Abstract
Alcohol use disorder (AUD) and related health conditions result from a complex interaction of genetic, neural and environmental factors, with differential impacts across the lifespan. From its inception, the Collaborative Study on the Genetics of Alcoholism (COGA) has focused on the importance of brain function as it relates to the risk and consequences of alcohol use and AUD, through the examination of noninvasively recorded brain electrical activity and neuropsychological tests. COGA's sophisticated neurophysiological and neuropsychological measures, together with rich longitudinal, multi‐modal family data, have allowed us to disentangle brain‐related risk and resilience factors from the consequences of prolonged and heavy alcohol use in the context of genomic and social‐environmental influences over the lifespan. COGA has led the field in identifying genetic variation associated with brain functioning, which has advanced the understanding of how genomic risk affects AUD and related disorders. To date, the COGA study has amassed brain function data on over 9871 participants, 7837 with data at more than one time point, and with notable diversity in terms of age (from 7 to 97), gender (52% female), and self‐reported race and ethnicity (28% Black, 9% Hispanic). These data are available to the research community through several mechanisms, including directly through the NIAAA, through dbGAP, and in collaboration with COGA investigators. In this review, we provide an overview of COGA's data collection methods and specific brain function measures assessed, and showcase the utility, significance, and contributions these data have made to our understanding of AUD and related disorders, highlighting COGA research findings.
Keywords: alcohol use disorder, EEG, ERO, ERP, family, genomics, neurocognitive, neurodevelopment, neurophysiology, neuropsychological
In this review, we provide an overview of the neurophysiological and neuropsychological measures that have been collected within the COGA sample. We also provide illustrative examples of how these data, in combination with the rich genetic and phenotypic longitudinal data available, have advanced our understanding of the etiology and consequences of alcohol use disorder and related disorders.
1. INTRODUCTION
Alcohol use disorder (AUD) and related health conditions result from a complex interaction of genetic, neural and environmental factors, with differential impacts across the lifespan. 1 , 2 , 3 , 4 The Collaborative Study on the Genetics of Alcoholism (COGA) is a multi‐site, interdisciplinary study that uses a family‐based research strategy focused on families densely affected with AUD and community comparison families. The goal of COGA is to elucidate the genetic and molecular mechanisms underlying the predisposition for AUD, uncovering neurobiological processes that potentially mediate genetic influences, and characterizing the interplay of genetic and environmental risk factors. 1 Briefly, multi‐modal data has been collected on family members (aged 7–97), including clinical and behavioral measures derived from an age‐tailored version of the Semi Structured Assessment for the Genetics of Alcoholism (SSAGA; see 1. Overview), various questionnaires on behavior, personality and social functioning, blood samples for genetic analyses, and a neuropsychological and neurophysiological battery to assess brain function (see 2. Sample and Clinical Data). Data were collected at seven study sites across the United States, each lab operating under the same protocol, resulting in collection of similar data across all sites that could be pooled in analyses. 5 , 6 , 7 , 8 An overview of the overarching objectives of COGA, as well as detailed description of the COGA study data collection are provided elsewhere in this issue (see 1. Overview; 2. Sample and Clinical Data; 4. Genetics; 5. Functional Genomics).
From its inception, COGA has focused on the importance of brain function as it relates to the risk and consequences of alcohol use and AUD, through the examination of noninvasively recorded brain electrical activity and neuropsychological tests. A landmark study in 1984 from Begleiter and Porjesz reported that sons of fathers affected with AUD displayed neurophysiological differences even before they ever consumed alcohol. 9 These findings, which were replicated in both males and females by several independent research groups throughout the world and within both male and female offspring of the original COGA participants, 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 changed the field's thinking from an assumption that all neural anomalies were a result of prolonged alcohol consumption to the hypothesis that neural differences before onset of use may also contribute to risk for AUD. Decades later, COGA's sophisticated neurophysiological and neuropsychological measures, together with the rich multi‐modal family and longitudinal data collection, have allowed us to disentangle brain‐related risk and resilience factors from the consequences of prolonged and heavy alcohol use in the context of genomic, psychosocial and environmental influences over the lifespan.
Alcohol use disorder is a complex and multifactorial disorder, making the understanding of pathways from genetic predispositions to phenotypic expression of AUD a huge challenge. It was initially theorized that alcohol‐related measures of brain function such as neurophysiological markers, described as “endophenotypes” or “intermediate phenotypes,” may reflect simpler genetic processes and be more proximal to the genes involved in the predisposition to AUD. 19 , 20 , 21 However, regarding AUD, research has found that brain‐based phenotypes are also complex, multi‐factorial traits that reflect the contribution of many genetic variants of small effect from across the genome. Nonetheless, measures of brain function associated with AUD, such as neurophysiological markers of risk, continue to be extremely useful in delineating underlying mechanisms (e.g., attention, impulsivity) associated with liability for complex conditions such as AUD. Over multiple decades, COGA has led the field in characterizing genetic variation associated with brain functioning and has advanced understanding of how genomic risk affects the development and course of AUD.
To date, the COGA study has amassed brain function data on over 9871 participants, including 7837 with data at more than one time point (Table 1). Please see 2. Sample and Clinical Data for an overview of the different data collection waves, the research question guiding the focus of the data collection, the numbers of participants assessed at each, and tabular summaries of their characteristics. Briefly, data collection has focused on different participant subsets over time, depending on the research emphasis. Table 1 presents a comparison of key characteristics across the different “waves” of data collection. For several different subsets of participants and data collections, there are multiple assessments available on the same individuals. However, only the “Prospective study” which focused on assessments of youth aged 12–22 at two‐year intervals was specifically designed as a longitudinal study. 85% of this subsample has at least three follow‐up assessments, and a “non‐response analysis” indicated that individuals who did not return for follow‐ups were younger, but no significant differences regarding other key characteristics have been observed. We continue to take measures to ensure minimal impact of “missing data” on our study's findings. There is notable diversity in age (ranging from 7 to 97), gender (52% female), self‐reported race and ethnicity (28% Black, 9% Hispanic). These data are available to the research community through several mechanisms, including directly through the NIAAA, through dbGAP, and in collaborations (see 1. Overview). In this review, we detail COGA's data collection methods for the specific brain function measures assessed, showcase their utility, significance and contributions to our understanding of AUD and related disorders, and highlight COGA research findings.
TABLE 1.
COGA Brain function subsample description (as of January 2023).
| COGA Studies | All | Initial study | Prospective study | Lifespan study (ongoing) | |
|---|---|---|---|---|---|
| Collection years | 1989–2023 | 1989–2004 | 2004–2019 | 2019–2023 **data updated 1/2023 | |
| Life stage (age range) | Lifespan (7–97) | Lifespan (7–79) | Adolescence and young adulthood (12–32) | Midlife (33–49)**prospective study participants | Later Life (50+)**initial study participants |
| Sample sizes | 9871 | 8098 | 2979 | 870 | 1335 |
| Longitudinal subsamples | |||||
| 1+ assessments | 7837 | 7449 | 2794 | 870 | 1083 |
| 2+ assessments | 644 | 200 | 2412 | 851 | 1255 |
| 3+ assessments | 505 | – | 1997 | 799 | 10 |
| 4+ assessments | 470 | – | 1486 | 745 | 1 |
| Genomic Data Available | 9076 | 6204 | 2872 | 835 | 1299 |
| Key characteristics | |||||
| Age range at baseline (mean) | 7–79 (38.9) | 7–79 (39.8) | 12–32 (16.9) | 33–41 (36.5) | 50–97 (65.5) |
| Female | 51.9% | 51.2% | 51.5% | 57.0% | 60.4% |
| Black (self‐identified) | 27.9% | 18.7% | 29.0% | 21.8% | 16.6% |
| Hispanic (self‐identified) | 8.9% | 6.0% | 11.8% | 11.7% | 5.9% |
| DSM‐IV alcohol dependence | 24.3% | 31.0% | 17.5% | 24.4% | 41.8% |
| DSM‐5 alcohol use disorder | 47.5% | 53.5% | 41.4% | 52.0% | 62.5% |
Note: Description of individuals within COGA that have neurophysiological and neuropsychological data. A full list and description of all measures can be found in the Supplemental Methods. A description of the full COGA sample can be found in 2. Sample and Clinical Data.
2. COGA'S MEASURES OF BRAIN FUNCTION
In this section, we provide a brief description of COGA's neurophysiological and neuropsychological assessment battery and highlight the unique information that each metric can offer to improve our understanding of the role of brain function in AUD. Tables 2 and 3 provide an overview of selected measures and findings from COGA. Detailed information about data collection and processing can be found in the Supplemental Material.
TABLE 2.
