Abstract
Whole slide imaging is revolutionizing the field of pathology and is currently being used for clinical, educational, and research initiatives by an increasing number of institutions. Pathology departments have distinct needs for digital pathology systems, yet the cost of digital workflows is cited as a major barrier for widespread adoption by many organizations. Memorial Sloan Kettering Cancer Center (MSK) is an early adopter of whole slide imaging with incremental investments in resources that started more than 15 years ago. This experience and the large-scale scan operations led to the identification of required framework components of digital pathology operations. The cost of these components for the 2021 digital pathology operations at MSK were studied and calculated to enable an understanding of the operation and benchmark the accompanying costs.
This paper describes the unique infrastructure cost and the costs associated with the digital pathology clinical operation use cases in a large, tertiary cancer center. These calculations can serve as a blueprint for other institutions to provide the necessary concepts and offer insights towards the financial requirements for digital pathology adoption by other institutions.
Keywords: Digital pathology, Whole slide imaging, Laboratory economics, Pathology innovation, Operations, Cost
Introduction
Digital pathology (DP) coupled with machine learning and big data acquisition is on track to revolutionize the pathology medical practice similar to the innovations spurred by immunohistochemistry and genomics.1 Yet, despite the promise of digital pathology, multiple barriers exist in adoption and implementation of DP in clinical practice. Of these, the investment in the infrastructure and costs of implementation are significant.2, 3, 4, 5, 6, 7 This is a major limiting factor in resource poor geographical locations and non-academic institutions.
As with other new technologies, there are limited data regarding the costs that are associated with digital pathology and the return on investment (ROI) that they offer for pathology operations and healthcare systems.5 In 2010, Dr. Walter H. Henricks, argued that “revenue from digital pathology activities is not likely to cover the costs, at least in the near term but possibly not ever.”8 In 2014, Ho et al projected 5-year total cost savings of $12.38 million based on anticipated improvements in pathology productivity and histology lab consolidation.9 Another cost saving of $5.35 million was suggested due to improved diagnoses and avoidance of unnecessary treatment costs. More recent studies describe future savings based on cost-saving calculations.2,5,9,10 Examples include >19 working hours that were saved per day with digital slides in the Netherlands,10 annual savings of $CA 26 000 in courier costs, $CA 60 000 in travel, and $CA 45 000 in accommodation, meals, and car rental expense in Canada11 and $24/courier trip between UCLA’s pathology sites.12
A previous study from our department, projected a 5-year $1.3 million savings based on comprehensive comparative cost analysis of a fully digital workflow that included off-site storage, slide retrieval costs, vendor labor, scanner hardware and software, whole slide image (WSI) storage, and support staff.2
Vodovnik13 reported that digital pathology may lead to shorter diagnosis time than traditional microscopy and suggested the potential of digital pathology to yield savings in both diagnostic and non-diagnostic tasks. Other publications highlighted other benefits of digital pathology transformation, yet many of these reported benefits were not quantifiable and are not leading to any cost reduction, additional revenue, or cost avoidance.5,14,15
Memorial Sloan Kettering (MSK) was an early adopter of whole slide imaging with initial exploration and incremental investments in resources that started more than 15 years ago.16 The institution is a 514-bed comprehensive cancer center, with more than 21 000 employees, including 1417 attending physicians and 150 institute member scientists. In 2021, the institution admitted more than 22 800 patients and had more than 781 900 outpatient visits at local and regional facilities. The Department of Pathology and Laboratory Medicine (DPLM) includes 123 attendings and 40 training fellows which include various subspecialties. In 2021, the histology laboratory processed more than 90 000 total accessions and more than a million glass slides.
With more than 6.1 million slides scanned to date, MSK Department of Pathology and Laboratory Medicine has been able to analyze historic operational and financial data to gain a better understanding of the true costs that are associated with a large-scale operation at a tertiary care academic medical center in New York City. Though it is challenging to quantify all tangible and intangible costs that are associated with a disruptive technology, every effort was made to explore and quantify them. This paper aims to describe the unique infrastructure cost and the costs associated with the use cases at MSK in regards to the digital pathology clinical operation, and may serve as a blueprint for other institutions to provide the necessary concepts and offer insights towards adoption of digital pathology.
Methods
This study is based on the experience of MSK, a high-volume, academic, tertiary care cancer center. While routine clinical prospective scanning started in 2020, the first full year to include clinical prospective scanning data was in 2021. The data used for this study is based on the experience obtained in the 2021 fiscal year.
Scanning operation (hardware and software)
In 2021, the DPLM at MSK had 25 whole slide scanners including Leica Aperio AT2 and GT450 (Leica Biosystems, Buffalo Grove, Illinois); 3DHistech Pannoramic 1000, and Philips Ultra-Fast Scanner (Philips, Amsterdam, the Netherlands). The predominant whole slide scanners used for the clinical operation during the time of this study were the Leica/Aperio AT2 and GT450. Glass slides generated from the laboratory at MSK follow the Department scanning protocols. Glass slides are scanned predominantly at ×40 (0.25 μm/pixel); however, ×20 (0.5 μm/pixel) or 63× (0.16 μm/pixel) scans were performed for specified scan needs. The WSI are transferred and stored in the institution’s data center. The Department’s network connection is 1 gigabit/s to each computer workstation and whole slide scanner. The whole slide scanners share a 10 gigabit/s connection to the institutional data center. The advanced barcoding and tracking module implemented at our institution uses 2D barcodes that are decoded by the whole slide scanners, and in turn interface the specific scanner vendors’ image management database software (e.g., Aperio eSlide Manager, Philips Intellisite, and 3DHistech CaseCenter) with the LIS (Cerner CoPath Plus, Cerner Corporation, Kansas City, Missouri). Whole slide images are launched from within the LIS and are viewed in a separate WSI viewer application (i.e., Web-based file format agnostic MSK Slide Viewer17).
Operational cost analysis
Digital scanning costs were analyzed based on actual costs (e.g., invoices) for departmental scanning of prospective, retrospective, and consult cases. Factors included personnel (i.e., slide file staff, scanning technicians), hardware, software, service agreements, information technology (IT) infrastructure, analog, and digital storage. Data analysis of MSK’s digital pathology operations included scan volumes, infrastructure costs, financial records of scanner capital acquisitions, and service contracts were obtained from the department’s accounting records.
Staffing records were obtained from our human resources system. For scan team time allocations, a survey was developed and distributed to the scan team members that collected self-reported scan-related task activity.
Vendor service call and scanner repair records were collected from the laboratory maintenance records and from our technical team logs. Data storage facility associated costs were collected from internal accounting records.
Assumptions in data generation
Every effort was made to include actual incurred costs obtained from departmental records. Aggregated data was used when required to protect proprietary MSK records and per vendor contractual restrictions. Internal data based on 2021 actuals were used for calculations based on hardware, personnel, vendor costs, and institutional digital file storage for that fiscal year.
For cost avoidance and savings, operational cost analysis was conducted. All assumptions used were based on actual departmental experience and diagnostics market forecast used for financial planning within the department.
Results
MSK digital pathology infrastructure
MSK is one of the earlier adopters of DP with early exploration that started in 2006 and incremental growth ever since.16, 17, 18 Current scanning operations at MSK includes clinical, research, and education needs.1,14,18 The DPLM clinical scanning is further divided between prospective scanning where all daily cases from histology laboratory get digitized before being reviewed by the pathologists, and retrospective scanning, where representative slides are digitized after pathologists review of glass slides. Non-routine scanning workflows provide solutions to clinical consultation slides, conference cases, academic research, and industry collaborations as well as patient needs and legal requests. Each of these workflow needs have different priorities and turnaround times but they all require trained staff across multiple teams, space, and other resources to provide timely quality WSI to the end user.
