Abstract
Objective
To compare the incidence of aspiration pneumonia, nausea, and vomiting after intravascular administration of non-ionic iodinated contrast media (ICM) between patients who fasted before contrast injection and those who did not.
Materials and Methods
Ovid-MEDLINE and Embase databases were searched from their inception dates until September 2022 to identify original articles that met the following criteria: 1) randomized controlled trials or observational studies, 2) separate reports of the incidence of aspiration pneumonia, nausea, and vomiting after intravascular injection of non-ionic ICM, and 3) inclusion of patients undergoing radiological examinations without fasting. A bivariate beta-binomial model was used to compare the risk difference in adverse events between fasting and non-fasting groups. The I2 statistic was used to assess heterogeneity across the studies.
Results
Ten studies, encompassing 308013 patients (non-fasting, 158442), were included in this meta-analysis. No cases of aspiration pneumonia were reported. The pooled incidence of nausea was 4.6% (95% confidence interval [CI]: 1.4%, 7.8%) in the fasting group and 4.6% (95% CI: 1.1%, 8.1%) in the non-fasting group. The pooled incidence of vomiting was 2.1% (95% CI: 0.0%, 4.2%) in the fasting group and 2.5% (95% CI: 0.7%, 4.2%) in the non-fasting group. The risk difference (incidence in the non-fasting group–incidence in the fasting group) in the incidence of nausea and vomiting was 0.0% (95% CI: -4.7%, 4.7%) and 0.4% (95% CI: -2.3%, 3.1%), respectively. Heterogeneity between the studies was low (I2 = 0%–13.5%).
Conclusion
Lack of fasting before intravascular administration of non-ionic ICM for radiological examinations did not increase the risk of emetic complications significantly. This finding suggests that hospitals can relax fasting policies without compromising patient safety.
Keywords: Contrast media, Fasting, Nausea, Vomiting, Aspiration pneumonia
See the invited Editorial by Choi et al., in volume 24(10) on page 944 to 946, https://doi.org/10.3348/kjr.2023.0751.
INTRODUCTION
Iodinated contrast media (ICM) are increasingly used in modern radiological examinations. Nausea and vomiting have been frequently reported after the intravascular administration of high-osmolar ionic ICM [1]. Fasting is usually required to prevent aspiration pneumonia should nausea and vomiting develop. With the introduction of iso- to low-osmolar non-ionic ICM, which has replaced ionic ICM, the incidence of emetic complications was reduced [2,3]. Indeed, in a systematic review published in 2012 [4], no aspiration pneumonia was noted in 2001 patients who had been exposed to non-ionic ICM after fluid ingestion.
Abolishing fasting before intravascular ICM injections can alleviate patients’ discomfort and reduce delays before examination, which is particularly beneficial in emergency situations. The recently published European Society of Urogenital Radiology (ESUR) and American College of Radiology (ACR) guidelines do not mandate fasting prior to intravascular ICM administration, given the lack of evidence indicating the need for fasting [5,6]. Nevertheless, the requirement for and duration of fasting before contrast-enhanced radiological examinations vary substantially across countries and institutions [4,7]. A potential reason for this variation in practice may be the absence of thorough evidence on whether abolishing the requirement for fasting affects emetic complications, including nausea, vomiting, and aspiration pneumonia.
Therefore, this systematic review and meta-analysis aimed to compare the incidence of nausea, vomiting, and aspiration pneumonia according to the fasting state in patients who underwent radiological examinations with intra-vascular administration of non-ionic ICM.
MATERIALS AND METHODS
This study was exempted from ethics review by the Seoul National University Hospital institutional review board, and informed consent was not necessary.
Literature Search
This study adhered to the reporting guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [8]. We performed a systematic electronic search of the Embase and OVID-Medline databases to identify relevant original research studies using the following search terms: fasting AND [CT OR computed tomography OR angiography OR catheter*] AND [vomiting OR nausea OR aspiration]. The search was conducted from the database inception dates up to September 30, 2022. The search protocol was designed by an experienced investigator (S.H.Y., 10 years of experience in meta-analysis) and the literature search was conducted independently by two reviewers (S.H.Y., and H.C.). The search was further supplemented by manual screening of the bibliographies of the retrieved articles.