Overview of neurophysiological and neuropsychological testing.
| COGA Studies | All | Initial Study | Prospective Study | Lifespan study (ongoing) | |
|---|---|---|---|---|---|
| Collection years | 1989–2023 | 1989–2004 | 2004–2019 | 2019–2023**data updated 1/2023 | |
| Life stage (age range) | Lifespan (7–97) | Lifespan (7–79) | Adolescence and young adulthood (12–32) | Midlife (33–49)**prospective study participants | Later Life (50+)**initial study participants |
| Neurophysiological experiments | |||||
| Resting‐state EEG a , b , c , d | ✓ | ✓ | ✓ | ✓ | ✓ |
| Visual oddball task a , b , c , d | ✓ | ✓ | ✓ | ✓ | ✓ |
| Auditory oddball a , b , c , d | ✓ | ✓ | ✓ | ✓ | ✓ |
| Semantic priming a , b , c , d | ✓ | ✓ | ✓ | ✓ | ✓ |
| Go‐NoGo task b , c | ✓ | ✓ | |||
| Monetary gambling task b , c | ✓ | ✓ | |||
| Continuous performance test b , c | ✓ | ✓ | |||
| Color/word stroop b , c | ✓ | ✓ | |||
| Auditory novel stimuli b , c | ✓ | ✓ | |||
| Cognitive/affective stroop b , c , d | ✓ | ✓ | ✓ | ||
| Mismatch negativity a | ✓ | ✓ | |||
| Bereitschafts potentials a | ✓ | ||||
| Contingent negative variation a | ✓ | ||||
| Object recognition a | ✓ | ||||
| Intertrial interference a | ✓ | ||||
| Neuropsychological tasks | |||||
| Tower of London b , c | ✓ | ✓ | |||
| Visual span test b , c | ✓ | ✓ | |||
| NIH Toolbox cognitive battery c , d | ✓ | ✓ | |||
| NIH Toolbox emotional battery c , d | ✓ | ✓ | |||
| Porteus maze test a , d | ✓ | ✓ | |||
| TRAILS A&B a , d | ✓ | ✓ | |||
| Wechsler Adult & Child Intelligence Scales‐revised a , d | ✓ | ✓ | |||
| California verbal learning test adult and child a , d | ✓ | ✓ | |||
| Ravens progressive matrices a , d | ✓ | ✓ | |||
| Wide range achievement test revised a , d | ✓ | ✓ | |||
Note: A full list and description of all measures can be found in the Supplemental Methods. A description of the full COGA sample is provided in: 2. Sample and Clinical Data.
Initial COGA study (1989–2004).
Prospective Study Sample: Multiple assessments every 2 years during adolescence and young adulthood from 2004 to 2019. Added Frontal Lobe Battery in Prospective study to assess aspects of frontal lobe in development during that period. Phenotypes derived from this data can be used for studies of neurodevelopmental trajectories.
Lifespan Project: Midlife (ML): Prospective Study sample as they enter early mid‐life/midlife (33–49);
Lifespan Project Latelife (LL): Initial sample now aged >50 (One or two previous assessments ~20 years ago).
TABLE 3.
Selected COGA Neurophysiological and Neuropsychological Findings.
| Task | Key Phenotypes: Findings and Significance |
|---|---|
| Resting EEG a , b , c , d |
Theta power (3–7 Hz):
Beta power (13–28 Hz):
Inter/Intrahemispheric EEG Coherence:
Functional Connectivity (eLORETA lagged connectivity)
|
| Visual oddball task (VPc) a , b , c , d |
ERP: P300 amplitude to targets (Pz):
ERO: Frontal Theta to targets:
ERO: Posterior Delta to targets:
|
| Auditory oddball task (AOD) a , b , c , d |
ERP: P300 amplitude to targets:
ERO: Theta to targets:
|
| Semantic priming task (ANT) a , b , c , d |
ERP: N400 amplitude to primed and unprimed words
|
| Go/NoGo task (GNG) b , c |
ERP: P300 and N200 amplitude during ‘Go’ and ‘NoGo’
ERO: Frontal Theta during ‘NoGo’:
|
| Monetary Gambling Task (MGT) b , c |
ERP: P300 and N200 amplitude
ERO: Frontal Theta
|
| CATs Tower of London Test (TOLT) b , c |
|
| CATs Visual Span Test (VST) b , c |
Initial COGA Sample (1989–2004).
Prospective Study Sample: Multiple assessments every 2 years during adolescence and young adulthood from 2004 to 2019. Added Frontal Lobe Battery in Prospective study to assess aspects of frontal lobe in development during that period. Phenotypes derived from this data can be used for studies of neurodevelopmental trajectories.
Lifespan Project: Midlife (ML): Prospective Study sample as they enter mid‐life (33–49).
Lifespan Project Latelife (LL): Initial sample now aged >50 (One or two previous assessments ~20 years ago).
2.1. Neurophysiological assessments
Using electroencephalography (EEG) techniques, COGA's neurophysiological battery records voltage oscillations originating from the cortical surface of the brain, by use of non‐invasive scalp electrodes (Figure 1A). These high‐temporal resolution recordings provide millisecond by millisecond indices of ensembles of neurons firing in synchrony during resting state (resting EEG) and during sensory, behavioral and cognitive tasks, from which event‐related potentials (ERPs) and event‐related oscillations (EROs) are derived. Although fMRI provides superior spatial (including subcortical) resolution to precisely pinpoint brain structures involved, the fine time‐scale and wide range of frequency bands provided by neurophysiological recordings may prove important for understanding subtle neural communication during sensory and cognitive processing relevant to neuropsychiatric outcomes. COGA's neurophysiological battery is designed to assess aspects of brain function that may be aberrant in AUD and/or involved in a vulnerability to increase risk to develop AUD. COGA's neurophysiological and neuropsychological batteries have evolved over time, both to stay current with state‐of‐the‐science development of brain function measures, and to the best capture brain functioning throughout the different stages of the lifespan focused on with each phase of data collection (e.g., adolescence and young adulthood, later‐life). Table 2 provides an overview of the specific assessments at each phase of data collection and Table 3 for an overview of selected measures and significant findings in COGA).
FIGURE 1.
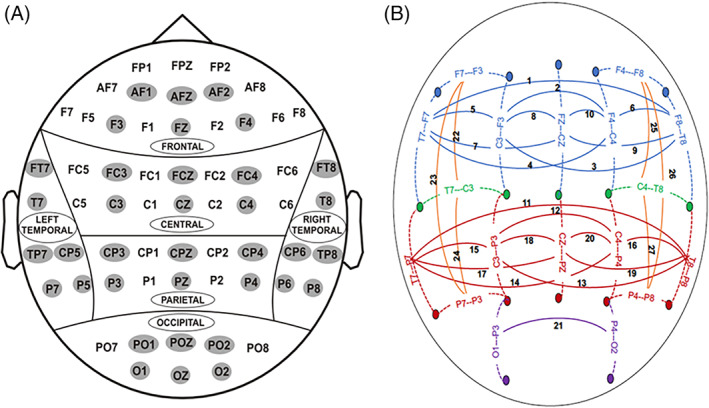
EEG Electrodes and Coherence Pairs used for Electrophysiological Recordings and Analyses in COGA. (A) Sixty‐one scalp electrodes are used to record EEG and ERPs in frontal (F), central (C), parietal (P), occipital (O), left‐temporal (LT) and right‐temporal (RT) regions. Even numbers signify the right side of the head, while odd numbers signify the left side, and Z signifies the center; the numbers indicate coordinates ascending from the center to the periphery of the scalp. (B) Bipolar electrode pairs used as the coherence measures of resting state EEG.
2.1.1. Resting state EEG
During “resting state,” defined here as when one is not engaged in a cognitive task, the brain still emits spontaneous, rhythmic activity. Resting EEG measures a complex signal of voltage oscillations comprising a wide range of spectral frequencies (i.e., number of waves per second, Hz), that are subdivided into bands: delta (1–3.5 Hz), theta (3.5–7.5 Hz), alpha (7.5–12.5), beta (12.5–28) and gamma (above 28 Hz). The resting EEG is stable and highly heritable across all frequency bands. 69 These EEG rhythms are indicators of global brain states from the alert‐awake state to drowsiness and stages of sleep. In healthy adults, alpha and beta frequencies predominate the awake resting EEG, with alpha rhythm dominating during relaxation parietal‐occipitally and beta seen throughout the scalp with mental activation. 70 In COGA, resting‐state EEG is recorded while participants have their eyes closed (4.25 min) and again while they have their eyes open (4.25 min). Measures of resting EEG analyzed in COGA are (1) EEG power (absolute and relative) in each frequency band, representing the amount of neural activity in these frequency bands, 71 and (2) metrics of EEG functional connectivity (coherence and source connectivity (eLORETA) that reflect the degree to which different brain regions/neural networks 71 communicate with each other.
2.1.2. Time‐locked EEG during cognitive tasks (ERPs, EROs)
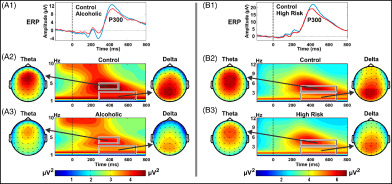
In contrast to resting state EEG recordings, COGA also records EEG when participants are engaged in cognitive/behavioral tasks. Studies focusing on neural responses to discrete stimuli or in relation to behavioral responses typically measure ERP responses and EROs. ERPs reflect changes in voltage across time following the presentation of a stimulus or the emission of a response, relative to a pre‐stimulus baseline in the time domain. 72 These small voltage fluctuations are difficult to see in a single trial, so by averaging this activity across trials, Activity that is time‐locked in relation to the event of interest summates while activity unrelated to the stimulus cancels out. ERP response is quantified in terms of positive‐ and negative‐going peaks or ‘components’ in the average signal waveform within particular time windows. Positive and negative ERP components are designated “P” and “N” respectively and numbered to reflect the approximate timing in milliseconds, or latency, of their peak (e.g., P300, N400). 72 Earlier ERP components are theorized to reflect sensory and more ‘automatic’ processes related to perceptual registration of an event, whereas later components are theorized to reflect cognitive processing of events. 73 , 74 ERPs are interpreted based on mode of delivery (auditory, visual), frequency (frequent, rare) and content of the eliciting stimuli. The amplitude (measured as height of an ERP peak or trough, in microvolts) indicates the magnitude of neural resources that contributed to process a stimulus or event, whereas the latency (time of peak occurrence) reflects neural processing time. 75 One of the most widely used ERP components in neuropsychiatric research is the P3 or P300, a large positive component, maximal at centroparietal electrodes and occurring between 300 and 700 ms after the stimulus onset 76 (Figure 2). The P300 is related to the “significance” of a stimulus in a task, and not its physical features, reflecting attentional allocation and context updating processes in working memory. 78 , 79 , 80 , 81
FIGURE 2.
