factor and may serve as a reservoir for continued phosphorylation of PMPA during the chase period.
FIG. 3.
Catabolism of PMPAp and PMPApp from R-PBMC (A) and PHA-PBMC (B) incubated with 1 μM [3H]bis(POC)PMPA (▾, ▪) or 10 μM [3H]PMPA (▴, •) for 24 h. At different intervals thereafter, cells were analyzed for PMPA metabolites as described in Materials and Methods. The measured t1/2 were calculated from the slopes of the weighted lines generated by linear analysis.
DISCUSSION
We present here a preclinical evaluation of bis(POC)PMPA as a potential prodrug of PMPA. Bis(POC)PMPA is very stable in buffer at acidic pH (pH 2.0) and 37°C, exhibiting a t1/2 of over 150 h. Thus, it is likely that bis(POC)PMPA may stay virtually intact in the gastric environment, when administered by the oral route. In preliminary studies, bis(POC)PMPA has demonstrated an oral bioavailability of 20 to 36% in mice and dogs, while PMPA, the parent compound, is poorly absorbed following oral administration (9, 16).
Bis(POC)PMPA exhibited increased activity against both laboratory-adapted and primary clinical isolates of HIV strains in different cell culture systems. The inhibition of HIV in PHA-PBMC was increased >100-fold over that of the unmodified parental drug and was accompanied with a proportional increase in cytotoxicity. The observation that the prodrug retained the same selectivity (∼6,000) as the parent drug shows that the protective group on the phosphonate was not toxic to the cells. Thus, the observed increases in the antiviral and cytotoxic activities of bis(POC)PMPA, without any loss of selectivity, are more likely the result of increased accumulation of PMPApp, which is both the active and cytotoxic form of the drug.
Surprisingly, bis(POC)PMPA was three- to fourfold more potent against HIV and ∼threefold less cytotoxic than its bis(POM) counterpart. The reason for the difference in the activities of the two prodrugs is unclear. Stimulated PBMC incubated with 1 μM bis(POC) for 16 h accumulated 16.3 and 48.8 mmol of PMPAp and PMPApp/106 cells, respectively, compared to 12.2 and 24.5 mmol of PMPAp and PMPApp/106 cells, respectively, accumulated by PBMC after bis(POM)PMPA treatment. Thus, it is possible that the greater PMPApp levels in bis(POC)PMPA-treated cells account for the slightly greater antiviral activity compared to that of bis(POM)PMPA. The increased toxicity, and concomitant reduction in selectivity, of bis(POM)PMPA relative to bis(POC)PMPA cannot be attributed to these anabolite differences. Both prodrugs are hydrolyzed to formaldehyde, carbon dioxide, and PMPA. Bis(POM)PMPA also generates pivalic acid, whereas the carbamate prodrug releases isopropanol. It is possible that the accumulation of pivalic acid contributes to the increased cytotoxicity of bis(POM)PMPA, although further studies are required to establish this point.
A major advantage of bis(POC)PMPA is its increased bioavailability after oral administration, thus obviating the need for intravenous infusions. In previous studies Bis(POC)PMPA had oral bioavailabilities of ∼20 and 40% in monkeys and in humans, respectively (7, 16). A peak level of PMPA in plasma of ∼0.3 μg/ml (t1/2, 8 h; area under the concentration-time curve, 4.7 to 5.7 mg/h/liter) was achieved after a single oral administration of bis(POC)PMPA (equivalent to 27 mg of PMPA/kg of body weight) in monkeys. Bis(POC)PMPA is highly susceptible to serum esterases. This limits the persistence of the prodrug in plasma and its availability to interact directly with target cells. Nevertheless, our results show that bis(POC)PMPA is taken by cells within minutes and provides significantly greater intracellular levels of PMPA and active metabolites. Thus, enhanced antiviral activity of the drug may be expected if even a minute fraction (<0.001%) of the orally administered bis(POC)PMPA can enter the circulation in its native form. It is also not clear whether the benefits of improved drug delivery can be realized by direct intravenous inoculation of bis(POC)PMPA. In vivo pharmacological studies need to be pursued to test this possibility.