Study Selection
The inclusion criteria were as follows: 1) randomized controlled trials or observational prospective or retrospective cohort studies, 2) studies that reported the separate incidences of aspiration pneumonia, nausea, and vomiting after intravascular administration of non-ionic ICM for an enhanced CT scan or angiography, 3) studies including patients undergoing the radiological examinations without fasting. The exclusion criteria were 1) non-English literature, 2) studies with oral contrast media, 3) review articles or systematic reviews, 4) studies in which all participants fasted before examinations, and 5) studies reporting the combined incidence of nausea and vomiting as an emetic complication in such a way that calculation of the individual complication incidences was precluded.
Data Extraction
Data were independently extracted by two authors (S.H.Y and H.C.) using a standardized form. We extracted 1) study characteristics (authors, country, publication year, study period, study design, definition of fasting and non-fasting states, and type of examination and contrast medium used); 2) patient characteristics (number of patients, mean or median age, and the proportion of participants in fasting and non-fasting states); and 3) the reported number and percentage of events of aspiration pneumonia, vomiting, and nausea. The definition of fasting and non-fasting in each study is summarized in a Supplementary Table 1. Regarding complications, when the incidence of nausea and vomiting was reported together, we contacted the authors and asked them to calculate the incidence of nausea and vomiting separately.
Quality Assessment
The Newcastle–Ottawa Quality Scale for cohort studies was applied to assess the quality of the included studies [9]. The scale allocates a maximum of 4 points for selection, 2 points for comparability, and 3 points for exposure or outcomes. The outcomes were the incidence of aspiration pneumonia, nausea, and vomiting, respectively. We considered a follow-up period of at least 4 days to be sufficient for monitoring aspiration pneumonia occurrence [10]. Study quality was assessed by one reviewer (H.C. under the supervision of another reviewer, S.H.Y.). Scores of more than 7 and 5–6 indicated high-quality and moderate-quality studies, respectively.
Statistical Analysis
The incidence of adverse events for individual studies was estimated with the Clopper–Pearson confidence interval by group. The incidence in patients who did not fast prior to the enhanced imaging examination was compared with that in patients who did fast using risk difference as the effect measure. Since adverse events in the included studies were rare, and one or both groups had zero events, a bivariate beta-binomial model was employed. Such a model is based directly on data from binomial distributions and can thus avoid bias from continuity correction and makes no explicit assumption of homogeneity [11]. When no adverse event occurred in the included studies, the Mantel–Haenszel method with continuity correction was performed, as proposed by Xu et al. [12]. Subgroup analysis was conducted exclusively for studies that adopted no restriction whatsoever in oral intake before the examination as the definition for non-fasting.
We used the I2 statistic to assess heterogeneity across the studies. In addition, funnel plots were visually inspected to assess potential publication bias. All analyses were performed with R version 4.1.2 (http://www.R-project.org) and SAS (version 9.4, SAS Institute).