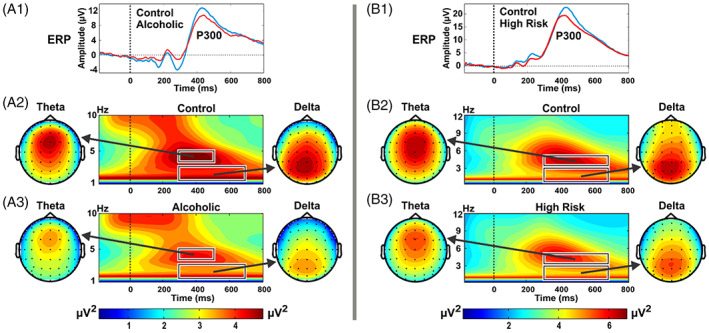
Brain wave characteristics in individuals with AUD (left panels, A1–A3) and their children, who are at high risk to develop AUD, due in part to genetic influences through family history (right panels, B1–B3) during a mental task called a “visual oddball task” in which they were asked to press a button only to a specific “target” image on a computer screen, while ignoring other more frequent images. Brain responses only to the target stimulus are illustrated in this figure. In A1 and B1 (top panels) you can see that both individuals with AUD 35 , 77 (red lines, left panel) and their high‐risk children 36 from AUD families (red line, right panel) had smaller P3 or P300 waves (the large peak in top panel occurring between 300 and 700 ms after the target) compared to those unaffected (blue lines, left panel) and at low risk from community comparison families (blue lines, right panel). In A2 and A3, and B2 and B3 (middle and bottom panels) you can see the lower magnitude of brain waves oscillating at theta (4–7 Hz) and delta (1–3 Hz) frequencies during the P300 response in both individuals with AUD (left panel) and their high‐risk children (right panel) compared to those unaffected (left panel) and offspring from comparison community families (right panel); note that theta oscillations have a frontal focus while delta oscillations have a more posterior focus. These differences in brain wave characteristics between individuals affected compared to unaffected with AUD, and between offspring from AUD families compared to offspring from community comparison families indicate less activation and/or weaker synchronization of neural activity during this cognitive task in those with AUD and their high‐risk children.
Event‐related oscillations are time–frequency measures of superimposed EEG activity at different frequency bands that are temporally related to sensory and cognitive processing that contribute to ERPs 82 (Further details can be found in the Supplementary Material and Figure 2). We can analyze these brain signals in terms of their time‐frequency components arising from various brain regions during sensory and cognitive tasks. 83 While EROs are partitioned in the same frequency bands as resting EEG (e.g., delta, theta, alpha, beta, gamma), they are functionally different from spontaneous rhythms, with each frequency band underlying specific cognitive processes, namely, delta: signal detection, decision making; theta: conscious awareness, recognition memory; alpha: attentional resources; beta and gamma: sensory, integrative processes. Faster frequency oscillations (gamma and beta) are involved in shorter range local neural communication while slower frequencies (delta, theta and alpha) are involved in longer range neural communication in the brain. 84 P300 responses are primarily the outcome of theta and delta EROs elicited during cognitive processing. 35 , 82 Theta oscillations peak earlier during the attentional and memory‐related aspects of the task and have a frontal focus, representing fronto‐limbic or cortico‐hippocampal interactions. 19 , 77 , 85 , 86 Delta oscillations peak at posterior regions and are involved with decision‐making and response selection aspects of the task and represent cortico‐cortical interactions. 19 , 77 , 86 , 87
2.1.3. Neuropsychological assessments
COGA has also assessed various aspects of cognitive function with neuropsychological batteries since its inception, with emphasis on different aspects of cognitive function depending on the life stage of the sample under study (Table 2 details specific neurocognitive assessments at different waves of COGA). In the initial COGA study, a comprehensive, wide range of neuropsychological assessments were obtained on the sample (aged 7–79) to assess child and adult verbal, non‐verbal and fluid intelligence, abstract reasoning, planning, verbal learning and memory, reading, comprehensive and mathematical abilities, and visual attention. These tests include: Wechsler Adult Intelligence Scale Revised (WAIS‐R), 88 Wechsler Intelligence Scale for Children Revised (WISC‐R), 89 Wide Range Achievement Test Revised (WRAT‐R), 90 Trail Making Test (TMT), 91 Porteus Maze Test (PMT), 92 and California Verbal Learning Test, Adult (CVLT‐A) 93 and Child (CVLT‐C). 94 During the subsequent waves of data collection (i.e., the prospective study of adolescents and young adults), we focused on neuropsychological assessments of frontal lobe function, which are undergoing maturation during adolescence and young adulthood. Neuropsychological assessments of frontal executive function, such as planning and problem‐solving abilities (Tower of London Task) and visuospatial working memory (Visual Span Test) from the Colorado Assessment Tests (CATs) 95 were implemented as part of the frontal lobe focused battery of neurophysiological and neuropsychological assessments. These assessments of frontal lobe executive function were repeated during the current wave of data collection (i.e., the Lifespan Study) in the prospective sample as they entered mid‐life (33–41 years old). In the ongoing Lifespan Study, the NIH Toolbox Cognitive battery (NIHT‐CB) 96 has been implemented to comprehensively assesses neurocognitive functions that are essential for effective daily life including, attention, processing speed, language, episodic memory, working memory and basic executive functions (inhibitory and attentional control, unconscious set‐shifting and maintenance aspect of cognitive flexibility). We have also implemented the NIH Toolbox Emotion Battery (NIHT‐EB) 97 to assesses negative affect (anger, fear and sadness), psychological well‐being (general life satisfaction, meaning and purpose, and positive affect), social relationships (friendship, loneliness, positive peer interaction, social withdrawal, empathic behavior, peer rejection, perceived hostility, perceived rejection, emotional support, and instrumental support) and stress and self‐efficacy (perceived stress, self‐efficacy).
3. HOW DO NEURAL SIGNATURES ASSOCIATED WITH AUD HELP ELUCIDATE THE ROLE OF BRAIN FUNCTION IN THE RISK AND CONSEQUENCES OF ALCOHOL USE AND AUD ACROSS THE LIFESPAN?
From its inception, COGA has recognized the importance of individual differences in brain function as both an antecedent and consequence of AUD. We have implemented a comprehensive battery of neurophysiological measures designed to assess activation and communication in neural networks in resting state and neural processing during cognitive tasks found to be affected in AUD (e.g., attention, response inhibition, reward processing). With this approach, we have identified neurophysiological measures that are associated with AUD as well as neurophysiological measures that differ based on family history of AUD and are present in some family members, even before alcohol exposure. The neurophysiological and neuropsychological COGA data have been instrumental in demonstrating that variations in brain function are both antecedents to, and consequences of, the effects of prolonged and heavy alcohol consumption and AUD (Table 3 provides specific results with neural measures during resting state and cognitive tasks and measures of neurocognitive function). COGA data have been particularly useful in studying the role of brain function as a neural liability for risk of AUD, and determining which of the measures of brain function have utility in genetic studies of AUD. As indicated in Section 2 above, EEG phenotypes are highly heritable, and several have been found to be aberrant in densely affected AUD families, including their offspring, compared to the comparison families, including related to severity and earlier age of onset.
Since its earliest studies, COGA has found that low P300 amplitude, associated with AUD is also found in high‐risk offspring. 11 The initial studies in COGA reported lower P300 amplitudes to target stimuli in both visual and auditory oddball tasks in those with AUD and their offspring, albeit more consistently in visual tasks. 77 COGA have demonstrated that low parietal P300 to visual targets is more prevalent in members of dense AUD families, including their offspring compared to members of community comparison families. 11 , 98 More recently, COGA has demonstrated that higher family history density of AUD was associated with lower amplitude parietal P300, higher likelihood of AUD, and earlier onset of regular drinking. 32 Thus, the longitudinal study of family members with multimodal measures can elucidate the important relationship between neural measures and family density of AUD in affecting risk of developing AUD as well as allow for examining how social environment may moderate risk factors (further detail provided in Section 3.3). COGA was a pioneer in developing and implementing ERO methods to understand neural mechanisms underlying P300 and AUD. Investigating the EROs in the visual oddball task, our investigators were the first to demonstrate that the low amplitude P300 in AUD was due to lower activation of frontal theta EROs and posterior delta EROs. 35 Subsequently, COGA was the first to demonstrate that lower theta and delta EROs underlying P300 in the same task were more sensitive than P3 in discriminating between high‐risk and low‐risk offspring. 36 Thus, reduced activation of P300 and the associated frontal theta and posterior delta ERO measures of brain function during the oddball task are indicative of problems with attention, memory and decision‐making processes, preceding the development of AUD. These visual oddball task measures are obtained in all COGA participants, from its inception through the current Lifespan project and are considered to be the “core” phenotypes that can be used for analyses on the whole sample as well as in longitudinal analyses (>7000 participants have data at more than one timepoint). Examining resting state EEG, COGA have reported increased EEG beta power (12–28 Hz) in individuals with AUD and in their offspring. 23 , 24 Beta rhythm is an index of neural excitability, 99 and the increased beta power in individuals with AUD and at‐risk family members is indicative of an imbalance in excitation/inhibition in neural networks. While increased beta was found for both genders, it was more pronounced in males than females, and related to family density of AUD, especially increased in those with two or more first‐degree relatives with AUD. 77 COGA has also found increased resting state EEG interhemispheric coherence (Figure 1B) in several frequency bands in individuals with AUD and their offspring; it was not found to be related to length of abstinence from alcohol. 19 , 28
When COGA launched its prospective study of adolescent and young adult offspring (2004–2019), a neurocognitive battery was included to assess frontal lobe function during this critical developmental time period (e.g., to examine executive control and reward networks). In a Go/NoGo task, individuals with AUD and their high‐risk offspring showed lower frontal N200, P300 and frontal theta ERO during response inhibition, indicating less activation in frontal areas. 55 , 56 , 57 , 58 , 59 , 60 A series of studies using a Gambling task reported low P300 amplitudes and less theta ERO activation in frontal regions during the loss condition in individuals with AUD and high‐risk offspring, indicating deficits in reward processing. 37 , 62 , 63 , 64 Taken together, these findings indicate frontal lobe deficits in executive control and reward networks in adolescents and young adults at risk for developing AUD. 58 , 64
In sum, resting state EEG, ERP and ERO phenotypes have been central to COGA's success in addressing the role of brain function and genomic risk in AUD. In the sections that follow, we highlight studies where these neurophysiological markers of risk have been instrumental in advancing understanding of how genomic risk affects AUD development and resilience across the lifespan.
