Abstract
Background and aims
It is suggested that the changes in atherosclerosis happen mainly under the influence of non-fasting lipids. To date, the studies in the postprandial state were primarily performed on healthy subjects. This exploratory, cross-sectional study investigates the change in lipid profile, inflammation, and platelet activation in patients with different cardiovascular risk profiles in the postprandial state.
Methods
The studied population consists of 66 patients with different cardiovascular risks: patients with a history of the chronic coronary syndrome (CCS) and diabetes mellitus type 2 (DM2) (n = 20), CCS without DM2 (n = 25), and a healthy control group (n = 21). Lipid variables and markers of platelet function and inflammation were assessed during the fasting state and three and 5 h after a standardized fat meal using a standardized oral fat tolerance test (OFTT), a milkshake with 90 g of fat.
Results
Patients with CCS and DM2 were significantly older and had the highest BMI. All patients with CCS were on acetylsalicylic acid, and 95% of CCS patients were on high-dose statins. The absolute leukocyte and neutrophile count increased significantly in the control group during the OFTT in comparison to CCS subjects. There was a significant decrease of HDL and increase of triglycerides during the OFTT, however with no difference between groups. There was no difference in the change of platelet activity between all groups.
Conclusion
This study showed that OFTT leads to an increased postprandial inflammation response in healthy group compared to CCS ± DM2 while there was no change in lipid profile and platelet activity.
Keywords: Oral fat tolerance test, Fat loading, High-fat meal, Chronic coronary syndrome, Platelet activity, Postprandial lipid profile, Postprandial inflammation
Highlights
-
•
The results support the opinion that the non-fasting lipid profile simplifies clinical practice for patients, laboratories, and clinicians.
-
•
high-fat meal diet causes a postprandial inflammatory response [[1], [2], [3]]. However, pervious studies were performed exclusively on healthy subjects, whereas our study expands the current knowledge on postprandial inflammation in patients at very-high cardiovascular risk.
-
•
Hence, our results suggest the anti-inflammatory and antiplatelet effect of statins and acetylsalicylic acid also in the postprandial phase.
1. Introduction
Previously, fasting for at least 8 h was required to evaluate the lipid profile. However, fasting for more than 8 h usually occurs only before breakfast, and most individuals remain presumably longer in the non-fasting state. During the fasting state, plasma contains only atherogenic lipoproteins of hepatic origin, whereas atherogenic lipoproteins of intestinal origin are also present in the non-fasting state. Therefore, evaluating a non-fasting lipid profile determines more accurately the total amount of atherogenic lipoproteins in plasma during the day [[4], [5], [6]].
The association between hyperlipidemia and a prothrombotic state is well known, and several mechanisms are involved. Very low-density lipoprotein (VLDL) particles and low-density lipoprotein cholesterol (LDL-c) particles can activate platelets by changing the lipid composition and interactions between lipoprotein and lipoprotein receptors on the platelet membrane [7]. Moreover, an increase in platelet cholesterol enhances the sensitivity of platelets to aggregate [8]. On the opposite, high-density lipoprotein cholesterol (HDL-c) removes cholesterol from peripheral tissues, therefore limiting the content of intraplatelet cholesterol and modulating signals of the platelet pathway [9].
Studies on postprandial lipemia have shown that postprandial triglycerides (TG) are a better predictor of cardiovascular disease (CVD) than fasting TG [5,10,11]. Furthermore, postprandial lipemia was reported to be associated with endothelial dysfunction, elevated oxidative stress, upregulation of proinflammatory genes in endothelial cells, increased production of leukocyte activation markers and stimulation of the complement system, respectively [12]. These processes are considered promoting factors in the development and progression of atherosclerosis [12]. Most studies on postprandial lipemia were performed in healthy subjects or in individuals with diabetes mellitus type 2 (DM2) without CVD. Accordingly, little is known about the lipid profile during a postprandial state in patients with established CVD [2,[13], [14], [15], [16], [17], [18], [19], [20]]. Therefore, our study aimed to investigate postprandial lipid profiles and markers of inflammation and platelet activation in high-risk chronic coronary syndrome (CCS) patients with/without DM2 and to compare these results with a healthy population.
2. Methods
This study is an explorative and hypothesis-generating longitudinal analysis of postprandial platelet activity and inflammation during an oral fat tolerance test (OFTT) in subjects with different cardiovascular risk profiles. The study was designed and performed according to the Helsinki criteria and evaluated by the local ethics committee (EC Number: 16-004-0316).
The study was conducted at the 3rd Medical Department, Cardiology and Intensive Care Medicine, at Clinic Ottakring (Wilhelminenhospital), Vienna, Austria.
2.1. Study population
The studied population consisted of sixty-six participants with different cardiovascular risk profiles: 1. Subjects with a history of CCS with DM2 (CCS + DM), 2. Subjects with CCS without DM2 (CCS-DM), and 3. A healthy control group.
Subjects with a history of ischemic heart disease, acute coronary syndrome, or percutaneous coronary intervention (PCI) at least one year prior to study inclusion were considered as having CCS. Obviously, healthy subjects consisted of the control group. In general, subjects older than 80 years or younger than 18 years, or patients with relevant hematologic disorders (haemoglobin <10 mg/dl, platelet count <100 × 109 cells/l or platelet count >600 × 109 cells/l), known platelet function disorder, a history of alcohol or drug abuse, ongoing antibiotics therapy, or on oral anticoagulants were excluded. All subjects gave their written informed consent for participation in the study.
There are no data on changes in platelet function in patients with CCS after OFTT assessed via multiplate available in the literature. Therefore, no exact sample size calculation could be performed on the given co-primary outcome variables. Alternatively, sample size estimation was performed according to the results on ADP-response of platelet-rich plasma after OFTT in patients with metabolic syndrome by Sinzinger et al. [15]. Accordingly, 21 patients would be needed to show a significant difference in platelet activation (given a desired power of 0.8 and a p-value of 0.05) per group.
2.2. Oral fat tolerance test
Study participants underwent three blood drawings and a standardized high-fat meal. The first blood collection was performed after a 10-h fasting period between 7 and 8 a.m. In case patients were on regularly prescribed drugs, such therapy was taken approximately 1 h before the first blood drawing. Subjects consumed a standardized high-fat meal during the oral fat tolerance test (OFTT) directly after the first blood draw. This high-fat meal was served as a milkshake and included 90 g fat, 62,35 g saturated fatty acid, and 42,7 g carbohydrates, with a corresponding caloric intake of 1032,8 kcal. The milkshake was consumed within 10 min under direct staff supervision. Subjects were not allowed to perform any physical activity during the whole course of OFTT and remained mainly in a sitting position. Subjects were not allowed to eat anything else, and only intake of water or nonfatty fluids was permitted during the 5 h after the fat loading. The second and third blood collections were performed three and 5 h after consuming the standardized high-fat meal.