RESULTS
Literature Search
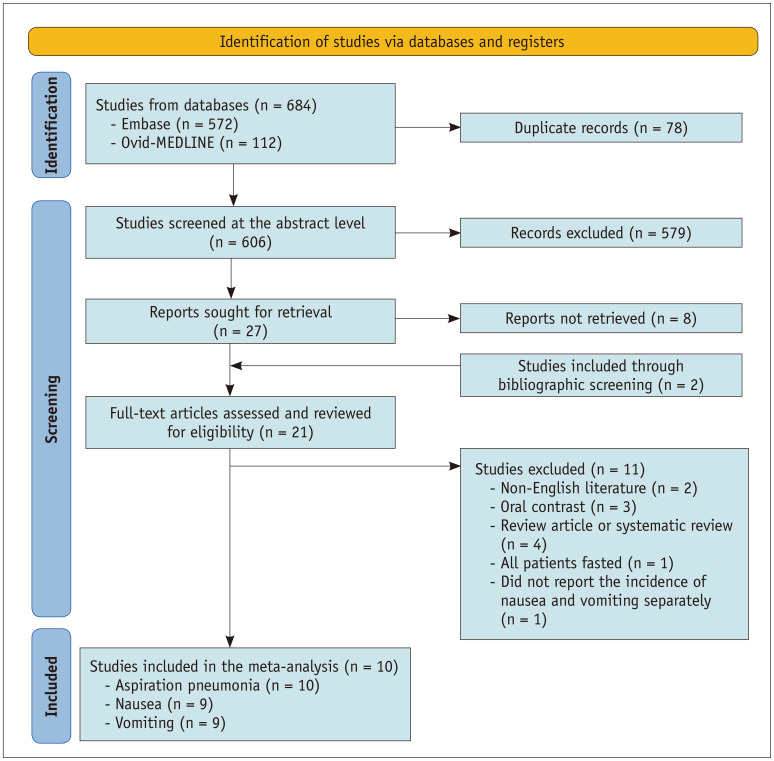
From the initial database search, 684 references were identified. After duplicates were removed, the remaining 606 articles underwent abstract-level review. By reviewing the titles and abstracts, 579 publications were excluded. Of the 27 remaining studies, eight articles were not retrievable, leaving 19 studies. To this, we added two fully accessible articles by screening bibliographies [13,14]. After a full-text review of these 21 publications, 10 studies were finally included in the meta-analysis (Fig. 1, Table 1) [3,13,15,16,17,18,19,20,21,22]. The other 11 studies were excluded for the following reasons: non-English literature (n = 2), use of oral contrast rather than intravascular injections (n = 3), review (n = 3) or systematic review (n = 1) articles, studies that only evaluated the incidence of adverse events in the fasting group (n = 1), and research reporting the merged incidence of nausea and vomiting (n = 1). Two of the included studies [16,17] reported the incidence of nausea and vomiting together as emetic complications, but we were able to calculate the incidence of nausea and vomiting separately after contacting the authors.
Fig. 1. Flow diagram of the study. Identification and selection of studies for inclusion in this meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses recommendations.
Table 1. Study characteristics.
| Studies | Country | Study design | Study period | Type of examination | Contrast media | Number of total patients | Number of patients in the fasting group | Number of patients in the non-fasting group | Incidence of nausea (fasting/non-fasting, %) | Incidence of vomiting (fasting/non-fasting, %) | Incidence of aspiration pneumonia |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liu et al. [22] | China | Retrospective | 2019.01 to 2020.12 | CT | LOCM | 127200 | 49676 | 77524 | 0.008/0.009 | 0.022/0.037 | 0 |
| IOCM | |||||||||||
| Neeman et al. [18]‡ | Israel | Prospective | 2018.04 to 2019.09 | CT | LOCM | 2091 | 1080 | 1011 | 6.6/7.6 | 2.6/3.0 | 0 |
| Tsushima et al. [20] | Japan | Retrospective | 2015.12 to 2020.03 | CT | LOCM | 58603 | 43927 | 14676 | 0.31/0.18* | 0.12/0.16 | 0 |
| Ha et al. [16]† | Republic of Korea | Retrospective | 2017.04 to 2019.07 | CT | LOCM | 864 | 276 | 588 | 0.7/0.7 | 1.1/1.5 | 0 |
| Barbosa et al. [3]‡ | Brazil | Prospective | 2011.11 to 2013.12 | CT | LOCM | 3206 | 1619 | 1587 | 1.1/0.9 | 0.1/0.2 | 0 |
| Li et al. [19] | China | Prospective | 2015.06 to 2017.06 | CT | LOCM | 110836 | 51807 | 59029 | 0.01/0.01 | 0.05/0.07 | 0 |
| IOCM | |||||||||||
| Hamid et al. [21] | United Kingdom | Retrospective | 2010.01 to 2013.12 | Angiography | N/A | 1916 | 0 | 1916 | N/A | N/A | 0 |
| Kwon et al. [17]† | Republic of Korea | Prospective | 2005.09 to 2009.03 | Angiography | LOCM | 2000 | 200 | 1800 | 1.5/0 | 1.0/1.1 | 0 |
| Kim et al. [15] | Republic of Korea | Prospective | 2017.01 to 2017.03 | CT | LOCM | 1175 | 948 | 227 | 2.85/3.08 | 0/0 | 0 |
| Park et al. [13] | Republic of Korea | Prospective | 2006.04 to 2006.08 | CT | N/A | 122 | 38 | 84 | 34.2/31.0 | 15.8/21.4 | 0 |
*There was statistically significant difference of incidence between the groups (P = 0.006), †The studies reported incidence of nausea and/or vomiting together, but we obtained the separate incidence of nausea and vomiting by contacting the authors, ‡Studies designed as randomized controlled studies.