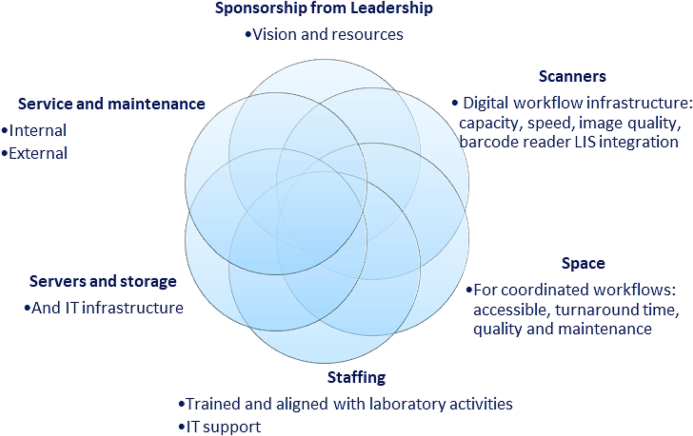
Digital pathology system infrastructure is required to ensure that pathology specimens get transformed into digital slides to be reviewed for reporting by a pathologist, and other downstream applications. These resources are institution and use case-dependent.1,3,17 In general, identified resources include hardware, software, data storage, staffing, space, and service agreements (Fig 1). A critical component in the 6 “S” requirements for successful digital pathology operations is sponsorship from leadership, to plant the seed for the vision and investment in the technology and the related innovations. Though most of the infrastructure have associated costs, in our experience, perhaps the most crucial factor is having leadership support with vision and commitment to resources. This is an example of an intangible cost and is a complex subject matter with varying structures at different institutions and will not be further discussed in this paper. The other S’ will be discussed later in this paper in their respective sections.
Fig 1.
The 6 “S” infrastructure requirements for successful WSI operations.
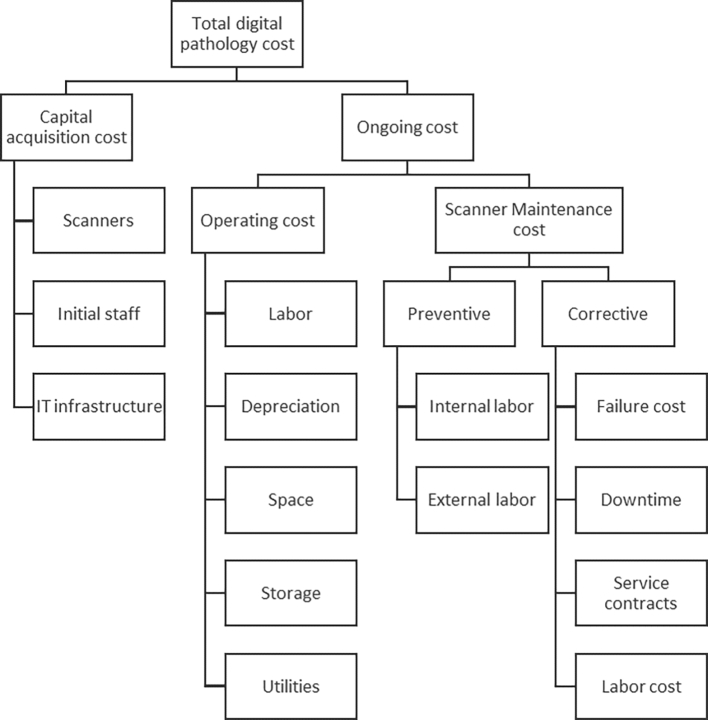
Acquisition vs ongoing costs
A distinction between capital investments costs (i.e., acquisition) and ongoing (i.e., ownership) costs is made in Fig. 2 (Fig. 2). The capital acquisition costs include scanning equipment, servers, workstations and software development, infrastructure-related employees, space configuration including furniture, network connectivity, and equipment depreciation. The ownership cost includes the operating costs of related staffing, training, space, and utilities as well as service and maintenance costs, both internal and external. The digital scanner downtime is included in the maintenance cost as well, as delays in service will have tangible consequences on clinical operations.
Fig 2.
Components of digital pathology operations cost.
The ownership cost was further divided between fixed costs that are incurred regardless of achieved scan volumes, and variable costs, which will fluctuate depending on the scanner output during the period studied. As with other laboratory operations, careful analysis of capital and operational budgets are conducted annually to ensure all costs associated with the operation are evaluated and accounted for, especially as these may change as the technology continues to develop.19
Some of the ongoing costs are fixed expenses (e.g., scanner depreciation, maintenance, full-time employees space) while others are variable costs that include the servers use, data storage, and network bandwidth.20 The stand-alone slide acquisition cost of DP is higher than that of analog microscopy due to the need for an additional digitization step (e.g., scanner, personnel, and software) and data storage infrastructure. This investment is often perceived as a barrier for introducing DP in pathology operations2,5,21 especially in resource poor settings.22 At the same time, there is an assumption of future cost-saving in the variable costs of running DP operations derived from slide handling and labor associated with analog microscopy.2,5,10,12,21,23
The specific infrastructure needs are institution and use case-dependent and will dictate the budget required for that specific operation.1 In this current study, the relevant MSK fixed infrastructure costs were included. The variable costs of the MSK DPLM digital scanning operation are derived from 2021 operations, using data obtained in a full year of routine daily scanning of clinical cases.
Scanner (whole slide scanner) cost
Use case-specific whole slide scanners are necessary for digital workflow infrastructure. As digital pathology operations expanded at MSK, there was an identified need for high throughput scanners. High scanning capacity and speed scanners were necessary for the large volume pathology operation that handles millions of glass slides a year, including slides generated at MSK and external slides that are shipped to the institution for consultation. These high-throughput scanner models cost tens to hundreds of thousands of dollars depending on the model and its capacity.5,24 For many years, the cost of the scanners seemed stagnant,24,25 yet starting in 2020, as with other electronic component devices, the global supply challenges resulted in increased cost of scanners.
Scanner models have different capacities and throughputs for routine histology slides with varying technical and quality attributes.1,3,24 Our high-throughput scanner capacity as featured in the vendors’ marketing materials ranges from 300 to 1000 slides per load.
The digital scanners cost detailed in this study is comprised of the annualized acquisition cost as well as the service and maintenance contract cost that was incurred in 2021. The aggregated data used is required in order to protect specific details from proprietary vendor contracts.
Acquisition cost
From the start of the operations in 2006, the cumulated estimated cost of MSK’s high throughput scanner purchases tops $7.5 million, but this number includes several obsolete scanner models, dedicated research scanners, and low throughput scanners that are not in clinical use. The cost of scanner hardware is part of MSK’s capital expenses, and as with other laboratory instruments, MSK assumes a 7-year straight line annual depreciation which should be accounted for in the capital budget. The 2021 operations that were evaluated in this study, included 15 Leica Aperio GT450 (Leica Biosystems, Buffalo Grove, Illinois), 5 Leica AT2 (Leica Biosystems, Buffalo Grove, Illinois), 3 Philips Ultra-Fast Scanners (Philips, Amsterdam, The Netherlands), and one 3DHistech P1000 (3DHistech, Budapest, Hungary) whole slide scanners.
Ongoing costs: Scanner service and maintenance
Whole slide scanners, as with other laboratory equipment, require scheduled maintenance as well as scanner annual service contracts with the different vendors vary and are calculated to cost between 7% and 20% of the cost of the scanners, with varying costs that are associated with response times, remote and onsite service visits, and parts replacement. This is part of the cost of the ownership of the scanner and needs to be budgeted based on vendor quotes that may be updated annually.