2.3. Analysis of laboratory parameters
Blood samples for routine lipid parameters and blood count were analysed at the general laboratory of the Clinic Ottakring within 1 h after each blood draw. Blood collections were performed using vacuum tubes (Vacuette, Greiner Bio-one GmbH, Germany), and the blood was centrifuged at 5 °C with 4000 rpm for 30 min. A particle-enhanced immunonephelometry (Atellica® NEPH 630 System, Siemens Healthineers, Germany) was used to determine the lipoprotein (a) (lp a) concentration. Total cholesterol, HDL-c, LDL-c, TGs, apolipoprotein A, and apolipoprotein B were determined from serum on a Siemens Dimension Vista platform (Dimension Vista 1500 Intelligent Lab System, Siemens Healthineers, Germany). Absolute leukocyte, neutrophil, lymphocyte and granulocyte counts, and platelet activation markers (mean platelet volume (MPV), platelet distribution width (PDWD), reticulated thrombocytes) were determined based on fluorescence flowcytometry (XN-3000, Sysmex, Germany).
Platelet function was assessed by multiple electrode aggregometry (MEA) using the Multiplate Analyzer system (Roche Diagnostics GmbH, Mannheim, Germany). Blood for the MEA assay was drawn into hirudin-prepared blood tubes (Multiplate® Hirudin Blood Tube, Double-Wall, Roche Diagnostics GmbH, Mannheim, Germany) and was analysed within 10 min after each blood draws from whole blood. The MEA assay detects a change in electrical impedance upon platelet aggregation on metal electrodes placed in the test cuvette. The increase in impedance as aggregation occurs was transformed and is presented as aggregation units (AU) in this study. As agonists in MEA we used adenosine diphosphate (ADP) at a concentration of 6.5 μmol/L (ADP test Reagent Kit, Roche Diagnostics GmbH, Mannheim, Germany), arachidonic acid (ASPI) at a concentration of 0.5 mmol/L (ASPI test Reagent Kit, Roche Diagnostics GmbH, Mannheim, Germany), and thrombin receptor-activated peptide (TRAP) at a concentration of 32 μmol/L (TRAP-6, TRAP test Reagent Kit, Roche Diagnostics GmbH, Mannheim, Germany), respectively.
2.4. Statistical analysis
The chi-square test of homogeneity was used to determine a potential difference between the binomial proportions of three studied groups on a dichotomous dependent variable (baseline characteristics, medication). We used one-way analysis of variance (ANOVA) to determine whether there are statistically significant differences between the means of continuous variables between the groups (baseline characteristic-age). If the data were not normally distributed, the Kruskal-Wallis H test was adopted (BMI). Normality analysis of laboratory values was performed visually through boxplot and the Shapiro-Wilk test. Normally distributed variables are presented as mean ± standard deviation. Not normally distributed variables are shown as median with an interquartile range (IQR). Differences in the laboratory parameters within the groups during the OFTT were determined using one-way repeated-measures ANOVA for normally distributed variables. The Friedman test was used for not normally distributed variables. Furthermore, we used a two-way mixed ANOVA to detect whether there is an interaction between a between-subjects factor (a group with different cardiovascular risk profile) and a within-subjects factor (time during the OFTT) on a lipid profile, inflammation, and markers of platelet activity. We used subjects and time as random factors. If there was a statistically significant difference in proportions during the above-mentioned statistical tests, we used a post hoc test to determine where the differences between these groups or between the different time points lie. Furthermore, Bonferroni correction was applied in pairwise comparison in post hoc analysis.
For all tests, a p-value of <0.05 was considered significant. Calculations were performed using the SPSS 27 statistical package for Macintosh (BM Corp. Released 2020, IBM SPSS Statistics for Macintosh, Version 27.0. Armonk, NY: IBM Corp, United States).
3. Results
3.1. Clinical characteristics
Subjects with CCS + DM2 were the oldest (age 66 ± 7 years), whereas the control group was the youngest (age of 53 ± 5 years). There was a significant difference in the percentage of male individuals between the three groups (p = 0.038), yet no difference was found after a post hoc chi-quadrat comparison between each group. Subjects with CCS with/without DM2 had higher BMI than the control group. Moreover, all patients from the CCS + DM and CCS-DM groups were on antiplatelet monotherapy with acetylsalicylic acid (100 mg daily), and at least 92% of CCS patients were on daily high-dose statin therapy. The baseline characteristics and regular drug intake stratified by the groups are presented in Table 1 and Table 2.
Table 1.
Baseline characteristics of the study population.
| CCS + DM N = 20 | CCS- DM N = 25 | Control group N = 21 | p-value | |
|---|---|---|---|---|
| Age, yrs (mean, ±SD) | 66 (7)a | 61 (7)b | 53 (5)c | < 0.001 |
| Male, n (%) | 14 (70.0) | 18 (72.0) | 8 (38.1) | 0.038 |
| BMI, kg/m2 (median, IQR) | 31.0 (6.2)a | 29.8 (8.0)a | 25.8 (5.6)b | 0.004 |
| Arterial hypertension, n (%) | 20 (100.0)a | 21 (84.0)a | 3 (14.3)b | < 0.001 |
| History of acute coronary syndrome, n (%) | 10 (50.0)a | 19 (76.0)a | 0 b | < 0.001 |
| History of PCI, n (%) | 20 (100.0)a | 24 (96.0)a | 0 b | < 0.001 |
| History of CABG, n (%) | 2 (10.0) | 1 (4.0) | 0 | 0.387 |
| Positive family history for CCS, n (%) | 6 (30.0) | 9 (36.0)a | 0 b | 0.025 |
| Peripheral artery occlusive disease, n (%) | 1 (5.0) | 2 (8.0) | 0 | 0.633 |
| Cerebral occlusive disease, n (%) | 1 (5.0) | 4 (16.0) | 0 | 0.148 |
| Chronic kidney disease, n (%) | 2 (10.0) | 1 (4.0) | 0 | 0.387 |
| Smoking, n (%) | 2 (10.0) | 6 (24.0) | 3 (14.3) | 0.461 |
BMI- body mass index, CABG-coronary artery bypass graft, CCS- chronic coronary syndrome, DM-diabetes mellitus type 2, IQR-interquartile range, PCI- percutaneous coronary intervention, yrs-years, SD - standard deviation.