3.1. How do genomic factors influence brain functioning across the lifespan and contribute to antecedents and resilience for AUD?
From its inception, COGA has successfully used heritable, reliable, quantitative measures of brain function, to identify key genes that are significantly associated with these neural endophenotypes, advancing our approaches as newer genomic research, technologies and methods emerge (see 2. Sample and Clinical Data). COGA is in a unique position to use its considerable multimodal data to contribute to, as well as move beyond GWAS. COGA's multidisciplinary research team has facilitated translation from genetic variants identified by GWAS to their influence on the developmental trajectories of EEG phenotypes and relationships to substance use and clinical outcomes in longitudinal studies, as well as to perform molecular and functional genetic studies to enhance understanding (see 4. Genetics and 5. Functional Genomics).
3.1.1. Early linkage and association studies of neurophysiological (endo)phenotypes
Our initial genetic studies took advantage of COGA's family history of AUD density and sensitive neurophysiological measures to perform linkage and association studies. We first reported significant linkage with resting EEG beta power and a GABAA receptor gene involved in inhibitory neural networks, 25 that was subsequently found to be associated with SNPs in GABRA2 and later with alcohol dependence, substance dependence, and related disorders, including precursor externalizing phenotypes. 100 , 101 , 102 These findings suggest an imbalance in excitation‐inhibition (hyperexcitability, disinhibition) in family members at risk for AUD. We identified several other genes in our COGA linkage families using neurophysiological phenotypes that were indices of risk for AUD. We found linkage and association between theta EROs to targets in the visual oddball task and SNPs in CHRM2 (cholinergic muscarinic receptor gene) 40 , 103 and GRM8 (metabotropic glutamate receptor gene) 41 that were also found to be associated with alcohol dependence and related phenotypes (e.g., depression 104 ). These early genetic findings have been replicated and extended in several other samples throughout the world. 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 For example, in an independent sample of high‐risk offspring from multiplex AUD families, the association of variants within CHRM2 and P300 amplitude was confirmed, particularly trajectories of P300 in young male offspring aged 8–12. 115 Further, variation in GABRA2 (and its interaction with BDNF) was associated with gray matter volumes, suggesting that inherited variation in these genes may promote early developmental differences in neuronal proliferation of the cerebellum in these high‐risk offspring. 116 GABRA2 was also implicated in a large meta‐analytic genetic association study of EEG beta power and remained significant independent of COGA data. 117 In an independent sample, GRM8 variants were also found to be associated with P300 amplitude during response inhibition, as well as with AUD and related disorders, with data suggesting that the association of GRM8 and AUD may be mediated through an inherited instability in brain function that affects cognitive control. 118 These early studies are still being evaluated in much larger GWAS studies of alcohol dependence and related phenotypes. We also found that increased interhemispheric resting state EEG coherence in AUD families, suggesting dysfunctional thalamo‐cortical and cortico‐cortical connectivity. 28 This measure of coherence was significantly associated with SNPs in GABRA2 at parieto‐occipital regions and SNPs in CHRM2 at centro‐parietal regions. The GABAergic and cholinergic systems interact in local inhibitory circuits, and therefore are likely to impact cortical synchronization (i.e., coherence), which animal models suggest may impair behavioral flexibility and contribute to memory deficits. 119 These early findings are now emerging in much larger GWAS studies of alcohol dependence and related phenotypes. For example, variants in GABRA2, CHRM2 and GRM8 have been implicated in large GWAS (>1 million participants) of alcohol consumption, risk‐taking behaviors, and related addictive phenotypes 120 , 121 , 122 (e.g., smoking). Variants in GABRA2 have also been associated with EEG‐based phenotypes, 123 but in relatively smaller samples (>10 thousand participants) given the uniqueness of these datasets.
3.1.2. Genome‐wide association studies (GWAS) of neurophysiological phenotypes
Genetic data from COGA's enriched and ancestrally diverse families have been used independently as well as in consortia efforts (e.g., ENIGMA‐EEG) to conduct GWAS of the EEG‐based phenotypes described in Table 2. In one example, a family‐based GWAS of frontal theta EROs during P300 to targets in the visual oddball task identified several genome‐wide significant non‐coding variants as well as a synonymous SNP (rs702859) within KCNJ6 (the gene encoding GIRK2, G protein‐activated inward rectifier potassium channel 2). 42 Converging data from other research groups has demonstrated that GIRK2 activation contributes to slow inhibitory postsynaptic potentials important in modulating neuronal excitability. GIRK channels are directly activated by ethanol 124 and play an important role in both ethanol‐ and opioid‐induced analgesia. Low frontal theta ERO activation is associated with AUD 35 and those at risk, 36 and these genetic findings suggest that GIRK2 activation accounts for some of the variations in frontal theta oscillations seen in COGA families. We used COGA's longitudinal data to examine the neurodevelopmental trajectories of these frontal theta ERO phenotypes in the same visual oddball task during adolescence and young adulthood 44 , 52 and found age‐ and sex‐specific effects of the KCNJ6 variants 52 between the aged of 12–25. In another example of this approach, in the same adolescent and young adult sample in a reward processing task, frontal theta power EROs increased as a function of the minor allele dose of KCNJ6 SNP rs702859 during the loss condition 65 (Figure S1) These findings have implications for understanding the mechanisms through which genetics can influence neuronal circuits and indirectly, reward related behaviors. Ongoing research aims to determine functional significance of these GIRK2 variants in iPSC studies in COGA families 125 (see 5. Functional Genomics).
COGA conducted the first neural AUD endophenotype GWAS among individuals of African ancestry (AA) and reported genome‐wide significant association between beta EEG power and chromosome 3 variants that influence the expression of BCHE (butyrylcholinesterase 27 ). Several of these variants were also associated with AUD in COGA, and the results replicated in the Yale‐Penn Study of Addiction, an independent sample of AA adults. 27 These variants were also associated with ‘heavy episodic drinking’ among adolescent COGA offspring, 27 suggesting a role of these loci in neural and behavioral disinhibition across different stages of the lifespan. COGA recently conducted the first EEG interhemispheric coherence GWAS, identifying loci in an intergenic region on chromosome 18 that were associated with resting state EEG theta coherence and had both age and sex‐specific effects. These chromosome 18 variants were also associated with higher number of drinks on one occasion and DSM‐5 AUD symptoms in COGA families and associated with alcohol drinker status and alcohol intake frequency in the UK Biobank, an independent sample. 126 These findings provide support for the role of genetic variants on chromosome 18q23 in regulating both neural connectivity and alcohol use behaviors, potentially via dysregulated myelination. Interestingly, these variants were also associated with corpus callosum volume in a subset of COGA participants and UK Biobank participants. COGA will continue leading efforts to replicate and expand this work independently and in collaboration with the ENIGMA Consortium‐EEG Workgroup 117 (11 studies, total N: 17,168).
3.1.3. Polygenic scores (PGS) and neurophysiological phenotypes
The advent of polygenic scores (PGS), note, also described as polygenic risk scores (PRS), 127 , 128 which are an aggregate of genetic information from GWAS, permits characterization of the complex interplay of genetic influences on neurodevelopmental trajectories of brain function and risk for AUD. COGA has examined whether polygenic score for DSM‐IV AD (AD PGS) 129 was associated with developmental trajectories of interhemispheric and intrahemispheric neural connectivity from adolescence to young adulthood (aged 12–32). AD PGS was found to affect development of frontal‐central alpha connectivity in young adult males, but not females, 130 and was also associated with decreased planning and problem solving skills and poorer visuospatial working memory (Figure S2). 131 In another recent study, COGA researchers examined the associations between P3 amplitude, PGS for behavioral dyscontrol (EXT PGS), and self‐report of externalizing behaviors. 122 , 132 Investigators examined these associations among adolescents (12–17) and young adults (18–32) of both European and African ancestry. Both the EXT PGS and P3 amplitude were associated with externalizing behaviors, but the EXT PGS was not significantly associated with P3 amplitude. The results suggest that genetic liability for behavioral dyscontrol and P3 amplitude are each uniquely contributing to the expression of externalizing behavior, perhaps indexing different facets of externalizing risk. 132
Across linkage studies, GWAS, and PGS, COGA is uniquely positioned to address multiple facets of complex clinical phenomena, with expertise in translation of findings between brain function, genomics and substance use. The consortia continues to work collaboratively internally and externally to enhance our understanding of mechanisms underlying AUD and related disorders, including in understudied ancestrally diverse populations.
3.2. How does the social–environmental context (and interaction with genetic risk factors) impact brain functioning and ultimately impact risk for AUD?