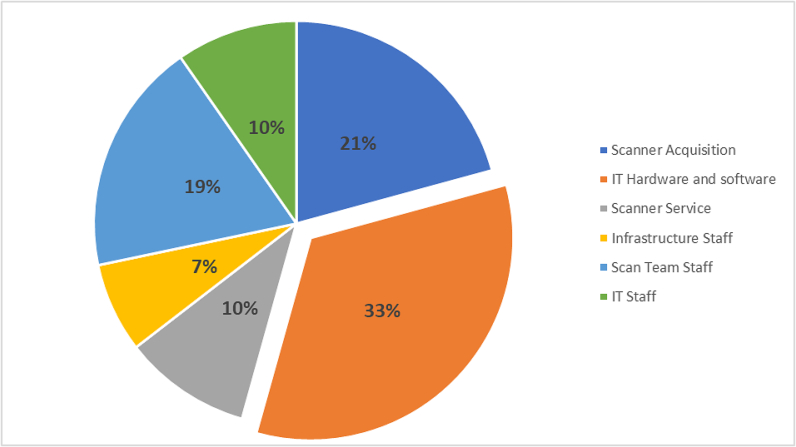
Taken together, this total annual scanner ownership, including hardware and maintenance cost for 2021 is $1.6 million, and it accounted for 31.5% of the total cost of the digital pathology operation at MSK (Fig 6).
Fig. 6.
Pie chart showing percent distribution of digital pathology cost categories annualized for the year 2021.
The cost of scanner ownership can be divided by the number of slides scanned on an instrument to calculate the contribution of the scanner hardware cost per slide. Using this approach, we calculated specific scanner cost contribution of each scanner to our respective 2021 scan volumes for each scanner. The calculated annual cost of scanner ownership included the depreciated annual cost of the scanner using a 7-year straight line depreciation and the scanner service cost. This was divided by the 2021 scan volumes for the specific high throughput scanners. The cost per scan was calculated to be between $0.55 and $19.53, with the number of slides scanned per scanner playing a critical role. Our lowest cost/scan was found in the high throughput scanners that were in routine daily use and exhibited no significant downtime through the 2021 year.
As scanners have high fixed costs as well as space requirements, maximizing their usage will result in a reduction in the fixed cost per slide. In our experience, scanners can allow daily scanning volumes that exceed their marketed intended capacity after careful planning and increasing operations time to 24 h/day. Continuous load scanner offers the benefit of undisrupted operations with minimal or no supervision. The identification of a true 24-h scanner capacity allowed the calculation of a theoretical cost per scan that could be achieved with maximal technician efficiency, and if no hardware malfunctions occured. Using an actual maximal productivity of slides scanned per day that was achieved in our archival scanning operations in 2020–2021 with our high-throughput scanners, the minimal cost for the hardware was calculated at $0.30/slide assuming annual scanning on 350 days/year. This can be used as a reference value, however, it is not realistic due to planned and unplanned service downtimes and device operator requirements.
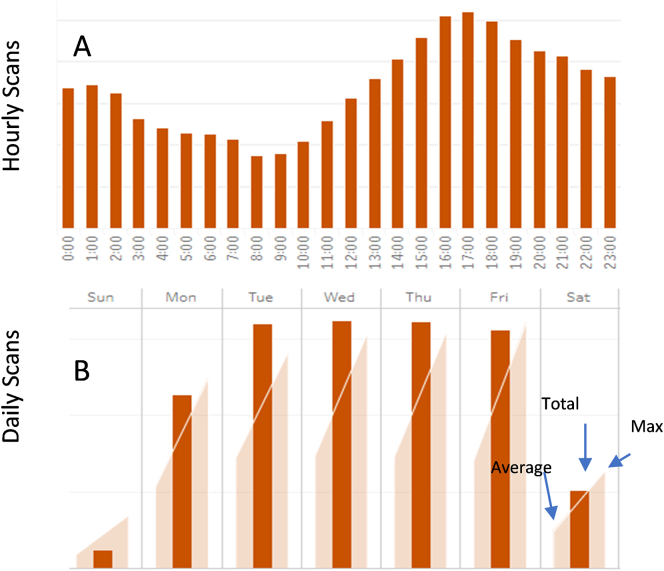
An analysis of hourly and daily scanner usage is a prerequisite to identifying available capacity and maximizing scanner use. At MSK, an operations dashboard was internally developed to support this need (Fig. 3). This dashboard is updated in close to real time with data extracted from HoBBiT (Honest Broker for Bioinformation Technology), which has been previously described.17 The dashboard is available for use by administrators, pathologists, and the laboratory staff for their specific needs. Aggregated data is used to identify weekly and hourly scanning trends and available capacity for the different use cases within the overall operation. Priority scanning is given to clinical prospective cases as their timing requirement is dictated by the need to maintain pathologist review and reporting turnaround times. Additional scanning for retrospective, research, and education cases are adjusted by the availability of scanners. Monitoring and analyzing the usage patterns of specific scanners results in periodic shifting of the scanners used for the time sensitive prospective scanning, thus maximizing overall available capacity. Available data allowed the expansion of our operations to 24 h/day with daytime coverage on the weekends. This scanning support is in alignment of the histology lab slide production and allows the minimization of downtimes in which slides are awaiting trainee or pathologist review. This extension to 24 h/day scanning resulted in a differential increase in wages for the overnight shifts but allowed the cost savings of sharing workstations and bench space by individuals on the different shifts. Additionally, this was seen as a benefit towards personal needs of staff members with family or school obligations, resulting in very low attrition among the team members. The cost of this dashboard development was added to the IT hardware and software cost discussed in “IT Infrastructure” below.
Fig 3.
Representative dashboard depiction of hourly (A) and daily (B) slide scan volumes at MSK. Internal dashboard was developed in response to the need to identify throughput and available scanner capacity.
Preventative maintenance
Scanners contain sensitive optical as well as robotic components, and major efforts need to be made to prevent failures and downtimes. Many of the failures that are observed are a result of mounting media, glass shards, and label fragments accumulation inside the scanners, and could be minimized with proper timely cleaning of the instruments. As a result, in the last few years, using the experience gained and anticipated failures, we developed standard operating procedures (SOPs) to address the intended worklow and mitigate failures, escalating issues internally before reaching out to the scanner vendor and service provider. We also added weekly preventive maintenance steps that are calculated at 10 full-time employees (FTEs) min/scanner/week. We estimate that this preventive maintenance dramatically reduced some common failures of the scanners alleviating the need for unscheduled downtimes of the scanners and better usage of the scanners. This cost was added to the digital scan team staffing category below.
Scanner downtime cost
The downtime that is associated with external service visits can be significant. We calculate the cost of scanner downtime interruptions based on the actual time the scanner is not operational. The annualized cost of the scanner is used to estimate the monetary cost of the idle scanner. That figure is multiplied by the number of downtime days per year and is estimated in our experience, to be at least $64 000/year for all scanners, an average of $2560 per scanner/year based on data collected in the 2021 fiscal year.
In addition to this cost, the effort of the institutional staff that is involved with the troubleshooting and repair of scanners should also be accounted for.