Significant differences were noted in the metabolism of PMPA and bis(POC)PMPA in R-PBMC and PHA-PBMC. The amounts of PMPA and its metabolites, derived from either prodrug or PMPA, were two- to threefold higher in R-PBMC than in PHA-PBMC. This situation is quite the opposite of what is seen with the dideoxynucleosides such as zidovidine or 2′, 3′-didehydro-3′-deoxythymidine (d4T), which are highly dependent on the activated state of the PBMC for their phosphorylation to the triphosphate (10). Several factors control the pool size of intracellular nucleotide analogs, and these include membrane transport, phosphorylation, and degradation of the triphosphates. PMPA is probably internalized by endocytosis like other structurally related acyclic nucleoside phosponates (e.g., PMEA and HPMPC) and accumulates rather slowly within cells (6, 17). Intracellular PMPA levels were ∼twofold greater in R-PBMC than in PHA-PBMC (Table 2 and Fig. 1). It is possible that endocytosis of PMPA in R-PBMC is more efficient than in PHA-PBMC. Alternatively, the relatively smaller size and cytosolic volume of R-PBMC, relative to PHA-PBMC, may translate to higher drug concentrations.
Several factors may explain the differences in the metabolic profile of PMPA, relative to that of other dideoxynucleosides. PMPA (and other nucleoside phosphonates) circumvent the initial phosphorylation required for most nucleosides, which phosphorylation is often a limiting factor in resting cells. We showed previously that PMPA and PMEA are phosphorylated to the mono- and diphosphate derivatives by the actions of adenylate kinase, a ubiquitous enzyme that is localized primarily to the mitochondria in human lymphocytes, and nucleoside diphosphate kinase, respectively (19, 20). Both are ubiquitous enzymes that show high levels of activity throughout the cell cycle. One may, therefore, expect that PMPA and related compounds are phosphorylated to similar extents in resting and dividing cells.
The identity of the enzymes involved in the intracellular catabolism of these nucleoside analogs is currently not known. Surprisingly, we found a significant difference between R-PBMC and PHA-PBMC in their clearance of PMPA metabolites. The t1/2 of clearance of PMPA metabolites ranged from 33 to 50 h in R-PBMC compared to 12 to 15 h in PHA-PBMC. These results clearly show that PMPApp catabolism is significantly slower in resting PBMC than in PHA-PBMC, and further studies are in progress to elucidate the mechanisms underlying this process. This is the first observation of such a difference in nucleotide catabolism between resting and replicating cells. This increased retention of PMPA metabolites in resting cells is likely to result in better antiviral activity, compared to other nucleoside analogs, in cells which have limited proliferative capacity (e.g., monocytes/macrophages and dendritic cells). Consistent with this idea, Balzarini et al. (4) showed that PMEA is more effective in inhibiting HIV replication in primary macrophages than in lymphocytes. Thus, the long persistence of PMPA metabolites in resting cells may be a highly desirable property of this drug, and it may translate into an improved therapeutic potential. Such persistence suggests that PMPA has the potential to be effective in suppressing HIV replication in dividing cells and to reduce the establishment of latently infected quiescent cells.
In summary, bis(POC)PMPA is a novel prodrug highly active against HIV in vitro in both established and primary peripheral blood lymphocytes. This improved efficacy was due to rapid intracellular uptake of the prodrug followed by anabolic phosphorylation to the pharmacologically active metabolites. The PMPApp levels in bis(POC)PMPA-treated cells exceeded the levels obtained in cells incubated with unmodified PMPA by >1,000-fold. Metabolic studies also showed that PMPApp accumulated to high levels in both resting and activated PBMC, and it persisted for a considerably longer period in resting cells. These results may explain the superior efficacy of PMPA in the SIV model. While these studies were being completed, studies in animal systems and with humans have shown that bis(POC)PMPA achieves pharmacologically active systemic concentrations of PMPA. The low toxicity and favorable pharmacokinetics of bis(POC)PMPA warrant further evaluation of this compound in HIV-infected individuals.
ACKNOWLEDGMENTS
This research was supported in part by PHS grants RO1 AI27652 and U01-AI32908 (Pediatric ACTU 065), Cancer Center Core Grant P30 CA21765 from the National Institutes of Health, and by the American Lebanese Syrian Associated Charities.