CCS + DM-subjects with a history of CCS and DM, CCS-DM- subjects with CCS but without DM2,. control group without CCS- control group.
If there was a significant difference, the post hoc analysis was performed. The significant difference in proportion in the post hoc analysis was shown between the groups with different letters (a,b,c).
Table 2.
Medication according to the study group.
| CCS + DM N = 20 | CCS- DM N = 25 | Control group N = 21 | p-value | |
|---|---|---|---|---|
| Beta blockers, n (%) | 17 (85.0) | 20 (80.0) | 1 (4.8) | < 0.001 |
| ACEI/ARB, n (%) | 15 (75.0) | 16 (64.0) | 3 (14.3) | < 0.001 |
| Calcium channel blockers, n (%) | 9 (45.0) | 3 (12.0) | 0 | < 0.001 |
| Diuretics, n (%) | 3 (15.0) | 4 (16.0) | 0 | 0.157 |
| Acetylsalicyl acid, n (%) | 20 (100.0) | 25 (100) | 0 | < 0.001 |
| High-dose statins, n (%) | 20 (100.0) | 23 (92.0) | 0 | < 0.001 |
| Diabetes medication, n (%) | ||||
| Metformin | 19 (95.0) | 0 | 0 | |
| Sulfonylurea | 4 (20.0) | 0 | 0 | |
| Pioglitazone | 1 (5.0) | 0 | 0 | |
| SGLT2-inhibitors | 3 (15.0) | 0 | 0 | |
| GLP-1 RA | 1 (5.0) | 0 | 0 | |
| DPP-4- inhibitor | 6 (30.0) | 0 | 0 | |
| Insulin | 7 (35.0) | 0 | 0 | |
ACEI- angiotensin-converting enzyme inhibitors, ARB- angiotensin II receptor blockers, CCS- chronic coronary syndrome, DM2-diabetes mellitus type 2, DPP4- dipeptidyl peptidase 4, GLP1 RA- Glucagon-like peptide-1 receptor antagonist, IQR-interquartile range, SGLT2-sodium-glucose cotransporter-2, SD - standard deviation.
CCS + DM-subjects with a history of CCS and DM, CCS-DM- subjects with CCS but without DM2,. control group without CCS- control group.
3.2. Lipid profile
3.2.1. Group differences in lipid profile during the whole course of OFTT
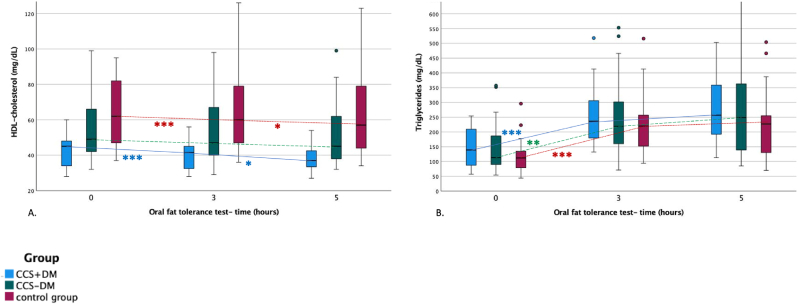
Total cholesterol concentration (TCC), LDL-c concentration, and apolipoprotein B concentration were significantly higher in the control group than in CCS + DM (TCC: +91.6 ± 12.5 mg/dL, p < 0.001, LDL-c: +62.6 ± 10.2 mg/dL, p < 0.001, apolipoprotein B: +0.31 ± 0.7 mg/d, p < 0.001) and CCS-DM (TCC: +60.0 ± 11.86 mg/dL, p < 0.001, LDL-c: +44.1 ± 9.7 mg/dL, p < 0.001, apolipoprotein B: +0.24 ± 0.7 mg/dL, p = 0.002) respectively. HDL-c concentration in CCS + DM was significantly lower compared to CCS-DM (-13.2 ± 5.3 mg/dL, p = 0.045) or the control group (-24.7 ± 5.5 mg/dL, p < 0.001) (Fig. 1A). There was no difference in TG concentration between all three groups (Fig. 1B), (Table 3.). The difference between study groups in total cholesterol, HDL-c and apolipoprotein A remained significant even after adjustment for age and BMI.
Fig. 1.
Lipid profile (A. HDL-c, and B. triglyceride concentration (mg/dL)) during the oral fat tolerance test.
FL-fat loading Blue boxplot-patients with chronic coronary syndrome and diabetes mellitus type 2, green boxplot-patients with the chronic coronary syndrome without diabetes mellitus type 2, red boxplot-control group Dots represent the outliers *- significant difference between two-time points **- significant difference between baseline and time point after 5 h ***- significant changes between baseline and time point after 3 h and after 5 h.
Table 3.
Laboratory values during the oral fat tolerance test.