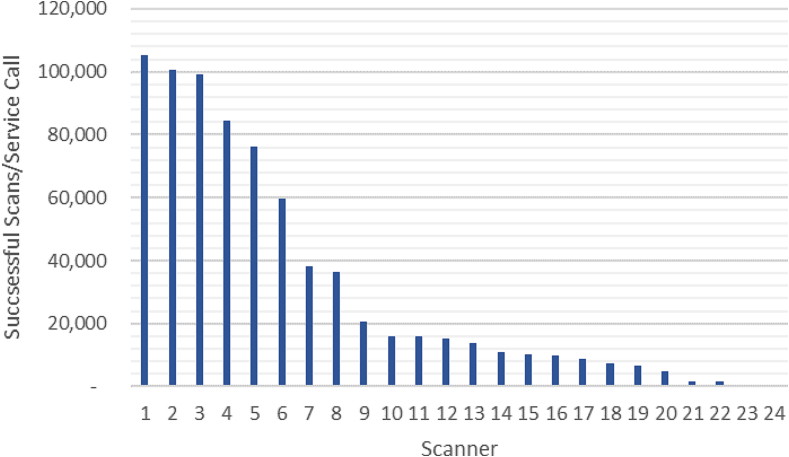
Our experience shows variation in the reliability of scanner hardware. There are differences across vendors and within scanner models. We have been monitoring failures and service calls for all our scanners from Aug 2020. Fig. 4 summarizes the number of successful scans per service call for our scanners in 2021 by summing all slide counts and tracking all vendor service calls. These are calls to report technical scanner issues that could not get resolved by the internal team and resulted in a pause (e.g., downtime with no clinical scanning) on that specific device. Analysis of the data demonstrated high variability between the reliability of our models and the specific scanners. The range of the successful scans/service calls varied from 69 to 106 000 slides/service call (higher ratios show better performing scanners). The frequency of the service calls and the downtime associated with the scanners in addition to the routine preventative maintenance (PM) are factors that are used when planning our daily operations. The reliability of our scanners also affects the procurement decisions of our operations. As the industry matures and newer high throughput scanners enter the market, the technologies and reliability of scanners are likely to improve. We have evaluated new models in the past and continue to evaluate new scanner models to identify solutions that may best fit scanning use cases within the department.
Fig. 4.
Number of successful scans per service call made to the vendor for each installed high throughput scanner at MSK in a 12-month period in 2021.
Space and overhead expenses
Space is essential for coordinated workflows and needs to be accessible to all users in the lab or in close proximity space to guarantee turnaround time, quality and ease of troubleshooting and maintenance.1,26. Placing scanners adjacent to the glass slide stainers and coverslipping instruments in the laboratory minimizes the slides transfer time, adding to the quality and to the overall ergonomics of the digital scan operators. Yet, depending on the scanner model, placing scanners in the laboratory can increase the overall noise in the laboratory from the instrument operation and require increased cooling and ventilation. They also need additional bench space around the instrument to allow for service and troubleshooting. Placing scanners in the laboratory also may be a challenge for the post scan quality control and monitoring needs. The continuous load scanners allowed us to increase our operations and align them to the laboratory scanning needs, to ensure availability to pathologists for clinical signout responsibilities.
Another underestimated space need that should be accounted for when transitioning to digital pathology operations is for the pre- and post-scan activities. Our WSI operations require slide prep areas, workstations for image reviews, and bookshelves racks for staging folders of cases for the different scan needs; especially for slides received in consultation from outside laboratories. These areas should be in close proximity to the scanning activities to maximize efficiency. Additional dedicated scanning areas are needed for separating between the clinical, research, and development activities.
The extension of scanning to 24 h/day, allowed MSK to achieve efficiencies and guarantee a better return on investment (ROI) for the scanning operation. This also allowed saving space for staff who are now working in variable shifts 24 h/day, 7 days/week.
Most pathology operations are under pressure to minimize their footprint, and MSK’s main campus where the laboratory is operating is located in New York City. Rent in large urban areas can be costly but pathology space is part of the hospital indirect cost and thus will not be added to this study calculations.
The glass slide storage expense at MSK was previously reported.2 Additional expense that is incurred by digital pathology operations may include space-associated expenses such as utilities as well as administrative salary allocation. This paper did not include any of these expenses as they are not accounted directly for the digital pathology operations and are part of the hospital overhead, however, mitigation of slide courier and delivery costs may account for a significant percent of cost savings.2,11
Staffing for digital pathology system operation
Digital scan team staff
The scanning process involves handoff between multiple teams, with the digital scan team being responsible to ensure successful quality scanning as well post-scan review. Image quality is a major deciding factor in our scan needs and contributes to a major staffing expense. Overall, we estimate a need of 1 FTE for 3–4 whole slide scanner devices. In 2021, there were 16 staff members including administration, training, and management. Scanning staff work in 8-h shifts 24 h/day with daytime coverage on weekends. Prospective scanning is done by the lab aides in the histology lab, and digital slide reviews are performed by the digital scan associates. Eight digital scanning assistants are tasked with pre-scan activities that include prepping slides for retrospective and prospective scanning, including slides submitted from outside institutions that need manual relabeling and cleaning. They also support the prospective scanning in the histology laboratory, by maintaining the equipment, troubleshooting, and communicating with the vendors as needed. The digital scan team handles all other scanning needs in the department including educational and research requests from trainees, pathologists, and other stakeholders.
Four digital scan associates review the WSI on an average day using the vendors’ image management viewing tools and ensure the image availability in the LIS. All team members are trained on the use of all scanner models, follow the different SOPs, and go through routine competency testing.
The daily tasks of the team members vary between shifts and the changing workload need, with clinical operations prioritized in the time allocation of the staff to maintain turnaround time. This time allocation will vary with changes in use cases, size of scanning operations, daily shift coverage, administrative support as well as the type of scanners used, and specific quality control (QC) needs.
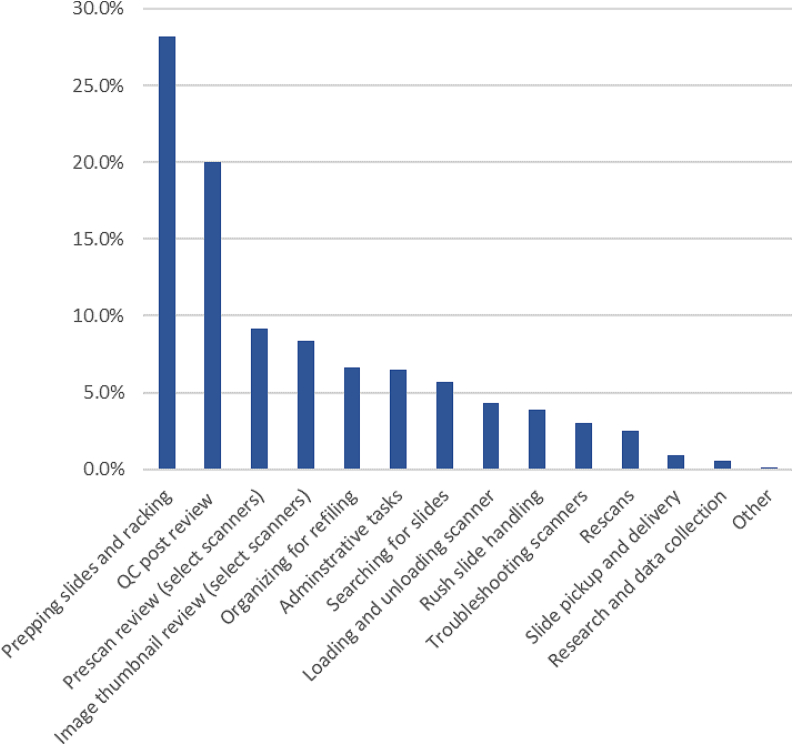
A time allocation of the different tasks of the team study looked at the average daily work distribution among the different team members during the 2021 year (Fig. 5). The study concluded that 28% of the team’s effort, the largest time allocation of all the identified tasks, was dedicated to the steps needed for prepping glass slides and racking slides in the scanner racks. These tasks were largely for consult slides received from other institutions. This task is very manually intensive and is essential for certain slides. We estimate consult glass slides that do not get manually inspected and prepped for the scan show a 50% higher likelihood of requiring a rescan, and without pre-scan preparation are likely to fail to produce quality WSI. The lack of this manual inspection and cleaning results in additional time before the WSI are available for clinical review as there is a need to find and retrieve the glass slides, return the glass slides to the team for an additional time-consuming manual handling of the slides for a successful scan.