H9/HIV-1IIIB and MT-2 cells were obtained from the NIH AIDS Research and Reference Reagent Program (Rockville, Md.) through the generosity of different contributors. We thank Jack Greenhaw for excellent technical assistance.
REFERENCES
- 1.Abrahamson K, Erikson B O, Holme E, Jodal U, Lindstedt S, Nordin I. Impaired ketogenesis in carnitine depletion caused by short term administration of pivalic acid prodrug. Biochem Med Metab Biol. 1994;52:18–21. doi: 10.1006/bmmb.1994.1028. [DOI] [PubMed] [Google Scholar]
- 2.Arimilli M N, Kim C U, Dougherty J, Mulato A, Oliyai R, Shaw J P, Cundy K C, Bischofberger N. Synthesis, in vitro evaluation and oral bioavailability of 9-(2-phosphonomethoxypropyl)adenine prodrugs. Antivir Chem Chemother. 1997;8(6):557–567. [Google Scholar]
- 3.Balzarini J, De Clercq E. Acyclic purine nucleoside phosphonates as retrovirus inhibitors. In: Jeffries D J, De Clercq E, editors. Antiviral chemotherapy. New York, N.Y: John Wiley & Sons; 1995. pp. 41–45. [Google Scholar]
- 4.Balzarini J, Holy A, Jindrich J, Naesens L, Snoeck R, Schols D, De Clercq E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob Agents Chemother. 1993;37:332–338. doi: 10.1128/aac.37.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balzarini J, Aquaro S, Perno C F, Witvrouw M, Holy A, De Clercq E. Activity of the (R)-enantiomers of 9-(2-phosphonylmethoxypropyl)-adenine and 9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine against human immunodeficiency virus in different human cell systems. Biochem Biophys Res Commun. 1996;219:337–341. doi: 10.1006/bbrc.1996.0234. [DOI] [PubMed] [Google Scholar]
- 6.Connelly M C, Robbins B L, Fridland A. Mechanism of uptake of the phosphonate analog (S)-1-(3-hydroxy-2-phosphonylmethopropyl) cytosine (HPMPC) in Vero cells. Biochem Pharmacol. 1993;46:1053–1057. doi: 10.1016/0006-2952(93)90670-r. [DOI] [PubMed] [Google Scholar]
- 7.Deeks, S. G., P. Barditch-Grovo, P. Lietman, F. Hwang, K. Cundy, J. Rooney, N. Nellman, and J. Khan. The safety, pharmacokinetics and antiretroviral activity of intravenous PMPA, a novel anti-HIV therapy, in HIV infected adults. Unpublished data. [DOI] [PMC free article] [PubMed]
- 8.Farquhar D, Srivastava D N, Kuttesh N J, Saunders P P. Biologically reversible phosphate protective groups. J Pharmacol Sci USA. 1983;72:324–325. doi: 10.1002/jps.2600720332. [DOI] [PubMed] [Google Scholar]
- 9.Fridland A, Robbins B L, Srinivas R V, Arimili M, Kim C, Bischofberger N. Abstracts of the Tenth International Conference on Antiviral Research. Atlanta, Ga: International Society for Antiviral Research; 1997. Antiretroviral activity and metabolism of bis(POC)PMPA, an oral bioavailable prodrug of PMPA, abstr. 27; p. A49. [Google Scholar]
- 10.Gao W Y, Agbaria R, Driscoll J S, Mitsuya H. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2′, 3′-dideoxynucleoside analogs in resting and activated cells. J Biol Chem. 1994;269:12633–12638. [PubMed] [Google Scholar]
- 11.Heijink R A, Kruining J, DeWilde G A, Balzarini J, De Clercq E, Schalm S W. Inhibitory effects of acyclic nucleoside phosphonates on human hepatitis B virus and duck hepatitis B virus infections in tissue culture. Antimicrob Agents Chemother. 1994;38:2180–2182. doi: 10.1128/aac.38.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer R P, Phillips L R, Biddle J A, Thaker D R, Egan W. Synthesis of acyloxyalkyl acylphosphonates as potential prodrugs of the antiviral, trisodium phosphonoformate (foscarnet sodium) Tetrahedron Lett. 1989;30:7141–7144. [Google Scholar]
- 13.Lacy S A, Cundy K C, Hitchcock M J M. 34th Annual Meeting of the Society of Toxicology, Baltimore, Md. 1995. Evaluation of the subchronic toxicity of oral prodrug of the anti-HIV nucleoside analog 9-(2-phosphonylmethoxyethyl) adenine (PMEA), abstr 15; p. 179A. [Google Scholar]
- 14.Myles, M.H., N. Bischofberger, and E. A. Hoover. 1997. Evaluation of 9-(2-phosphonylmethoxypropyl) adenine antiviral therapy for acute feline immunodeficiency virus infection, abstr. 70A. Presented at the Third International Feline Retrovirus Symposium, Fort Collins, Colo. 70A.