| Group effect p-value | Time effect p-value | Interaction | |
|---|---|---|---|
| Lipid profile | |||
| Total cholesterol | <0.001 | 0.918 | F (4, 126) = 0.449, p = 0.773, partial η2 = 0.014 |
| HDL-c | <0.001 | <0.001 | F (4, 126) = 0.429, p = 0.788, partial η2 = 0.013 |
| LDL-c | <0.001 | 0.057 | F (4, 126) = 2.365, p = 0.056, partial η2 = 0.070 |
| Triglycerides | 0.406 | <0.001 | F (3.521, 126) = 2.267, p = 0.075, partial η2 = 0.067 |
| Apolipoprotein A | <0.001 | 1.000 | F (3.976, 124) = 0.707, p = 0.588, partial η2 = 0.022 |
| Apolipoprotein B | <0.001 | 0.675 | F (3.805, 124) = 1.183, p = 0.322, partial η2 = 0.037 |
| Inflammation | |||
| Abs. leukocyte count | 0.131 | <0.001 | F (3.626, 124) = 4.280, p = .004, partial η2 = 0.121 |
| Abs. neutrophile count | 0.063 | <0.001 | F (2.927, 124) = 5.892, p = 0.001, partial η2 = 0.160 |
| Abs. lymphocyte count | 0.907 | <0.001 | F (4, 124) = 0.737, p = 0.568, partial η2 = 0.023 |
| Abs. granulocyte count | 0.090 | 0.003 | F (3.850, 122) = 0.331, p = 0.850, partial η2 = 0.011 |
| NLR | 0.674 | <0.001 | F (3.11, 124) = 0.290, p = 0.839, partial η2 = 0.009 |
| Platelet activity | |||
| Abs. Thrombocyte count | 0.525 | <0.001 | F (4, 124) = 1.303, p = 0.273, partial η2 = 0.040 |
| MPV | 0.896 | 0.342 | F (4, 122) = 1.023, p = 0.398, partial η2 = 0.032 |
| PDW | 0.634 | 0.207 | F (4, 124) = 1.022, p = 0.399, partial η2 = 0.032. |
| Reticulated thrombocytes | 0.832 | 0.163 | F (4, 122) = 1.190, p = 0.319, partial η2 = 0.038 |
| ADPtest | 0.021 | 0.003 | F (4, 126) = 0.751, p = 0.555, partial η2 = 0.023 |
| ASPItest | <0.001 | 0.006 | F (4, 126) = 2.149, p = 0.079, partial η2 = 0.064 |
| TRAPtest | 0.037 | 0.001 | F (3.641, 122) = 0.412, p = 0.782, partial η2 = 0.013 |
Abs.-absolute, ADPtest-adenosine diphosphate test performed using Multiplate test, ASPItest-arachidonic acid test performed using multiplate test, HDL-c- high-density lipoprotein cholesterol, LDL-c- low-density lipoprotein cholesterol, MPV- mean platelet volume, NLR-neutrophile-lymphocyte ratio, PDW- platelet distribution width, TRAPtest-thrombin receptor-activated peptide performed using multiplate test.
3.2.2. Postprandial changes in lipid profile
The TCC, LDL-c, apolipoprotein A1, and apolipoprotein B did not change during the OFTT. OFTT caused a significant decrease in HDL-c, with the lowest values observed after 5 h in all groups. HDL-c decreased from 42.1 ± 8.8 mg/dL pre-intervention to 38.8 ± 8.6 mg/dL 5 h after intervention in CCS + DM (p < 0.001). In CCS-DM, HDL-c fell from 49.0 (IQR, 17.6) mg/dL pre-intervention to 45.0 (IQR, 28.0) mg/dL 5 h after intervention (p < 0.001), respectively. In the control group, HDL-c changed from 62.0 (IQR, 37.0) mg/dL pre-intervention to 57.0 (IQR, 36.0) mg/dL 5 h after fat loading (p < 0.001) (Fig. 1A). TG increased significantly with the greatest TG level 5 h after fat loading in all three groups. TG increased from 139.0 (IQR, 124.0) mg/dL pre-intervention to 257.0 (IQR, 185.3) mg/dL 5 h after intervention in CCS + DM (p < 0.001), from 113.0 (IQR, 108.0) mg/dL pre-intervention to 249.0 (IQR, 252.5) mg/dL 5 h thereafter in CCS-DM (p < 0.001), and from 112.0 (IQR, 59.0) mg/dL pre-intervention to 227.0 (IQR, 149.0) mg/dL 5 h after fat loading in the control group (p < 0.001) (Fig. 1B). However, as documented by the interaction terms, we observed no significantly different change in cholesterol values after OFTT among groups (Table 3.).
3.3. Inflammatory markers
3.3.1. Group differences in inflammatory markers during the whole course of OFTT
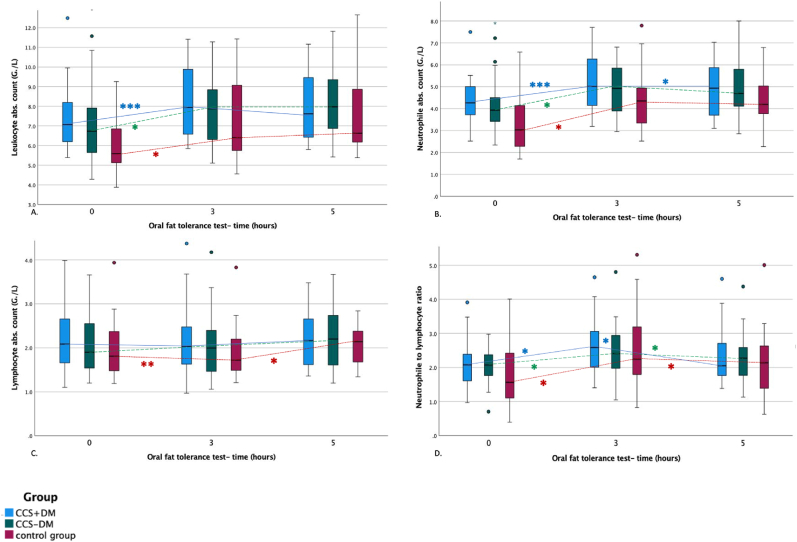
At baseline, leukocyte absolute count was significantly lower in the control group than in CCS + DM (-1.4 ± 0.6 G/L, p = 0.008) and CCS-DM (-1.2 ± 0.6 G/L, p = 0.020). There was no difference in leukocyte count three and 5 h after fat loading between the three groups (Fig. 2. A). The baseline values of neutrophile absolute count before fat loading were significantly lower in the control group than in CCS + DM (-1.08 ± 0.4 G/L, p = 0.008) and CCS-DM (-0.97 ± 0.37 G/L, p = 0.012) (Fig. 2. B). However, after adjustment for age and BMI, there was no difference in leukocyte and neutrophile absolute count between the three groups. There was no significant difference in absolute lymphocyte and granulocyte count and neutrophile to lymphocyte ratio (NLR) between the three groups (Fig. 2. C and Fig. 2D).
Fig. 2.
Postprandial inflammation (A. absolute leukocyte count, B. absolute neutrophile count, C. absolute lymphocyte count (G/L), and D. neutrophile to lymphocyte ratio (%)) during the oral fat tolerance test.
FL-fat loading Blue boxplot-patients with chronic coronary syndrome and diabetes mellitus type 2, green boxplot-patients with the chronic coronary syndrome without diabetes mellitus type 2, red boxplot-control group Dots represent the outliers *- significant difference between two-time points **- significant difference between baseline and time point after 5 h ***- significant changes between baseline and time point after 3 h and after 5 h.
