Summary
The Báthory family was one of the most powerful noble families in the medieval Hungarian Kingdom. Their influence peaked during the Ottoman occupation of Hungary, when the only partially autonomous region of the country was Transylvania, under Turkish protectorate. Several members of the family became Princes of Transylvania, and one of them, István Báthory, was also the elected King of Poland. We hereby present the first genetic data about this extinct family. Archaeological excavations in Pericei, a settlement now part of Romania, revealed the former family chapel of the Báthory family. Through this work, two Báthory family members were successfully identified among the 13 skeletons found at the site. The presence of Y chromosome haplogroup R-S498 fits the historical account describing the family’s German (Swabian) origins. Their genomic composition also indicates a family of Germanic origin that intermixed with medieval Hungarians.
Subject areas: Genomics, Human Genetics, Paleogenetics, Phylogenetics
Graphical abstract
Highlights
-
•
4th degree genetic relation of two Báthory members matched historical sources
-
•
Y chromosome haplogroup data align with the documented Swabian origin of the family
-
•
Genome data indicate a Germanic origin admixed with the local Hungarian population
Genomics; Human Genetics; Paleogenetics; Phylogenetics
Introduction
Noble families have played essential roles in History, sometimes even having a greater impact on events than royal families. This was particularly true during the Ottoman Occupation Period of the medieval Hungarian Kingdom (1541–1699), which ultimately resulted in the country being split into three parts for almost 150 years: the Habsburg Royal Hungary in the north and west, the Ottoman Hungary in the central and south, and the Principality of Transylvania under Turkish protectorate in the east.
The Báthory (or Báthori/Bátori) family was undoubtedly one of the most important noble families in medieval Hungary, holding extensive landholdings in the territories that now comprise Hungary, Poland, Slovakia, and Romania. Members of the Báthory family held administrative, military, and ecclesiastical leadership positions and played a significant role in the history of the Principality of Transylvania.1,2,3
According to written sources, the Báthory family can be traced back to the Swabian Gutkeled family of the 11th century CE, with their names appearing in Hungarian chronicles: " … it is certain that King Péter brought in the Gutkeled lineage when he fled to Emperor Henry. They originated from the Stauf castle in Swabia … The Gutkeled lineage rose to prominence during the reigns of Kings Salamon, László, and Géza".4
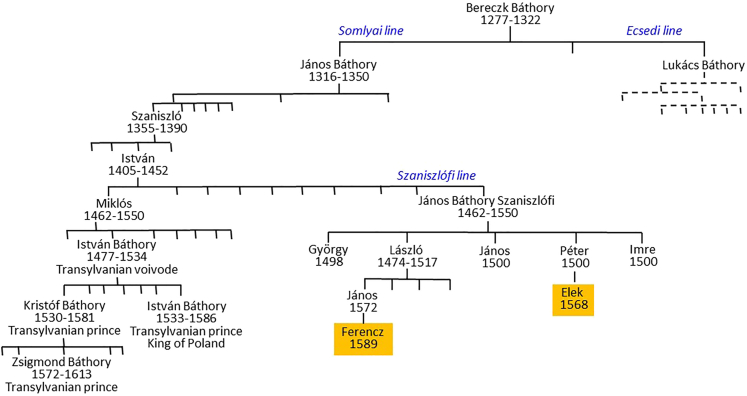
However, it was Bereczk (mentioned in written sources between 1277 and 1322), who is considered the founder of the noble dynasty, who was awarded land donations by King László IV, and began to use the Báthory name permanently.2,3 The family subsequently split into two main branches based on their land possessions (Figure 1): the Ecsedi line, who claimed territories in the northeast part of the kingdom (i.e., in Partium and Upper Hungary), and the Somlyói (or Somlyai) line in Transylvania, which emerged rapidly in the 16th century CE. Several of their descendants were even elected Princes of Transylvania.1,2,3
Figure 1.
Part of the Báthory family tree illustrating the main branches (based on Wertner, 1900)
Some family members' birth and death dates are unknown; instead, we recorded the dates they were mentioned in the documents. Adult members that were buried in the Pericei church are colored in yellow.
The most prominent member of the Somlyói line was undoubtedly István Báthory (1533–1586), who, in addition to being the Prince of Transylvania, was also the elected King of Poland.5 In the 15th century CE, another sub-branch of the Somlyói line was established, known as the Szaniszlófi, or younger branch. Descendants of János Báthory Szaniszlófi (ca. 1462–1500), grandson of Szaniszló Báthory (1355–1390), belonged to this sub-branch3 (Figure 1).
According to written sources, all branches of the Báthory family became extinct in the first third of the 17th century CE, as both Gábor and András from the Somlyói line, and István from the Ecsedi line, died without a male heir.2,3 While the genealogy of the Báthory family is a well-known topic validated by numerous written sources such as diplomas and private letters, many questions and debates still remain regarding the family and its members.6 A significant portion of the written sources from their library and tombs were lost or destroyed after their extinction, making it the task of future interdisciplinary research to explore and reconstruct the lost information about this prominent noble family. So far, there have been no genetic studies conducted on the members of the Báthory family. The remains of the Transylvanian Prince and Polish King, István Báthory, are located in Krakow, while the burials of several known Báthory Transylvanian rulers were desecrated, and their bones were either placed in mass graves or lost. The identification of the burial sites of other potential family members has not yet been carried out.
A recent archaeological investigation has uncovered potential new information about the Báthory family in Pericei (Szilágyperecsen, Szilágy/Sălaj county, Transylvania/Romania). The family’s presence in Pericei began with the Szaniszlófi branch, that acquired numerous possessions in the area, including a large house mentioned in a 1559 document,7 a domus et curia nobiliaris referenced in a pre-1602 document,8 a castle called “castello Perechen” mentioned in 1625, and a chapel where two adult Báthory members were buried.2,9 Studies on historical documents concluded that Elek and Ferencz Báthory were the two members interred in the chapel9,10 (Figure 1).
Although the family chapel was destroyed during Ottoman invasions in 1658 and 1660, two Renaissance funerary slabs, undoubtedly related to the Báthory family in Pericei, had been discovered. These slabs are currently housed in the Reformed Church in Pericei and were dated to the second half of the 16th century. The slabs depict two deceased males lying on their backs dressed in knights' armor (Figure 2A), and the Báthory family’s coat of arms is carved on one of the gravestones.11
Figure 2.
Remnants of the Báthori family's church and tombstones
(A) The two tomb slabs of two members of the Báthory family in the current Reformed Church in the village of Pericei.
(B) Traces of the medieval church discovered in 2020 (photo archive of the History and Art Museum Zalău).