- 15.Naesens L, Balzarini J, Bischofberger N, De Clercq E. Antiretroviral activity and pharmacokinetics in mice of oral bis(pivaloyloxymethyl)-9-(2-phosphonylmethoxyethyl)adenine, the bis(pivaloyloxymethyl) ester prodrug of 9-(2-phosphonylmethoxyethyl)adenine. Antimicrob Agents Chemother. 1996;40:22–28. doi: 10.1128/aac.40.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naesens L, Bischofberger N, Arimili M, Kim C, De Clercq E. Abstracts of the Tenth International Conference on Antiviral Research. Atlanta, Ga: International Society for Antiviral Research; 1997. Antiretrovirus activity and pharmacokinetics in mice of bis(POC)PMPA, the bis(isopropyloxycarbonyloxymethyl) oral prodrug of PMPA, abstr. 28; p. A50. [Google Scholar]
- 17.Palu G, Stefanelli S, Rassu M. Cellular uptake of phosphonylmethoxy alkylpurines. Antiviral Res. 1991;16:115–119. doi: 10.1016/0166-3542(91)90063-w. [DOI] [PubMed] [Google Scholar]
- 18.Robbins B L, Connelly M C, Marshall D R, Srinivas R V, Fridland A. A human T lymphoid cell variant resistant to the acyclic nucleoside phosphonate 9-(2-phosphonylmethoxyethyl)adenine shows a unique combination of a phosphorylation defect and increased efflux of the agent. Mol Pharmacol. 1995;47:391–397. [PubMed] [Google Scholar]
- 19.Robbins B L, Fridland A. Metabolism and therapeutic activity of acyclic adenine phosphonate analogs. Int Antivir News. 1996;4:57–59. [Google Scholar]
- 20.Robbins B L, Greenhaw J, Connelly M C, Fridland A. Metabolic pathways for the activation of the antiviral agent 9-(2-phosphonylmethoxyethyl)adenine in human lymphoid cells. Antimicrob Agents Chemother. 1995;39:2304–2308. doi: 10.1128/aac.39.10.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto F, Ikeda S, Tsukamoto G. Studies on prodrugs. I. Preparation and characterization of acyloxyallylester of ampicillin. Chem Pharm Bull. 1983;31:2698–2707. doi: 10.1248/cpb.31.2698. [DOI] [PubMed] [Google Scholar]
- 22.Srinivas R V, Hurwitz J L. HIV growth, inhibition and measurement. In: Lefkovitz I, editor. The immunology methods manual. New York, N.Y: Academic Press; 1997. pp. 1959–1973. [Google Scholar]
- 23.Srinivas R V, Robbins B L, Connelly M C, Gong Y F, Bischofberger N, Fridland A. Metabolism and in vitro antiviral activities of bis(pivaloyloxymethyl) prodrugs of acyclic nucleoside phosphonates. Antimicrob Agents Chemother. 1993;37:2247–2250. doi: 10.1128/aac.37.10.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivas R V, Robbins B L, Connelly M C, Gong Y F, Bischofberger N, Fridland A. Pivaloyloxymethyl esters of acyclic nucleoside phosphonates. Int Antivir News. 1994;2:53–55. doi: 10.1128/aac.37.10.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai C C, Follis K E, Sabo A, Beck T W, Grant R F, Bischofberger N, Benveniste R E, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl) adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 26.Tsai C C, Follis K E, Beck T W, Sabo A, Bischofberger N, Daily P J. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy on chronic SIV infection in macaques. AIDS Res Hum Retroviruses. 1997;13:707–712. doi: 10.1089/aid.1997.13.707. [DOI] [PubMed] [Google Scholar]