3.3.2. Postprandial changes in inflammatory markers
During OFTT, absolute leukocyte count increased significantly from 7.1 (IQR, 2.1) G/L pre-intervention to a maximum of 7.9 (IQR, 3.5) G/L 3 h after intervention in the CCS + DM (p < 0.001), from 6.7 (IQR, 2.3) G/L pre-intervention to maximum of 7.8 (IQR, 2.9) G/L 5 h after fat loading in CCS-DM (p = 0.001), and from 5.6 (IQR, 2.1) G/L pre-intervention to a maximum of 6.6 (IQR, 3.0) G/L 5 h after intervention in the control group (p < 0.001), respectively (Fig. 2A). Neutrophile absolute count increased significantly during the OFTT from 4.3 (IQR, 1.3) G/L pre-intervention to a maximum of 5.0 (IQR, 2.3) G/L 3 h after fat loading in CCS + DM (p < 0.001), from 4.0 (IQR, 1.0) G/L pre-intervention to a maximum of 4.9 (IQR, 2.1) G/L 3 h after intervention in CCS-DM (p < 0.001) and from 3.3 ± 1.3 G/L pre-intervention to a maximum of 4.5 ± 2.0 G/L 5 h after intervention in the control group (p < 0.001) (Fig. 2B). Absolute lymphocyte count increased significantly during the OFTT only in the control group (Fig. 2. C). Absolute granulocyte count did not change after fat loading in any group. NLR increased during the OFTT with the maximum after 3 h in all three groups, (Fig. 2D, Table 3.). As documented by the interaction terms, we observed significantly different behaviour only in the absolute leukocyte and neutrophile count after OFTT among groups. The change of absolute leukocyte count after fat loading was higher in the control group (1.6 ± 0.25 G/L) than in subjects with CCS-DM (0.8 ± 0.21 G/L) and CCS + DM (1.0 ± 0.24 G/L) (Table 3.).
3.4. Postprandial platelet activity
3.4.1. Group differences in platelet activity during the whole course of OFTT
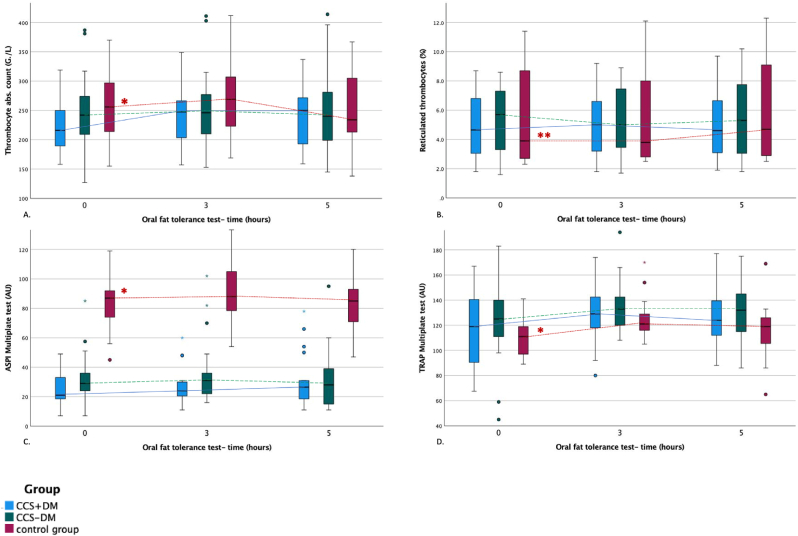
There was no difference concerning the concentration of platelet count, MPV, PDSWD, or reticulated thrombocytes between groups (Fig. 3). However, in response to ADP, platelet activity was significantly lower in the control group than in CCS-DM (-13.12 ± 4.93 AU, p = 0.030). There was no difference between the other groups. Platelet activity in response to ASPI was significantly higher in the control group compared to CCS + DM (+58.73 ± 4.9 AU, p < 0.001) and to CCS-DM (+58.06 ± 4.6 AU, p < 0.001) (Fig. 3. C). This difference remained significant even after adjustment for age and BMI. Platelet activity in response to ASPI was comparable in CCS + DM and CCS-DM. The platelet activity in response to TRAP was significantly lower in the control group than in CCS-DM (-14.6 ± 15.51 AU, p = 0.031) (Fig. 3D). There was no difference between the other groups.
Fig. 3.
Platelet activity (A. thrombocyte absolute count (G./L), B. platelet aggregation measured by ADPtest, C. ASPItest, and D. TRAPtest (AU)) during the oral fat tolerance test.
FL-fat loading Blue boxplot-patients with chronic coronary syndrome and diabetes mellitus type 2, green boxplot-patients with the chronic coronary syndrome without diabetes mellitus type 2, red boxplot-control group Dots represent the outliers *- significant difference between two-time points **- significant difference between baseline and time point after 5 h.
3.4.2. Postprandial changes in platelet activity
A significant increase in platelet activity during OFTT was shown by increased platelet count, reticulated thrombocytes, and increased platelet activity in response to ASPI and TRAP tests in the control group (Fig. 3.B, 3.C, 3.D). However, as documented by the interaction terms, we observed no significantly different change in the markers of platelet activity after OFTT among groups (Table 3.).
4. Discussion
This study aimed to analyse changes in the lipid profile, platelet activity and inflammation during the postprandial phase after a high-fat meal in patients with CCS. We observed 1) a significant decrease of HDL-c and a significant increase of TG in all studied groups, but no difference in the increase between all groups; 2) a postprandial increase in inflammatory markers shown by increased absolute leukocyte and neutrophil counts with significantly higher increase of inflammation in the control group; and c) an increased postprandial platelet activity only in the healthy control group, however no difference in the change of platelet activity among the groups.
Several large population studies revealed that the lipid profile changes minimally after normal food intake. Maximal mean changes for random, non-fasting versus fasting levels are +26 mg/dl for TG, −8 mg/dl for total cholesterol, −8 mg/dl for LDL, +8 mg/dl for remnant cholesterol, and −8 mg/dl for non–HDL. Apolipoprotein B, Lp (a), and HDL cholesterol are largely unaffected [[21], [22], [23], [24], [25]]. Moreover, it is suggested that the slight reduction in total cholesterol, LDL, and non-HDL is caused due to fluid intake and not food intake. Hence, this phenomenon might also occur also during the fasting state, when nonfatty fluids intake is allowed [6]. These results supported the opinion that the non-fasting lipid profile simplifies clinical practice for patients, laboratories, and clinicians without implications for prognostic, diagnostic, and therapeutic options for cardiovascular disease prevention. Therefore, guidelines and statements from many societies recommend the evaluation of lipid profiles in the non-fasting state [[26], [27], [28], [29], [30]]. In our study, HDL decreased, and TG increased significantly during the OFTT while the rest of the lipid profile remained stable irrespective of the present cardiovascular risk profile. However, the change of cholesterol values after the fat loading was not statistically different among the studied groups. Hence, our findings support the evidence mentioned above that the lipid profile should be evaluated in the nonfasting state [[21], [22], [23], [24], [25]]. The significant change in HDL-c in our study could be interpreted by the use of a high-fat meal, whereas the studies from Mora et al. and Lansgted et al. were performed after normal food intake [21,24]. The reason for using a high-fat meal in our study was to achieve the most pronounced changes in the postprandial state. Lower basal levels of lipoproteins in the CCS groups compared to healthy controls in our study were caused by lipid-lowering treatment in the majority of patients.