The remains of the family chapel were rediscovered during archaeological excavations conducted between 2017 and 2021, which aimed to develop infrastructure around the Reformed Church (Figure 2B). Among several medieval graves under the ruins of the chapel, two burials were found that contained exceptional archaeological funerary inventory that could identify the presumed Báthory members.12 One of these graves (Figure 3) is particularly notable, containing a helmet, armor decorated in the niello technique with gold-plated ornaments, sabers, spurs, and a mace decorated with geometric elements. These archaeological findings were a clear indication of the high status of the buried nobleman.
Figure 3.
Funerary context that depicts a noble man with armor burried in a lead coffin (photo archive of the History and Art Museum Zalău)
We conducted an archaeogenetic analysis of the skeletal remains in order to genetically identify the presumed Báthory members and determine the paternal Y chromosome lineage of the family. The main challenge we faced was the absence of genetic reference data from Báthory members. Therefore, our study aims to provide the first genetic information on potential Báthory members. We also attempted to investigate the population structure of the graveyard by sequencing the genomes of 13 out of the 14 human remains found in the old chapel and determining their genetic relationships using autosomal and Y chromosomal markers.
The genetic relatedness between the prominent individual and another neighboring individual in the graveyard was consistent with the familial relationships of Elek and Ferencz Báthory, who were reportedly buried in the chapel. The agreement between the genetic and historical evidences supports the identification of these two individuals as Báthory members.
Results
Next Generation Sequence (NGS) data
We obtained genome coverages ranging from 0.16 to 3.25 (Table 1 and S1), with an average coverage of 1.07x, and mitogenome coverages ranging from 30 to 361x. Most samples had low mitochondrial contamination estimated by Schmutzi (1–2%), with one sample (PER04B) having 8% contamination. X contamination was estimated to be under 1% in all samples except for PER02, which had 5.6% contamination. Further details of the NGS results can be found in Table S1; sex determination data are provided in Table S2; the Y defining single-nucleotide polymorphisms (SNPs) are given in Table S3; and mitogenome SNP data are shown in Table S4.
Table 1.
Summary of NGS data
| Sample ID/PER | 01 | 02 | 03–1 | 04A | 04B | 05 | 08 | 09 | 09–1 | 09B | 10 | 11 | 22 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample source | petrosa | tooth | petrosa | petrosa | petrosa | tooth | tooth | petrosa | petrosa | petrosa | petrosa | petrosa | petrosa |
| Endogenous DNA | 27.7% | 20% | 20.2% | 13% | 49.5% | 58.4% | 64% | 12% | 9.4% | 23.3% | 14.6% | 74.3% | 82.2% |
| Total no of reads(M.) | 222.7 | 49.8 | 366.7 | 67.8 | 112.4 | 44.5 | 190.3 | 1647.0 | 182.7 | 653.0 | 33.0 | 70.7 | 30.7 |
| No of unique reads (M.) | 50.8 | 9.1 | 40.0 | 28.2 | 56.8 | 26.6 | 23.4 | 128.5 | 42.2 | 89.8 | 22.2 | 54.9 | 24.4 |
| Avg. genome coverage (fold) | 1.11 | 0.16 | 0.71 | 0.65 | 1.33 | 0.56 | 0.74 | 3.25 | 1.18 | 1.92 | 0.39 | 1.29 | 0.63 |
| Avg. X-chr. coverage | 0.61 | 0.09 | 0.39 | 0.35 | 0.71 | 0.57 | 0.4 | 1.71 | 1.21 | 1.96 | 0.22 | 0.69 | 0.64 |
| Avg. Y-chr. coverage | 0.50 | 0.07 | 0.28 | 0.26 | 0.53 | 0.02 | 0.29 | 1.02 | 0.04 | 0.06 | 0.13 | 0.44 | 0.02 |
| Mitochondrial bps covered >5x | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 99% | 100% | 100% |
| Schmutzi mitochondrial | 1% | 2% | 1% | 1% | 8% | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 0% |
| contamination ANGSD X contamination | 0.7% | 5.6% | 0.7% | 1.1% | 0.5% | N/A | 0.9% | 0.6% | N/A | N/A | 0.9% | 0.4% | N/A |
Family relationships within the cemetery
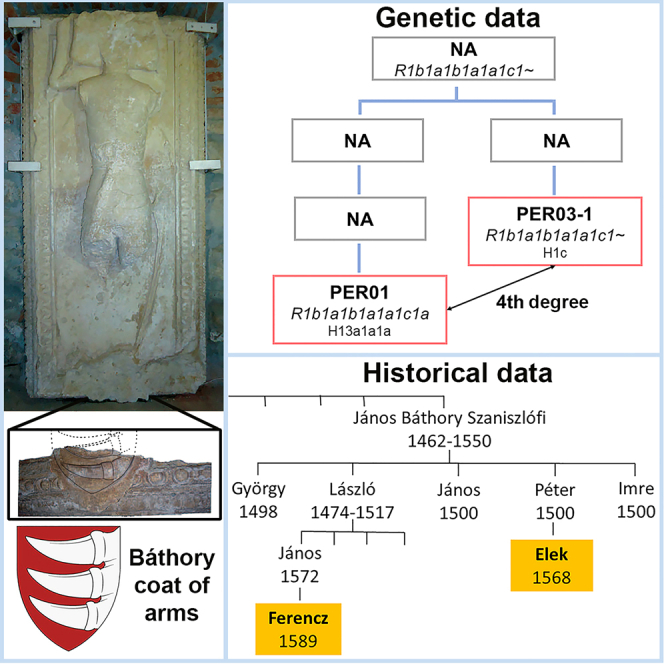
The armored individual, sample PER01, and the individual from the neighboring grave PER03-1 showed 4th-degree relatedness and shared the same Y lineage, haplogroup R1b-S498 (R1b1a1b1a1a1c1a) (Table S5). This compelling evidence strongly suggests that they could be the remains of the Báthory family members Elek and Ferencz, as mentioned in historical written sources.
Kinship analysis also revealed several other related individuals in the medieval church in Pericei (Table S5). Individuals PER02 and PER11, who had identical mitochondrial haplotypes, were found to be 4th-degree relatives, and PER02 was also found to be a 3rd degree relative of sample PER04A, while the relationship between PER11 and PER04A was uncertain (>4th degree). All three individuals were buried in graves next to each other, indicating that the church was indeed the burial place of local noble families, a common custom during that period.