Social–environmental experiences throughout the life course, such as interpersonal influences (parenting, peers, romantic relationships) and traumatic stress, may alter neurophysiological and behavioral development, thereby increasing risk for AUD and related psychopathology over and above the contributions of genetics and neurophysiology. 133 , 134 COGA has examined the influence of social‐environmental context on neurocognitive function and AUD as well as how genetic risk may moderate these associations. For example, COGA examined the association of childhood traumatic experiences with developmental trajectories of brain function during response inhibition. 66 Data were drawn from the COGA prospective cohort, comprising offspring from high‐risk and comparison families who were aged 12–22 at enrollment, with follow ups at two‐year intervals since 2004 (see 2. Sample and Clinical Data). Individuals exposed to sexual assaultive trauma prior to age 10 had slower rates of change in developmental trajectories of frontal theta during response inhibition. Importantly, these effects remained significant after accounting for other traumatic exposures, parental history of AUD and participants' substance use, but not measures of impulsivity. Lagged frontal neurophysiological development during response inhibition may reflect delays in frontal lobe development, synaptic pruning and/or cortical maturation involving neural circuits. These same areas were associated with increased risk for internalizing psychopathology and symptoms of AUD in young adulthood. 66 These findings support the hypothesis that changes in neurocognitive development related to early sexual trauma exposure may increase risk for mental health and substance use problems in young adulthood. Building on this work, COGA 67 observed that female, but not male, participants who experienced a sexual assaultive trauma, and were from families more densely affected with AUD, had higher rates of PTSD symptoms. In addition, exposure to nonsexual assaultive trauma was associated with poorer neurocognitive performance (planning and problem‐solving skills on the Tower of London test) in young adulthood. Parenting has been found to impact offspring brain development, neurocognitive function, risk and resilience for AUD via both genetic and socio‐environmental factors. Using data from the COGA prospective cohort, COGA demonstrated that greater ‘closeness with father’ was associated with larger P300 amplitude and higher frontal theta power in offspring. 33 Also, ‘closeness with mother’ was associated with less binge drinking in offspring. Importantly, these associations remained significant beyond other relevant risk factors such as parental AUD, other substance use, income, education and offspring characteristics such as impulsivity. These findings suggest that close relationships with parents, during the critical period of adolescence, may mitigate the “neurodevelopmental lag” in individuals with heightened vulnerability to AUD and may contribute to more efficient neurocognitive functioning. 33
3.3. How do these multi‐modal risk and protective factors fit together to influence the development and course of AUD?
Taking advantage of the wealth of our multimodal data and our interdisciplinary expertise, COGA has used machine learning (ML) methods as one approach to understand the interplay of complex factors involved in risk and resilience to as well as recovery from AUD. ML methods take an atheoretical approach to aggregating high‐dimensional data. 135 COGA has used ML algorithms to build models with the goal of identifying individuals at higher risk of developing AUD. For example, we reported that ML models combining EEG and AUD associated genetic variants outperformed other models based on a single type of data, suggesting that each contributed unique and significant information. 136 Using a Random Forests method, we found that EEG hyperconnectivity across the default mode network regions, PGS for AUD, alcohol consumption and related health consequences, elevated neuroticism, increased harm avoidance, and fewer positive life events could all be used to classify individuals who would develop alcohol induced memory problems 20 years later. 31 More recently, COGA has used ML algorithms to predict the difference in AUD recovery status, identifying several discriminative features, including PGS related to alcohol use, personality and psychopathology, psychosocial factors and electrophysiological indicators including lower default mode network and fusiform connectivity and higher insula connectivity 137 (Figure S3). Taken together, these ML findings highlight the strength of the multidomain data and analytical expertise in COGA and provide examples of how this information can be synthesized across modalities to identify risk factors and improve prediction of the development of AUD, and patterns of persistence, resistance and recovery from AUD, across the lifespan.
4. CONCLUSIONS AND FUTURE DIRECTIONS
Alcohol use disorder and related health conditions result from a complex interaction of genetic, neural and environmental factors, with differential impacts across the lifespan. From its inception, COGA has focused on the importance of brain function as it relates to the risk and consequences of alcohol use and AUD, through the examination of noninvasively recorded brain electrical activity and neuropsychological tests. COGA's sophisticated neurophysiological and neuropsychological measures, together with other rich multi‐modal family and longitudinal data, have allowed us to disentangle brain‐related risk and resilience factors from the consequences of prolonged and heavy alcohol use in the context of genomic and social‐environmental influences over the lifespan. COGA has led the field in identifying genetic variation associated with brain functioning, which has advanced the understanding of how genomic risk affects AUD. To date, COGA has amassed an impressive collection of neurophysiological and neuropsychological data on close to 10,000 participants (Table 1) who have also been carefully assessed with diagnostic interviews, behavioral questionnaires, and for whom DNA samples have been collected—all of which are available to the research community. As the complexity of genetic analyses has grown, so too have the tools used in the investigation of brain function. Building upon the past and looking towards the future, COGA is in a unique position to continue to contribute to the field by further investigating the neurophysiological and neuropsychological brain function data and its critical role in linking genetic influences on neural systems to the development and course of AUD and related disorders.
(B) Bipolar electrode pairs used as the coherence measures of resting state EEG.
Supporting information
Data S1. Supporting Information
ACKNOWLEDGMENTS
The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators: B. Porjesz, V. Hesselbrock, T. Foroud. Scientific Director: A. Agrawal. Translational Director: D. Dick, includes 10 different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, T. Foroud, Y. Liu, M.H. Plawecki); University of Iowa Carver College of Medicine (S. Kuperman, J. Kramer); SUNY Downstate Health Sciences University (B. Porjesz, J. Meyers, C. Kamarajan, A. Pandey); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, D. Dick, R. Hart, J. Salvatore); The Children's Hospital of Philadelphia, University of Pennsylvania (L. Almasy); Icahn School of Medicine at Mount Sinai (A. Goate, P. Slesinger); and Howard University (D. Scott). Other COGA collaborators include L. Bauer (University of Connecticut); J. Nurnberger Jr., L. Wetherill, X., Xuei, D. Lai, S. O'Connor, (Indiana University); G. Chan (University of Iowa; University of Connecticut); D.B. Chorlian, J. Zhang, P. Barr, S. Kinreich, G. Pandey (SUNY Downstate); N. Mullins (Icahn School of Medicine at Mount Sinai); A. Anokhin, S. Hartz, E. Johnson, V. McCutcheon, S. Saccone (Washington University); J. Moore, F. Aliev, Z. Pang, S. Kuo (Rutgers University); A. Merikangas (The Children's Hospital of Philadelphia and University of Pennsylvania); H. Chin and A. Parsian are the NIAAA Staff Collaborators. We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co‐PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting‐ Kai Li, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA). We are indebted to Arthur Stimus for his invaluable assistance in coordinating and supervising data collection and management of the COGA Brain Function data and would like to express our deepest gratitude for his many contributions to COGA since its inception.
Meyers JL, Brislin SJ, Kamarajan C, et al. The collaborative study on the genetics of alcoholism: Brain function. Genes, Brain and Behavior. 2023;22(5):e12862. doi: 10.1111/gbb.12862
Jacquelyn L. Meyers and Sarah J. Brislin have contributed equally to this work and have agreed to share first authorship.
This is the 3rd paper in a series of linked papers: 1. The Collaborative Study on the Genetics of Alcoholism: Overview. 2. The Collaborative Study on the Genetics of Alcoholism: Sample and Clinical Data. 3. The Collaborative Study on the Genetics of Alcoholism: Brain Function. 4. The Collaborative Study on the Genetics of Alcoholism: Genetics. 5. The Collaborative Study on the Genetics of Alcoholism: Functional Genomics.
Contributor Information
Jacquelyn L. Meyers, Email: Jacquelyn.Meyers@downstate.edu.
Sarah J. Brislin, Email: sarah.brislin@rutgers.edu.
Bernice Porjesz, Email: Bernice.Porjesz@downstate.edu.
DATA AVAILABILITY STATEMENT
COGA data are available in dbGaP (phs000125, phs000763, phs000976, phs001208), or via an application to the National Institute on Alcohol Abuse and Alcoholism (https://www.niaaa.nih.gov/research/major‐initiatives/collaborative‐studies‐genetics‐alcoholism‐coga‐study), or a COGA investigator sponsored secondary analysis proposal.