CT = computed tomography, LOCM = low-osmolar contrast media, IOCM = iso-osmolar contrast media, N/A = not available
Study Characteristics and Quality Assessments
The characteristics of the included studies are summarized in Table 1. In total, 308013 patients were included, with 149571 in the fasting group and 158442 in the non-fasting group. Six of the 10 studies were designed prospectively, and two studies were randomized controlled trials. Seven studies (7/10, 70%) were conducted in Asia: four in Korea, two in China, and one in Japan. As outcomes, all but one of the studies compared the incidence of adverse events between fasting and non-fasting groups. Hamid et al. [21] only investigated the incidence of outcomes in non-fasting patients. All studies considered aspiration pneumonia, and the incidence of nausea and vomiting was assessed in nine of the 10 studies. Quality assessment using the Newcastle–Ottawa scale identified six studies as high-quality and two as moderate-quality. The results of the quality assessment are presented in Supplementary Table 2.
Definition of Fasting and Non-fasting
Among the included studies, five (50%, 5/10) defined non-fasting as involving no restriction in oral intake at all. The other definitions were neither restriction nor encouragement of food ingestion (n = 1), fasting from solid food for less than 4 hours (n = 1) or for less than 6 hours (n = 1), and no more than a light meal or fluid administration within 1 hour before the CT scan (n = 2). Regarding fasting, six of nine studies defined fasting as more than 4 hours of solid food restriction, while the other three studies defined fasting as more than 6 hours of solid food restriction (Supplementary Table 1).
Incidence of Nausea, Vomiting, and Aspiration Pneumonia
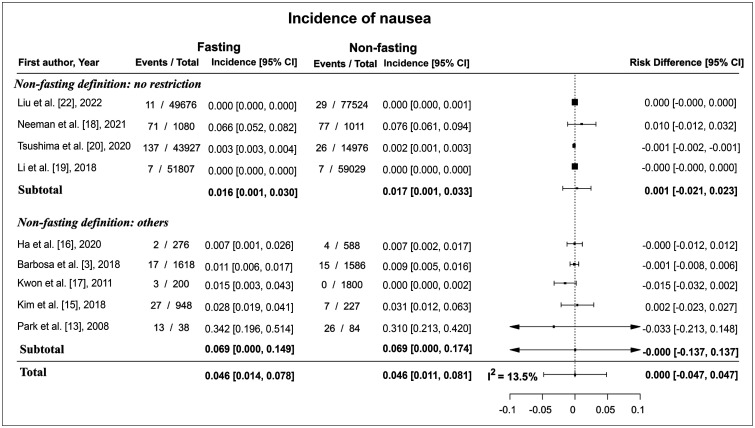
Across the nine studies, the incidence of nausea in the fasting group ranged from 0.01% to 34%, while that in the non-fasting group ranged from 0.01% to 31%. The pooled incidence of nausea was 4.6% (95% confidence interval [CI]: 1.4%, 7.8%) in the fasting group and 4.6% (95% CI: 1.1%, 8.1%) in the non-fasting group. The differences in incidence between the fasting and non-fasting groups (incidence in the non-fasting group–incidence in the fasting group) ranged from -3.0% to 0.0%. The pooled risk difference was 0.0% (95% CI: -4.7%, 4.7%), which indicated no statistically significantly difference between the two groups (Fig. 2). The I2 statistic was 13.5%.
Fig. 2. Forest plot of the risk difference in the incidence of nausea between fasting and non-fasting group. There were no risk differences between the two groups and the pooled risk difference was 0.0% (95% CI: -4.7%, 4.7%). CI = confidence interval.
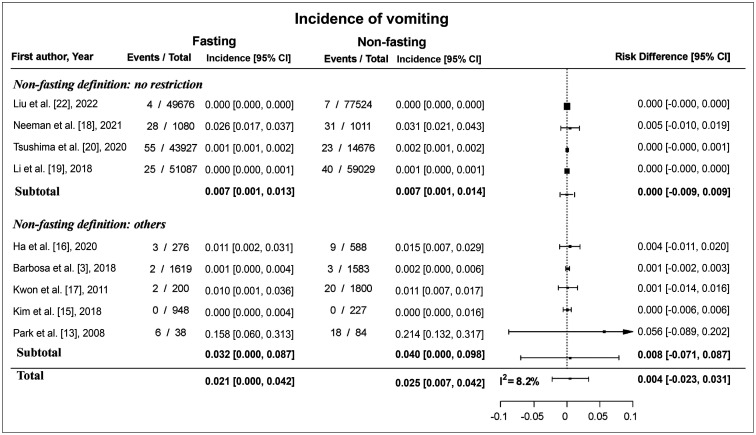
The incidence of vomiting ranged from 0% to 16% in the fasting group and from 0% to 21% in the non-fasting group. The pooled incidence of vomiting was 2.1% (95% CI: 0.0%, 4.2%) in the fasting group and 2.5% (95% CI: 0.7%, 4.2%) in the non-fasting group. The difference in incidence between the fasting and non-fasting groups (incidence in the non-fasting group–incidence in the fasting group) ranged from 0.0% to 6.0%. The pooled risk difference was 0.4% (95% CI: -2.3%, 3.1%) (Fig. 3), and the I2 statistic was 8.2%.