Y chromosome and mitochondrial haplogroups
Table 2 summarizes both the International Society of Genetic Genealogy (ISOGG) based and family tree-based Y haplogroup (Hg) classifications for each sample. The two Báthory individuals share the same R1b1a1b1a1a1c1 (R1b-S264) Y lineage with consistent Hg defining markers, although the R-S498 marker, which defines the R1b1a1b1a1a1c1a subgroup, was not covered in the PER03-1 genome due to its lower coverage. Ancient individuals published in the literature belonging to subgroups of R1b-S264 include three Vikings (VK396, VK143, and VK323),13 one medieval Roman (R58),14 and one medieval Bavarian (ALH-1).15 According to the Y-Full database (https://www.yfull.com), the vast majority of modern individuals belonging to the R1b-S264 branch are from Germanic-speaking regions, such as Germany, the United Kingdom, Sweden, Denmark, Norway, and the Netherlands. These data are in line with the reported German-Swabian origin of the Báthory family.
Table 2.
Y chromosomal and mitochondrial Hgs of the studied samples
| Sample ID | Y chr. Hg (Family Tree) | Y chr. Hg (ISOGG, Yleaf) | Mitochondrial Hg (Haplogrep) |
|---|---|---|---|
| PER01 | R-S498 | R1b1a1b1a1a1c1a | H13a1a1a |
| PER02 | I-CTS10936 | I2a1a2b1 | H7c4 |
| PER03-1 | R-S264 | R1b1a1b1a1a1c1∗ | H1c |
| PER04A | J-PF7394 | J2a1a1a2b2a1b1∼ | J1c10 |
| PER04B | R-S1690 | R1b1a1b1a1a1c2a1∼ | T2 |
| PER05 | woman | woman | U5a1b |
| PER08 | R-S206 | R1b1a1b1a1a2b2 | H7e |
| PER09 | R-Y4383 | R1a1a1b1a2b3a3a2f∼ | A26 |
| PER09A | woman | woman | H1n6 |
| PER09B | woman | woman | T2b5 |
| PER10 | Q-YP821 | Q1a2a1a4a∼ | J1c5a |
| PER11 | I-BY25361 | I2a1b2a2b2b | H7c4 |
| PER22 | woman | woman | U4c1 |
In the cemetery, two other males belonged to different sub-branches of the R-U106 Hg, two others belonged to I2a, and one to R1a, Q1a, and J2a (Table 2). I2 is one of the oldest Hgs in Europe and originated in the Late Paleolithic period,16 so it is not very indicative of a regional origin. The R-Y4383 lineage of PER09 is typical in central-eastern Europe. The J-PF7394 Hg of PER04A has a Near Eastern origin, and sequences that match our sample were found in Turkey.17 The Q-YP821 Hg of PER10 is found today with low frequencies in Hungary, Poland, and Russia and was brought to Europe with the Hun and Mongolian migrations. Q1a had also been detected in Hun, Avar, and Conquering Hungarian samples.18,19 The maternal lineages of the 13 individuals belonged to western Eurasian Hgs (H, J, T and U), except for PER09, who carried the East-Eurasian A26 lineage (Table 2 and S4). A26 is a rare derivative of macro-Hg A, clearly of East Asian origin, being most frequent in China, Siberia, and the Americas.20 Although we only have information on the A26 subclade from two Danish samples, this is likely due to the overrepresentation of Danish mitogenomes in databases, thanks to the extensive Danish mitogenome project.21 This Hg most likely arrived in Europe with the Eastern immigrants as it was also present in several Avar and Conquering Hungarian individuals.19
The genome structure of the individuals
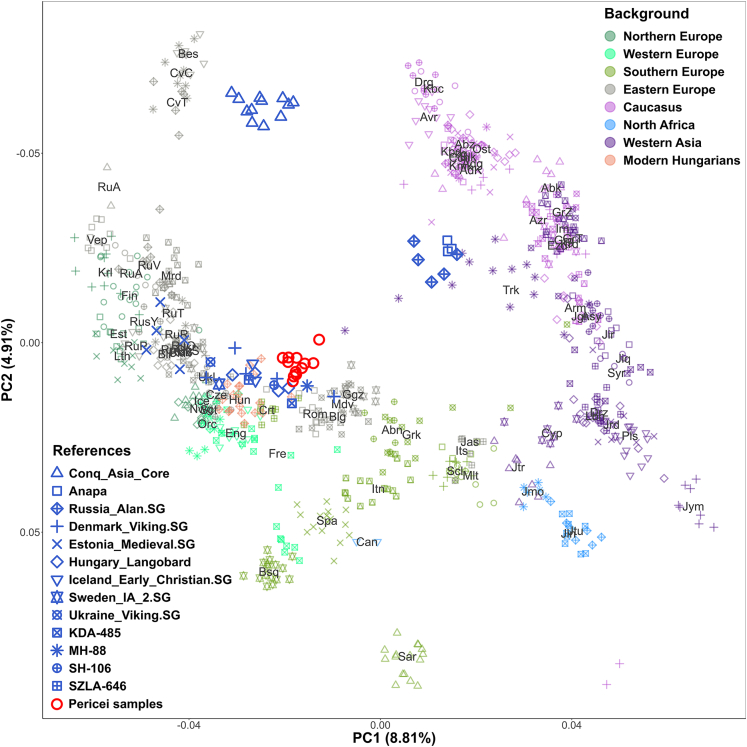
We conducted a principal-component analysis (PCA) by projecting our ancient genomes onto the axes computed from modern European individuals (Figure 4). We had to exclude one sample (PER02) from the population genetic analyses as it had negligible genome coverage.
Figure 4.
PCA of the Pericei individuals (red circles) projected onto axes determined by 784 modern genomes
3-letter codes of modern populations are defined in Table S6. We also depicted the PCA position of ancient reference genomes that were utilized in the qpAdm analyses.
The resulting PCA plot shows that the studied individuals are clustered closely together, in the proximity of modern Eastern-European populations such as Czechs, Hungarians, Croatians, and Romanians. However, their PCA position is slightly shifted from these modern groups, suggesting that they carried additional ancestry, likely originating from Eastern Eurasia.
Next, we used F3-outgroup statistics to determine which ancient or modern European populations shared the highest genetic drift with the Pericei individuals. The results, shown in Table S7, consistently indicated that the Pericei individuals had the greatest shared drift with ancient and modern individuals from North-Western Europe (such as Iceland, Norway, Sweden, Denmark, Britain, Estonia, and Lithuania) as well as with individuals from medieval Hungary.