REFERENCES
- 1. Dick DM, Kendler KS. The impact of gene‐environment interaction on alcohol use disorders. Alcohol Res. 2012;34(3):318‐324. [PMC free article] [PubMed] [Google Scholar]
- 2. Begleiter H. The collaborative study on the genetics of alcoholism. Alcohol Health Res World. 1995;19(3):228‐236. [PMC free article] [PubMed] [Google Scholar]
- 3. Reich T, Edenberg HJ, Goate A, et al. Genome‐wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81(3):207‐215. doi:10.1002/(SICI)1096‐8628(19980508)81:3<207::AID‐AJMG1>3.0.CO;2‐T [PubMed] [Google Scholar]
- 4. Nurnberger JI Jr, Wiegand R, Bucholz K, et al. A family study of alcohol dependence: coaggregation of multiple disorders in relatives of alcohol‐dependent probands. Arch Gen Psychiatry. 2004;61(12):1246‐1256. doi: 10.1001/archpsyc.61.12.1246 [DOI] [PubMed] [Google Scholar]
- 5. Cohen HL, Wang W, Porjesz B, et al. Visual P300: an interlaboratory consistency study. Alcohol. 1994;11(6):583‐587. doi: 10.1016/0741-8329(94)90087-6 [DOI] [PubMed] [Google Scholar]
- 6. Alexander JE, Polich J, Bloom FE, et al. P300 from an auditory oddball task: inter‐laboratory consistency. Int J Psychophysiol. 1994;17(1):35‐46. doi: 10.1016/0167-8760(94)90053-1 [DOI] [PubMed] [Google Scholar]
- 7. Kuperman S, Porjesz B, Arndt S, et al. Multi‐center N400 ERP consistency using a primed and unprimed word paradigm. Electroencephalogr Clin Neurophysiol. 1995;94(6):462‐470. doi: 10.1016/0013-4694(94)00312-9 [DOI] [PubMed] [Google Scholar]
- 8. Rohrbaugh JW, Dunham DN, Stewart PA, et al. Slow brain potentials in a visual‐spatial memory task: topographic distribution and inter‐laboratory consistency. Int J Psychophysiol. 1997;25(2):111‐122. doi: 10.1016/s0167-8760(96)00714-3 [DOI] [PubMed] [Google Scholar]
- 9. Begleiter H, Porjesz B, Bihari B, Kissin B. Event‐related brain potentials in boys at risk for alcoholism. Science. 1984;225(4669):1493‐1496. doi: 10.1126/science.6474187 [DOI] [PubMed] [Google Scholar]
- 10. Polich J, Pollock VE, Bloom FE. Meta‐analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115(1):55‐73. [DOI] [PubMed] [Google Scholar]
- 11. Porjesz B, Begleiter H, Reich T, et al. Amplitude of visual P3 event‐related potential as a phenotypic marker for a predisposition to alcoholism: preliminary results from the COGA project. Collaborative study on the genetics of alcoholism. Alcohol Clin Exp Res. 1998;22(6):1317‐1323. doi: 10.1111/j.1530-0277.1998.tb03914.x [DOI] [PubMed] [Google Scholar]
- 12. Benegal V, Jain S, Subbukrishna DK, Channabasavanna SM. P300 amplitudes vary inversely with continuum of risk in first degree male relatives of alcoholics. Psychiatr Genet. 1995;5(4):149‐156. [DOI] [PubMed] [Google Scholar]
- 13. Van Der Stelt O. Visual P3 as a potential vulnerability marker of alcoholism: evidence from the Amsterdam study of children of alcoholics. Alcohol Alcohol. 1999;34(3):267‐282. doi: 10.1093/alcalc/34.3.267 [DOI] [PubMed] [Google Scholar]
- 14. Hill SY, Steinhauer SR, Locke‐Wellman J, Ulrich R. Childhood risk factors for young adult substance dependence outcome in offspring from multiplex alcohol dependence families: a prospective study. Biol Psychiatry. 2009;66(8):750‐757. doi: 10.1016/j.biopsych.2009.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ehlers CL, Wall TL, Garcia‐Andrade C, Phillips E. Visual P3 findings in Mission Indian youth: relationship to family history of alcohol dependence and behavioral problems. Psychiatry Res. 2001;105(1–2):67‐78. doi: 10.1016/s0165-1781(01)00313-4 [DOI] [PubMed] [Google Scholar]
- 16. Iacono WG, Carlson SR, Malone SM, McGue M. P3 event‐related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59(8):750‐757. doi: 10.1001/archpsyc.59.8.750 [DOI] [PubMed] [Google Scholar]
- 17. Hill SY, Steinhauer S, Park J, Zubin J. Event‐related potential characteristics in children of alcoholics from high density families. Alcohol Clin Exp Res. 1990;14(1):6‐16. [DOI] [PubMed] [Google Scholar]
- 18. Hill SY, Steinhauer SR. Event‐related potentials in women at risk for alcoholism. Alcohol. 1993;10(5):349‐354. doi: 10.1016/0741-8329(93)90019-k [DOI] [PubMed] [Google Scholar]
- 19. Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. ScientificWorldJournal. 2007;7:131‐141. doi: 10.1100/tsw.2007.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salvatore JE, Gottesman II, Dick DM. Endophenotypes for alcohol use disorder: an update on the field. Curr Addict Rep. 2015;2(1):76‐90. doi: 10.1007/s40429-015-0046-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636‐645. doi: 10.1176/appi.ajp.160.4.636 [DOI] [PubMed] [Google Scholar]
- 22. Rangaswamy M, Porjesz B, Chorlian DB, et al. Theta power in the EEG of alcoholics. Alcohol Clin Exp Res. 2003;27(4):607‐615. doi: 10.1097/01.ALC.0000060523.95470.8F [DOI] [PubMed] [Google Scholar]
- 23. Rangaswamy M, Porjesz B, Chorlian DB, et al. Beta power in the EEG of alcoholics. Biol Psychiatry. 2002;52(8):831‐842. doi: 10.1016/s0006-3223(02)01362-8 [DOI] [PubMed] [Google Scholar]
- 24. Rangaswamy M, Porjesz B, Chorlian DB, et al. Resting EEG in offspring of male alcoholics: beta frequencies. Int J Psychophysiol. 2004;51(3):239‐251. doi: 10.1016/j.ijpsycho.2003.09.003 [DOI] [PubMed] [Google Scholar]
- 25. Porjesz B, Almasy L, Edenberg HJ, et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci U S A. 2002;99(6):3729‐3733. doi: 10.1073/pnas.052716399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meyers JL, Zhang J, Manz N, et al. A genome wide association study of fast beta EEG in families of European ancestry. Int J Psychophysiol. 2017;115:74‐85. doi: 10.1016/j.ijpsycho.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meyers JL, Zhang J, Wang JC, et al. An endophenotype approach to the genetics of alcohol dependence: a genome wide association study of fast beta EEG in families of African ancestry. Mol Psychiatry. 2017;22(12):1767‐1775. doi: 10.1038/mp.2016.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain Res. 2008;1235:153‐171. doi: 10.1016/j.brainres.2008.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meyers JL, Chorlian DB, Johnson EC, et al. Association of Polygenic Liability for alcohol dependence and EEG connectivity in adolescence and Young adulthood. Brain Sci. 2019;9(10):280. doi: 10.3390/brainsci9100280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyers JL, Chorlian DB, Bigdeli TB, et al. The association of polygenic risk for schizophrenia, bipolar disorder, and depression with neural connectivity in adolescents and young adults: examining developmental and sex differences. Transl Psychiatry. 2021;11(1):54. doi: 10.1038/s41398-020-01185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kamarajan C, Pandey AK, Chorlian DB, et al. Predicting alcohol‐related memory problems in older adults: a machine learning study with multi‐domain features. bioRxiv 2023:2022.2012.2030.522330. doi: 10.1101/2022.12.30.522330 [DOI] [PMC free article] [PubMed]
- 32. Pandey G, Seay MJ, Meyers JL, et al. Density and dichotomous family history measures of alcohol use disorder as predictors of behavioral and neural phenotypes: a comparative study across gender and race/ethnicity. Alcohol Clin Exp Res. 2020;44(3):697‐710. doi: 10.1111/acer.14280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pandey G, Kuo SI, Horne‐Osipenko KA, et al. Associations of parent‐adolescent closeness with P3 amplitude, frontal theta, and binge drinking among offspring with high risk for alcohol use disorder. Alcohol: Clin Exp Res. 2023;47(1):155‐167. doi: 10.1111/acer.14973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen AC, Manz N, Tang Y, et al. Single‐nucleotide polymorphisms in corticotropin releasing hormone receptor 1 gene (CRHR1) are associated with quantitative trait of event‐related potential and alcohol dependence. Alcohol Clin Exp Res. 2010;34(6):988‐996. doi: 10.1111/j.1530-0277.2010.01173.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones KA, Porjesz B, Chorlian D, et al. S‐transform time‐frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin Neurophysiol. 2006;117(10):2128‐2143. doi: 10.1016/j.clinph.2006.02.028 [DOI] [PubMed] [Google Scholar]
- 36. Rangaswamy M, Jones KA, Porjesz B, et al. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int J Psychophysiol. 2007;63(1):3‐15. doi: 10.1016/j.ijpsycho.2006.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamarajan C, Rangaswamy M, Manz N, et al. Topography, power, and current source density of theta oscillations during reward processing as markers for alcohol dependence. Hum Brain Mapp. 2012;33(5):1019‐1039. doi: 10.1002/hbm.21267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen AC, Porjesz B, Rangaswamy M, et al. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol Clin Exp Res. 2007;31(1):156‐165. doi: 10.1111/j.1530-0277.2006.00277.x [DOI] [PubMed] [Google Scholar]
- 39. Kamarajan C, Rangaswamy M, Chorlian DB, et al. Theta oscillations during the processing of monetary loss and gain: a perspective on gender and impulsivity. Brain Res. 2008;1235:45‐62. doi: 10.1016/j.brainres.2008.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jones KA, Porjesz B, Almasy L, et al. A cholinergic receptor gene (CHRM2) affects event‐related oscillations. Behav Genet. 2006;36(5):627‐639. doi: 10.1007/s10519-006-9075-6 [DOI] [PubMed] [Google Scholar]
- 41. Chen AC, Tang Y, Rangaswamy M, et al. Association of single nucleotide polymorphisms in a glutamate receptor gene (GRM8) with theta power of event‐related oscillations and alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):359‐368. doi: 10.1002/ajmg.b.30818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kang SJ, Rangaswamy M, Manz N, et al. Family‐based genome‐wide association study of frontal theta oscillations identifies potassium channel gene KCNJ6. Genes Brain Behav. 2012;11(6):712‐719. doi: 10.1111/j.1601-183X.2012.00803.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zlojutro M, Manz N, Rangaswamy M, et al. Genome‐wide association study of theta band event‐related oscillations identifies serotonin receptor gene HTR7 influencing risk of alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(1):44‐58. doi: 10.1002/ajmg.b.31136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chorlian DB, Rangaswamy M, Manz N, et al. Gender modulates the development of theta event related oscillations in adolescents and young adults. Behav Brain Res. 2015;292(1):342‐352. doi: 10.1016/j.bbr.2015.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chorlian DB, Rangaswamy M, Manz N, et al. Genetic and neurophysiological correlates of the age of onset of alcohol use disorders in adolescents and young adults. Behav Genet. 2013;43(5):386‐401. doi: 10.1007/s10519-013-9604-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Padmanabhapillai A, Porjesz B, Ranganathan M, et al. Suppression of early evoked gamma band response in male alcoholics during a visual oddball task. Int J Psychophysiol. 2006;60(1):15‐26. doi: 10.1016/j.ijpsycho.2005.03.026 [DOI] [PubMed] [Google Scholar]