Postprandial hyperglycemia and hyperlipemia are associated with several chronic systemic low-grade inflammatory states such as obesity, DM2, atherosclerosis, non-alcohol fatty liver disease, and rheumatoid arthritis [31,32]. Indeed, in our study, CCS was associated with higher baseline levels of absolute leukocyte and neutrophil count, with the highest values in CCS + DM patients compared to the control group. Furthermore, fat loading led to a postprandial increase in inflammatory markers, as shown by increased absolute leukocyte and neutrophil count and NLR in the whole study population. Our findings support the outcome of previous studies which demonstrate that a high-fat meal diet causes postprandial inflammatory response associated with elevated levels of interleukin-6, tumor necrosis factor- α, C-reactive protein and leukocyte count [[1], [2], [3]]. However, these studies were performed exclusively on healthy subjects, whereas our study expands the current knowledge on postprandial inflammation in patients at very-high cardiovascular risk. While CCS patients showed a higher level of inflammation compared to the control group, the delta increase in inflammation markers in the postprandial state was significantly higher in the control study group. A possible explanation could be the pleiotropic effects of statins in CCS patients beyond lipid-lowering. Such effects include anti-inflammatory mechanisms, improvement of endothelial function, as well as reduction of antioxidant stress and platelet activation [[33], [34], [35]].
Prior investigations examining the platelet activity in the postprandial state show conflicting evidence [15,16,20,36,37]. The reason for these discrepancies includes differences in methodology, different types of meals with different fat concentrations, as well as different measuring methods or various concentrations of agonists used in the tests, respectively. Furthermore, the type of fatty acid composition plays a substantial role in platelet activation. Saturated fatty acids and trans-fatty acids have been shown to increase platelet aggregation, while cis-, mono-, and polyunsaturated fatty acids have been shown to decrease platelet aggregation [38]. In our study, oral fat loading composed mainly of saturated fatty acids (2/3 saturated fatty acids, 1/3 unsaturated fatty acids) caused no significant change of platelet activity between the groups. This may be surprising since CCS and DM2 are considered to be associated with a higher prothrombotic state expecting also a higher platelet activity in these patients [39,40], but it can be explained by the fact that all CCS patients in our study were on antiplatelet and almost all on lipid-lowering therapy. Our finding is further supported by Oostrom et al., who demonstrated reduced postprandial inflammation and platelet count in the postprandial phase when on rosuvastatin therapy [41].
The findings of our study suggest that the fat loading led to higher postprandial inflammation in the control group. The possible explanation may be the treatment for CCS such as acetylsalicylic acid and statins which may inhibit postprandial inflammation and therefore lead to a more stable postprandial state in patients with CCS ± DM in comparison to a healthy control population. This finding would further strengthen the importance of the CCS therapy. However, such a hypothesis should be proven by studies investigating the OFTT in CCS patients with and without medication. However, such a study would be unethical due to current recommendations for the treatment of CCS [42].
5. Limitations and strengths
Our study's strength was to focus on subjects with high cardiovascular risk and strict supervision during the OFTT. Potential limitations include the relatively small size of the study population. However, the number of subjects included in this study was higher than in other previously published studies focused on the postprandial state. As this study is explorative and hypothesis-generating, the results need to be confirmed in larger studies. Furthermore, the groups in this study are not homogenous. The reason why we included inhomogeneous groups for this study was that most of the previously published studies were performed in healthy subjects and we wanted to show the difference in postprandial reaction between groups with different cardiovascular risks and different baseline therapy to show the real-world differences in the postprandial state. Moreover, Bonferroni correction was not applied, and the generalizability of some results may be questioned. Another potential limitation of our study is the difficulty of generalising the results of this highly controlled laboratory-based trial by use of a standardized very high-fat meal. However, only a standardized high-fat meal can demonstrate potential effects on variables of lipid profile, inflammation and platelet activation, effects that were not shown in previous studies with non-standardized meals.