Fig. 3. Forest plot of the risk difference in the incidence of vomiting between fasting and non-fasting group. The pooled risk difference was 0.4% (95% CI, -2.3%, 3.1%) and the risk difference was 0% (95% CI, -0.9%, 0.9%) among the studies that defined non-fasting as no restriction. CI = confidence interval.
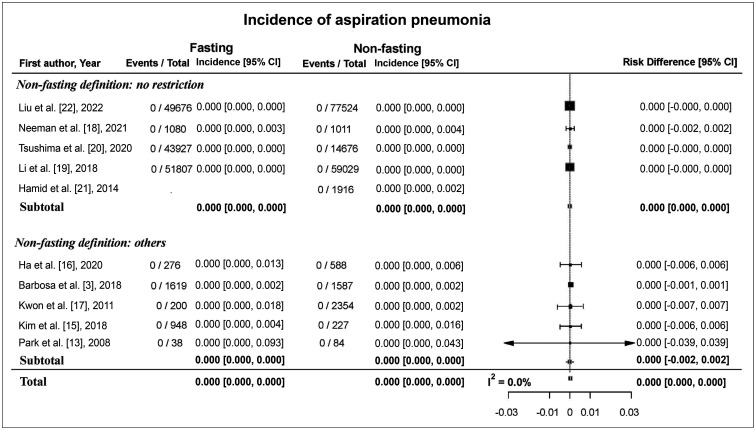
No cases of aspiration pneumonia were reported in any of the 10 studies. Three of the studies reported an adequate follow-up period of more than 96 hours for detecting cases of aspiration pneumonia (Supplementary Table 2). The estimated pooled risk difference between the fasting and non-fasting groups was 0.0 (95% CI: 0.0, 0.0) (Fig. 4), and the I2 statistic was 0.0%.
Fig. 4. Forest plot of the risk difference in the incidence of aspiration pneumonia between fasting and non-fasting group. No cases of aspiration pneumonia were reported in any of the 10 studies. CI = confidence interval.
Subgroup Analysis of Nausea and Vomiting According to the Fasting Definition
Four studies, which defined non-fasting as no restriction in oral intake at all, compared the incidence of nausea and vomiting according to fasting status. The risk difference in the incidence of nausea between the fasting and non-fasting groups ranged from -0.1% to 1.0% and the pooled risk difference was 0.1% (95% CI: -2.1%, 2.3%) (Fig. 2). The risk difference in the incidence of vomiting between the fasting and non-fasting group ranged from 0.0% to 0.5%, and the pooled risk difference was 0.0% (95% CI: -0.9%, 0.9%) (Fig. 3).
Publication Bias
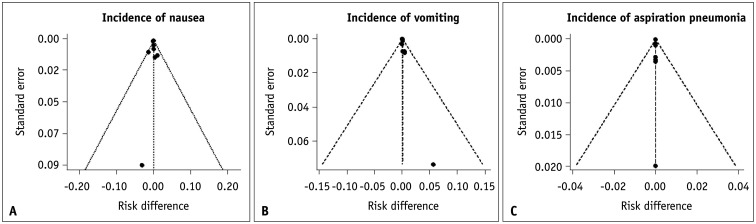
A visual assessment of funnel plots showed no asymmetry, indicating a low likelihood of publication bias (Fig. 5).
Fig. 5. Funnel plots of the incidence of adverse events in fasting and non-fasting groups. A-C: There was low likelihood of publication bias based on the symmetry of the plots.