- 47. Padmanabhapillai A, Tang Y, Ranganathan M, et al. Evoked gamma band response in male adolescent subjects at high risk for alcoholism during a visual oddball task. Int J Psychophysiol. 2006;62(2):262‐271. doi: 10.1016/j.ijpsycho.2006.05.012 [DOI] [PubMed] [Google Scholar]
- 48. Hada M, Porjesz B, Begleiter H, Polich J. Auditory P3a assessment of male alcoholics. Biol Psychiatry. 2000;48(4):276‐286. doi: 10.1016/s0006-3223(00)00236-5 [DOI] [PubMed] [Google Scholar]
- 49. Hada M, Porjesz B, Chorlian DB, Begleiter H, Polich J. Auditory P3a deficits in male subjects at high risk for alcoholism. Biol Psychiatry. 2001;49(8):726‐738. doi: 10.1016/S0006-3223(00)01049-0 [DOI] [PubMed] [Google Scholar]
- 50. Suresh S, Porjesz B, Chorlian DB, et al. Auditory P3 in female alcoholics. Alcohol Clin Exp Res. 2003;27(7):1064‐1074. doi: 10.1097/01.ALC.0000075549.49800.A0 [DOI] [PubMed] [Google Scholar]
- 51. Ramachandran G, Porjesz B, Begleiter H, Litke A. A simple auditory oddball task in young adult males at high risk for alcoholism. Alcohol Clin Exp Res. 1996;20(1):9‐15. doi: 10.1111/j.1530-0277.1996.tb01035.x [DOI] [PubMed] [Google Scholar]
- 52. Chorlian DB, Rangaswamy M, Manz N, et al. Genetic correlates of the development of theta event related oscillations in adolescents and young adults. Int J Psychophysiol. 2017;115:24‐39. doi: 10.1016/j.ijpsycho.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roopesh BN, Rangaswamy M, Kamarajan C, Chorlian DB, Pandey AK, Porjesz B. Reduced resource optimization in male alcoholics: N400 in a lexical decision paradigm. Alcohol Clin Exp Res. 2010;34(11):1905‐1914. doi: 10.1111/j.1530-0277.2010.01279.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roopesh BN, Rangaswamy M, Kamarajan C, et al. Priming deficiency in male subjects at risk for alcoholism: the N4 during a lexical decision task. Alcohol Clin Exp Res. 2009;33(12):2027‐2036. doi: 10.1111/j.1530-0277.2009.01042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pandey AK, Kamarajan C, Tang Y, et al. Neurocognitive deficits in male alcoholics: an ERP/sLORETA analysis of the N2 component in an equal probability go/NoGo task. Biol Psychol. 2012;89(1):170‐182. doi: 10.1016/j.biopsycho.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kamarajan C, Porjesz B, Jones KA, et al. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a go/No‐go task. Biol Psychol. 2005;69(3):353‐373. doi: 10.1016/j.biopsycho.2004.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kamarajan C, Porjesz B, Jones KA, et al. Spatial‐anatomical mapping of NoGo‐P3 in the offspring of alcoholics: evidence of cognitive and neural disinhibition as a risk for alcoholism. Clin Neurophysiol. 2005;116(5):1049‐1061. doi: 10.1016/j.clinph.2004.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pandey AK, Kamarajan C, Manz N, Chorlian DB, Stimus A, Porjesz B. Delta, theta, and alpha event‐related oscillations in alcoholics during go/NoGo task: neurocognitive deficits in execution, inhibition, and attention processing. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:158‐171. doi: 10.1016/j.pnpbp.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kamarajan C, Porjesz B, Jones K, et al. Event‐related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry. 2006;59(7):625‐634. doi: 10.1016/j.biopsych.2005.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kamarajan C, Porjesz B, Jones KA, et al. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. Int J Psychophysiol. 2004;51(2):155‐180. doi: 10.1016/j.ijpsycho.2003.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meyers J, McCutheon V, Pandey A, et al. Early sexual assaultive trauma, polygenic risk for AUD/CUD, and neural response inhibition in adolescence and Young adults: trajectories of frontal oscillations during a go/Nogo task. Eur Neuropsychopharmacol. 2019;29:S951‐S952. doi: 10.1016/j.euroneuro.2017.08.303 [DOI] [Google Scholar]
- 62. Kamarajan C, Rangaswamy M, Tang Y, et al. Dysfunctional reward processing in male alcoholics: an ERP study during a gambling task. J Psychiatr Res. 2010;44(9):576‐590. doi: 10.1016/j.jpsychires.2009.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kamarajan C, Pandey AK, Chorlian DB, et al. Reward processing deficits and impulsivity in high‐risk offspring of alcoholics: a study of event‐related potentials during a monetary gambling task. Int J Psychophysiol. 2015;98(2 Pt 1):182‐200. doi: 10.1016/j.ijpsycho.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kamarajan C, Pandey AK, Chorlian DB, et al. Deficient event‐related theta oscillations in individuals at risk for alcoholism: a study of reward processing and impulsivity features. PloS One. 2015;10(11):e0142659. doi: 10.1371/journal.pone.0142659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kamarajan C, Pandey AK, Chorlian DB, et al. A KCNJ6 gene polymorphism modulates theta oscillations during reward processing. Int J Psychophysiol. 2017;115:13‐23. doi: 10.1016/j.ijpsycho.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meyers J, McCutcheon VV, Pandey AK, et al. Early sexual trauma exposure and neural response inhibition in adolescence and young adults: trajectories of frontal theta oscillations during a go/No‐go task. J Am Acad Child Adolesc Psychiatry. 2019;58(2):242‐255 e242. doi: 10.1016/j.jaac.2018.07.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Subbie‐Saenz de Viteri S, Pandey A, Pandey G, et al. Pathways to post‐traumatic stress disorder and alcohol dependence: trauma, executive functioning, and family history of alcoholism in adolescents and young adults. Brain Behav. 2020;10(11):e01789. doi: 10.1002/brb3.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pandey AK, Meyers JL, Kamarajan C, et al. Impaired fluid cognition, developmental trajectories, and relation to polygenic risk scores in COGA study of individuals with family history of alcohol dependence. Alcohol Clin Exp Res. 2019;43:52A. [Google Scholar]
- 69. van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. Am J Hum Genet. 1996;58(3):562‐573. [PMC free article] [PubMed] [Google Scholar]
- 70. Niedermeyer E. The Normal EEG of the waking adult. In: Niedermeyer E, Lopes da Silva F, eds. Electroencephalography: Basic Principles, Clinical Applications and Related Fields. Lippincott Williams & Wilkins; 1999:149‐173. [Google Scholar]
- 71. Nunez PL, Srinivasan R. Electric Fields of the Brain: the Neurophysics of EEG. 2nd ed. Oxford University Press; 2006. [Google Scholar]
- 72. Coles MGH, Rugg MD. Event‐related brain potentials: an introduction. In: Rugg MD, Coles MGH, eds. Electrophysiology of Mind: Event‐Related Brain Potentials and Cognition. Oxford University Press; 1995:1‐26. [Google Scholar]
- 73. Hillyard SA, Kutas M. Electrophysiology of cognitive processing. Annu Rev Psychol. 1983;34(1):33‐61. doi: 10.1146/annurev.ps.34.020183.000341 [DOI] [PubMed] [Google Scholar]
- 74. Donchin E, Ritter W, McCallum C. Cognitive psychophysiology: the endogenous components of the ERP. In: Callaway E, Tueting P, Koslow S, eds. Brain Event‐Related Potentials in Man. Academic Press; 1978:349‐441. [Google Scholar]
- 75. Luck SJ. An introduction to event‐related potentials and their neural origins. An Introduction to the Event‐Related Potential Technique. MIT Press; 2005:1‐50. [Google Scholar]
- 76. Sutton S, Braren M, Zubin J, John ER. Evoked‐potential correlates of stimulus uncertainty. Science. 1965;150(3700):1187‐1188. doi: 10.1126/science.150.3700.1187 [DOI] [PubMed] [Google Scholar]
- 77. Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116(5):993‐1018. doi: 10.1016/j.clinph.2004.12.016 [DOI] [PubMed] [Google Scholar]
- 78. Donchin E. Surprise!? Surprise? Psychophysiology. 1981;18(5):493‐513. [DOI] [PubMed] [Google Scholar]
- 79. Donchin E, Coles GH. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11:357‐374. [Google Scholar]
- 80. Polich J. Overview of P3a and P3b. In: Polich J, ed. Detection of change: event‐related potential and fMRI findings. Kluwer Academic Press; 2003:83‐98. [Google Scholar]
- 81. Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128‐2148. doi: 10.1016/j.clinph.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Karakas S, Erzengin OU, Basar E. The genesis of human event‐related responses explained through the theory of oscillatory neural assemblies. Neurosci Lett. 2000;285(1):45‐48. [DOI] [PubMed] [Google Scholar]
- 83. Basar E, Basar‐Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39(2–3):241‐248. [DOI] [PubMed] [Google Scholar]
- 84. von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38(3):301‐313. [DOI] [PubMed] [Google Scholar]
- 85. Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11(6):739‐744. doi: 10.1016/s0959-4388(01)00278-1 [DOI] [PubMed] [Google Scholar]
- 86. Pandey AK, Kamarajan C, Rangaswamy M, Porjesz B. Event‐related oscillations in alcoholism research: a review. J Addict Res Ther. 2012;Suppl 7(1):1‐13. doi: 10.4172/2155-6105.S7-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Guntekin B, Basar E. Review of evoked and event‐related delta responses in the human brain. Int J Psychophysiol. 2016;103:43‐52. doi: 10.1016/j.ijpsycho.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 88. Wechsler D. Manual for the Wechsler Adult Intelligence Scale‐Revised (WAIS‐R). The Psychological Corporation; 1981. [Google Scholar]
- 89. Wechsler D. Wechsler Intelligence Scale for Children‐Revised. The Psychological Corporation; 1972. [Google Scholar]
- 90. Jastak S, Wilkinson GS. Wide Range Achievement Test Revised (WRAT‐R). Jastak Associates; 1984. [Google Scholar]
- 91. Reitan RM. Trail Making Test: Manual for Administration and Scoring. Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- 92. Porteus S. The Porteus Maze Test Manual. Harrap; 1952. [Google Scholar]
- 93. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test (CVLT). The Psychological Corporation; 1987. [Google Scholar]