6. Conclusion
This study showed that fatty meal causes no significant changes in the lipid profile and platelet activity and leads to increased postprandial inflammation in healthy subjects. Despite the higher risk profile, these changes in inflammation were not expressed in CCS ± DM2 patients, which most obviously can be explained by the consequent use of antiplatelet therapy and statins in these patients.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Emerson SR, Kurti SP, Harms CA, Haub MD, Melgarejo T, Logan C, Rosenkranz SK. Magnitude and Timing of the Postprandial Inflammatory Response to a High-Fat Meal in Healthy Adults: A Systematic Review 1-3. Available from: https://academic.oup.com/advances/article/8/2/213/4558153. [DOI] [PMC free article] [PubMed]
- 2.Herieka M., Erridge C. High-fat meal induced postprandial inflammation. Mol Nutr Food Res. 2014;58:136–146. doi: 10.1002/mnfr.201300104. [DOI] [PubMed] [Google Scholar]
- 3.Meessen E.C.E., Warmbrunn M.V., Nieuwdorp M., Soeters M.R. Human postprandial nutrient metabolism and low-grade inflammation: a narrative review. Nutrients [Internet] 2019;11 doi: 10.3390/nu11123000. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/31817857/ [cited 2022 May 13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordestgaard B.G. A test in context: lipid profile, fasting versus nonfasting. J Am Coll Cardiol. 2017;70:1637–1646. doi: 10.1016/j.jacc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Langsted A., Nordestgaard B.G. Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology. 2019;51:131–141. doi: 10.1016/j.pathol.2018.09.062. [DOI] [PubMed] [Google Scholar]
- 6.Simundic A.M., Cornes M., Grankvist K., Lippi G., Nybo M. Standardization of collection requirements for fasting samples: for the working group on preanalytical phase (WG-PA) of the European federation of clinical chemistry and laboratory medicine (EFLM) Clin Chim Acta. 2014;432:33–37. doi: 10.1016/j.cca.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Pedreño J., Hurt-Camejo E., Wiklund O., Badimón L., Masana L. Platelet function in patients with familial hypertriglyceridemia: evidence that platelet reactivity is modulated by apolipoprotein E content of very-low-density lipoprotein particles. Metabolism. 2000;49:942–949. doi: 10.1053/meta.2000.6742. [DOI] [PubMed] [Google Scholar]
- 8.Ravindran R., Krishnan L.K. Increased platelet cholesterol and decreased percentage volume of platelets as a secondary risk factor for coronary artery disease. Pathophysiol Haemostasis Thrombosis. 2007;36:45–51. doi: 10.1159/000112639. [DOI] [PubMed] [Google Scholar]
- 9.Nofer J.-R., Brodde M.F., Kehrel B.E. High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clin Exp Pharmacol Physiol. 2010;37:726–735. doi: 10.1111/j.1440-1681.2010.05377.x. [DOI] [PubMed] [Google Scholar]
- 10.Borén J., Matikainen N., Adiels M., Taskinen M.R. Postprandial hypertriglyceridemia as a coronary risk factor. Clin Chim Acta [Internet] 2014;431:131–142. doi: 10.1016/j.cca.2014.01.015. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/24508990/ [cited 2022 May 29] [DOI] [PubMed] [Google Scholar]
- 11.Keirns B.H., Sciarrillo C.M., Koemel N.A., Emerson S.R. Fasting, non-fasting and postprandial triglycerides for screening cardiometabolic risk. J Nutr Sci. 2021;10:1–14. doi: 10.1017/jns.2021.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmid J.A., Nakamura K., Klop B., Cauduro Oliveira Macedo R., Wu M., Zhao Y., Liu L., Yang S., Liu G., Pan L., et al. Mechanisms of atherosclerosis induced by postprandial lipemia. Front Cardiovasc Med. 2021;1 doi: 10.3389/fcvm.2021.636947. www.frontiersin.org [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazidi M., Valdes A.M., Ordovas J.M., Hall W.L., Pujol J.C., Wolf J., Hadjigeorgiou G., Segata N., Sattar N., Koivula R., et al. Meal-induced inflammation: postprandial insights from the Personalised REsponses to DIetary Composition Trial (PREDICT) study in 1000 participants. Am J Clin Nutr. 2021;114:1028–1038. doi: 10.1093/ajcn/nqab132. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/34100082/ [cited 2022 May 13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulet M.M., Cheillan D., Di Filippo M., Lelekov-Boissard T., Buisson C., Lambert-Porcheron S., Nazare J.A., Tressou J., Michalski M.C., Calzada C., et al. Postprandial triglyceride-rich lipoproteins from type 2 diabetic women stimulate platelet activation regardless of the fat source in the meal. Mol Nutr Food Res. 2020;64 doi: 10.1002/mnfr.202000694. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/32844550/ [cited 2021 Nov 25] [DOI] [PubMed] [Google Scholar]
- 15.Sinzinger H., Berent R. Platelet function in the postprandial period [Internet] Thromb J. 2012;10 doi: 10.1186/1477-9560-10-19. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/22943574/ [cited 2021 Jul 4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyson D.A., Paglieroni T.G., Wun T., Rutledge J.C. Postprandial lipemia is associated with platelet and monocyte activation and increased monocyte cytokine expression in normolipemic men. Clin Appl Thromb Hemost. 2002;8:147–155. doi: 10.1177/107602960200800211. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/12121056/ [cited 2022 Jan 28] [DOI] [PubMed] [Google Scholar]
- 17.Spectre G., Mobarrez F., Stålesen R., Östenson C.G., Varon D., Wallen H., Hjemdahl P. Meal intake increases circulating procoagulant microparticles in patients with type 1 and type 2 diabetes mellitus. Platelets [Internet] 2019;30:348–355. doi: 10.1080/09537104.2018.1445837. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/29547014/ [cited 2021 Nov 25] [DOI] [PubMed] [Google Scholar]
- 18.Schauren B.C., Portal V.L., Beltrami F.G., Dos Santos T.J., Pellanda L.C. Postprandial metabolism and inflammatory markers in overweight adolescents. J Dev Orig Health Dis. 2014;5:299–306. doi: 10.1017/S2040174414000269. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/24965136/ [cited 2021 May 17] [DOI] [PubMed] [Google Scholar]
- 19.Madec S., Corretti V., Santini E., Ferrannini E., Solini A. Effect of a fatty meal on inflammatory markers in healthy volunteers with a family history of type 2 diabetes. Br J Nutr. 2011;106:364–368. doi: 10.1017/S0007114511000286. [DOI] [PubMed] [Google Scholar]
- 20.Bröijersén A., Karpe F., Hamsten A., Goodall A.H., Hjemdahl P. Alimentary lipemia enhances the membrane expression of platelet P- selectin without affecting other markers of platelet activation. Atherosclerosis. 1998;137:107–113. doi: 10.1016/s0021-9150(97)00260-8. [DOI] [PubMed] [Google Scholar]
- 21.Mora S., Rifai N., Buring J.E., Ridker P.M. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993–1001. doi: 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langsted A., Freiberg J.J., Nordestgaard B.G. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation [Internet] 2008;118:2047–2056. doi: 10.1161/CIRCULATIONAHA.108.804146. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/18955664/ [cited 2022 Jan 27] [DOI] [PubMed] [Google Scholar]