Fig 5.
Percent of digital scan staff time allocated to different tasks on an average work day in 2021 (% of time spent on task).
Other tasks include the post-scan QC review process, the AT2 and P1000 prescan review, and the image thumbnail review on the scanners. Altogether, the pre- and post-scan QC-related activities are the majority of the time allocation for scan team members. Other tasks are administrative, involving moving slides between the different teams in the department, and research-related activities by the team.
Most laboratories calculate labor costs such as salaries and benefits as 60%–70% of the total expense budget.19 Based on our monthly volumes and total scanning operations compensation, we calculated that the total digital scan team staffing fixed and variable costs contributes $0.79–0.92 to the cost per slide scanned. These costs include auxiliary staff and varies monthly depending on the total scan volume in the month. The volume variation is derived from monthly case volume fluctuations based on actual patient hospital visits, number of work days in a month, holiday schedule, etc.
An additional factor in the total staffing cost is the need for 24-h coverage as mentioned above. The added cost of evening, night, and weekends operation differential is needed for our clinical operations.
Additional support staff
Pathology administrative staff
Additional staff from other pathology teams support some aspects of the digital scanning operations. These include slide file room attendants, and administrative staff. These tasks include providing slides for scanning, archival retrieval, and the return of slides after scanning.
IT support staff
Digital pathology requires adequate staff to support the clinical digital workflow including slide viewing solutions, workstation support, LIS integration support, networking, and new instrument onboarding processes. The staff includes individuals from pathology IT who directly support the scanning operations.17
Research and development cost
All our new model scanners are routinely evaluated on site prior to commitment to purchase to guarantee the suitability of the equipment to our specific needs and the potential for their integration into our clinical use in the laboratory, guaranteeing barcode readability and LIS integration. These evaluations require shifting some of the laboratory available resources, mainly staff time and space but result in cost avoidance for the purchase of unsuitable equipment. We estimate the effort of scanner evaluation to require an average of 6 weeks of a technician to install, train, scan, and review images. Estimated time for a senior scientist and a pathologist to plan, evaluate, compare, analyze data, and develop the needed documentation is a minimum of 40 h for each scanner. This will also include vendor meetings and coordination. We typically find research and development to be iterative in nature, and this time needs to be accounted for when planning resources and timelines.
Clinical validation and regulatory cost
A sometimes overlooked expense that should be considered is that of the establishment and validation of digital workflows to ensure quality clinical operations, as with any other new test and laboratory equipment. Additional costs may be associated with scanner validation and any related research and development work before clinical scanning initiates. The clinical digital transformation requires planning and verification/validation studies including writeup and submission for regulatory agencies per CAP guidelines.18,27 This effort involves input from multiple pathologists, technicians, and regulatory personnel. The regulatory cost includes periodic testing of the scanners, establishing PM schedules and training modules require planning for additional staff time. We estimate this effort to account for 0.5 FTE from the digital scan team and at least 30 h of pathologists’ time. This time is typically dedicated to pathologist glass and digital slide review as well as discordance analysis and documentation. The estimated pathologist and scan team time will be dependent on the scale of the validation.
Infrastructure staff
The initial investment in digital scanning effort required input from administrators, pathologists, finance, project management, and IT for identifying needs, technologies, planning, and onboarding of the WSI technologies. This effort was essential for obtaining leadership support and for successful adoption of digital pathology before implementing clinical grade scanning. An estimate of the time commitment of the individuals involved in the years before the large-scale scanning and integration of digital scanning into the clinical workflows in 2020 was calculated. The annualized cost for 2021 was estimated at $370 000.
IT infrastructure
Servers and data storage
One of the largest costs associated with WSI is the IT infrastructure required to obtain, move, and store the WSI.2,5 This infrastructure includes networks, data storage solutions, workstations, and peripheral equipment such as barcode scanners, label printers, etc.
Depending on retention guidelines and hospital operation decisions, digital pathology may require the preservation of many WSIs for a long time. The estimated size of a 1×3” digital pathology image scanned in one z-plane is 2 GB. Sun et al estimates storage requirements in China which has as many as 50 million new pathological examinations per year, requiring 50–250 PB of digital storage capacity (Sun et al., 2022). This storage cost will vary among institutions and their different use cases for the WSI as data storage considerations depend on institutional policy regarding the longevity of patient data.
MSK WSI are hosted on a centralized data share in the hospital’s data center.17 MSK allocated a total of 9 PB for slide storage, with similar storage for redundancy (i.e., disaster recovery). The cost of this on premise storage infrastructure amounts to $1 M per petabyte (PB), including redundant storage. To reduce storage cost, a 2-tier storage cluster has been employed keeping the equivalent number of slides for 6 months scanning on a tier-1 flash storage for fast access in the viewer. Older slides are automatically stored on less expensive tier-2 disk storage. The ready-access availability of these 2-tier storage is similar, however the tiers are related to the hard drive specifications (e.g., read/write speeds). Differences between those are negligible and do not affect viewing speeds.
The data center costs are capital expenditures with a 5-year straight line depreciation and the need for increased data storage is ongoing. The annualized cost of this storage in 2021, was $1.6 million.
Future cloud storage vendors may offer a reduction in the cost of long-term storage, waived egress costs, and discounts based on high volume storage capacities, however the need for immediate availability of data requires a more expensive, storage tier.
Software solutions infrastructure cost
DP operations at scale require multiple software solutions to enable efficiency and maintain quality operations. As commercial tools to enable digital pathology workflows were not widely available, additional solutions were developed internally at MSK for integrating digital workflows in diagnostics, research, and educational systems. These included a single vendor-agnostic digital slide viewer and a service linking pathology data with research to enable the compilation of large-scale datasets for computational pathology.17 Other tools were identified after the 2020 NYSDOH approval for remote clinical reporting during the public health emergency in 2020, which resulted in the integration of clinical prospective scanning. These include a pathologist clinical worklist, a scanning dashboard, and image QC review dashboard. With the gap in commercially available solutions, these were developed internally and deployed at MSK.
With the gradual anticipated expansion of our operations, and novel technologies that are being integrated, there are additional efficiency and quality digital tools that are identified by the different stakeholders and are in different phases of development. We anticipate the continued need for internal tool development and the employment of data scientists, solutions architects, and software programmers to allow the development and maintenance of these innovative tools. The estimated direct costs of these tool developments and maintenance are $630K/year.
Total incurred cost distribution
Identifying the actual incurred MSKs DP related costs from the start of the DP journey, allowed us to aggregate the cost data and calculate the estimated contribution of each of those costs to the total cost of DP operations in the 2021 budget year, the first full budget year since the prospective scanning operations began in the department.
These costs were grouped into 6 categories: scanner acquisition, related IT infrastructure, vendor scanner service, initial staffing for the infrastructure stages, IT staff, and the digital scan team. The infrastructure cost, including IT, and initial staff was annualized using a 5-year depreciation. The scanner acquisition was adjusted using a 7-year depreciation. All other annual expenses for service contracts and staff (both IT and scan team) were derived from 2021 records, as appearing in our finance system.