The qpAdm analysis showed that the Pericei individuals' ancestry primarily originated from either Medieval Hungarian or Northern European populations, with minor contributions ranging from 1% to 10% from eastern immigrant groups such as Conq_Asia_Core or Avar_Asia_Core (Table S8). Alternative models suggested minor Iranian ancestry from the Caucasus (Alan or Anapa), ranging from 10% to 30%. Notably, all of these minor ancestries were present in the genomes of the medieval Hungarian population.19 These findings support the theory that the Pericei individuals had Germanic origins, with genetic admixture from local populations in the Carpathian Basin, including the genetic legacy of the Hun, Avar, and conquering Hungarian immigrants from the eastern steppe.
Discussion
The Báthory family was a prominent aristocratic family in medieval Hungary that wielded significant political influence during the Ottoman Occupation Period, particularly in Transylvania. This study focused on the genetic identification of Báthory family members among the 14 remains discovered during archaeological excavations in the Báthory family chapel in Pericei.
Unfortunately, we did not have any comparative genetic material from the Báthory family, so we had to rely solely on archaeological and historical data. Based on the rich funerary offerings found with the deceased, especially in one of the graves, and considering the location of these graves within the former church, we could identify the most likely candidates to be potential members of the Báthory family. Furthermore, the written historical records indicate that the Báthory family had Swabian origins, and two fourth-degree relatives from this family were interred in the Pericei temple.
We successfully identified fourth-degree kinship between the armored individual and another individual from the neighboring grave after sequencing the genomes of 13 individuals excavated in Pericei. These results support that the two individuals found in the altar zone of the chapel were most likely Elek and Ferencz Báthory from the Szaniszflófi sub-branch of the Somlyói line. The Y lineage of these individuals was R1b-S498 (R1b1a1b1a1a1c1a), typical for Germanic peoples, which is consistent with the Swabian origin of the family.
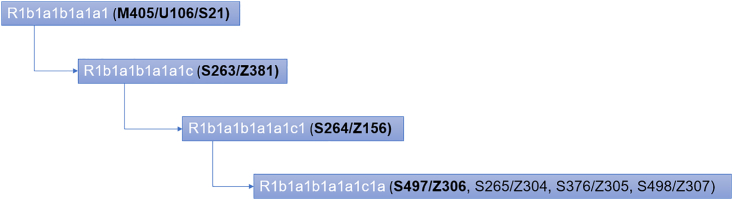
This Y lineage is derived from R1b-S263/Z381 (R1b1a1b1a1a1c), which is derived from R1b- M405/U106/S21 (R1b1a1b1a1a1) (Figure 5). R1b-S21 is considered the "North-Western R1b-Hg" or the "Germanic" Hg as it is found with the highest frequencies in Germany, Denmark, Belgium, Austria, and Switzerland. The spread of this lineage in Europe can be attributed to the Germanic migrations that took place between the 3rd and 10th century.22 In ancient samples, sub-branches of Hg R1b (R1b1a2, R1b1a2a1a1c2b2b, R1b1a2a1a1c2b2b1a1) were found almost exclusively in medieval males discovered in an Alemanni cemetery in Germany.23 These findings corroborate the Germanic origin of the Báthory family and are consistent with the ethnic demographics of Transylvania, specifically the presence of the Saxons.
Figure 5.
Branches of the Hg R1b- M405/U106/S21 (R1b1a1b1a1a1)
Our genome analysis results revealed that after settling in Hungary, the Báthorys mixed with the local Hungarian population, which by this time incorporated eastern Eurasian genome elements of the Huns, Avars, and conquering Hungarians. Our data also suggest that the church was a family burial place and may have contained other noble family members likely connected to the Báthorys. This study is an example of interdisciplinary research, combining archaeological, historical, and genetic analysis, and provides a foundation for future studies of the Báthory family and other noble families in the region.
Limitations of the study
The population genetic analysis is limited by the available reference genomes. Furthermore, in addition to their main European component, the examined genomes contained so small minor components (Asian and Caucasian) whose precise identification is not possible with the available methods.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human archaeological remains | This paper | N/A |
| Critical commercial assays | ||
| MinElute PCR Purification Kit | QIAGEN | Cat No./ID: 28006 |
| Accuprime Pfx Supermix | ThermoFisher Scientific | Cat. No: 12344040 |
| Deposited data | ||
| Newly published ancient genomes | This paper | https://www.ebi.ac.uk/ena/browser/view/PRJEB63184 |
| Human reference genome NCBI build 37, GRCh37 | Genome Reference Consortium | http://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/human/ |
| Ancient comparison dataset | Allen Ancient DNA Resource (Version v54.1) | https://reich.hms.harvard.edu/allen-ancient-dna-resource-aadr-downloadable-genotypes-present-day-and-ancient-dna-data |
| Ancient comparison dataset | Maróti et al., 202219 | https://www.ebi.ac.uk/ena/browser/view/PRJEB499771 |
| Oligonucleotides | ||
| Illumina specific adapters | Custom synthetized | https://www.sigmaaldrich.com/HU/en/product/sigma/oligo?lang=en®ion=US&gclid=CjwKCAiAgvKQBhBbEiwAaPQw3FDDFnRPc3WV75qapsXvcTxxzBXy48atqyb6Xi5f_8e6Df2EJI0NNhoCmzIQAvD_BwE |
| Software and algorithms | ||
| Cutadapt | Martin, 201124 | https://cutadapt.readthedocs.io/en/stable/# |
| Burrow-Wheels-Aligner | Li and Durbin, 200925 | http://bio-bwa.sourceforge.net/ |
| samtools | Li et al., 200926 | http://www.htslib.org/ |
| PICARD tools | Broad Institute, 201627 | https://github.com/broadinstitute/picard |
| ATLAS software package | Link et al., 201728 | https://bitbucket.org/wegmannlab/atlas/wiki/Home |
| MapDamage 2.0 | Jónsson et al., 201329 | https://ginolhac.github.io/mapDamage/ |
| Schmutzi software package | Renaud et al., 201530 | https://github.com/grenaud/schmutzi |
| ANGSD software package | Korneliussen et al., 201431 | https://github.com/ANGSD/angsd |
| HaploGrep 2 | Weissensteiner et al., 201632 | https://haplogrep.i-med.ac.at/category/haplogrep2/ |
| Yleaf software tool | Ralf et al., 201833 | https://github.com/genid/Yleaf |
| mosdepth software | Pedersen and Quinlan34 | https://github.com/brentp/mosdepth |
| PCAangsd software | Meisner and Albrechtsen35 | https://github.com/Rosemeis/pcangsd |
| RcppCNPy R package | N/A | https://rdocumentation.org/packages/RcppCNPy/versions/0.2.10 |
| smartpca | Patterson et al., 200636 | https://github.com/chrchang/eigensoft/blob/master/POPGEN/README |
| ADMIXTURE software | Alexander et al., 200937 | https://dalexander.github.io/admixture/ |
| R 3.6.3 | R core development team38 | https://cran.r-project.org/bin/windows/base/old/3.6.3/ |
| ADMIXTOOLS software package | Patterson et al., 201239 | https://github.com/DReichLab/AdmixTools |
| DATES algorithm | Narasimhan et al., 201940 | https://github.com/priyamoorjani/DATES/tree/v753 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Tibor Török (torokt@bio.u-szeged.hu).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Ancient samples
We present 13 whole genomes from the 16th century from Transylvania, Romania, associated with the Báthory family, one of the most powerful noble families in the medieval Hungarian Kingdom. Samples were collected from the family chapel that was discovered during the archaeological excavations conducted between 2017 and 2021, in Pericei, Romania.12
Method details
Historical identification of the possible Báthory members
According to medieval documents, the presence of the Báthory family in Pericei began with the descendants of János Báthory Szaniszlófi (1462–1500). The possessions and history of the Báthory family in Pericei, particularly the life of Ferencz Báthory are well described.3 Historical records indicate that two family members were buried in the family chapel in Pericei: Elek and Ferencz Báthory.9,10 The Wesselényi family archive contains various documents printed in Pericei bearing Ferencz’s signature and the ring seal with his monograms (Hungarian National Archive Section P, No. 702). Based on their genealogical data,3 Ferencz’s grandfather (László Báthory 1474–1517) was the brother of Elek’s father (Péter Báthory 1500), so Ferencz and Elek were 4th-degree relatives on the paternal side (Figure 1.).