- 94. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test‐Children's Version (CVLT‐C). The Psychological Corporation; 1993. [Google Scholar]
- 95. Davis HP, Keller F. Colorado Assessment Tests (CATs), Version 1.2. Colorado Springs; 2002. [Google Scholar]
- 96. Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH toolbox. Neurology. 2013;80(11 Suppl 3):S54‐S64. doi: 10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Babakhanyan I, McKenna BS, Casaletto KB, Nowinski CJ, Heaton RK. National Institutes of Health toolbox emotion battery for English‐ and Spanish‐speaking adults: normative data and factor‐based summary scores. Patient Relat Outcome Meas. 2018;9:115‐127. doi: 10.2147/PROM.S151658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Porjesz B, Begleiter H, Litke A, et al. Visual P3 as a potential phenotypic marker for alcoholism: evidence from the COGA national project. In: Ogura C, Koga Y, Shimokochi M, eds. Recent Advances in Event‐Related Brain Potential Research. Elsevier Science; 1996:539‐549. [Google Scholar]
- 99. Whittington MA, Faulkner HJ, Doheny HC, Traub RD. Neuronal fast oscillations as a target site for psychoactive drugs. Pharmacol Ther. 2000;86(2):171‐190. [DOI] [PubMed] [Google Scholar]
- 100. Edenberg HJ, Dick DM, Xuei X, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(a) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74(4):705‐714. doi: 10.1086/383283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dick DM, Bierut L, Hinrichs A, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36(4):577‐590. doi: 10.1007/s10519-005-9041-8 [DOI] [PubMed] [Google Scholar]
- 102. Agrawal A, Edenberg HJ, Foroud T, et al. Association of GABRA2 with drug dependence in the collaborative study of the genetics of alcoholism sample. Behav Genet. 2006;36(5):640‐650. doi: 10.1007/s10519-006-9069-4 [DOI] [PubMed] [Google Scholar]
- 103. Jones KA, Porjesz B, Almasy L, et al. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53(2):75‐90. doi: 10.1016/j.ijpsycho.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 104. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903‐1911. doi: 10.1093/hmg/ddh194 [DOI] [PubMed] [Google Scholar]
- 105. Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129B(1):104‐109. doi: 10.1002/ajmg.b.30091 [DOI] [PubMed] [Google Scholar]
- 106. Lappalainen J, Krupitsky E, Remizov M, et al. Association between alcoholism and gamma‐amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29(4):493‐498. [DOI] [PubMed] [Google Scholar]
- 107. Fehr C, Sander T, Tadic A, et al. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype‐specific analysis. Psychiatr Genet. 2006;16(1):9‐17. doi: 10.1097/01.ypg.0000185027.89816.d9 [DOI] [PubMed] [Google Scholar]
- 108. Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B. GABA‐A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res. 2008;42(3):184‐191. doi: 10.1016/j.jpsychires.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 109. Philibert RA, Gunter TD, Beach SR, et al. Role of GABRA2 on risk for alcohol, nicotine, and cannabis dependence in the Iowa adoption studies. Psychiatr Genet. 2009;19(2):91‐98. doi: 10.1097/YPG.0b013e3283208026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lydall GJ, Saini J, Ruparelia K, et al. Genetic association study of GABRA2 single nucleotide polymorphisms and electroencephalography in alcohol dependence. Neurosci Lett. 2011;500(3):162‐166. doi: 10.1016/j.neulet.2011.05.240 [DOI] [PubMed] [Google Scholar]
- 111. Villafuerte S, Strumba V, Stoltenberg SF, Zucker RA, Burmeister M. Impulsiveness mediates the association between GABRA2 SNPs and lifetime alcohol problems. Genes Brain Behav. 2013;12(5):525‐531. doi: 10.1111/gbb.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Heitzeg MM, Villafuerte S, Weiland BJ, et al. Effect of GABRA2 genotype on development of incentive‐motivation circuitry in a sample enriched for alcoholism risk. Neuropsychopharmacology. 2014;39(13):3077‐3086. doi: 10.1038/npp.2014.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Brown‐Rice KA, Scholl JL, Fercho KA, et al. Neural and psychological characteristics of college students with alcoholic parents differ depending on current alcohol use. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:284‐296. doi: 10.1016/j.pnpbp.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Villafuerte S, Heitzeg MM, Foley S, et al. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Mol Psychiatry. 2012;17(5):511‐519. doi: 10.1038/mp.2011.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hill SY, Jones BL, Holmes B, Steinhauer SR, Zezza N, Stiffler S. Cholinergic receptor gene (CHRM2) variation and familial loading for alcohol dependence predict childhood developmental trajectories of P300. Psychiatry Res. 2013;209(3):504‐511. doi: 10.1016/j.psychres.2013.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hill SY, Wang S, Carter H, et al. Cerebellum volume in high‐risk offspring from multiplex alcohol dependence families: association with allelic variation in GABRA2 and BDNF. Psychiatry Res. 2011;194(3):304‐313. doi: 10.1016/j.pscychresns.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Smit DJA, Wright MJ, Meyers JL, et al. Genome‐wide association analysis links multiple psychiatric liability genes to oscillatory brain activity. Hum Brain Mapp. 2018;39(11):4183‐4195. doi: 10.1002/hbm.24238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bauer LO, Covault JM. GRM8 genotype is associated with externalizing disorders and greater inter‐trial variability in brain activation during a response inhibition task. Clin Neurophysiol. 2020;131(6):1180‐1186. doi: 10.1016/j.clinph.2020.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Frodl‐Bauch T, Bottlender R, Hegerl U. Neurochemical substrates and neuroanatomical generators of the event‐related P300. Neuropsychobiology. 1999;40(2):86‐94. [DOI] [PubMed] [Google Scholar]
- 120. Karlsson Linner R, Biroli P, Kong E, et al. Genome‐wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet. 2019;51(2):245‐257. doi: 10.1038/s41588-018-0309-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Saunders GRB, Wang X, Chen F, et al. Genetic diversity fuels gene discovery for tobacco and alcohol use. Nature. 2022;612:720‐724. doi: 10.1038/s41586-022-05477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Karlsson Linner R, Mallard TT, Barr PB, et al. Multivariate analysis of 1.5 million people identifies genetic associations with traits related to self‐regulation and addiction. Nat Neurosci. 2021;24(10):1367‐1376. doi: 10.1038/s41593-021-00908-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Smit DJA, Andreassen OA, Boomsma DI, et al. Large‐scale collaboration in ENIGMA‐EEG: a perspective on the meta‐analytic approach to link neurological and psychiatric liability genes to electrophysiological brain activity. Brain Behav. 2021;11(8):e02188. doi: 10.1002/brb3.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Aryal P, Dvir H, Choe S, Slesinger PA. A discrete alcohol pocket involved in GIRK channel activation. Nat Neurosci. 2009;12(8):988‐995. doi: 10.1038/nn.2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Popova D, Gameiro‐Ros I, Youssef MM, et al. Alcohol reverses the effects of KCNJ6 (GIRK2) noncoding variants on excitability of human glutamatergic neurons. Mol Psychiatry. 2023;28(2):746‐758. doi: 10.1038/s41380-022-01818-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Meyers JL, Zhang J, Chorlian DB, et al. A genome wide association study of interhemispheric theta EEG coherence: implications for neural connectivity and alcohol use behavior in families of European and African ancestry. Transl Psychiatry. 2021;26(9): 5040‐5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. International Schizophrenia C , Purcell SM, Wray NR, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748‐752. doi: 10.1038/nature08185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dima D, Breen G. Polygenic risk scores in imaging genetics: usefulness and applications. J Psychopharmacol. 2015;29(8):867‐871. doi: 10.1177/0269881115584470 [DOI] [PubMed] [Google Scholar]
- 129. Walters RK, Polimanti R, Johnson EC, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21(12):1656‐1669. doi: 10.1038/s41593-018-0275-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Meyers JL, Chorlian DB, Zhang J, et al. Polygenic Risk Scores from alcohol dependence affects developmental trajectories of EEG alpha coherence in young adult males. in preparation.
- 131. Pandey AK, Meyers JL, Kamarajan C, et al. Deficits in cognitive planning, problem‐solving ability, visuospatial immediate memory span and working memory, their developmental trajectories and association with polygenic risk scores in the families of individuals with alcohol use disorder: Evidence from the COGA study. in preparation.
- 132. Brislin S, Salvatore J, Meyers J , et al. (2023). Examining associations between genetic and neural risk for externalizing behaviors in adolescence and early adulthood. Psychol Med. 2023;1‐11. doi: 10.1017/S0033291723001174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bick J, Nelson CA. Early adverse experiences and the developing brain. Neuropsychopharmacology. 2016;41(1):177‐196. doi: 10.1038/npp.2015.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17(10):652‐666. doi: 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- 135. Baldi P, Brunak S. Bioinformatics: the Machine Learning Approach. MIT press; 2001. [Google Scholar]
- 136. Kinreich S, Meyers JL, Maron‐Katz A, et al. Predicting risk for alcohol use disorder using longitudinal data with multimodal biomarkers and family history: a machine learning study. Mol Psychiatry. 2021;26(4):1133‐1141. doi: 10.1038/s41380-019-0534-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kinreich S, McCutcheon VV, Aliev F, et al. Predicting alcohol use disorder remission: a longitudinal multimodal multi‐featured machine learning approach. Transl Psychiatry. 2021;11(1):166. doi: 10.1038/s41398-021-01281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Data Availability Statement
COGA data are available in dbGaP (phs000125, phs000763, phs000976, phs001208), or via an application to the National Institute on Alcohol Abuse and Alcoholism (https://www.niaaa.nih.gov/research/major‐initiatives/collaborative‐studies‐genetics‐alcoholism‐coga‐study), or a COGA investigator sponsored secondary analysis proposal.