- 23.Steiner M.J., Skinner A.C., Perrin E.M. Fasting might not Be necessary before lipid screening: a nationally representative cross-sectional study. Pediatrics [Internet] 2011;128:463–470. doi: 10.1542/peds.2011-0844. [cited 2022 May 13] /pediatrics/article/128/3/463/30693/Fasting-Might-Not-Be-Necessary-Before-Lipid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langsted A., Nordestgaard B.G. Nonfasting lipids, lipoproteins, and apolipoproteins in individuals with and without diabetes: 58 434 individuals from the Copenhagen general population study. Clin Chem. 2011;57:482–489. doi: 10.1373/clinchem.2010.157164. https://academic.oup.com/clinchem/article/57/3/482/5621219 [cited 2022 May 13] [DOI] [PubMed] [Google Scholar]
- 25.Sidhu D., Naugler C. Fasting time and lipid levels in a community-based population: a cross-sectional study. Arch Intern Med. 2012;172:1707–1710. doi: 10.1001/archinternmed.2012.3708. [DOI] [PubMed] [Google Scholar]
- 26.Nordestgaard B.G. Initiative for the EAS (EAS) and the EF of CC and LM (EFLM) joint consensus, Langsted A, initiative for the EAS (EAS) and the EF of CC and LM (EFLM) joint consensus, Mora S, initiative for the EAS (EAS) and the EF of CC and LM (EFLM) joint consensus, Kolovou G, initiative for the EAS (EAS) and the EF of CC and LM (EFLM) joint consensus, Baum H, initiative for the EAS (EAS) and the EF of CC and LM (EFLM) joint consensus, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points—a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J. 2016;37:1944–1958. doi: 10.1093/eurheartj/ehw152. https://academic.oup.com/eurheartj/article/37/25/1944/1749006 [cited 2022 May 13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catapano A.L., Graham I., De Backer G., Wiklund O., John Chapman M., Drexel H., Hoes A.W., Jennings C.S., Landmesser U., Pedersen T.R., et al. ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. https://academic.oup.com/eurheartj/article/37/39/2999/2414995 2016 [cited 2022 May 13] [DOI] [PubMed] [Google Scholar]
- 28.Anderson T.J., Grégoire J., Pearson G.J., Barry A.R., Couture P., Dawes M., Francis G.A., Genest J., Grover S., Gupta M., et al. Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263–1282. doi: 10.1016/j.cjca.2016.07.510. 2016. [DOI] [PubMed] [Google Scholar]
- 29.Leung A.A., Nerenberg K., Daskalopoulou S.S., McBrien K., Zarnke K.B., Dasgupta K., Cloutier L., Gelfer M., Lamarre-Cliche M., Milot A., et al. Hypertension Canada's 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569–588. doi: 10.1016/j.cjca.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 30.Jellinger P.S., Handelsman Y., Rosenblit P.D., Bloomgarden Z.T., Fonseca V.A., Garber A.J., Grunberger G., Guerin C.K., Bell D.S.H., Mechanick J.I., et al. American association of clinical endocrinologists and American college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23:1–87. doi: 10.4158/EP171764.APPGL. [DOI] [PubMed] [Google Scholar]
- 31.Bozzetto L., Annuzzi G., Ragucci M., Di Donato O., Della Pepa G., Della Corte G., Griffo E., Anniballi G., Giacco A., Mancini M., et al. Insulin resistance, postprandial GLP-1 and adaptive immunity are the main predictors of NAFLD in a homogeneous population at high cardiovascular risk. Nutr Metab Cardiovasc Dis. 2016;26:623–629. doi: 10.1016/j.numecd.2016.01.011. https://pubmed.ncbi.nlm.nih.gov/27134062/ [cited 2022 May 17] [DOI] [PubMed] [Google Scholar]
- 32.Tang M.W., Koopman F.A., Visscher J.P.M., de Hair M.J., Gerlag D.M., Tak P.P. Hormone, metabolic peptide, and nutrient levels in the earliest phases of rheumatoid arthritis-contribution of free fatty acids to an increased cardiovascular risk during very early disease. Clin Rheumatol. 2017;36:269–278. doi: 10.1007/s10067-016-3456-x. https://pubmed.ncbi.nlm.nih.gov/27807638/ [cited 2022 May 17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wassmann S. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002;22:300–305. doi: 10.1161/hq0202.104081. [DOI] [PubMed] [Google Scholar]
- 34.Palinski W. New evidence for beneficial effects of statins unrelated to lipid lowering. Arterioscler Thromb Vasc Biol. 2001;21:3–5. doi: 10.1161/01.atv.21.1.3. [DOI] [PubMed] [Google Scholar]
- 35.Undas A., Brummel-Ziedins K.E., Mann K.G. Statins and blood coagulation. Arterioscler Thromb Vasc Biol. 2005;25:287–294. doi: 10.1161/01.ATV.0000151647.14923.ec. [DOI] [PubMed] [Google Scholar]
- 36.Wiens L., Lutze G., Luley C., Westphal S. Platelet count and platelet activation: impact of a fat meal and day time. Platelets. 2007;18:171–173. doi: 10.1080/09537100600930946. [DOI] [PubMed] [Google Scholar]
- 37.Santilli F., Formoso G., Sbraccia P., Averna M., Miccoli R., Di Fulvio P., Ganci A., Pulizzi N., Lattanzio S., Ciabattoni G., et al. Postprandial hyperglycemia is a determinant of platelet activation in early type 2 diabetes mellitus. J Thromb Haemost. 2010;8:828–837. doi: 10.1111/j.1538-7836.2010.03742.x. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/20088941/ [cited 2021 Nov 25] [DOI] [PubMed] [Google Scholar]
- 38.Krekels J.P., Verhezen P.W., Henskens Y.M. Platelet aggregation in healthy participants is not affected by smoking, drinking coffee, consuming a high-fat meal, or performing physical exercise. https://us.sagepub.com/en-us/nam/open-access-at-sage [DOI] [PMC free article] [PubMed]
- 39.Kok M.G.M., Meijers J.C.M., Pinto-Sietsma S.J. Individuals with coronary artery disease at a young age and features of the metabolic syndrome have an increased prothrombotic potential. Thromb Haemost. 2014;111:458–464. doi: 10.1160/TH13-07-0587. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/24306178/ [cited 2022 May 18] [DOI] [PubMed] [Google Scholar]
- 40.Grant P.J. Diabetes mellitus as a prothrombotic condition. J Intern Med. 2007;262:157–172. doi: 10.1111/j.1365-2796.2007.01824.x. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/17645584/ [cited 2022 May 18] [DOI] [PubMed] [Google Scholar]
- 41.Van Oostrom A.J.H.H.M., Plokker H.W.M., Van Asbeck B.S., Rabelink T.J., Van Kessel K.P.M., Jansen E.H.J.M., Stehouwer C.D.A., Cabezas M.C. Effects of rosuvastatin on postprandial leukocytes in mildly hyperlipidemic patients with premature coronary sclerosis. Atherosclerosis. 2006;185:331–339. doi: 10.1016/j.atherosclerosis.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 42.Neumann F.J., Sechtem U., Banning A.P., Bonaros N., Bueno H., Bugiardini R., Chieffo A., Crea F., Czerny M., Delgado V., et al. ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2019;41:407–477. doi: 10.1093/eurheartj/ehz425. https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/31504439/ 2020 [cited 2023 Jun 15] [DOI] [PubMed] [Google Scholar]