The two skeletons containing rich inventory were found inside the church, in the altar zone. As the burial place can indicate the deceased’s social status, the funerary inventory and the two skeletons buried next to each other, presumably can be identified as Ferencz and Elek Báthory (Figure 1).
Sample preparation and DNA extraction
The samples analyzed in this study have been deposited at the Museum of History and Art in Zalau, Romania. They were collected during archaeological excavations using gloves and facemasks to minimize the risk of contamination with modern human DNA. Samples were taken from 14 individuals, and DNA was successfully extracted from 13 of them.
Multi-root teeth or petrous bone were collected for molecular analysis (Table 1), and osteological materials were decontaminated with bleach, followed by UV irradiation. DNA extraction and library preparation were performed in sterile laboratories dedicated to aDNA work at the Department of Archaeogenetics of the Institute of Hungarian Research and the Department of Genetics, University of Szeged, Hungary. The soaking method was used for DNA extraction from teeth, which preserves the morphological integrity of teeth.41 For petrous bones, 200 mg of bone powder was used. A pre-digestion step was performed in 1.5 mL of extraction buffer containing 0.5M EDTA and 100 μg/mL Proteinase K. Samples were incubated with the pre-digestion buffer for 30 min at 48°C, followed by a 72-h digestion in extraction buffer containing 0.45 EDTA, 250 μg/mL Proteinase K, and 1% Triton X-100 at 48°C. Next, 7.5 mL of binding buffer containing 5 M GuHCl, 90 mM NaOAc, 40% isopropanol, and 0.05% Tween 20 was added to the extract, and DNA was purified using Qiagen MinElute columns. The quantity of DNA extracts was measured with a Qubit 3.0 Fluorometer (Invitrogen) using the dsDNA High Sensitivity Assay kit.
NGS library preparation, DNA sequencing and NGS data processing
The details of library preparation, sequencing, and sequence analysis are provided in Neparáczki et al. 2017.42 Partial uracil-DNA glycosylase (UDG)-treated DNA extracts were utilized for library construction, as described in.43 50 μl DNA extract was subjected to partial uracil-DNA-glycosylase (UDG) treatment for 30 min at 37°C in the following mix: 6 μl 10X Tango buffer, Thermo Scientific, 0.25 μl 25mM dNTPs, Thermo Scientific, 0.6 100 mM ATP, Thermo Scientific and 1.8 μl USER enzyme (1U/μl−1. After 30 min 1.2 μl of UGI (2 U/μl, UDG inhibitor) was added and put back in the incubation chamber at 37°C for another 30 min.
We made double-stranded libraries44 using double indexing,45 with few modifications.Blunt-End repair was performed with 3 μl of T4 polynucleotide kinase (10 U/ μl) and 1.2 μl of T4 DNA polymerase (5 U/μl) at 25°C for 15 min and 12°C for 5 min. DNA was then purified on MinElute column (Qiagen) in 20 μl Elution buffer, and double stranded library was made with 4 μl of T4 DNA ligase buffer 10X, 4 μl PEG-4000 (50%), 1 μl T4 DNA ligase (5 U/μl) and 0.5 μl adapter mix for 22°C for 30 min. DNA was then purified on MinElute column (Qiagen) in 20 μl Elution buffer.
Adapter Fill-in step was made in 4 μl ThermoPol reaction buffer (10X), 0.4 μl 25mM dNTPs, 1.5 μl Bst polymerase, large fragment (8 U/μl) for 20 min at 37°C. DNA was then purified on MinElute column (Qiagen) in 15 μl Elution buffer.
We omitted the preamplification step, and the libraries were directly double-indexed in one PCR step using Accuprime Pfx Supermix, which contained 10 mg/mL BSA and 200 nM indexing P5 and P7 primers, using the following cycles: 95°C for 5 min, 12 times, 95°C for 15 s, 60°C for 30 s, and 68°C for 3 s, followed by a 5-min extension at 68°C. The libraries were purified using MinElute columns and eluted in 20 μL EB buffer (Qiagen). The DNA concentration was measured with the dsDNA High Sensitivity Assay kit on a Qubit 3.0 Fluorometer, and the fragment size was determined using the TapeStation automated electrophoresis system (Agilent). To estimate the amount of endogenous human DNA in each library, low coverage shotgun sequencing was performed using the iSeq 100 platform from Illumina. Whole-genome sequencing was conducted on a NovaSeq 6000 (Illumina) using the paired-end sequencing method (2x150bp), following the manufacturer’s guidelines.
Quantification and statistical analysis
Bioinformatical processing
The paired-end reads' adapters were removed using Cutadapt [doi:10.14806/ej.17.1.200]24 and the quality of the reads was assessed using FastQC.46 Only reads longer than 25 nucleotides were included in the study. BWA v.0.7.9 software25 was used to align the reads to the human genome reference sequence GRCh37.75, which also contains the revised Cambridge Reference Sequence (rCRS, NC 012920.1) of the mitochondrial genome. The BWA mem algorithm was used in paired mode with default parameters. We applied an additional filter to remove exogenous DNA,19 that is only molecules with >90% similarity to the reference genome were considered. Samtools v1.126 was used to merge sequences from several lanes. Duplicate data were identified using PICARD tools,27 and paired-end reads in the BAM files were merged using the "mergeReads" task from the ATLAS package.28
Quality assessment of ancient sequences
MapDamage 2.029 was used to analyze the ancient DNA damage patterns, and the Rescale option was used to adjust the read quality scores to account for postmortem damage. The Schmutzi algorithm30 was used to estimate mitochondrial genome contamination, and the ANGSD program31 was used to assess contamination in male samples.