A review of the aggregated data of the 2021 operations costs demonstrates that the highest costs that are associated with our digital pathology operations stem from the infrastructure costs, those of IT hardware and software (33%), the staff who was involved in setting the infrastructure (7%), while the scanner acquisition contributed only 21% to the annualized costs of the 2021 operations. Additional ongoing costs include those of the digital scan team staff (21%), IT staff (10%), and a sometimes ignored cost of scanner service agreements (10%).
These are almost all fixed costs that are independent of the volume of slides scanned. Maximizing the number of scans and resources and aligning them with the departmental scan needs will result in lower cost/slide cost for the fixed costs. The variable costs will be volume dependent and should be allocated according to the anticipated scan volume.
Cost avoidance and savings
The benefits of WSI are also associated with cost avoidance and cost savings that are derived from using WSI instead of glass slides. Several costs were identified that should be reviewed to identify potential cost avoidance or savings. These costs could be subtracted from the actual incurred DP costs when building a business case for DP. Institutions that are transitioning to DP are incurring some of these costs while using hybrid manual–digital pathology workflows. This is the case that was observed at MSK.
Glass slide search
The hybrid digital pathology workflow that is ongoing at MSK requires the allocation of resources to support the digital workflow, yet some of the time allocation is derived from the analog microscopy slide handling by multiple teams. Tasks such as organizing for refiling, searching for slides, rush slide handling, slide pickup and delivery that amount to a total of 17.2% of our digital scan team time allocation, would be minimized once our operations are fully digital.
Our study surveyed the tasks that additional teams from office coordinators, laboratory staff, and slide file room individuals are involved in the manual slide handling. An example is the daily search for slides by the digital scan team as well as by 3 other pathology teams. The direct labor cost that is involved with these slide searches was calculated at 10 000 h/year in 2021. This effort disrupts normal workflows, is usually time sensitive, and involves staff members in different shifts, depending on the search need. We estimate that there are 47 staff members in pathology with varying participation in slides searches at some point during a given month.
Additional slide handling by pathologist office coordinators was calculated at 4 h a day for each, an estimated cost of >$1.32 million to the department in 2021. These costs could be substantially minimized or avoided as we migrate to digital workflows. At this current transition period, while we still use a hybrid of manual and digital pathology workflows, these costs will likely to continue.
Slide requests from slide file room
The hybrid digital and analog pathology workflows at MSK resulted in a reduced need to pull out slides from the slide file room in our hospital location. The requests were reduced to ~540/month (40 min each ~6500/year) that were estimated to cost ~$142K per year.
Manual case assembly
With digital workflow, glass slides can be digitized soon after slides are coverslipped and dry. With compatible slide racks that can go directly from the drying chamber to a digital slide scanner, a tedious step of slide collation and case assembly can be avoided. Glass slides can get filed directly after scanning, avoiding the need to manually sort and collate the slides after the pathologists’ review. This task can only be eliminated when the digital transformation is complete as any hybrid workflow will result in manual sorting and handling of slides. Currently, laboratory aides are performing this task for all cases, with an estimate of 18 h/day, resulting in an annual expense of $180K.
Legal request slide handling
There is an occasional need to produce glass slides for legal cases that are submitted to our department. These requests require manual search, slide transportation from external storage to our facility and manual handling of glass slides that are being reviewed by expert pathologists. The use of digital slides proved to be an alternative to the glass slides for these legal cases, avoiding the tasks of slide requests, searches, and onsite appointment with a legal secretary. By calculating the cost of obtaining glass slides vs digital slides, we found that the migration to digital slides will save 77% of the cost of total legal glass slide handling. Our study also demonstrated a digital workflow time savings of 85% compared to manual analog one resulting in the reduction of the turnaround time for fulfilling these legal requests.
Space savings
With a fully digital workflow, there are opportunities for space savings such as multiheaded scope rooms, pathologists’ offices not needing to be near the laboratory except for a core group to guide grossing complex specimen, glass slide storage, etc. This cost was not included in this publication, and will be a topic for future discussions once the digital slide transformation will allow the reimagination of departmental work space.
Additional financial benefits of digital pathology
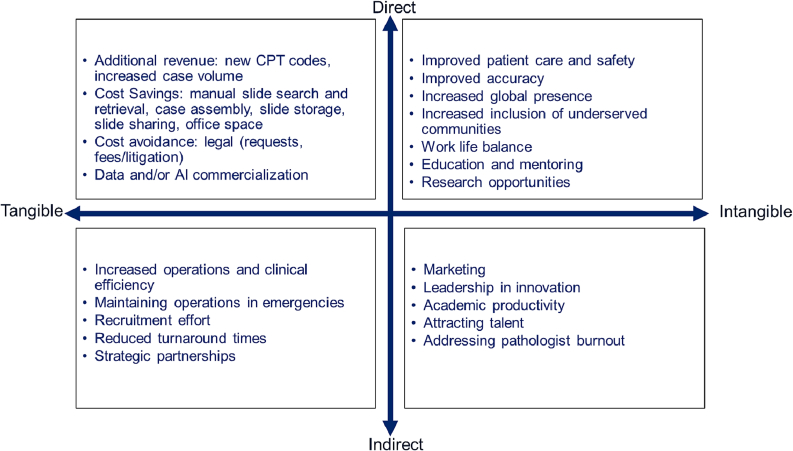
The calculation of digital pathology benefits and ROI should include the direct benefits to an institution and the indirect benefits that are associated with the migration to digital pathology. Fig. 7 summarizes the direct benefits of DP, including additional revenue and cost savings, improved patient care, and the indirect benefits, those of increased efficiency, emergency preparedness, recruitment, collaborations, and leadership in innovation. These are further divided between tangible and intangible benefits.
Fig. 7.
Digital pathology benefits quadrant. Direct benefits vs indirect ones, tangible benefits vs intangible benefits that are associated with digital pathology operations.
The indirect benefits include unknown fiscal benefits. Tangible benefits are easier to account for than those of intangible benefits, those that are non-physical in nature. These are based on knowledge assets that are acquired through research and development, human capital, supply chain volume and product distribution systems, brands, software investments, and the organization of a business that are increasing components of the assets of modern firms.28 Despite the growth in importance, few or none of most organizations intangibles appear as assets on balance sheets.28 Intangible benefits consist of subjective perceptions and attitudes towards an organization are not reported in formal accounting, even though they affect an organization value. It is often hard to measure and quantify the value of intangible benefits of many disruptive technologies, including those of DP.
Indirect benefits to institutions are sometimes overlooked yet should be included in the financial discussions of DP adoption and expansion. At MSK, we identified additional indirect benefits of WSI that included both tangible and intangible benefits. An example of an intangible benefit is being able to easily attract innovative faculty and staff, adding job satisfaction and mitigating burnout among the pathologists. In addition, there is the promise of potential revenue to the institution from innovative digital tools, increased global presence and patient outreach in underserved communities. As an academic institution, the added benefit of using WSI for education and research opportunities, including the development of artificial intelligence tools, is intangible, and the ability to provide state-of-the-art patient care cannot be quantified but is in line with MSK’s mission of innovation and advancement. This can also lead to quality faculty and trainee applicants who can further increase their department’s value through leadership and productivity.
Discussion
In the past few years, there is greater DP adoption, with increasing number of publications that include pandemic induced adoption that highlighted the benefits of DP for continuous pathology operations in public health emergencies.5,14,15,29 The high cost of digital infrastructure and resources has been cited as a barrier for entry for digital pathology implementation, yet there have only been few recent studies that looked at the cost of digital pathology at scale.2,9,10,18,30 Our DP experience at MSK in the last 15 years highlighted the multiple institutional needs for a successful DP ecosystem and the costs that are associated with implementing a large-scale DP operation. The 2021 historical costs and metrics of our MSK specific operations were used in this study to allow perspective of the magnitude of the expense, though they may not accurately translate to other institutions’ use cases.