DISCUSSION
This meta-analysis had two principal findings. First, the incidence of adverse emetic complications associated with intravascular contrast media administration for radiographic examinations was low. Across 308013 patients, the pooled incidence of nausea was 4.6% in both the fasting and non-fasting groups, while the pooled incidence of vomiting was 2.1% in the fasting group and 2.5% in the non-fasting group. Notably, no cases of aspiration pneumonia were reported. Second, the development of adverse emetic complications did not differ according to fasting status. The risk difference in the incidence of nausea and vomiting between the two groups was 0.0% (95% CI: -4.7%, 4.7%) and 0.4% (95% CI: -2.3%, 3.1%), respectively. The results were maintained in subgroup analysis, in which we included only studies with no restriction on food ingestion prior to imaging at all, and the CI became narrower: the risk difference was 0.1% (95% CI: -2.1%, 2.3%) for nausea and 0.0% (95% CI: -0.9%, 0.9%) for vomiting. The finding of low heterogeneity between the studies supports the credibility of our results (I2 = 0%–13.5%).
The ESUR guideline states that fasting is not recommended prior to administration of non-ionic low-osmolar contrast medium or iso-osmolar contrast medium [6], and the recently updated ACR guideline published in 2021 added a new chapter on fasting policy, stating that fasting is not required, given its potential negative effects and lack of preventive effects for emetic complications [5]. However, despite these guidelines, many institutions still ask patients to fast for periods substantially exceeding the recommended fasting threshold [23]. Our results provided further support for these guidelines, as the meta-analysis findings indicated that fasting has no added benefit for preventing emetic complications. Additionally, studies such as that of Lee et al. [4] showed little association between fluid ingestion prior to enhanced CT and the development of aspiration pneumonia. Oowaki et al. [2] concluded that fasting, rather than non-fasting, increased the risk of nausea and vomiting after administration of ICM.
Shortening or abolishing the fasting period before enhanced radiological examination offers several advantages from both physiological and economic perspectives. Simplified preparation can reduce patients’ discomfort, inconvenience, and the risk of hypoglycemia in patients with diabetes mellitus. Prolonged fasting can induce insulin resistance [24] can increase catabolism, and can have adverse effects on the metabolic and hemodynamic responses to stress [24]. Fasting can also decrease gastric pH levels and increase gastric volume, putting patients at risk of reflux [18]. Dehydration and hypoglycemia are particularly concerning for older patients, infants, and children [25,26]. For the same reason, policies have changed regarding the fasting time for procedures that require fasting, even before general anesthesia [27]. Patients may also stop their routine medications during the fasting period, which may increase risk in patients with hypertension or diabetes mellitus [25]. Furthermore, the abolition of unnecessary fasting can simplify the radiology workflow in a context where enhanced CT examinations are increasingly being performed.
On the other hand, for particular CT protocols, fasting is beneficial. For example, to evaluate the gall bladder adequately, sufficient distension is required. In studies evaluating bowel loops, such as CT colonography or enterography, bowel cleansing is important, and fasting is essential for effective lesion detection. Furthermore, the impact of non-fasting on specific populations susceptible to nausea and vomiting or who are at risk of aspiration due to their general condition, has not been investigated adequately. Even though no cases of aspiration pneumonia were involved in the studies included in this meta-analysis, several potential risk factors for emetic complications have been identified. Kim et al. [15] demonstrated drug hypersensitivity (odds ratio 4.3, 95% CI: 1.9, 17.5), and Ha et al. [16] identified ongoing chemotherapy (odds ratio 4.3, 95% CI: 1.4, 13.1), along with certain types of contrast media, as independent predisposing factors for emetic complications. As such, the decision to fast or not before enhanced CT scans should be determined considering the risks and benefits pertinent to the patient’s situation and the CT indications.
While no consensus about the selection of effect-measures for rare events exist [12], in this meta-analysis, we opted to use the risk difference, instead of relative effect measures such as the odds ratio, for the following reasons. Firstly, we included studies in which no adverse events were reported in one or both groups, which makes it difficult to calculate the relative effect measures properly. Moreover, total aspiration pneumonia cases in the included studies were zero. Although using relative effect measures with continuity correction can be employed, this would lead to large bias when the sample sizes of the two arms are imbalanced [12,28], as was the case in some of the included studies.