Uniparental haplogroup assignment
Mitochondrial Hgs were determined using HaploGrep2 (v2.4.0).32 The Yleaf software tool33 was used to determine the Y chromosome Hg, updated with the ISOGG2020 Y-tree dataset.
Genetic sex determination
The biological sex of the individuals was determined by calculating the X/Y ratio of the shotgun sequencing reads, as described in Skoglund et al., 2013.47
Estimation of genetic relatedness
The ANGSD software tool (version: 0.931–10-g09a0fc5)31 was used to perform haplotype calling on whole genome samples, using the "-doHaploCall 1 -doCounts 1 -sites" parameters and the HumanOrigins and 1240K site coordinates from the Reich laboratory datasets. Variants were then transferred to plink format.
Population genetic analyses
PCA
To uncover population structure based on genomic data, we conducted PCA analysis.36 PCA is a dimensionality reduction method used to visualize multidimensional data, and the first and second principal components (PCs) capture the highest data variability. In simpler terms, PCA groups samples based on genomic similarity, bringing together genomes that are similar to each other. We projected our ancient samples onto PCA axes determined by a set of carefully selected references. We designed the PCA analysis to determine population structure in a presumably highly stratified late Medieval European population, and therefore, we restricted the references from which the background was constructed to strictly Western Eurasian populations (populations west of the Ural) with some populations from Northern Africa (Table S6). Our Western Eurasian population list is a slight modification of that in.48 Individuals were selected from the Allen Ancient Data Resource (AADR), 1240K + HO dataset (version v54.1) from the Reichlab repository. We selected 784 suitable individuals belonging to 85 populations from the 10,513 contemporary individuals present in the AADR dataset. PCA Eigen vectors were calculated from 784 pseudo-haploidized modern genomes using smartpca (EIGENSOFT version 7.2.1).36 Our ancient genomes were projected onto the modern background using the "lsqproject: YES and inbreed: YES" options. The PC2 axis was reversed to better represent the real geographical arrangement of the displayed populations.
F3-statistics
We used outgroup F3-statistics49 to measure shared drift between our samples and ancient and modern European populations. The highest F3-values indicate shared evolutionary past between populations. We only examined post-CE European populations from the AADR dataset (version v52.2), and also added all medieval individuals from the Carpathian Basin published in.19 Altogether 502 ancient and modern populations were tested, as listed in Table S6. We used African Mbuti genomes as an outgroup and applied ADMIXTOOLS39 to calculate F3-statistics. This analysis was also done with the HO dataset, and we removed populations below 50 thousand overlapping markers.
qpAdm analysis
We used qpAdm50 from the ADMIXTOOLS software package39 for modeling our genomes as admixtures of 2 source populations and estimating ancestry proportions. We used the HO dataset for this analysis, as it allowed us to include modern populations as references, which were not available in other formats. We set the details: YES parameter to evaluate Z-scores for the goodness of fit of the model, which was estimated using a Block Jackknife. To exclude suboptimal models, we used the "model-competition" approach described in.19 As majority sources in the “Left-populations” we used the "Eur_Core" populations from Maróti et al., 2022,19 which were identified as representing local medieval populations of the Carpathian Basin. Additionally, we included 10 ancient populations with the highest outgroup F3-values, as they were deemed suitable candidates for representing majority sources as well. We selected those 10 ancient populations, that appeared most frequently among the top 30 results of the F3-outgroup statistics across all analyzed samples in Table S7. For the presumed minority sources in the “Left-populations”, we used representative samples from the previously identified medieval immigrant groups: Conq_Asia_Core, Avar_Asia_Core, Hun_Asia_Core, and Alan.19 As Right (Reference) populations we used Iran_GanjDareh_N, Anatolia_N, Latvia_HG, Baikal_EN (Lokomotiv, Shamanka), WSHG (Tyumen, Sosnoviy), Russia_MLBA_Sintashta, Finnish, Han and Ket, which proved to be appropriate for modeling medieval Hungarian populations.19
In Table S8 all passing models are shown which received significant P-values after model competition runs in the format shown below. Column LEFT lists the source populations which were tested in each 2-way combinations for the given Test populations. From the large number of plausible models with significant P-values (above >0.05 not shown) each plausible source was moved to the RIGHT one by one in model competition runs, and the "total" column indicates the number of competition runs repeated for each plausible model. Column "valid" shows how many times the given model passed, while "excluded" shows how many times it was excluded. In latter case the excluding extra Right population is shown in the "exclude pops" column. "neg" column indicates the number of models in which any of the sources produced a negative fraction. "nested P″ column indicates the number of models with significant (>0.05) nested p values, when the Rank-1 model is more plausible. "max p" indicates the maximum P-value obtained from the competition runs, while "max P right pop" shows the extra Right population in this model. "min P″ and "min P Right pop" means the same for the lowest p value model, while "avg P″ means the average p value of the valid models. Models are arranged according to max p and min p values.
Estimation of genetic relatedness
To investigate the kinship relationships between individuals, we utilized the kinship analysis developed by Nyerki et al. 2023,51 which has been shown to reliably identify relatedness up to the 4th degree from low coverage genome data. We performed the kinship analysis using the 1240K dataset and the PCAngsd software (version 0.99) from the ANGSD package,35 with the “-inbreed 1 -kinship” options. We used the R (version 4.1.2)38; the RcppCNPy R package (version 0.2.10) to import the Numpy output files of PCAngsd.
Acknowledgments
We are grateful to Imre Küzmös for collecting relevant data from the Y-Full database and to Luca Kis for her help in drawing the graphical abstract.
Funding: This research was funded by grants from the National Research, Development and Innovation Office (K-124350 to T.T), the Competence Centre of the Life Sciences Cluster of the Centre of Excellence for Interdisciplinary Research, Development and Innovation of the University of Szeged to T.T. (the author is a member of the Ancient and modern human genomics competence center research group), and University of Szeged Open Access Fund, a Grant number 6388 to T.T. E.N. was supported by TUDFO/5157-1/2019-ITM and TKP2020-NKA-23; K.M. was supported by UNKP-22-3-SZTE-422 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund.