Our data demonstrated the need for a large investment in IT hardware and software that should be accounted for in clinical DP operations. This includes investment in capital expenses that are part of the systems hardware and software infrastructure, and the ongoing needs for IT solutions to support new scanner technologies and operational needs, periodic scanner and network updates, and updated digital storage migration. This IT hardware and software category, contributed to the largest cost of our annualized 2021 operation, and accounted for 33% of the total budget. Additional IT support staff was involved in ongoing technical support and contributed to 10% of the DP effort cost.
The scanner acquisition and service categories were the second largest category of our costs for 2021 with 21% and 10% of the total operation cost respectively. This cost includes all scanners used in our operations in 2021. Maximizing the use of the available scanners is key in obtaining better ROI for DP operations. A dashboard was developed to review the scanned image upload in the LIS and monitor the scanning activities in real time to maximize scanner productivity.
The next largest cost category was the total direct labor cost of the digital pathology team, which accounted for 21% of the total annual cost of the operation. Our current DP operations rely on manual handling of the slides and equipment needed for the digitization steps. The digital scan team responsibilities include handling of slides, maintaining and troubleshooting instruments, ensuring quality image upload to the LIS, and providing customer service to all stakeholders. The analysis of the staff time allocation highlighted the large requirement of manual labor during the pre- and post-scan QC efforts. These are required QC pre- and post-scan image review that are attractive candidates for future automation development.
This current study also evaluated the additional cost of handling slides that could be minimized or eliminated with digital workflows. These include searches for lost slides, legal glass slide requests, transfer of slides by pathologist office coordinators, collation of slides before pathologist review, and any legal costs associated with lost slides. We estimate these institutional costs to total more than $2.5 million a year.
Pathology departments traditionally have an offsite archive for long-term storage.2 These storage requirements to “Retain histopathology slides for at least 10 years from the date of examination” are a federal regulation.31 One of the advantages of having WSIs interfaced and readily available on the LIS is the increased ease of access to patient material that can be reviewed by the pathologist on demand.2In a previous paper from our group, Hanna et al.2 calculated that glass slide requests from the department slide archive showed a 93% decrease. With this decrease in slide requests from the department slide library, 3 full-time employees from the slide file room were redistributed and incorporated into the DP operations workflow. This savings in FTE need has been maintained to date. Real estate storage costs in large metropolitan areas such as NYC, are at a premium, and with a decrease in demand for physical glass slides, a decrease in archival glass slide transport between offsite storage facilities could be achieved. Additional savings could be achieved by moving archival storage facilities further away to less costly locations.
An additional labor cost category was identified during our study, and that was the cost of the staff and faculty engagement in the initial DP initiative, identifying technologies, evaluating vendor solutions, and securing institutional funding. These innovators dedicated time and efforts that resulted in the early adoption of DP at MSK. We chose to annualize this cost and add the 2021 contribution of this category to our calculations. This infrastructure team’s cost is estimated to be 7% of the total 2021 operations cost.
Established pathology departments need to temporarily incur the traditional costs associated with analog microscopy while migrating to a fully digital pathology operation. Currently, the MSK pathology operations uses a hybrid workflow in which both DP and analog pathology are maintained, and staffing expense are reflecting that. In this study, an attempt was made to separate between transitional digital pathology costs and the actual costs of running a digital operation. We anticipate that the total cost of our pathology operations will decrease (adjusted for inflation), as we minimize the analog pathology operation.
In the future, direct tangible benefits of DP will include revenue generated from additional CPT codes and cost savings from labor that were associated with traditional manual microscopy. In addition, indirect cost savings that could be achieved include recruitment costs for faculty and staff, marketing costs and costs that are associated with conducting research and educational activities. These costs were not evaluated in our current study and will be revisited in future studies.
There were many quality improvements to patient care that were recognized by developing DP capabilities at MSK. These include the integration of pathology in the patient digital records, adding to pathologist diagnostic accuracy by allowing integration of computational pathology, improving TAT to case signout, gaining instantaneous availability of pathology images for clinician enquiries, expertise availability on weekends for difficult and emergencies requiring expedited treatment, making world class pathology available to underserved populations, ability to consult for rare pathologies, ability to operate during times of emergency and more.14 The return on investment for this disruptive technology cannot simply account for these capabilities, and the effect on patient care is one of the most the dominant factors in the adoption of DP at MSK.
Taken together, the need for investment in infrastructure as a prerequisite to a clinical digital pathology operations, results in a barrier for adoption for many institutions. Our study, with the strength of real-world data from the digital pathology operations at MSK, demonstrates the different cost categories that contribute to the total cost of operations. Other departments may have different operational initiatives and prerequisites for implementing a DP operation. While not all costs may have been identified and accounted for in this assessment, it can serve as a guide to institutions entertaining digital pathology transformation. Additional benefits of DP to patient care should be a guiding force to all institutions when contemplating the investment in infrastructure and planning for a phased growth of DP.
Conflict of interest disclosure
OA is a consultant for Techcyte.
KEJ is Co-Founder and Advisor of Genomenon, Inc. and he has research support from Thermo-fisher MGH is a consultant for PaigeAI, VolastraTx, and advisor for PathPresenter.
All other authors have nothing to disclose.
Acknowledgments
The authors would like to thank the Warren Alpert Center for Digital and Computational Pathology for supporting clinical and investigational use of digital pathology at our center and for the funding for the development of the MSKViewer and HoBBiT. We also acknowledge the work of the digital scan team and the DigITs team, laboratory and pathology IT staff for continuous support throughout the Department's digital pathology journey. This work was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Contributor Information
Orly Ardon, Email: ardono@mskcc.org.
Eric Klein, Email: kleine@mskcc.org.
Allyne Manzo, Email: manzoa@mskcc.org.
Christine England, Email: cisekc@mskcc.org.
Allix Mazzella, Email: mazzela2@mskcc.org.
Luke Geneslaw, Email: geneslal@mskcc.org.
John Philip, Email: philipj@mskcc.org.
Peter Ntiamoah, Email: ntiamoap@mskcc.org.
Jeninne Wright, Email: wrightj2@mskcc.org.
Sahussapont Joseph Sirintrapun, Email: sirintrs@mskcc.org.
Oscar Lin, Email: lino@mskcc.org.
Kojo Elenitoba-Johnson, Email: elenitk@mskcc.org.
Victor E. Reuter, Email: reuterv@mskcc.org.
Meera R. Hameed, Email: hameedm@mskcc.org.
Matthew G. Hanna, Email: hannam@mskcc.org.