While our meta-analysis provides valuable insights into the relationship between fasting and adverse emetic complications associated with intravascular contrast medium administration, several limitations should be noted. First, the meta-analysis included some retrospective studies, which may have been subject to some confusion regarding fasting time and the development of adverse events. Second, due to insufficient data on the incidence of nausea and vomiting according to the specific types of ICM used, we were unable to perform a subgroup analysis based on ICM type. The incidence of emetic complications might vary according to the types of contrast media used. Third, inconsistent definitions of fasting and non-fasting groups were used across the studies, and most non-fasting groups received instructions without restrictions, rather than being encouraged to consume solids and fluids, which may have led to inaccurate information about their fasting status. Fourth, considering that only cooperative patients can provide informed consent and be enrolled in a prospective study, the results of the included prospective studies may not be applicable to uncooperative patients, such as those whose consciousness is impaired and who are at risk for aspiration.
In conclusion, our meta-analysis demonstrated that allowing free ingestion before the intravascular administration of non-ionic ICM for radiological examinations did not significantly increase the risk of emetic complications, including nausea and vomiting. These results provided further evidence to support the ACR and ESUR guidelines that recommend against fasting policies and may help hospitals to relax their fasting policies to improve patients’ experiences, without compromising patient safety.
Acknowledgments
The authors gratefully acknowledge Andrew Dombrowski, PhD (Compecs, Inc.) for his assistance in improving the use of English in this manuscript.
Footnotes
Conflicts of Interest: Soon Ho Yoon is the chief medical officer for Medical IP and holds a stock option in the firm, however this do not affect to publish this manuscript. All remaining authors have declared no conflicts of interest.
- Conceptualization: Soon Ho Yoon.
- Data curation: Soon Ho Yoon, Hyewon Choi.
- Formal analysis: Hyunsook Hong.
- Investigation: Soon Ho Yoon, Hyewon Choi.
- Methodology: Soon Ho Yoon, Hyunsook Hong.
- Supervision: Min Jae Cha, Soon Ho Yoon.
- Writing—original draft: Hyewon Choi, Hyunsook Hong.
- Writing—review & editing: Soon Ho Yoon, Min Jae Cha.
Funding Statement: None
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2023.0399.
References
- 1.Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the safety of contrast media. Radiology. 1990;175:621–628. doi: 10.1148/radiology.175.3.2343107. [DOI] [PubMed] [Google Scholar]
- 2.Oowaki K, Saigusa H, Ojiri H, Ariizumi M, Yamagisi J, Fukuda K, et al. [Relationship between oral food intake and nausea caused by intravenous injection of iodinated contrast material] Nihon Igaku Hoshasen Gakkai Zasshi. 1994;54:476–479. Japanese. [PubMed] [Google Scholar]
- 3.Barbosa PNVP, Bitencourt AG, Tyng CJ, Cunha R, Travesso DJ, Almeida MFA, et al. Preparative fasting for contrast-enhanced CT in a cancer center: a new approach. AJR Am J Roentgenol. 2018;210:941–947. doi: 10.2214/AJR.17.19061. [DOI] [PubMed] [Google Scholar]
- 4.Lee BY, Ok JJ, Abdelaziz Elsayed AA, Kim Y, Han DH. Preparative fasting for contrast-enhanced CT: reconsideration. Radiology. 2012;263:444–450. doi: 10.1148/radiol.12111605. [DOI] [PubMed] [Google Scholar]
- 5.ACR Committee on Drugs and Contrast Media. ACR manual on contrast media: fasting prior to intravascular contrast media administration. Reston: American College of Radiology; 2022. p. 14. [Google Scholar]
- 6.European Society of Urogenital Radiology. ESUR guidelines on contrast agents (version 10.0) [accessed on April 10, 2023]. Available at: https://www.esur.org/esur-guidelines-on-contrast-agents .