Author contributions
Conceptualization, A.G., E.N., and T.T.; methodology, B.K. and E.N.; software, Z.M. and O.S.; formal analysis, E.N. and Z.M.; investigation, A.G., K.M., B.T., D.B.K., T.K., and G.I.B.V.; resources, E.N. and T.T.; data curation, A.G., Z.M., E.N., and O.S.; writing—original draft preparation, A.G.; writing—review and editing, B.T, E.N., and T.T.; visualization, A.G. and O.S.; supervision, E.N. and T.T.; funding acquisition, E.N. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: September 14, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107911.
Supplemental information
Data and code availability
-
•
Aligned sequence data have been deposited at European Nucleotide Archive (http://www.ebi.ac.uk/ena) under accession number PRJEB63184 and are publicly available as of the date of publication. This paper analyzes existing, publicly available data. These accession numbers for the datasets are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Jenei F. 1968. Az Ecsedi Báthoriakról. A Nyíregyházi Jósa András Múzeum Évkönyve 10; pp. 103–107. [Google Scholar]
- 2.Nagy I. Friebeisz István; Pest: 1857. Magyarország Családai Czimerekkel És Nemzedékrendi Táblákkal I. [Google Scholar]
- 3.Wertner M. A Báthoryak családi történetéhez (Egy genealogiai táblával) Turul. 1900;18:6–29. [Google Scholar]
- 4.Scriptores rerum Hungaricarum. Tempore ducum regumque stirpis Arpadianae gestarum I. Budpestini, Academia Litter. Hungarica atque Societate Histor. Hungarica – Typographiae Reg. Universitatis Litter. Szentpétery E., editor. Hung. Sumptibus. 1937 [Google Scholar]
- 5.Pálffy G. 2000. A Tizenhatodik Század Története (Pannonica Kiadó) [Google Scholar]
- 6.Tóth N. Ki kicsoda az ecsedi Bátori családban. A Bátori család ecsedi ágának tagjai, 1377-1541. Szabolcs-Szatmár-Beregi Szle. 2009;44:1:5–47. [Google Scholar]
- 7.Hungarian National Archive Section P, No. 702 Wesselényi Family Archive. File 1. No. 69
- 8.Hungarian National Archive Section P, No. 108 Eszterházy Family Archive. Repositorium 25/B., Fasc. A. 186, No. 90: “Date in Castello Perechen 16 July Anno 1625.”
- 9.Emődi T. Artă romănească – artă europeană. Centenar Virgil Vătăşianu. Marius Porumb – Aurel Chiriac eds. Academia Română – Institutul de arheologie şi Istoria Artei. Cluj Napoca – Muzeul Ţării Crişurilor; Oradea, Oradea: 2002. Monumente funerare figurative renascentiste din Transilvania; pp. 135–142. [Google Scholar]
- 10.Bunyitay V. 1896. Schematismus historicus venerabilis Cleri Dioecesis Magno-Varadiensis Latinorum pro anno Domini et millennari MDCCCXCV1. [Google Scholar]
- 11.Culic D. Monumente ulitate (I). Bisericile medievale dispărute din județul Sălaj. Forgotten Monuments (I). Lost Medieval Churches in Sălaj County. ACTA MVSEI POROLISSENSIS. 2016;XXXVIII:409–418. [Google Scholar]
- 12.Băcueț- Crișan D., Keresztes T. ACTA MVSEI POROLISSENSIS. XLIII; 2021. Cercetările arheologice preventive de la Pericei, str. Góc, nr. 668 (jud. Sălaj). Date preliminare privinid urmele unei biserici medievale dispărute; pp. 203–214. [Google Scholar]
- 13.Margaryan A., Lawson D.J., Sikora M., Racimo F., Rasmussen S., Moltke I., Cassidy L.M., Jørsboe E., Ingason A., Pedersen M.W., et al. Population genomics of the Viking world. Nature. 2020;585:390–396. doi: 10.1038/s41586-020-2688-8. [DOI] [PubMed] [Google Scholar]
- 14.Antonio M.L., Gao Z., Moots H.M., Lucci M., Candilio F., Sawyer S., Oberreiter V., Calderon D., Devitofranceschi K., Aikens R.C., et al. Ancient Rome: A genetic crossroads of Europe and the Mediterranean. Science. 2019;366:708–714. doi: 10.1126/science.aay6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veeramah K.R., Rott A., Groß M., van Dorp L., López S., Kirsanow K., Sell C., Blöcher J., Wegmann D., Link V., et al. Population genomic analysis of elongated skulls reveals extensive female-biased immigration in Early Medieval Bavaria. Proc. Natl. Acad. Sci. USA. 2018;115:3494–3499. doi: 10.1073/pnas.1719880115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones E.R., Gonzalez-Fortes G., Connell S., Siska V., Eriksson A., Martiniano R., McLaughlin R.L., Gallego Llorente M., Cassidy L.M., Gamba C., et al. Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat. Commun. 2015;6:8912. doi: 10.1038/ncomms9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skourtanioti E., Erdal Y.S., Frangipane M., Balossi Restelli F., Yener K.A., Pinnock F., Matthiae P., Özbal R., Schoop U.-D., Guliyev F., et al. Genomic History of Neolithic to Bronze Age Anatolia, Northern Levant, and Southern Caucasus. Cell. 2020;181:1158–1175.e28. doi: 10.1016/j.cell.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Neparáczki E., Maróti Z., Kalmár T., Maár K., Nagy I., Latinovics D., Kustár Á., Pálfi G., Molnár E., Marcsik A., et al. Y-chromosome haplogroups from Hun, Avar and conquering Hungarian period nomadic people of the Carpathian Basin. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-53105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maróti Z., Neparáczki E., Schütz O., Maár K., Varga G.I.B., Kovács B., Kalmár T., Nyerki E., Nagy I., Latinovics D., et al. The genetic origin of Huns, Avars, and conquering Hungarians. Curr. Biol. 2022;32:2858–2870.e7. doi: 10.1016/j.cub.2022.04.093. [DOI] [PubMed] [Google Scholar]
- 20.Volodko N.V., Starikovskaya E.B., Mazunin I.O., Eltsov N.P., Naidenko P.V., Wallace D.C., Sukernik R.I. Mitochondrial Genome Diversity in Arctic Siberians, with Particular Reference to the Evolutionary History of Beringia and Pleistocenic Peopling of the Americas. Am. J. Hum. Genet. 2008;82:1084–1100. doi: 10.1016/j.ajhg.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raule N., Sevini F., Li S., Barbieri A., Tallaro F., Lomartire L., Vianello D., Montesanto A., Moilanen J.S., Bezrukov V., et al. The co-occurrence of mtDNA mutations on different oxidative phosphorylation subunits, not detected by haplogroup analysis, affects human longevity and is population specific. Aging Cell. 2014;13:401–407. doi: 10.1111/acel.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucotte G. The Major Y-Chromosome Haplogroup R1b-M269 in West-Europe, Subdivided by the Three SNPs S21/U106, S145/L21 and S28/U152, Shows a Clear Pattern of Geographic Differentiation. Adv. Anthropol. 2015;05:22–30. doi: 10.4236/aa.2015.51003. [DOI] [Google Scholar]