References
- 1.Hanna M.G., Ardon O., Reuter V.E., Sirintrapun S.J., England C., Klimstra D.S., et al. Integrating digital pathology into clinical practice. Mod Pathol. 2022;35(2):152–164. doi: 10.1038/s41379-021-00929-0. [DOI] [PubMed] [Google Scholar]
- 2.Hanna M.G., Reuter V.E., Samboy J., England C., Corsale L., Fine S.W., et al. Implementation of digital pathology offers clinical and operational increase in efficiency and cost savings. Arch Pathol Lab Med. 2019;143(12):1545–1555. doi: 10.5858/arpa.2018-0514-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanna M.G., Parwani A., Sirintrapun S.J. Whole slide imaging: technology and applications. Adv Anat Pathol. 2020;27(4):251–259. doi: 10.1097/PAP.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 4.Jahn S.W., Plass M., Moinfar F. Digital pathology: advantages, limitations and emerging perspectives. J Clin Med. 2020;9(11):3697. doi: 10.3390/jcm9113697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lujan G., Quigley J.C., Hartman D., Parwani A., Roehmholdt B., Van Meter B., et al. Dissecting the business case for adoption and implementation of digital pathology: a white paper from the digital pathology association. J Pathol Inform. Published Online. 2021:18. doi: 10.4103/jpi.jpi_67_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams B.J., Bottoms D., Treanor D. Future-proofing pathology: the case for clinical adoption of digital pathology. J Clin Pathol. 2017;70(12):1010–1018. doi: 10.1136/jclinpath-2017-204644. [DOI] [PubMed] [Google Scholar]
- 7.Zarella M.D., Bowman D., Aeffner F., Farahani N., Xthona A., Absar S.F., et al. A practical guide to whole slide imaging: a white paper from the digital pathology association. Arch Pathol Lab Med. 2018;143(2):222–234. doi: 10.5858/arpa.2018-0343-RA. [DOI] [PubMed] [Google Scholar]
- 8.Henricks W. Advancing Practice, Instruction, and Innovation Through Informatics. 2009. Not so fast? Concrete considerations for digital move.http://www.captodayonline.com/Archives/0110/0110ha_not_so_fast.html Published January 2010. Accessed April 7, 2022. [Google Scholar]
- 9.Ho J., Ahlers S.M., Stratman C., Aridor O., Pantanowitz L., Fine J.L., et al. Can digital pathology result in cost savings? A financial projection for digital pathology implementation at a large integrated health care organization. J Pathol Inform. 2014;5(1):33. doi: 10.4103/2153-3539.139714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baidoshvili A., Bucur A., van Leeuwen J., van der Laak J., Kluin P., van Diest P.J. Evaluating the benefits of digital pathology implementation: time savings in laboratory logistics. Histopathology. 2018;73(5):784–794. doi: 10.1111/his.13691. [DOI] [PubMed] [Google Scholar]
- 11.Evans A.J., Brown R.W., Bui M.M., Chlipala B.S., Lacchetti C., Milner D.A., et al. Validating whole slide imaging systems for diagnostic purposes in pathology: guideline update from the College of American Pathologists in Collaboration with the American Society for Clinical Pathology and the Association for Pathology Informatics. Arch Pathol Lab Med. Published online. May 18, 2021 doi: 10.5858/arpa.2020-0723-CP. [DOI] [Google Scholar]
- 12.Chong T., Palma-Diaz M.F., Fisher C., Gui D., Ostrzega N.L., Sempa G., et al. The California telepathology service: UCLA’s experience in deploying a regional digital pathology subspecialty consultation network. J Pathol Inform. 2019;10:31. doi: 10.4103/jpi.jpi_22_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vodovnik A. Diagnostic time in digital pathology: a comparative study on 400 cases. J Pathol Inform. 2016;7(1):4. doi: 10.4103/2153-3539.175377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ardon O., Reuter V.E., Hameed M., Corsale L., Manzo A., Sirintrapun S.J., et al. Digital pathology operations at an NYC tertiary cancer center during the first 4 months of COVID-19 pandemic response. Acad Pathol. 2021;8 doi: 10.1177/23742895211010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yousif M., Hassell L., Pantanowitz L. In: Digital Innovation for Healthcare in COVID-19 Pandemic. Information Technologies in Healthcare Industry. de Pablos P.O., Chui K.T., Lytras M.D., editors. Academic Press; 2022. Chapter 7 - Impact of COVID-19 on the adoption of digital pathology; pp. 95–107. [DOI] [Google Scholar]
- 16.Reuter V. Presented at: Pathology Visions 2009: Digital Pathology Solutions Conference. 2009. Technology in the modern surgical pathology laboratory: digital pathology and beyond. San Diego, CA. [Google Scholar]
- 17.Schüffler P.J., Geneslaw L., Yarlagadda D.V.K., Hanna M.G., Samboy J., Stamelos E., et al. Integrated digital pathology at scale: a solution for clinical diagnostics and cancer research at a large academic medical center. J Am Med Inform Assoc. 2021;28(9):1874–1884. doi: 10.1093/jamia/ocab085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanna M.G., Reuter V.E., Ardon O., Kim D., Sirintrapun S.J., Schüffler P.J., et al. Validation of a digital pathology system including remote review during the COVID-19 pandemic. Mod Pathol. 2020;33(11):2115–2127. doi: 10.1038/s41379-020-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacMillan D.H. Elements of a typical laboratory budget. Lab Med. 2003;34(7):515–519. doi: 10.1309/99F7NNJ09M3TGQHW. [DOI] [Google Scholar]
- 20.Sun K., Gao Y., Xie T., Wang X., Yang Q., Chen L., et al. A low-cost pathological image digitalization method based on 5 times magnification scanning. Quant Imaging Med Surg. 2022;12(5):2813–2829. doi: 10.21037/qims-21-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Pantanowitz L. Digital pathology: review of current opportunities and challenges for oral pathologists. J Oral Pathol Med. 2019;48(4):263–269. doi: 10.1111/jop.12825. [DOI] [PubMed] [Google Scholar]
- 22.Dawson H. Digital pathology – rising to the challenge. Front Med. 2022:9. doi: 10.3389/fmed.2022.888896. Accessed August 17, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans A.J., Vajpeyi R., Henry M., Chetty R., et al. Establishment of a remote diagnostic histopathology service using whole slide imaging (digital pathology) J Clin Pathol. Published online. July 1, 2020 doi: 10.1136/jclinpath-2020-206762. [DOI] [PubMed] [Google Scholar]
- 24.Patel A., Balis U.G.J., Cheng J., Li Z., Lujan G., McClintock D.S., et al. Contemporary whole slide imaging devices and their applications within the modern pathology department: a selected hardware review. J Pathol Inform. 2021;12:50. doi: 10.4103/jpi.jpi_66_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isse K., Lesniak A., Grama K., Roysam B., Minervini M.I., Demetris A.J., et al. Digital transplantation pathology: combining whole slide imaging, multiplex staining, and automated image analysis. Am J Transplant. 2012;12(1):27–37. doi: 10.1111/j.1600-6143.2011.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eloy C., Vale J., Curado M., Polonia A., Campelos S., Caramelo A., et al. Digital pathology workflow implementation at IPATIMUP. Diagnostics (Basel). 2021;11(11):2111. doi: 10.3390/diagnostics11112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantanowitz L., Sinard J.H., Henricks W.H., Fatheree L.A., Carter A.B., Contis L., et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137(12):1710–1722. doi: 10.5858/arpa.2013-0093-CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker R., Lennard A., Penman S., Teixeira A., et al. Accounting for intangible assets: suggested solutions. Account Busin Res. Published online. September 7, 2021:1–30. doi: 10.1080/00014788.2021.1938963. [DOI] [Google Scholar]
- 29.Williams B.J., Treanor D. Practical guide to training and validation for primary diagnosis with digital pathology. J Clin Pathol. 2020;73(7):418. doi: 10.1136/jclinpath-2019-206319. [DOI] [PubMed] [Google Scholar]
- 30.Retamero J.A., Aneiros-Fernandez J., del Moral R.G. Complete digital pathology for routine histopathology diagnosis in a multicenter hospital network. Arch Pathol Lab Med. 2019;144(2):221–228. doi: 10.5858/arpa.2018-0541-OA. [DOI] [PubMed] [Google Scholar]
- 31.42 CFR 493.1105 -- Standard: Retention requirements https://www.ecfr.gov/current/title-42/chapter-IV/subchapter-G/part-493/subpart-J/section-493.1105 Accessed September 9, 2022.