- 7.Han S, Yoon SH, Lee W, Choi YH, Kang DY, Kang HR. Management of adverse reactions to iodinated contrast media for computed tomography in Korean referral hospitals: a survey investigation. Korean J Radiol. 2019;20:148–157. doi: 10.3348/kjr.2017.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 9.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [accessed on April 10, 2023]. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 10.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665–671. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 11.Guo J, Xiao M, Chu H, Lin L. Meta-analysis methods for risk difference: a comparison of different models. Stat Methods Med Res. 2023;32:3–21. doi: 10.1177/09622802221125913. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Furuya-Kanamori L, Zorzela L, Lin L, Vohra S. A proposed framework to guide evidence synthesis practice for meta-analysis with zero-events studies. J Clin Epidemiol. 2021;135:70–78. doi: 10.1016/j.jclinepi.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Park JH, Rhee JE, Kim KS, Shin JH. Is the six-hour fasting before abdominal computed tomography necessary to prevent gastrointestinal adverse events in patients with abdominal pain? J Korean Soc Emerg Med. 2008;19:333–338. [Google Scholar]
- 14.Wagner HJ, Evers JP, Hoppe M, Klose KJ. [Must the patient fast before intravascular injection of a non-ionic contrast medium? Results of a controlled study] Rofo. 1997;166:370–375. doi: 10.1055/s-2007-1015444. German. [DOI] [PubMed] [Google Scholar]
- 15.Kim YS, Yoon SH, Choi YH, Park CM, Lee W, Goo JM. Nausea and vomiting after exposure to non-ionic contrast media: incidence and risk factors focusing on preparatory fasting. Br J Radiol. 2018;91:20180107. doi: 10.1259/bjr.20180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha JY, Choi YH, Cho YJ, Lee S, Lee SB, Choi G, et al. Incidence and risk factors of nausea and vomiting after exposure to low-osmolality iodinated contrast media in children: a focus on preparative fasting. Korean J Radiol. 2020;21:1178–1186. doi: 10.3348/kjr.2019.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon OK, Oh CW, Park H, Bang JS, Bae HJ, Han MK, et al. Is fasting necessary for elective cerebral angiography? AJNR Am J Neuroradiol. 2011;32:908–910. doi: 10.3174/ajnr.A2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neeman Z, Abu Ata M, Touma E, Saliba W, Barnett-Griness O, Gralnek IM, et al. Is fasting still necessary prior to contrast-enhanced computed tomography? A randomized clinical study. Eur Radiol. 2021;31:1451–1459. doi: 10.1007/s00330-020-07255-0. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Liu H, Zhao L, Liu J, Cai L, Zhang L, et al. The effect of preparative solid food status on the occurrence of nausea, vomiting and aspiration symptoms in enhanced CT examination: prospective observational study. Br J Radiol. 2018;91:20180198. doi: 10.1259/bjr.20180198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsushima Y, Seki Y, Nakajima T, Hirasawa H, Taketomi-Takahashi A, Tan S, et al. The effect of abolishing instructions to fast prior to contrast-enhanced CT on the incidence of acute adverse reactions. Insights Imaging. 2020;11:113. doi: 10.1186/s13244-020-00918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamid T, Aleem Q, Lau Y, Singh R, McDonald J, Macdonald JE, et al. Pre-procedural fasting for coronary interventions: is it time to change practice? Heart. 2014;100:658–661. doi: 10.1136/heartjnl-2013-305289. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Zhao L, Liu J, Lan F, Cai L, Fang J, et al. Change the preprocedural fasting policy for contrast-enhanced CT: results of 127,200 cases. Insights Imaging. 2022;13:29. doi: 10.1186/s13244-022-01173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorita A, Thongprayoon C, Ahmed A, Bates RE, Ratelle JT, Rieck KM, et al. Frequency and appropriateness of fasting orders in the hospital. Mayo Clin Proc. 2015;90:1225–1232. doi: 10.1016/j.mayocp.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Awad S, Constantin-Teodosiu D, Macdonald IA, Lobo DN. Short-term starvation and mitochondrial dysfunction - a possible mechanism leading to postoperative insulin resistance. Clin Nutr. 2009;28:497–509. doi: 10.1016/j.clnu.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Wickerham AL, Schultz EJ, Lewine EB. Nil per os orders for imaging: a teachable moment. JAMA Intern Med. 2017;177:1670–1671. doi: 10.1001/jamainternmed.2017.3943. [DOI] [PubMed] [Google Scholar]
- 26.Dennhardt N, Beck C, Huber D, Sander B, Boehne M, Boethig D, et al. Optimized preoperative fasting times decrease ketone body concentration and stabilize mean arterial blood pressure during induction of anesthesia in children younger than 36 months: a prospective observational cohort study. Paediatr Anaesth. 2016;26:838–843. doi: 10.1111/pan.12943. [DOI] [PubMed] [Google Scholar]
- 27.American Society of Anesthesiologists. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Task Force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126:376–393. doi: 10.1097/ALN.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.3. [updated February 2022]. [accessed on April 3, 2023]. Available at: https://training.cochrane.org/handbook .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.