- 23.O’Sullivan N., Posth C., Coia V., Schuenemann V.J., Price T.D., Wahl J., Pinhasi R., Zink A., Krause J., Maixner F. Ancient genome-wide analyses infer kinship structure in an Early Medieval Alemannic graveyard. Sci. Adv. 2018;4:eaao1262. doi: 10.1126/sciadv.aao1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. j. 2011;17:10–12. [Google Scholar]
- 25.Li H., Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broad Institute Picard tools. 2016. https://broadinstitute.github.io/picard/
- 28.Link V., Kousathanas A., Veeramah K., Sell C., Scheu A., Wegmann D. ATLAS: Analysis Tools for Low-depth and Ancient Samples. bioRxiv. 2017 doi: 10.1101/105346. [DOI] [Google Scholar]
- 29.Jónsson H., Ginolhac A., Schubert M., Johnson P.L.F., Orlando L. mapDamage2.0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics. 2013;29:1682–1684. doi: 10.1093/bioinformatics/btt193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renaud G., Slon V., Duggan A.T., Kelso J. Schmutzi: estimation of contamination and endogenous mitochondrial consensus calling for ancient DNA. Genome Biol. 2015;16:224. doi: 10.1186/s13059-015-0776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korneliussen T.S., Albrechtsen A., Nielsen R. ANGSD: Analysis of Next Generation Sequencing Data. BMC Bioinf. 2014;15:356. doi: 10.1186/s12859-014-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissensteiner H., Pacher D., Kloss-Brandstätter A., Forer L., Specht G., Bandelt H.-J., Kronenberg F., Salas A., Schönherr S. HaploGrep 2: mitochondrial haplogroup classification in the era of high-throughput sequencing. Nucleic Acids Res. 2016;44:W58–W63. doi: 10.1093/nar/gkw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ralf A., Montiel González D., Zhong K., Kayser M. Yleaf: Software for Human Y-Chromosomal Haplogroup Inference from Next-Generation Sequencing Data. Mol. Biol. Evol. 2018;35:1291–1294. doi: 10.1093/molbev/msy032. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen B.S., Quinlan A.R. Mosdepth: quick coverage calculation for genomes and exomes. Bioinformatics. 2018;34:867–868. doi: 10.1093/BIOINFORMATICS/BTX699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meisner J., Albrechtsen A. Inferring Population Structure and Admixture Proportions in Low-Depth NGS Data. Genetics. 2018;210:719–731. doi: 10.1534/genetics.118.301336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson N., Price A.L., Reich D. Population Structure and Eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander D.H., Novembre J., Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team . R Foundation for Statistical Computinge; 2015. R: A Language and Environment for Statistical Computing.http://www.r-project.org Doc. Free. available internet. [Google Scholar]
- 39.Patterson N., Moorjani P., Luo Y., Mallick S., Rohland N., Zhan Y., Genschoreck T., Webster T., Reich D. Ancient Admixture in Human History. Genetics. 2012;192:1065–1093. doi: 10.1534/genetics.112.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narasimhan V.M., Patterson N., Moorjani P., Rohland N., Bernardos R., Mallick S., Lazaridis I., Nakatsuka N., Olalde I., Lipson M., et al. The formation of human populations in South and Central Asia. Science. 2019;365 doi: 10.1126/science.aat7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harney É., Cheronet O., Fernandes D.M., Sirak K., Mah M., Bernardos R., Adamski N., Broomandkhoshbacht N., Callan K., Lawson A.M., et al. A minimally destructive protocol for DNA extraction from ancient teeth. Genome Res. 2021;31:472–483. doi: 10.1101/gr.267534.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neparáczki E., Kocsy K., Tóth G.E., Maróti Z., Kalmár T., Bihari P., Nagy I., Pálfi G., Molnár E., Raskó I., Török T. Revising mtDNA haplotypes of the ancient Hungarian conquerors with next generation sequencing. PLoS One. 2017;12:e0174886. doi: 10.1371/journal.pone.0174886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohland N., Harney E., Mallick S., Nordenfelt S., Reich D. Partial uracil – DNA – glycosylase treatment for screening of ancient DNA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015;370:20130624. doi: 10.1098/rstb.2013.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer M., Kircher M. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harb. Protoc. 2010;2010 doi: 10.1101/pdb.prot5448. pdb.prot5448. [DOI] [PubMed] [Google Scholar]
- 45.Kircher M., Sawyer S., Meyer M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 2012;40:e3–e8. doi: 10.1093/nar/gkr771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data [Online] 2010. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 47.Skoglund P., Storå J., Götherström A., Jakobsson M. Accurate sex identification of ancient human remains using DNA shotgun sequencing. J. Archaeol. Sci. 2013;40:4477–4482. doi: 10.1016/j.jas.2013.07.004. [DOI] [Google Scholar]
- 48.Mittnik A., Wang C.-C., Pfrengle S., Daubaras M., Zariņa G., Hallgren F., Allmäe R., Khartanovich V., Moiseyev V., Tõrv M., et al. The genetic prehistory of the Baltic Sea region. Nat. Commun. 2018;9:442. doi: 10.1038/s41467-018-02825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raghavan M., Skoglund P., Graf K.E., Metspalu M., Albrechtsen A., Moltke I., Rasmussen S., Stafford T.W., Jr., Orlando L., Metspalu E., et al. Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature. 2014;505:87–91. doi: 10.1038/nature12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harney É., Patterson N., Reich D., Wakeley J. Assessing the performance of qpAdm: a statistical tool for studying population admixture. Genetics. 2021;217 doi: 10.1093/genetics/iyaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nyerki E., Kalmár T., Schütz O., Lima R.M., Neparáczki E., Török T., Maróti Z. correctKin: an optimized method to infer relatedness up to the 4th degree from low-coverage ancient human genomes. Genome Biol. 2023;24:38. doi: 10.1186/s13059-023-02882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Aligned sequence data have been deposited at European Nucleotide Archive (http://www.ebi.ac.uk/ena) under accession number PRJEB63184 and are publicly available as of the date of publication. This paper analyzes existing, publicly available data. These accession numbers for the datasets are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.